Identification of a metabolic bottleneck for cold acclimation in Arabidopsis thaliana
Summary
Central carbohydrate metabolism of Arabidopsis thaliana is known to play a crucial role during cold acclimation and the acquisition of freezing tolerance. During cold exposure, many carbohydrates accumulate and a new metabolic homeostasis evolves. In the present study, we analyse the diurnal dynamics of carbohydrate homeostasis before and after cold exposure in three natural accessions showing distinct cold acclimation capacity. Diurnal dynamics of soluble carbohydrates were found to be significantly different in cold-sensitive and cold-tolerant accessions. Although experimentally determined maximum turnover rates for sucrose phosphate synthase in cold-acclimated leaves were higher for cold-tolerant accessions, model simulations of diurnal carbohydrate dynamics revealed similar fluxes. This implied a significantly higher capacity for sucrose synthesis in cold-tolerant than cold-sensitive accessions. Based on this implication resulting from mathematical model simulation, a critical temperature for sucrose synthesis was calculated using the Arrhenius equation and experimentally validated in the cold-sensitive accession C24. At the critical temperature suggested by model simulation, an imbalance in photosynthetic carbon fixation ultimately resulting in oxidative stress was observed. It is therefore concluded that metabolic capacities at least in part determine the ability of accessions of Arabidopsis thaliana to cope with changes in environmental conditions.
Introduction
As sessile organisms, plants have to adapt to environmental changes that affect their performance, growth and distribution. Low temperature plays a key role in this context, and thus has a great impact on crop productivity (Janskáet al., 2010).
Upon exposure to low non-freezing temperatures, Arabidopsis thaliana, like many other plants, is able to increase its freezing tolerance during a complex process termed cold acclimation. Low temperature influences enzyme-catalysed reactions via thermodynamic effects, slows down transport processes across membranes through reduction of membrane fluidity, and influences various other cellular events. As a consequence, cold acclimation incorporates numerous physiological and biochemical changes (Xin and Browse, 2000; Stitt and Hurry, 2002), and the reorganization of cellular metabolism affects gene expression, protein composition and membrane structure as well as the metabolite profile (Gilmour et al., 2000; Kaplan et al., 2007).
In several studies, it has been shown that acquisition of freezing tolerance is closely linked to changes in the regulation of primary carbohydrate metabolism (Guy et al., 1992; Sasaki et al., 2001; Klotke et al., 2004). During development of A. thaliana leaves in the cold, re-direction of newly fixed carbon towards sucrose synthesis rather than starch accumulation was observed (Strand et al., 1997, 1999), and the resulting higher content of sucrose in cold-acclimated leaves has also been found in various other plants. Sucrose may play a direct role as a cryoprotectant of membranes or more indirectly as a substrate for other cryoprotectants such as raffinose, which was shown to protect the photosynthetic machinery from freezing damage (Knaupp et al., 2011). Because of the complex nature of cold acclimation, a simple correlation between development of freezing tolerance and changes in metabolism cannot be expected (Hannah et al., 2006). Multigenic traits such as cold acclimation thus cannot be explained intuitively based on changes in concentrations of a single or only a few metabolites.
In this context, systems biology offers the possibility of investigating interactions among components of complex biological networks, and helps in elucidating the underlying regulatory mechanisms (Yuan et al., 2008). To study metabolic pathways, representation of enzyme-catalysed reactions by ordinary differential equations has proved to be a versatile tool. In a system of equations representing a metabolic pathway, incorporation of experimentally determined concentrations of metabolites and enzyme parameters can be used not only to validate the model but also to predict the effects that modifications of enzymatic parameters would have on metabolism (Nägele et al., 2010).
In the present study, the behaviour of three accessions of A. thaliana during exposure to low temperatures was examined. Low temperatures have direct thermodynamic effects on metabolism on the one hand, but on the other hand they also trigger cold acclimation. The acclimation capacity of European accessions of Arabidopsis was demonstrated to correlate with minimum habitat temperatures (Hannah et al., 2006). Therefore, we used accessions varying in acclimation capacity in order to allow separation of thermodynamic from acclimation effects. To analyse temperature effects on metabolic regulation, a simplified model of primary carbohydrate metabolism was constructed, comprising interconversions of the most abundant soluble sugars in mesophyll cells (Figure 1). Identification of kinetic parameters for metabolic reactions of primary carbohydrate metabolism was based on metabolite concentrations recorded over complete diurnal cycles. Model simulations revealed significant differences in the capacity for sucrose and raffinose synthesis during cold acclimation. Sucrose synthesis was found to be a metabolic bottleneck under challenging environmental conditions.
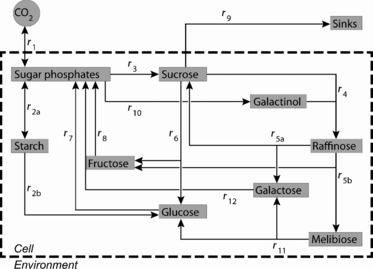
Reaction network of the primary carbohydrate metabolism in leaves of Arabidopsis thaliana. Reaction rates (r) represent central enzymatic steps of carbon assimilation, metabolite interconversion and carbon export.
Results
Net carbon uptake and enzyme activities of central metabolite interconversions
Rates of net photosynthesis were recorded for the three Arabidopsis accessions C24, Col and Rsch over a complete diurnal cycle before and after cold acclimation (Figure 2). Mean rates of CO2 exchange of non-acclimated plants were recorded at 22°C in a greenhouse with natural light supplemented to a minimum of 80 μmol photons m−2 sec−1. The accessions showed similar diurnal dynamics, with a steep increase in CO2 assimilation during the first 3–5 h of the light phase, followed by a decline in the afternoon and a constant nocturnal rate of respiration. Rates of net photosynthesis of cold-acclimated plants were determined at 4°C in a growth chamber with a light intensity of 50 μmol photons m−2 sec−1. A steep increase of CO2 fixation during the first hour of the light phase was followed by a plateau at 25–35 μmol CO2 h−1 g FW−1 until the end of the light period. Dark respiration at 4°C was significantly decreased in all accessions when compared to greenhouse plants (P < 0.05). C24 displayed the highest dark respiration rate (5.7 ± 0.5 μmol CO2 h−1 g FW−1), followed by Rsch (4.8 ± 0.4 μmol CO2 h−1 g FW−1) and Col (4.4 ± 0.1 μmol CO2 h−1 g FW−1).
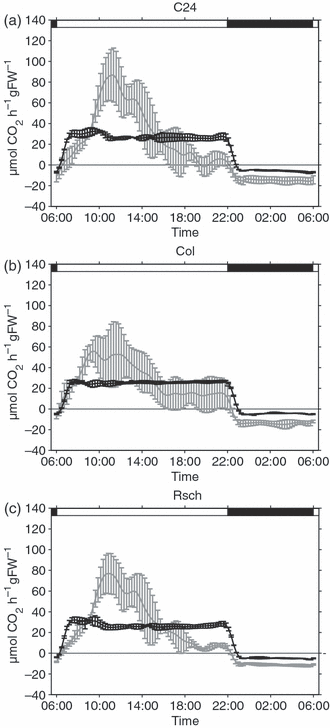
Rates of net photosynthesis. Net carbon uptake was recorded for non-acclimated (grey) and cold-acclimated (black) plants of the Arabidopsis accessions C24 (a), Col (b) and Rsch (c). Day/night transitions are indicated by the bars at the top. Solid lines represent means of three measurements ± SD. Day/night transitions are indicated by the bars at the top.
Enzyme activities catalysing central metabolite interconversions in sucrose and raffinose metabolism were determined every 4 h over a diurnal cycle starting at 06:00 (Figure 3). Activities were determined for non-acclimated and cold-acclimated plants. To enable simulation of biochemical reactions at 4°C, enzyme activities of cold-acclimated plants were determined at 4°C. For non-acclimated plants, sucrose phosphate synthase (SPS), catalysing the synthesis of sucrose-6-phosphate from UDP-glucose and fructose-6-phosphate, showed an increase during the first 4 h in the light, a plateau during the afternoon and a slight decrease during the night in all three accessions (Figure 3a–c). Peak values of approximately 75 μmol fructose-6-phosphate h−1 g FW−1 were reached in Col between 10:00 and 14:00. At 4°C, SPS activities were significantly decreased. Col showed a significantly higher SPS activity than C24 during almost the entire diurnal cycle, while SPS activity in Rsch was significantly higher than in C24 at all time points. Hydrolytic sucrose cleavage in leaves is catalysed by different isoforms of invertase with neutral or acidic pH optima. As all contribute to sucrose cleavage, the sum of neutral, acid vacuolar and cell wall-bound acid invertase activity was determined (Figure 3d–f). C24 showed a mean invertase activity of approximately 300 μmol sucrose h−1 g FW−1, while Col and Rsch displayed invertase activities of 200 μmol sucrose h−1 g FW−1. At 4°C, invertase activity was significantly higher in C24 when compared to Col and Rsch (P < 0.05). The hexose phosphorylation capacity was determined by measuring activities of glucokinase and fructokinase (Figure 3g–l). In non-acclimated plants, hexokinase activities in Col and Rsch increased during the first 8 h of the light phase and declined until 02:00, while activities in C24 were not significantly altered during the diurnal cycle. At 4°C, activities in C24, Col and Rsch decreased to 30–50% and showed a slight dampening of diurnal dynamics. In addition to these central enzyme activities of sucrose metabolism, raffinose metabolism was characterized by measuring activities of raffinose synthase and melibiase, which cleaves off the galactosyl unit from either raffinose or melibiose, i.e. before or after removal of the fructose by invertase. These activities showed very low dynamics and were at least tenfold lower when compared to other enzymes of sucrose metabolism (Figure 3m–r). Activities were similar for all accessions before acclimation, reaching values of 0.05–0.1 μmol sucrose h−1 g FW−1 for raffinose synthase and 0.2–0.3 μmol melibiose h−1 g FW−1 for melibiase, respectively. Melibiase activities further decreased at 4°C to 0.01–0.025 μmol melibiose h−1 g FW−1. Raffinose synthase activity in Rsch at 4°C was higher than in C24 and Col, reaching peak values of 0.05–0.075 μmol sucrose h−1 g FW−1 between 10:00 and 14:00, but did not significantly differ from C24 and Col over the whole diurnal cycle.
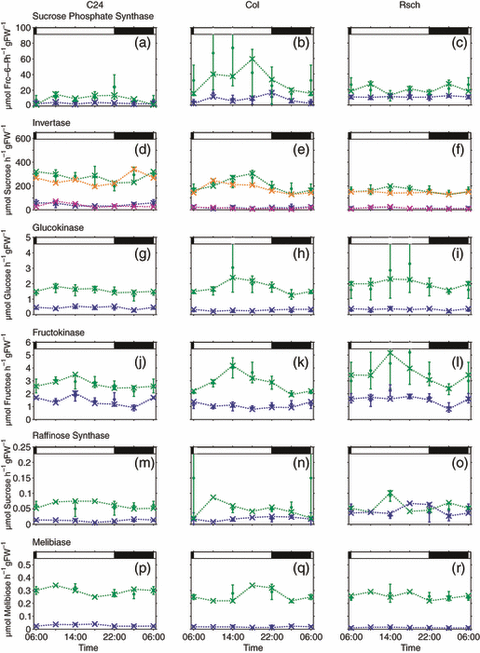
Diurnal dynamics of central enzyme activities. Maximum enzyme activities were experimentally determined for non-acclimated plants (green error bars; n > 3) and for cold-acclimated plants at 4°C (blue error bars; n > 3): sucrose phosphate synthase (a–c), invertase (d–f), glucokinase (g–i), fructokinase (j–l), raffinose synthase (m–o), melibiase (p–r). Green and blue crosses interlinked by dashed lines represent the enzyme activities that yielded the best simulation result for metabolite dynamics in non-acclimated and cold-acclimated plants. Maximum invertase activity was used to simulate degradation of sucrose and raffinose (d–f). Green (22°C) and blue (4°C) error bars represent experimentally determined maximum activities. Dashed lines interlinking green crosses (22°C, sucrose degradation) and blue crosses (4°C, sucrose degradation) yielded the best simulation result for metabolite dynamics. Orange crosses (22°C) and pink crosses (4°C) yielded the best simulation result for metabolite dynamics with respect to raffinose degradation. Day/night transitions are indicated by the bars at the top.
Diurnal metabolite dynamics
In contrast to a mathematical model developed for non-acclimated Col plants that focussed on sucrose metabolism (Nägele et al., 2010), the model presented here also accounts for raffinose metabolism in leaves of Arabidopsis starting from galactinol, which is the first metabolite exclusively dedicated to raffinose synthesis and is easy to measure. This model extension is indispensable for modelling carbohydrate metabolism during cold exposure, because raffinose plays an important role in the metabolic cold response of Arabidopsis thaliana (Klotke et al., 2004; Kaplan et al., 2007; Knaupp et al., 2011). Thus, the extended model comprises ten ordinary differential equations describing time-dependent changes of central metabolites (Figure 1). Except for synthesis and degradation of starch, all metabolite interconversions were described using the Michaelis–Menten equation that represents the dominating rate law in biochemical networks. Raffinose synthesis, catalysed by raffinose synthase, was modelled using a bi-substrate kinetic including concentrations of sucrose and galactinol (Kaplan et al., 2007). The rate of raffinose degradation was determined by allowing two alternative reactions, both involving invertase and melibiase but with different sequential arrangement (Kaplan et al., 2007). Rates of sucrose synthesis, sucrose degradation and hexose phosphorylation were assumed to follow the kinetics of the rate-limiting enzymes sucrose phosphate synthase, invertase and glucokinase/fructokinase, respectively (Sturm, 1999; Strand et al., 2003; Claeyssen and Rivoal, 2007). Experimentally determined Vmax values for these enzymes were used to constrain identification of kinetic parameter sets, which were determined by a process of parameter identification based on diurnal metabolite data (Tables S1–S6). The calculated sets of kinetic parameters and a starting concentration of each of the considered metabolites, pre-set to the experimentaly determined concentrations at timepoint 6:00, were then used to simulate entire diurnal dynamics of metabolite concentrations. This simulation yielded virtual metabolite concentration courses that remained within the standard deviations for most of the experimentally determined concentrations (4, 5).
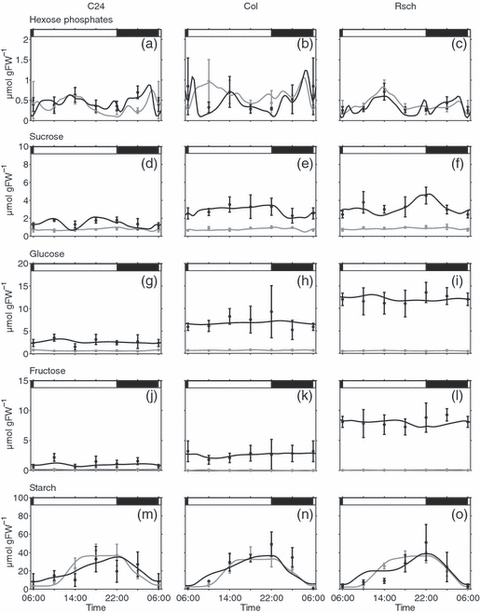
Diurnal concentration changes in sucrose and starch metabolism. Concentrations of hexose phosphates (a–c), as well as sucrose (d–f), glucose (g–i) and fructose (j–l) were experimentally determined for Rsch, Col and C24. Starch content was also determined for a whole diurnal cycle (m–o). Closed circles with error bars represent means of five measurements ± SD. Solid lines indicate the best simulation results for metabolite concentrations for non-acclimated (grey lines) and cold-acclimated plants (black lines). Day/night transitions are indicated by the bars at the top.
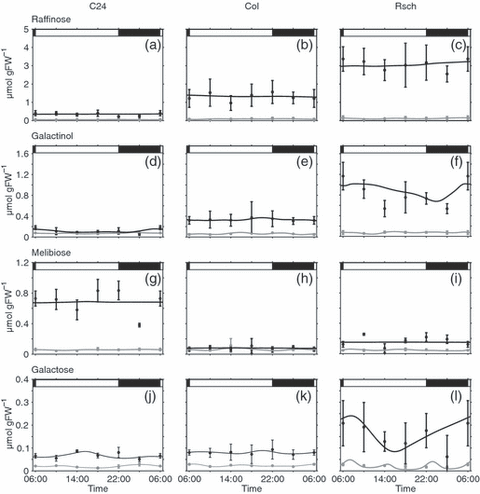
Diurnal concentration changes in the central raffinose metabolism. Concentrations of raffinose (a–c) as well as galactinol (d–f), melibiose (g–i) and galactose (j–l) were determined for Rsch, Col and C24. Closed circles with error bars represent means of five measurements ± SD. Solid lines indicate the best simulation results for metabolite concentrations for non-acclimated (grey lines) and cold-acclimated plants (black lines). Day/night transitions are indicated by the bars at the top.
Diurnal dynamics and concentrations of hexose phosphates were successfully described by the model for all accessions, and were found to be similar in non-acclimated and cold-acclimated plants (Figure 4a–c). In contrast to hexose phosphates, sucrose content increased significantly in cold-acclimated plants, and simulations as well as experimental data showed elevated levels of sucrose in Rsch and Col compared to C24 (Figure 4d–f). Like sucrose, concentrations of glucose and fructose were significantly increased in all accessions after cold acclimation (Figure 4g–l). Mean levels of hexoses were highest in Rsch (glucose, approximately 12.5 μmol g FW−1; fructose, approximately 8 μmol g FW−1) followed by Col (glucose, approximately 7.5 μmol g FW−1; fructose, approximately 2.5 μmol g FW−1) and C24 (glucose, approximately 2.5 μmol g FW−1; fructose, approximately 1.5 μmol g FW−1).
Levels of starch were significantly elevated during the light phase, reaching peak mean values of 50–60 μmol C6 g FW−1 in cold-acclimated Rsch (Figure 4m–o). During the night, starch levels decreased to basal levels of 5–10 μmol C6 g FW−1 in both non-acclimated and cold-acclimated plants. As an immediate product of photosynthesis, transitory leaf starch is only synthesized during the light phase and degraded during the subsequent night to sustain respiration and nocturnal carbohydrate metabolism. As this is a stand-alone bi-directional pathway, we did not perform kinetic modelling of starch metabolism, and instead calculated rates of synthesis (06:00–22:00) and degradation (22:00–06:00) to match the experimentally determined starch levels. Because dark respiration was reduced in cold-acclimating leaves at 4°C, an accumulation of starch would have resulted at low temperature, possibly influencing soluble sugar metabolism (Guy et al., 2008). To minimize such a starch suppression effect, which would not have been accessible to mathematical modelling, light intensities at 20°C and 4°C were adjusted in such a way that differences in diurnal starch content were minimized (see Experimental procedures). Hexose phosphates, representing the output of the Calvin–Benson cycle, were taken as substrates of starch synthesis, while degradation was modelled in two ways, yielding either glucose or hexose phosphates (Zeeman et al., 2007).
Raffinose and galactinol, which have been implicated in cold acclimation in many studies, were significantly increased during cold acclimation in all accessions (Figure 5a–f). Raffinose levels were highest in the cold-tolerant accession Rsch, followed by Col and C24. Galactinol was also most elevated in Rsch. Melibiose, resulting from raffinose degradation by invertase, was most significantly elevated in C24, but Col did not show an increase after cold acclimation. Galactose produced by melibiase-driven degradation of raffinose was significantly elevated after cold acclimation in all accessions, reaching values of 0.2 μmol g FW−1 in Rsch.
Simulated rates of metabolite interconversion
Enzymatic parameters identified in model simulations were used to calculate metabolic flux rates of reactions in sucrose and raffinose metabolism (6, 7). The calculated rate of sucrose synthesis as well as export to sink organs and other metabolic pathways were the most dynamic fluxes in both non-acclimated and cold-acclimated plants (Figure 6, r3 and r9). In the three accessions C24, Col and Rsch, both fluxes significantly increased during the first 4 h of the light phase, followed by a constant decline until the end of the night (Figure 6a–c). In Rsch, r3 and r9 were significantly increased between 22:00 and 02:00 before declining until the end of the night (Figure 6c). Particularly in non-acclimated plants, r9 became negative at the end of the night, indicating a flux of carbon from other metabolic pathways into soluble sugar metabolism. Rates of sucrose cleavage (r6) showed a significant increase during the first 8 h of the light phase, reaching a peak of 4.5 μmol C6 h−1 g FW−1 in the second half of the day, followed by a subsequent decline until the end of the night (Figure 6b). In contrast to Col, such dynamics were not observed in C24 and Rsch, which had rather constant rates of 3 and 4 μmol C6 h−1 g FW−1, respectively, with non-significant oscillations during the night (Figure 6a,c). In all three accessions, the rate of sucrose cleavage exceeded sucrose synthesis at the end of the night, and rates of sucrose synthesis became similar to those of hexose phosphorylation (r7 and r8). Compared to non-acclimated plants, fluxes of sucrose cleavage and hexose phosphorylation were significantly decreased at 4°C (Figure 6d–f). In Col and C24, rates of sucrose synthesis were significantly decreased during the light phase, while nocturnal rates were similar to those of non-acclimated plants. In Rsch, a cold-induced significant decrease was only detectable during the first 4 h of the day (Figure 6f).
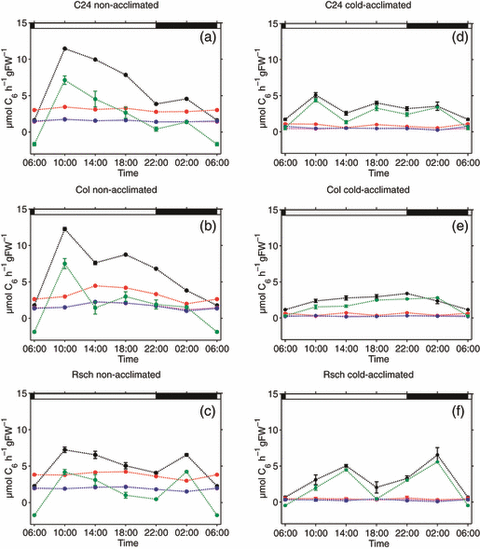
Diurnal dynamics of simulated fluxes in sucrose metabolism. Rates of sucrose synthesis (black), sucrose cleavage (red), sucrose export (green) and glucose/fructose phosphorylation (pink/blue) were determined for non-acclimated (a–c) and cold-acclimated plants (d–f) of C24, Col and Rsch. Closed circles with error bars represent means of 15 simulations ± SD for non-acclimated plants and 20 simulations ± SD for cold-acclimated plants. Day/night transitions are indicated by the bars at the top.
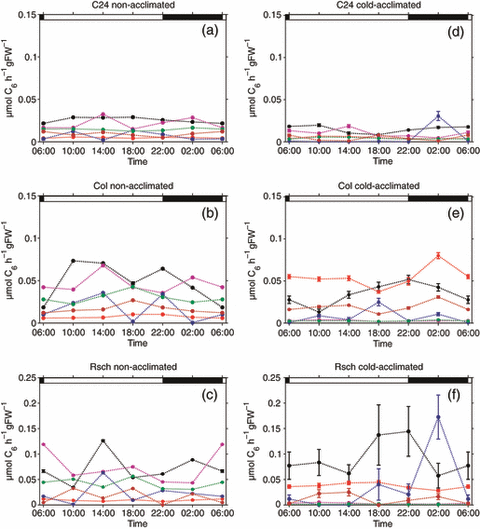
Diurnal dynamics of simulated fluxes in raffinose metabolism. Rates of raffinose synthesis (black), raffinose degradation by melibiase (red) and invertase (pink), galactinol synthesis (blue), melibiose degradation (green) and galactose phosphorylation (brown) were determined for non-acclimated (a–c) and cold-acclimated plants (d–f) of C24, Col and Rsch. Closed circles with error bars represent means of 15 simulations ± SD for non-acclimated plants and 20 simulations ± SD for cold-acclimated plants. Day/night transitions are indicated by the bars at the top.
In comparison to flux rates of sucrose metabolism, those involved in raffinose synthesis and degradation were at least 20 times lower in non-acclimated plants and 5–10 times lower in cold-acclimated plants (Figure 7a–f). Except for Rsch, most fluxes were rather constant during the whole diurnal cycle. The highest values of and most significant changes in rates of raffinose synthesis (r4 and r10) were observed in both non-acclimated and cold-acclimated Rsch plants (Figure 7c,f). As indicated by simulation results, rates of raffinose synthesis were up to 10 times higher in cold-acclimated Rsch plants than in C24 and Col plants.
Simulation of SPS activity at freezing temperatures
To evaluate possible relationships between cold tolerance and regulation of primary carbon metabolism, we compared calculated flux rates for the various accessions using the Vmax values determined for cold-acclimated plants. While flux rates were very similar among the accessions, the proportion of Vmax differed between the cold-sensitive C24 plants and the two tolerant accessions Col and Rsch in the case of SPS, reaching extreme values of 0.5 in C24 and 0.25 in Rsch at 4°C (Figure 8a,b). Because SPS activity in photosynthetically active leaves represents a rate-limiting step in carbon fixation, we thus assumed C24 to be more sensitive towards environmental changes that either cause a reduction in SPS activity or an increase in photosynthetic input of carbon into primary metabolism. A limitation in the capacity of SPS to catalyse sucrose synthesis would result in accumulation of phosphorylated intermediates and cause a disequilibrium between primary and secondary reactions of photosynthesis, promoting generation of reactive oxygen species. To test this hypothesis, we first assessed the impact that a change in temperature would have on the ratio of photosynthetic input and maximum SPS activity by calculating enzyme activities at different temperatures according to the Arrhenius equation (Figure 8c,d). The temperature at which Vmax of SPS would become rate-limiting for carbon fixation was found to be −3 to −5°C in C24, but not in Rsch, at a light intensity of 50 μmol m−2 sec−1 (Figure 8c,d). As reported by Strand et al. (2003), an increase of irradiance from 50 to 200 μmol m−2 sec−1 should result in an increase of CO2 uptake by approximately 25% in cold-acclimated plants. Assuming that the increase of 25% in CO2 uptake affects the rate of sucrose synthesis to the same extent, this would exceed the capacity of SPS in C24, but not in Rsch. Therefore, we exposed plants to temperatures of 4°C and −4°C and light intensities of both 50 and 200 μmol m−2 sec−1 for a period of 4 h (Figure 9). As an indicator of a redox disequilibrium that would trigger an increase in reactive oxygen species scavenging activities, we determined activity of peroxidase that catalyses the disproportionation of H2O2. Although there was no significant difference in peroxidase activity in control experiments at 4°C for both accessions (Figure 9a,b), a significant elevation of peroxidase activity was detected for C24 when the irradiance was elevated at −4°C (P < 0.01; Figure 9c,d). Peroxidase activity in Rsch was not affected by irradiance and was significantly lower at −4°C and 200 μmol m−2 sec−1 than in C24 (P < 0.01).
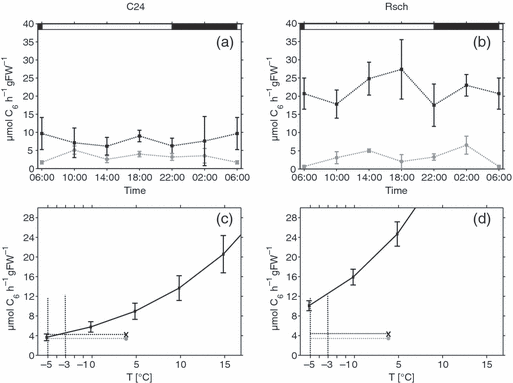
Limitation of sucrose synthesis by SPS activity at low temperature. Diurnal maximum SPS activities (black error bars) were compared to simulated rates of sucrose synthesis (grey error bars) in cold-acclimated plants of C24 (a) and Rsch (b). Mean rates of diurnal sucrose synthesis at 50/200 μmol m−2 sec−1 (grey filled circles/black crosses) were determined for C24 (c) and Rsch (d). Calculated sub-zero SPS activities (black error bars, continuous line) were compared to reaction rates, and the temperature regime at which SPS activity cannot compensate for sucrose synthesis was determined for C24 (black dotted lines). Day/night transitions are indicated by the bars at the top.
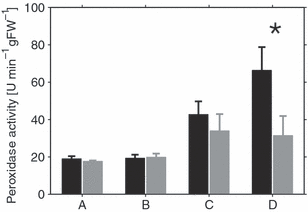
Peroxidase activity as a function of temperature and irradiance in C24 and Rsch. Peroxidase activity was determined for C24 (black bars) and Rsch (grey bars) at an irradiance of 50/200 μmol m−2 sec−1 at 4°C (a, b) and −4°C (c, d). The asterisk indicates significantly different peroxidase activity in C24 and Rsch (P < 0.01).
Discussion
A mathematical model is presented that, using ordinary differential equations, can simulate diurnal dynamics of central carbohydrate metabolism under non-acclimating and acclimating conditions based on identified kinetic enzyme parameters and pre-set starting concentrations of metabolites. The model was used to simulate diurnal dynamics of metabolite concentrations and enzyme activities (6, 7), and the simulations were compared with experimental data (3-5) recorded at six time points during a complete diurnal cycle in three accessions of Arabidopsis that have been shown to represent different cold-acclimation capacities (Hannah et al., 2006). Diurnal profiles of net photosynthesis, i.e. the carbon input function into the model, revealed a significantly higher rate of nocturnal leaf respiration in the cold-sensitive accession C24 compared to rates in Col and Rsch (Figure 2). Although we found a reduction in nocturnal respiration of warm-grown leaves during a 14-day exposure to 4°C as previously described by Talts et al. (2004), this reduction was different for the differentially cold-tolerant accessions. The higher rates of leaf respiration found in the cold-sensitive C24 accession cause larger maintenance costs resulting from an increased number of mitochondria per cell and increased enzyme activities (Miroslavov and Kravkina, 1991; Talts et al., 2004). Talts et al. (2004) proposed this as a mechanism to avoid bottlenecks at near-critical temperatures, allowing rapid reorganization of metabolism. Although C24 was not metabolically impaired at 4°C, it appeared to be closer to a critical temperature than the cold-tolerant accessions. The 50% lethality temperature (LT50) for C24 is only 10°C below the treatment temperature, compared with 15°C for Rsch (Hannah et al., 2006), which may explain the differences in nocturnal leaf respiration rates recorded at 4°C.
Mathematical modelling and simulation of diurnal metabolite dynamics represents an attractive tool for identification of metabolic bottlenecks resulting from temperature constrains on enzymatic turnover rates, because modelling allows a comprehensive estimation of metabolite fluxes that cannot be determined based on metabolite concentrations when cycling and/or redundant pathways are involved. Using modelling, it is possible to compare maximum enzyme activities, which can be assessed experimentally in a statistically robust manner, with rates of metabolite interconversion, which are difficult to determine experimentally. To identify steps in central carbohydrate metabolism that may create bottlenecks at low temperatures, we calculated metabolic flux rates based on kinetic parameters identified in the model simulation, and compared these flux rates with activities of rate-limiting enzymes involved in carbohydrate metabolism. Enzyme activities catalysing hexose phosphorylation and raffinose degradation displayed similar diurnal profiles in all three accessions. Significant differences occurred for SPS, invertase and raffinose synthase. At 4°C, the capacity for raffinose synthesis was highest in Rsch, showing up to tenfold higher rates than C24, and was intermediate in Col (Figure 3). Although raffinose does not appear to contribute to protection of the plasma membrane during freeze–thaw cycles (Zuther et al., 2004), stabilization of the photosynthetic machinery has been shown to play a physiological role in cold tolerance (Knaupp et al., 2011). It has been demonstrated that, under various abiotic stress conditions, raffinose synthesis is stimulated by an elevation of galactinol synthase activity, which provides the substrate for raffinose synthase (Taji et al., 2002). Indeed, concentrations of the precursors sucrose and galactinol were elevated in Rsch, and, under conditions of low raffinose synthase activity, high substrate concentrations may bring about raffinose accumulation via high saturation of the enzyme.
Over-expression of SPS, which catalyses the rate-limiting step of sucrose synthesis in leaves of Arabidopsis, was demonstrated to increase freezing tolerance in the accession Columbia (Strand et al., 2003). Consistently, we found that differences in SPS activity contribute to the differential cold tolerance of natural accessions of Arabidopsis, with the cold-tolerant accession Rsch having significantly elevated SPS activity during the entire diurnal cycle compared to C24 at 4°C (Figure 8). However, in contrast to SPS enzyme activities, flux rates of sucrose synthesis calculated on the basis of model simulations did not differ in cold-acclimated plants of C24, Col and Rsch because of similar rates of net photosynthesis and starch formation, leading to similar concentrations of hexose phosphates, which represent the substrate pool for sucrose synthesis. This demonstrates that, although metabolite levels differ between cold-sensitive and cold-tolerant accessions after 14 days of cold acclimation, the metabolic dynamics are similar and apparently do not correlate with cold tolerance. This is not surprising given that all accessions are chilling-tolerant and are able to establish a metabolic homeostasis that enables them to grow at non-freezing temperatures. However, this finding does not exclude physiological implications of the observed difference in maximum SPS activities of cold-sensitive and tolerant accessions. As already mentioned above, a high capacity of SPS would help to avoid metabolic bottlenecks occurring under rapidly changing environmental conditions. When sucrose synthesis becomes limiting, phosphate limitation of triose phosphate export from the plastids will result, and this must be compensated for by increased starch synthesis in order to prevent a collapse of the Calvin–Benson cycle (Poolman et al., 2000). When starch metabolism cannot compensate for insufficient export of triose phosphates because of low ambient temperature, linear electron transport is impaired (Schneider et al., 2002), and this in turn will result in a redox disequilibrium and potentially in production of reactive oxygen species. It has been demonstrated for Chlamydomonas reinhardtii that a slow down of the Calvin–Benson cycle enhances the extent of photoinactivation of photosystem II (Takahashi and Murata, 2005). This also occurs during exposure to high light, which again causes generation of reactive oxygen species that inhibit the repair of or even inactivate the reaction center of photosystem II (Murata et al., 2007). Our model simulations yielded kinetic parameters for sucrose synthesis in C24 and Rsch that predicted a possible enzymatic constraint at −4°C for C24 but not Rsch. To analyse whether the predicted higher susceptibility of cold-acclimated C24 plants to an overload of photosynthetic input at low temperature could be verified experimentally, cold-acclimated C24 and Rsch plants, which represented the extremes of cold tolerance in this study, were exposed to a temperature that, according to thermodynamic properties, should override the capacity for sucrose synthesis in C24, thus imposing a redox imbalance. We did indeed determine a redox disequilibrium based on induction of peroxidase activity in C24 at a temperature of −4°C and a light intensity of 200 μmol m−2 sec−1, thus verifying model predictions. It is interesting to note that a critical temperature of −4°C was also calculated for C24 based on an independent method, i.e. fluorescence analysis of photosystem II function (Mishra et al., 2011). In that study it was demonstrated that, at −4°C, fluorescence transients of C24 and the tolerant accession Tenela vary significantly, again indicating a disequilibrium of primary and secondary reactions of photosynthesis in C24 at this temperature. However, the critical temperature calculated for Rsch (−15°C) could not be tested, because photosynthetic light reactions are completely abolished at that temperature and absorbed quanta are disipated by fluorescence (Mishra et al., 2011). This implies that, because of the higher enzymatic capacity, Rsch would not encounter a metabolic limitation imposed by sucrose synthesis.
Based on experimental results and model simulations, we therefore conclude that a central feature of cold-tolerant accessions of Arabidopsis is their ability to avoid bottlenecks in central carbohydrate metabolism caused by rapid changes of environmental conditions.
Experimental procedures
Plant material
Arabidopsis thaliana plants of the accessions used in this study were grown in a 1:1 mixture of GS90 soil and vermiculite (Gebr. Patzer GmbH, www.einheitserde.de). Three plants each were raised in 10 cm pots in a growth chamber under 8 h light (50 μmol m−2 sec−1; 22°C)/16 h dark (16°C) for 4 weeks, and then transferred to a greenhouse with a temperature of 22°C during the day (16 h) and 16°C during the night (8 h). In the greenhouse, natural light was supplemented to an intensity of at least 80 μmol m−2 sec−1. The relative humidity was 70%. Plants were watered daily and fertilized every 2 weeks with standard nitrogen/phosphorus/potassium fertilizer. After 42 days, a set of plants was harvested. Leaf samples consisting of one or two rosette leaves (corresponding to approximately 100 mg fresh weight) were randomly taken from nine individual plants grown in three different pots every 4 h during a full day.
Another set of greenhouse-grown plants was shifted after 42 days to a growth chamber with a 16 h/8 h light/dark phase at 4°C, and a light intensity of 50 μmol m−2 sec−1 for 14 days prior to harvesting. Leaf samples were then taken as described above.
At harvesting, the aerial part of the plant was exclusively composed of rosette leaves, allowing direct comparison of metabolite with CO2 exchange data. Leaf samples were weighed, immediately frozen in liquid nitrogen and stored at −80°C until further processing.
Gas exchange measurement
Exchange rates of CO2 were measured using an infra-red gas analysis system (Uras 3 G, Hartmann & Braun AG, Hartmann & Braun AG, www.abb.com). A whole-rosette cuvette design was used as described by Nägele et al. (2010). Gas exchange was measured in the respective growth chambers shortly before plant harvest. Means of raw data for gas exchange were converted to flux rates per g FW (obtained at the end of the exposure by weighing complete rosettes). Experiments were repeated three times for each accession and condition.
Analysis of metabolite concentrations
Frozen leaf samples were homogenized using an MM20 ball mill (Retsch, Retsch GmbH, www.retsch.de). The homogenate was extracted twice in 400 μl of 80% ethanol at 80°C. Extracts were then dried and dissolved in 500 μl water. Contents of glucose, fructose, sucrose and raffinose, as well as galactose, galactinol and melibiose, were analysed via high-performance anion exchange chromatography (HPAEC). A CarboPac PA1 column (glucose, fructose, sucrose, raffinose) or CarboPac MA1 column (galactose, galactinol, melibiose) on a DX-500 gradient chromatography system (Dionex, Sunnyvale, CA, USA, www.dionex.com) coupled with pulsed amperometric detection by a gold electrode was applied.
For starch extraction, pellets from the ethanol extraction were solubilized by incubation in 0.5 m NaOH at 95°C for 30 min. After acidification with 1 m CH3COOH, the suspension was digested with amyloglucosidase for 2 h at 55°C. The glucose content of the supernatant that was subsequently used to assess the starch content of the sample was determined as described below.
Glucose-6-phosphate and fructose-6-phosphate were measured as described previously (Jelitto et al., 1992; Gibon et al., 2002).
Measurement of enzyme activities
Enzyme activities were determined in crude extracts of leaf samples.
For determination of the activities of soluble acid invertase and neutral invertase as well as cell wall-bound invertase, frozen leaf tissue was homogenized in 50 mm HEPES/KOH (pH 7.4), 5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm phenylmethanesulfonyl fluoride, 0.1% Triton X-100 and 10% glycerol. Suspensions were centrifuged for 25 min at 17 000 g and 4°C. The supernatants were used to determine acid and neutral invertase activities as previously described (Nägele et al., 2010).
Activity of sucrose phosphate synthase (SPS) was determined in homogenates of frozen leaf tissue in 50 mm HEPES/KOH (pH 7.5), 15 mm MgCl2, 1 mm EDTA, 2.5 mm dithiothreitol, 1 mm phenylmethanesulfonyl fluoride and 0.1% Triton X-100. Suspensions were centrifuged at 17 000 g and 4°C for 5 min. SPS activity was assayed in supernatants as described previously (Nägele et al., 2010).
Activity of glucokinase and fructokinase was measured as described previously (Wiese et al., 1999) at ambient temperature (22°C) and 4°C.
Activity of raffinose synthase and melibiase was determined in homogenates of frozen leaf tissue in 50 mm HEPES/KOH (pH 7.5), 5 mm MgCl2, 1 mm EDTA, 0.1% Triton X-100, 1 mm phenylmethanesulfonyl fluoride and 0.5 mm dithiothreitol. Suspensions were centrifuged at 17 000 g and 4°C for 5 min. Four volumes of 50 mm HEPES/KOH (pH 7.5), 5 mm MgCl2, 25 mm sucrose, 25 mm galactinol and 0.5 mm dithiothreitol (raffinose synthase), or 50 mm citrate phosphate buffer (pH 5.0), 5 mm MgCl2, 25 mm melibiose and 0.5 mm dithiothreitol (melibiase), were added to samples. After incubation for 20 h at 30°C or 48 h at 4°C, the reaction was stopped by adding 500 μl 100% ethanol. Controls were stopped directly after addition of the extract. The samples were dried and dissolved in 500 μl water. The changes in myo-inositol (in the case of raffinose synthase) and galactose (melibiase) contents were analysed via HPAEC on a DX-500 gradient chromatography system using a CarboPac MA1 column as described above.
Activity of peroxidase was determined in homogenates of frozen leaf tissue in phosphate buffer (pH 7). Homogenates were centrifuged at 1500 g and 4°C for 10 min and 500 μl of the supernatant was incubated with 250 μl of 1% v/v guaiacol. The reaction was started by addition of 125 μl of 0.066% v/v H2O2, and the change in absorption was determined photometrically at 470 nm for 10 min.
Mathematical modelling, parameter identification and simulation
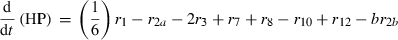 (1)
(1)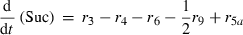 (2)
(2)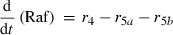 (3)
(3)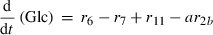 (4)
(4)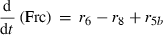 (5)
(5)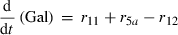 (6)
(6)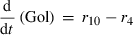 (7)
(7)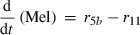 (8)
(8)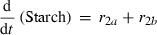 (9)
(9)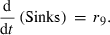 (10)
(10)The rate of net photosynthesis (r1) was approximated by a smoothing spline interpolation of measurements over whole diurnal cycles. It was calculated in units of μmol C1 h−1 g FW−1, and was included in the differential equation for hexose phosphates (HP) with a stoichiometric factor of 1/6 (Eqn 1). Rates of starch synthesis and degradation (r2a and r2b) were determined by identification of interpolation parameters. Interpolation was performed using a cubic spline. Although starch synthesis was unambiguously derived from the pool of hexose phosphates, nocturnal starch degradation was modelled by two alternative pathways yielding either glucose (ar2b) or hexose phosphates (br2b) where a + b = 1. The rate of sucrose export (r9) leaving the system to either sink organs or other metabolic pathways was calculated as the difference between carbon equivalents entering the system by net photosynthesis and the rate of concentration changes of carbohydrate pools.
Alterations of hexose phosphates (HP), sucrose (Suc), raffinose (Raf), glucose (Glc), fructose (Frc), galactose (Gal), galactinol (Gol), melibiose (Mel) depended on the rates of metabolite interconversion ri(t) catalysed by rate-limiting enzymes. Reaction rates were modelled by Michaelis–Menten kinetics. A possible diurnal modulation of substrate affinity in the reactions catalysed by invertase (r5b and r6), glucokinase (r7) and fructokinase (r8) was incorporated via concentration changes of allosteric effectors, which were considered as follows. The reaction rate of sucrose cleavage (enzyme invertase) was modelled as an irreversible Michaelis–Menten enzyme kinetic incorporating mixed inhibition by the products glucose and fructose. Glucose was modelled as a non-competitive inhibitor and fructose as a competitive inhibitor (Sturm, 1999). Reaction rates of glucose and fructose phosphorylation (enzyme hexokinase) were modelled as Michaelis–Menten enzyme kinetics non-competitively inhibited by hexose phosphates (Claeyssen and Rivoal, 2007). The synthesis of raffinose was modelled by a bi-substrate kinetic describing a double-displacement catalytic mechanism as suggested by Peterbauer et al. (2002) for stachyose synthesis A detailed description of reaction kinetics as well as complete model structures are given in Files S1 and S2.
Values of Vmax were considered adjustable during diurnal cycles within the standard deviation of measurements, whereas values of Km were defined as constant. Identification of unknown parameters was performed by minimizing the cost function, i.e. the sum of squared errors between simulated and measured states, by variation of the model parameters. The identification process was performed using a particle swarm pattern search method for bound constrained global optimization as described previously (Vaz and Vicente, 2007).
The model was implemented in the numerical software matlab (version 7.9.0, R2009b) using the software packages ‘systems biology toolbox2’ and the ‘sbpd extension package’ as described previously (Schmidt and Jirstrand, 2006).
Statistics
To determine the minimum number of parameter identification runs needed for a reliable mean value of simulation results, we applied an approach of randomly choosing k out of n calculated flux rates at each of the time points at which metabolite concentrations and enzyme activities were measured. We calculated the mean of the chosen flux rates and repeated the procedure 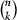 times. Then we built the variance of the
times. Then we built the variance of the 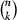 mean values. During the next steps, we chose (k + 1), (k + 2), …, n out of n flux rates. Identification runs were performed until variances did not differ significantly any more. t tests were performed using the matlab software (version 7.9.0, R2009b).
mean values. During the next steps, we chose (k + 1), (k + 2), …, n out of n flux rates. Identification runs were performed until variances did not differ significantly any more. t tests were performed using the matlab software (version 7.9.0, R2009b).
Calculation of sub-zero SPS activities using the Arrhenius equation
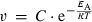 (11)
(11)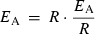 (12)
(12)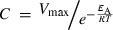 (13)
(13)Using these values of C and EA, we calculated SPS activities by solving Eqn 11 for a temperature regime of 25°C (298.15 K) to −5°C (268.15 K).
Acknowledgements
We would like to thank Annika Allinger for excellent plant cultivation.




