Specific changes in total and mitochondrial proteomes are associated with higher levels of heterosis in maize hybrids
Summary
The phenomenon of hybrid vigor (heterosis) has long been harnessed by plant breeders to improve world food production. However, the changes that are essential for heterotic responses and the mechanisms responsible for heterosis remain undefined. Large increases in biomass and yield in high-heterosis hybrids suggest that alterations in bioenergetic processes may contribute to heterosis. Progeny from crosses between various inbred lines vary in the extent of vigor observed. Field-grown maize F1 hybrids that consistently exhibited either low or high heterosis across a variety of environments were examined for changes in proteins that may be correlated with increased plant vigor and yield. Unpollinated ears at the time of flowering (ear shoots) were selected for the studies because they are metabolically active, rich in mitochondria, and the sizes of the ears are diagnostic of yield heterosis. Total protein and mitochondrial proteomes were compared among low- and higher-heterosis hybrids. Two-dimensional difference gel electrophoresis was used to identify allelic and/or isoform differences linked to heterosis. Identification of differentially regulated spots by mass spectrometry revealed proteins involved in stress responses as well as primary carbon and protein metabolism. Many of these proteins were identified in multiple spots, but analysis of their abundances by label-free mass spectrometry suggested that most of the expression differences were due to isoform variation rather than overall protein amount. Thus, our proteomics studies suggest that expression of specific alleles and/or post-translational modification of specific proteins correlate with higher levels of heterosis.
Introduction
Heterosis or hybrid vigor describes the improved performance of the first filial (F1) generation over their inbred parents in both quantitative and qualitative aspects (Shull, 1908). Heterotic responses are usually quantified in adult plants by measuring differences in biomass, yield, growth rate, flowering time and other traits (Flint-Garcia et al., 2009). However, the phenomenon may be observed early in embryo, seedling and root development (Hoecker et al., 2006; Jahnke et al., 2010). Trait enhancement in a hybrid is often measured in comparison with the mean of two parents or the better parent value (Springer and Stupar, 2007). Heterosis is prevalent in several crops including maize (Zea mays) and is exhibited in several traits (Duvick, 1999; Flint-Garcia et al., 2009). Various organs of the same hybrid exhibit different levels of heterosis, and there is also variation in the amount of heterosis for the same sets of traits among several hybrids. The phenomenon of heterosis has long been harnessed by plant breeders to improve world food production (Duvick, 2001).
Several models have been formulated based on various modes of gene action, such as dominance, overdominance, pseudo-overdominance and epistasis, to explain the genetic basis of heterosis (Lippman and Zamir, 2007). It is widely agreed that parental genetic diversity is important for the extent of the heterotic response. F1 hybrids generated from closely related inbred parents generally show less hybrid vigor than those from more distantly related inbred parents within the same genus (Birchler et al., 2003; Chen, 2010). However, diploid plants exhibit higher heterosis than haploid plants, and polyploids display even higher levels of heterosis than inbred plants or diploid hybrids with similar genetics (Riddle and Birchler, 2008). None of the currently existing hypotheses account for progressive heterosis. Therefore, these models alone are not enough to explain heterosis, and no single unifying mechanism has yet emerged (Birchler et al., 2010; Krieger et al., 2010).
Various studies have been performed to gather information on possible molecular mechanisms correlating with heterosis. Both additive and dominant genetic effects as well as interactions among loci were found by analyzing quantitative trait loci (QTL) associated with heterosis in various plant species, including maize (Frascaroli et al., 2007; Meyer et al., 2010; Schon et al., 2010). Comparative genome analyses of maize inbred lines found a high degree of non-collinearity in their genomes, which has been interpreted as supporting the dominance hypothesis (Fu and Dooner, 2002; Brunner et al., 2005; Springer et al., 2009). Gene expression profiling of selected genes and high-throughput transcriptome analysis showed differential regulation of various genes in diverse hybrids compared to their corresponding parental lines (Swanson-Wagner et al., 2006; Wang et al., 2006; Song et al., 2007; Li et al., 2009; Wei et al., 2009). Some of these studies suggested roles for CO2 assimilation (Bao et al., 2005), energy metabolism (Wei et al., 2009), circadian rhythm (Ni et al., 2009), epigenetic modification and small RNA-directed gene regulation (He et al., 2010). Most studies showed various non-additive and additive gene expression patterns (Guo et al., 2004; Swanson-Wagner et al., 2006; Meyer et al., 2007; Stupar et al., 2007, 2008; Uzarowska et al., 2007; Li et al., 2009; Andorf et al., 2010; Riddle et al., 2010). To summarize, these genetic, genomic and transcriptomic studies compared parental inbred lines to their corresponding F1 hybrids but no uniform global expression patterns were observed that clearly explain heterosis.
The levels of transcripts and protein abundance are not well correlated (Gygi et al., 1999). Moreover, many post-translational modifications are crucial for the regulation of protein function, which may be important for determining heterosis. Therefore, a proteomics approach should be useful in defining the end products of gene expression, i.e. proteins, and thus elucidating the biological/cellular processes underlying heterosis. Previous proteomics studies compared the proteomes of inbred parents to their F1 hybrids. Diverse findings were reported, including non-additive protein accumulation patterns and apparent dominant, overdominant and partially dominant effects. Proteins of various pathways (e.g. carbon, protein and energy metabolism; development; stress and disease responses; signal transduction) were suggested to contribute to heterosis (Hoecker et al., 2006; Wang et al., 2008; Song et al., 2009; Marcon et al., 2010; Fu et al., 2011; Zhang et al., 2012). However, in order to identify those changes that are essential for hybrid vigor, comparative proteomics studies across a variety of hybrids exhibiting various degrees of heterosis are required rather than comparison of inbred plants with their corresponding hybrids. Identification of proteins that are consistently altered in expression across higher-heterosis hybrids may suggest a common machinery that underlies heterosis. The greater biomass and yield of heterotic hybrids in maize suggest that mitochondrial functions may be important in the heterotic response.
Maize is often used as a model plant to study heterosis (Candela and Hake, 2008). A wide range of natural genetic diversity exists, and the B73 inbred line has been sequenced (Flint-Garcia et al., 2005; Schnable et al., 2009). We present an analysis of the proteins present in total extracts and mitochondrially enriched fractions of ear shoots from a series of maize hybrids that differ in their levels of heterosis. Two-dimensional difference gel electrophoresis (2D DIGE) coupled with mass spectrometry (MS) was used to separate and identify heterosis-related proteins, and gel-based label-free MS was used to identify global changes in the overall protein amount of selected proteins revealed by the 2D DIGE analyses.
Results
Three maize hybrids were chosen to differentiate between low and high heterosis based on a number of traits (Flint-Garcia et al., 2009). Each hybrid had the same female parent, B73, but a different male parent. For the low-heterosis hybrid, an inbred related to B73, N192, was used as the male parent (Figure 1a, left panel). For one of the higher-heterosis samples, Mo17 was used as the male parent (Figure 1a, middle panel). The resulting B73 × Mo17 hybrid has been used in most of the previous studies on the molecular basis of heterosis (e.g. Springer and Stupar, 2007). NC350, a tropical line adapted to US growth conditions, was used to generate the second high-heterosis hybrid (Figure 1a, right panel). Figure 1(b) shows a direct comparison of the three hybrids at maturity, and illustrates the differences in heterosis that are the basis of our experiments. It is clear that the B73 × N192 ‘low-heterosis’ hybrids are much shorter and less vigorous than the ‘high-heterosis’ hybrids B73 × Mo17 and B73 × NC350.
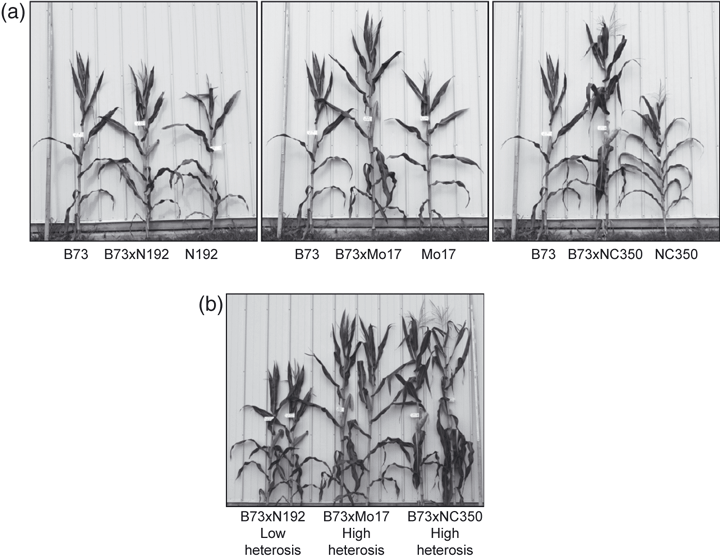
Plant materials used. F1 hybrids were generated by crossing three male parents with a common female parent, B73. (a) Comparison of F1 hybrids with parent inbred plants. The hybrid plant is in the center of each panel, with the B73 female on the left and the male inbred parent on the right. The left panel shows the low-heterosis hybrid (B73 × N192) used in this study, the middle (B73 × Mo17) and right panels (B73 × NC350) illustrate high-heterosis hybrids. (b) Two plants from each hybrid cohort are shown, illustrating the comparisons performed in this study: B73 × N192, our low-heterosis standard, versus B73 × Mo17 and B73 × NC350, the higher-heterosis hybrids.
Whereas previous studies compared inbred parents to their corresponding heterotic hybrids, this study compared a low-heterosis hybrid to two high-heterosis hybrids. However, our ultimate goal was to find common proteins that are altered in higher-heterosis hybrids irrespective of the inbred male parent. To that end, we concentrated our efforts on comparing low- and high-heterosis hybrid plants to identify those changes that may be correlated with high levels of heterosis.
Ear shoots harvested within 2 days of emergence of the first silks were chosen for the total and mitochondrial proteome analyses. Ear shoots develop into fully mature ears following pollination. The size of an ear shoot correlates with the final size of the ear, which is a measurable component of yield (Flint-Garcia et al., 2009). Thus, ear shoot size correlates with yield heterosis. Ear shoots are also very metabolically active, are a good source of highly respiring mitochondria, and have been used in several previous studies of mitochondrial proteins in maize (Newton and Walbot, 1985).
A combination of MALDI-TOF/TOF MS and LC-Q-TOF MS was used to identify differentially expressed 2D gel spots. Due to naming and accession number redundancy in the NCBInr Viridiplantae database, only protein hits corresponding to Zea mays with the highest protein score were reported. If the database search yielded hypothetical proteins, BLASTP (http://blast.ncbi.nlm.nih.gov) searches (Blossum62 matrix) against the NCBInr Viridiplantae database were performed to identify known homologous proteins.
Comparison of total proteins in various hybrids
Total proteins extracted from hybrid ear shoots were separated on 24 cm pH 3–10 immobilized pH gradient (IPG) strips, followed by SDS–PAGE using isocratic (12% acrylamide) gels. In all cases, protein from the low-heterosis hybrid was labeled with Cy3 (false-colored red) and protein from the two high-heterosis hybrids was labeled with Cy5 (false-colored green). Figure 2 shows the comparisons between the low-heterosis hybrid (B73 × N192) and each of the two high-heterosis hybrids, B73 × Mo17 (Figure 2a) and B73 × NC350 (Figure 2b). DeCyder software (http://www.gelifesciences.com/webapp/wcs/stores/servlet/productById/en/GELifeSciences-us/28996435) was used to detect and quantify protein spots on these gels, and a mean of 1052 protein spots were detected in each of the six gels (three biological replicates of low versus B73 × Mo17; three biological replicates of low versus B73 × NC350, Figure S1). The abundance of six protein spots changed consistently in both of the higher-heterosis hybrids compared to the low-heterosis hybrid (2, 3). These spot changes were reproduced in all three biological replicates of each hybrid and were greater than 1.5-fold (Table 1). LC-Q-TOF MS analysis revealed their identity as the translation elongation factor 2 (TEF2) subunit (spot b1), t-complex protein 1 subunit γ (TCP1γ, spot b5), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, spot b12), r40c1-like protein (gi|226507242, spot b15), an unknown protein (spot b16), and glutathione S-transferase III B (GSTIIIB, spot b18). Figure 3 shows magnified images of the total protein gels to further illustrate the changes in protein abundance. The spots labeled b1, b5 and b16 showed decreased expression in both of the high-heterosis hybrids (i.e. were more abundant in the low-heterosis samples). In contrast, spots b12, b15 and b18 showed increased expression in both of the high-heterosis hybrids.
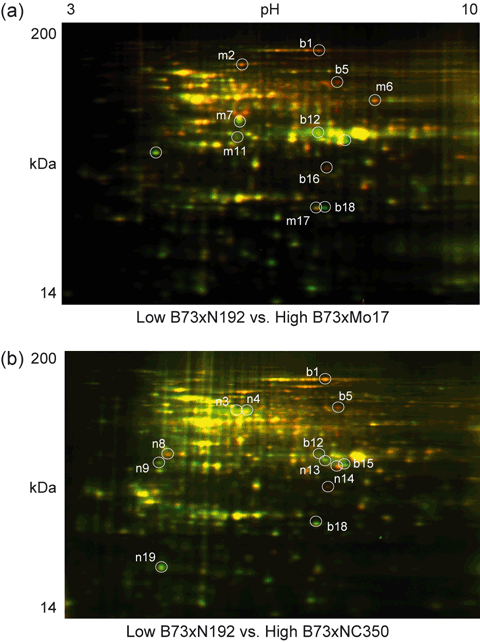
Two-dimensional difference gel electrophoresis of total proteins from ear shoots. Proteins extracted from maize ear shoots were separated using pH 3–10 IPG strips and isocratic (12% acrylamide) SDS–PAGE gels. In all cases, the low-heterosis samples were labeled with Cy3 (false-colored red) whereas the higher-heterosis samples were labeled with Cy5 (false-colored green). Protein spots with a differential abundance ≥1.5-fold that were consistently changed in all three biological replicates are indicated. The green and red spots indicate increased and decreased protein spot abundance with higher heterosis, respectively. Molecular mass (kDa) and isoelectric point (pH) ranges are shown. Protein spots differentially expressed in both high-heterosis hybrids are indicated by the prefix ‘b’, those differentially expressed in high-heterosis B73 × Mo17 hybrids only are indicated by the prefix ‘m’, and those differentially expressed in high-heterosis B73 × NC350 hybrids only are indicated by the prefix ‘n’. (a) Comparison of the low-heterosis standard B73 × N192 with the higher-heterosis hybrid B73 × Mo17. (b) Comparison of the low-heterosis standard B73 × N192 with the higher-heterosis hybrid B73 × NC350.
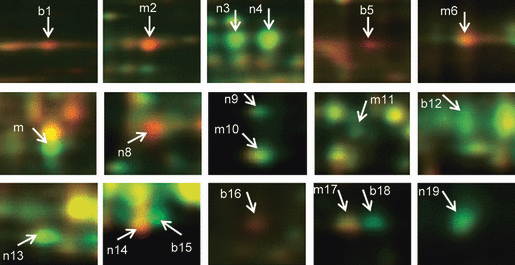
Consistently changed total protein spots chosen for MS identification. Magnified view of individual protein spots showing consistent differential abundance for both high-heterosis hybrid comparisons (spots labeled ‘b’). Spots that are differentially abundant for the B73 × Mo17 comparison only are labeled ‘m’, and those that are differentially abundant for the B73 × NC350 comparison only are labeled ‘n’. The protein identities of these 19 differential spots are provided in Table 1.
| Spot number | Protein name | Chromosomea | Accession number | Experimental pI/Mrb | MS/MS peptidec | Unique peptided | Protein abundance (fold change)e | Localizationf | |
|---|---|---|---|---|---|---|---|---|---|
| High (B73 × Mo17)/low | High (B73 × NC350)/low | ||||||||
| b1 | Translation elongation factor EF2 | 1 | gi|226503399 | 6.9/177.1 | 4 | 3 | −1.55 ± 0.27 | −1.55 ± 0.33 | U/U/C |
| m2 | ATP-dependent CLP protease | 10 | gi|293332601 | 5.9/115.0 | 16 | 0 | −1.51 ± 0.23 | −1.36 ± 0.24 | M/P/P |
| n3 | β-glucosidase | 10 | gi|1352081 | 5.7/71.3 | 4 | 0 | −1.26 ± 0.16 | 1.79 ± 0.30 | P/P/P |
| n4 | β-glucosidase | 10 | gi|1352081 | 5.9/74.6 | 5 | 3 | 1.41 ± 0.17 | 1.50 ± 0.17 | P/P/P |
| b5 | T-complex protein 1γ | 1 | gi|195655091 | 7.2/76.9 | 2 | 2 | −2.34 ± 0.34 | −2.42 ± 0.28 | U/M/C |
| m6 | Serine hydroxymethyltransferase | 4 | gi|195622500 | 7.7/52.0 | 14 | 3 | −1.50 ± 0.09 | −1.06 ± 0.04 | U/U/C |
| m7 | 3-phosphoglycerate kinase, cytosolic | 5 | gi|28172915 | 5.8/44.2 | 15 | 0 | 1.81 ± 0.07 | −0.78 ± 0.39 | S/U/V |
| n8 | [Salt tolerance protein] | 1 | gi|212274681 | 4.8/37.1 | 4 | 4 | −1.07 ± 0.11 | −1.50 ± 0.11 | U/U/C |
| Adenosine kinase | gi|4582787 | 5 | 5 | ||||||
| n9 | Fructokinase 1 | 3 | gi|162460362 | 4.6/33.9 | 13 | 6 | 1.39 ± 0.13 | 1.61 ± 0.12 | S/ER/C |
| n10 | DREPP2 protein | 5 | gi|195635963 | 4.6/31.7 | 8 | 8 | 1.58 ± 0.22 | 1.33 ± 0.15 | S/M/N |
| m11 | Malate dehydrogenase, cytoplasmic | 1 | gi|162464321 | 5.8/35.3 | 2 | 1 | 1.64 ± 0.12 | 0.60 ± 0.82 | U/U/C |
| b12 | [Glyceraldehyde 3-phosphate dehydrogenase] | 4 | gi|238015186 | 6.9/37.8 | 7 | 2 | 2.24 ± 0.43 | 1.65 ± 0.10 | U/M/C |
| n13 | [Malate dehydrogenase] | 6 | gi|226502058 | 6.9/34.1 | 10 | 5 | 1.44 ± 0.17 | 1.68 ± 0.12 | M/M/M |
| n14 | [r40c1-like protein] | 1 | gi|226507242 | 7.1/32.7 | 10 | 10 | −1.43 ± 0.09 | −1.63 ± 0.17 | U/U/C |
| b15 | [r40c1-like protein] | 1 | gi|226507242 | 7.2/33.7 | 11 | 8 | 3.36 ± 0.80 | 2.12 ± 0.20 | U/U/C |
| b16 | Unknown protein | 6 | gi|238012508 | 7.0/28.3 | 3 | 1 | −2.88 ± 0.32 | −3.08 ± 0.12 | M/U/P |
| FH protein interacting protein 1 | gi|195606108 | 3 | 3 | ||||||
| m17 | Glutathione S-transferase 3a | 3 | gi|4468792 | 6.9/22.8 | 4 | 4 | −1.55 ± 0.22 | 00 ± 00 | U/ER/P |
| b18 | Glutathione S-transferase 3b | 3 | gi|162460516 | 7.0/22.9 | 3 | 4 | 3.84 ± 1.33 | 1.62 ± 0.11 | U/ER/P |
| n19 | Actin-depolymerizing factor 3 | 1 | gi|162459533 | 4.7/16.1 | 2 | 0 | 1.34 ± 0.02 | 1.58 ± 0.10 | U/U/P |
- Protein spots differentially expressed in two high-heterosis hybrids compared to a low-heterosis hybrid standard when separated by pH 3–10 IPG strips and isocratic 12% acrylamide SDS–PAGE. Only spots showing ≥1.5-fold increase or decrease in abundance that was consistent in all three biological replicates are shown. Spot numbers correspond to the numbers shown in the gel images (2, 3). Prefixes ‘b’, ‘m’ and ‘n’ are added to each spot number to indicate that they are changed in both higher-heterosis hybrids, only in the B73 × Mo17 high-heterosis hybrid, or only in the B73 × NC350 high-heterosis hybrid, respectively. Protein names inside brackets indicate annotation based on matching orthologous proteins in the NCBI Viridiplantae database using BLASTP. The gene entry identified by the MASCOT search, for which the accession number is provided, was annotated as hypothetical, uncharcterized, or unknown.
- aChromosome location of the maize protein.
- bPhoretix 2D Evolution v2004 software (http://www.nonlinear.com) was used to calculate isoelectric point (pI) and molecular mass (Mr) data for 2D gel spots.
- cNumber of MS/MS peptides matched to the identified protein.
- dNumber of matching peptides unique to the identified protein.
- eFold change of protein abundance in two high-heterosis hybrids relative to the low-heterosis standard. Plus and minus symbols indicate increases and decreases in the abundance of protein, respectively. Values are means ± standard errors.
- fProtein localization predicted using TargetP (http://www.cbs.dtu.dk/services/TargetP), Predotar (http://urgi.versailles.inra.fr/predotar/predotar.html) and WolfPSORT (http://wolfpsort.org): M, mitochondria; S, secretory; C, cytosolic; P, plastid or chloroplast; N, nucleus; V, vacuole; ER, endoplasmic reticulum; U, unknown location.
In addition to the proteins whose level changed in both of the higher-heterosis hybrids, 13 more proteins were differentially accumulated in one or other of the two higher-heterosis hybrids. Six spots (m2, m6, m7, m10, m11 and m17 in Figure 3) were changed only in the B73 × Mo17 hybrid, and seven spots (n3, n4, n8, h9, n13, n14 and n19 in Figure 3) were changed only in the B73 × NC350 hybrid. These changes were consistent across all three biological replicates. The B73 × Mo17 hybrids showed elevation of 3-phosphoglycerate kinase (PGK, spot m7), DREPP2 protein (spot m10) and cytosolic malate dehydrogenase (cMDH, spot m11), and reduction of ATP-dependent CLP protease (spot m2), serine hydroxymethyltransferase (SHMT, spot m6), and GSTIIIA (spot m17). In the B73 × NC350 hybrids, levels of β-glucosidase (spots n3 and n4), fructokinase 1 (FRK, spot n9), mitochondrial MDH (mMDH, spot n13) and actin-depolymerizing factor (ADF3, spot n19) were increased, while level of r40c1-like protein (spot n14) and an uncharacterized maize protein homologous to salt tolerance protein (spot n8) were decreased. Full details of MS identification and spot quantification are provided in Tables S1 and S3.
Analysis of mitochondria-enriched proteomes in various hybrids
Proteins extracted from a mitochondria-enriched fraction of ear shoots displayed a mean of 846 spots in 2D maps in all six gels (Figure 4a,b). In total, 27 proteins exhibited consistently different amounts in the higher-heterosis hybrids compared with the low-heterosis hybrid (Figure 4a,b). The identities of these proteins are shown in Table 2 and magnified images are shown in Figure 5. Fourteen spots (b1, b3, b6, b8, b13–17, b20, b22, b23, b26 and b27) differentially accumulated in the same direction in both of the higher-heterosis hybrids. Nine were increased: an isoform of succinate dehydrogenase flavoprotein (SDH1, spot b3), aldehyde dehydrogenase 2 (ALDH2, spot b6), dihydrolipoamide dehydrogenase (DLDH, spot b8), formate dehydrogenase 1 (FDH1, spot b14), the NADH dehydrogenase (complex I) 24 kDa subunit (spot b20), a lipase domain-containing hypothetical protein (gi|242089565, spot b22), glycine-rich RNA-binding protein 7 (GRBP7, spot b27) and two uncharacterized proteins (spots b13 and b17). Six other proteins were decreased in abundance: the complex I 75 kDa subunit (spot b1), jasmonate-induced protein (JIP, spot b23), GRBP7 (spot b26) and two isoforms of an uncharacterized protein (gi|226507242, spots b15 and b16). In all cases, these spots showed consistent changes across biological triplicate analyses (Figure S2).
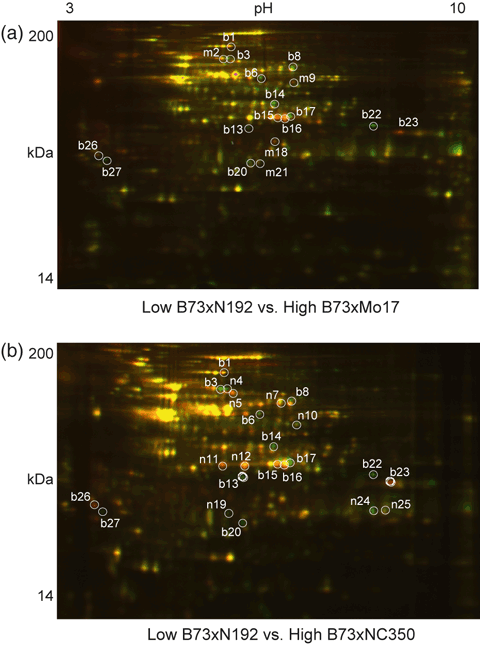
Two-dimensional difference gel electrophoresis of mitochondrial proteins from ear shoots. Mitochondria-enriched proteins isolated from maize ear shoots were separated by 2D DIGE as for total proteins. In all cases, the low-heterosis samples were labeled with Cy3 (false-colored red), whereas the two higher-heterosis samples were labeled with Cy5 (false-colored green). Protein spots with a differential abundance ≥1.5-fold that were consistently changed in all three biological replicates are indicated. The green and red spots indicate increased and decreased protein spot abundance with higher heterosis, respectively. Molecular mass (kDa) and isoelectric point (pH) ranges are shown. Protein spots differentially expressed in both high-heterosis hybrids are indicated by the prefix ‘b’, those differentially expressed in high-heterosis B73 × Mo17 hybrids only are indicated by the prefix ‘m’, and those differentially expressed in high-heterosis B73 × NC350 hybrids only are indicated by the prefix ‘n’. (a) Comparison of the low-heterosis standard B73 × N192 with the higher-heterosis hybrid B73 × Mo17. (b) Comparison of the low-heterosis standard B73 × N192 with the higher-heterosis hybrid B73 × NC350.
| Spot number | Protein name | Chromosomea | Accession number | Experimental pI/Mrb | MS/MS peptidec | Unique peptided | Protein abundance (fold change)e | Localizationf | |
|---|---|---|---|---|---|---|---|---|---|
| High (B73 × Mo17)/low | High (B73 × NC350)/low | ||||||||
| b1* | Ubiquinone oxidoreductase 75 kDa subunit | 5 | gi|195648210 | 5.9/91.5 | 3 | 2 | −1.69 ± 0.05 | −1.54 ± 0.08 | M/M/P/M |
| m2* | Succinate dehydrogenase flavoprotein | 2 | gi|195647178 | 5.7/71.4 | 3 | 3 | −1.50 ± 0.09 | 0.42 ± 0.79 | M/M/M/M |
| b3* | Succinate dehydrogenase flavoprotein | 2 | gi|195647178 | 5.8/70.8 | 3 | 3 | 2.45 ± 0.64 | 4.45 ± 0.80 | M/M/M/M |
| n4 | Succinate dehydrogenase flavoprotein | 2 | gi|195647178 | 5.9/70.8 | 4 | 4 | 1.29 ± 0.08 | 3.55 ± 0.73 | M/M/M/M |
| b5* | Succinate dehydrogenase flavoprotein | 2 | gi|195647178 | 5.9/65.9 | 3 | 3 | −1.25 ± 0.07 | −1.64 ± 0.18 | M/M/M/M |
| b6 | Aldehyde dehydrogenase 2 | 9 | gi|162464260 | 6.3/58.5 | 7 | 1 | 2.16 ± 0.52 | 2.96 ± 0.81 | M/M/M/M |
| n7 | [Dihydrolipoamide dehydrogenase] | 8 | gi|238010756 | 6.7/62.4 | 14 | 1 | −0.53 ± 0.79 | 1.57 ± 0.21 | U/U/C/U |
| b8 | [Dihydrolipoamide dehydrogenase] | 8 | gi|238010756 | 6.9/64.1 | 6 | 1 | 2.05 ± 0.34 | 3.01 ± 0.43 | U/U/C/U |
| m9 | Serine hydroxymethyltransferase | 1 | gi|195622620 | 6.8/56.8 | 10 | 5 | −1.50 ± 0.48 | −0.84 ± 1.07 | M/M/M/M |
| n10 | [Fumarate hydratase1] | 1 | gi|219886541 | 7.0/55.2 | 11 | 6 | −0.35 ± 0.84 | 1.56 ± 0.26 | M/M/P/M |
| n11 | Malate dehydrogenase, mitochondrial | 6 | gi|195628708 | 5.8/40.0 | 4 | 1 | 0.25 ± 0.83 | −1.59 ± 0.17 | M/M/M/M |
| n12* | Malate dehydrogenase, mitochondrial | 6 | gi|195628708 | 6.1/39.6 | 3 | 3 | −0.51 ± 0.77 | −1.57 ± 0.30 | M/M/M/M |
| b13 | Uncharacterized protein | 3 | gi|226533140 | 6.1/36.0 | 2 | 2 | 2.75 ± 0.30 | 5.30 ± 1.67 | U/U/C/U |
| b14 | Formate dehydrogenase 1 | 9 | gi|195640660 | 6.5/46.6 | 4 | 2 | 3.10 ± 0.45 | 4.39 ± 0.48 | M/M/M/M |
| b15 | [r40c1-like protein] | 1 | gi|226507242 | 6.2/39.2 | 14 | 9 | −1.66 ± 0.39 | −1.61 ± 0.18 | U/U/C/U |
| b16 | [r40c1-like protein] | 1 | gi|226507242 | 6.7/39.8 | 16 | 10 | −2.14 ± 0.90 | −2.04 ± 0.20 | U/U/C/U |
| b17 | [r40c1-like protein] | 1 | gi|226507242 | 6.8/40.1 | 11 | 7 | 6.54 ± 1.33 | 4.01 ± 0.94 | U/U/C/U |
| m18 | Unknown protein | 6 | gi|238012508 | 6.5/33.3 | 5 | 3 | −2.31 ± 0.38 | −1.32 ± 0.30 | M/U/P/M |
| n19 | [Ascorbate peroxidase] | 10 | gi|223947673 | 5.97/31.1 | 2 | 1 | 1.34 ± 1.27 | 4.77 ± 1.68 | M/M/P/M |
| b20 | Ubiquinone oxidoreductase 24 kDa subunit | 6 | gi|195638060 | 6.11/30.20 | 4 | 2 | 3.54 ± 0.62 | 3.80 ± 0.41 | M/M/P/M |
| m21 | Ubiquinone oxidoreductase 24 kDa subunit | 6 | gi|195638060 | 6.3/30.4 | 4 | 3 | −1.83 ± 0.19 | −0.45 ± 0.75 | M/M/P/M |
| b22 | Hypothetical protein | 8 | gi|242089565 | 8.1/36.7 | 2 | 2 | 18.33 ± 4.99 | 5.89 ± 2.56 | U/U/C/U |
| b23* | Jasmonate-induced protein | 6 | gi|226494783 | 8.4/35.0 | 4 | 4 | −2.94 ± 1.06 | −2.88 ± 0.56 | P/U/C/U |
| n24 | Voltage-dependent anion channel protein 2 | 6 | gi|162459730 | 8.1/31.4 | 5 | 3 | 0.77 ± 0.99 | 4.27 ± 0.64 | U/U/C/U |
| n25* | Voltage-dependent anion channel protein 1a | 2 | gi|162460904 | 8.3/31.0 | 2 | 2 | −1.41 ± 0.05 | 1.72 ± 0.41 | U/U/C/U |
| b26 | Glycine-rich RNA-binding protein 7 | 10 | gi|226532482 | 3.8/31.8 | 1 | 1 | −1.97 ± 0.19 | −2.21 ± 0.44 | M/M/P/M |
| b27 | Glycine-rich RNA-binding protein 7 | 10 | gi|226532482 | 3.9/30.8 | 1 | 1 | 9.58 ± 0.27 | 5.85 ± 1.55 | M/M/P/M |
- Protein spots differentially expressed in two high-heterosis hybrids compared to a low-heterosis hybrid standard when separated by pH 3–10 IPG strips and isocratic 12% SDS–PAGE. Only spots showing a ≥1.5-fold increase or decrease in abundance that was consistent in all three biological replicates are shown. Spot numbers correspond to the numbers shown in the gel images (4, 5). Prefixes ‘b’, ‘m’ and ‘n’ are added to each spot number to indicate that they are changed in both higher-heterosis hybrids, only in the high-heterosis B73 × Mo17 hybrid, or only in the high-heterosis B73 × NC350 hybrid, respectively. *These gel spots were identified using MALDI TOF-TOF MS + MS/MS. All others were identified with LC-Q-TOF MS + MS/MS. Protein names inside brackets indicate annotation based on matching orthologous proteins in the NCBI Viridiplantae database using BLASTP. The gene entry identified by the MASCOT search, for which the accession number is provided, was annotated as hypothetical, uncharcterized, or unknown.
- aChromosome location of maize protein
- bPhoretix 2D Evolution v2004 software (www.nonlinear.com) was used to calculate isoelectric point (pI) and molecular mass (Mr) data for 2D gel spots.
- cNumber of MS/MS peptides matched to the identified protein.
- dNumber of matching peptides unique to the identified protein.
- eFold change in protein abundance in two high-heterosis hybrids relative to the low-heterosis standard. Plus and minus symbols indicate increases and decreases in the abundance of protein, respectively. Values are means ± standard errors.
- fProtein localization predicted using TargetP, Predotar and WolfPSORT: M, mitochondria; S, secretory; C, cytosolic; P, plastid; U, unknown location.
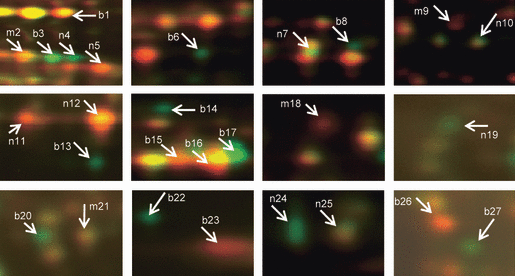
Consistently changed mitochondrial protein spots chosen for MS identification. Magnified view of individual protein spots showing consistent differential abundance for both the high-heterosis hybrid comparisons (spots labeled ‘b’). Spots that are differentially abundant for the B73 × Mo17 comparison only are labeled ‘m’, and those that are differentially abundant for the B73 × NC350 comparison only are labeled ‘n’. The protein identities of these 27 differential spots are provided in Table 2.
Analysis of the mitochondria-enriched proteomes showed that 13 proteins differentially accumulated in one or the other high-heterosis hybrid. Four spots (Figure 5: m2, m9, m18 and m21) showed reduced abundance specifically in the B73 × Mo17 hybrid. They were identified as an isoform of SDH1 (spot m2), SHMT (spot m9), an unknown protein (spot m18) and the complex I 24 kDa subunit (spot m21). Nine spots (Figure 5: n4, n5, n7, n10–12, n19, n24 and n25) were changed in the B73 × NC350 hybrid. Six were increased: an isoform of SDH1 (spot n4), DLDH (spot n7), fumarate hydratase 1 (spot n10), ascorbate peroxidase (spot n19), and voltage-dependent anion channel proteins 2 and 1a (VDAC, spots n24 and n25). Three spots showed decreased abundance: an isoform of SDH1 (spot n5) and two isoforms of mMDH (spots n11 and n12). Full details of MS identification and spot quantification are provided in Tables S2 and S4.
Isoform differences between hybrids
It is clear from the 2D DIGE data that a number of the differentially expressed proteins are represented as isoforms or ‘isoelectric trains’ of spots in which the same protein was identified in multiple spots with different isoelectric points. In order assess changes in overall protein abundance for such proteins in the various hybrids, we decided to use a gel-based label-free approach (‘geLC-MS’). This approach separates proteins by one-dimensional SDS–PAGE (Mr-based separation only) followed by trypsin digestion of excised bands and nanoHPLC MS of the digests. MS/MS spectral counts were examined for the following set of proteins in all hybrids: the top five proteins changed in the total 2D proteome map (Figure 6a), and the top ten proteins changed in the mitochondrial 2D proteome map (Figure 6b). Interestingly, the overall abundance of most of these proteins was not changed at all (Student’s t-test, P ≤ 0.05), particularly for those proteins exhibiting multiple isoelectric isoforms. The following proteins showed no statistically significant changes in overall protein abundance: TCP1, GAPDH, r40c1-like protein, an unknown protein (gi|238012508, spot b16) and GSTIIIA for the total proteome (Figure 6a), and SDH1, ALDH, DLDH, FDH1, r40c1-like protein, the 24 kDa subunit of mitochondria complex I and GRBP7 for the mitochondrial proteome (Figure 6b and Tables S5 and S6). Only the lipase domain-containing hypothetical protein (gi|242089565, spot b22), an uncharacterized protein (gi|226533140, spot b13) and JIP (spot b23) identified on the mitochondria-enriched 2D gels as ‘singletons’ showed concomitant changes in overall protein abundance by geLC-MS. The levels of the lipase-domain-containing hypothetical protein (gi|242089565) and an uncharacterized protein (gi|226533140) were increased whereas that of JIP was decreased in both higher-heterosis mitochondrial proteomes (Figure 6b), and these changes corroborated the 2D gel data (Tables S5 and S6). These differential expression patterns based on isoform differences would not be revealed by many of the standard protein quantification techniques such as Western blotting and geLC-MS. Additionally, assuming in many cases that the isoelectric trains are the result of post-translational modifications, a transcriptomic approach would not reveal these changes either. These points demonstrate the utility of the 2D gels in assessing changes in protein abundance in response to heterosis.
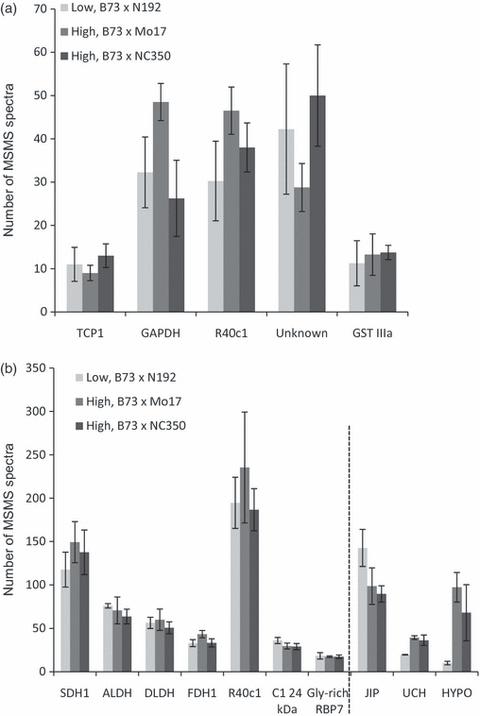
Histograms showing the spectral count quantification of selected differentially abundant proteins in the low-heterosis hybrid and two high-heterosis hybrids. Proteins represented in 2D DIGE spots with greater than twofold changes in protein abundance (see Tables 1 and 2) were chosen for evaluation by geLC-MS. Most do not show gross changes in their overall amounts between the low-heterosis hybrid and both high-heterosis hybrids. Only three mitochondrial proteins show consistent overall changes in protein amounts. The mean spectral counts of biological replicates are shown on the y axis; four replicates for total proteins and three replicates for mitochondria-enriched proteins. Error bars indicate the standard error of the mean. Most of the proteins shown to be differentially expressed as isoforms by 2D DIGE do not show gross changes in overall protein expression. Only three proteins, represented by isolated ‘singleton’ spots, show consistent gross changes in expression in (b), and these are indicated to the right of the dotted line. TCP1, t-complex protein 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; R40c1, r40c1-like hypothetical protein (gi|226507242); Unknown, unknown protein; GSTIIIA, glutathione S-tranferase IIIA; SDH1, succinate dehydrogenase flavoprotein subunit; ALDH, aldehyde dehydrogenase 2; DLDH, dihydrolipoamide dehydrogenase; FDH1, formate dehydrogenase; C1 24 kDa, 24 kDa subunit of complex I; Gly-rich RBP7, glycine-rich RNA binding protein 7; JIP, jasmonate-induced-protein; UCH, uncharacterized protein; HYPO, lipase domain-containing uncharacterized protein. (a) Data for the five proteins in total protein samples showing the greatest differential abundances on 2D DIGE gels. (b) Data for the top ten proteins present as differentially expressed spots in mitochondria-enriched 2D DIGE.
Discussion
We examined the total and mitochondrial proteomes for three F1 hybrids and identified proteins that are changed consistently in two high-heterosis hybrids, B73 × Mo17 and B73 × NC350, relative to a low-heterosis hybrid, B73 × N192. The selection of suitable samples is important because the level of heterosis may vary not only with different tissues/organs but also with their developmental stages (Flint-Garcia et al., 2009). Ear shoots were chosen because their sizes correlate well with final ear sizes and hence yield. The 2D DIGE proteome maps showed that only 2–3% of all the observed protein spots showed changed expression in the high- versus low-heterosis comparisons. Some of the differentially expressed proteins were found to be unique to one of the higher-heterosis hybrids. This probably reflects alleles from the inbred parent. Interestingly, many of the differentially accumulated spots had a common expression pattern in both of the higher-heterosis samples, despite their having genetically distant male parents (Mo17 and NC350), and these are the focus of this paper. Compared with the total proteome analyses, we observed more differentially expressed proteins in the mitochondria-enriched samples. The enrichment of mitochondria before proteomic analysis enabled identification of mitochondrially targeted proteins that were not detected in the unfractionated total proteome samples. Additionally, mitochondrial respiration is hypothesized to play crucial role in heterotic plants, so it was important to examine the mitochondrial proteome.
Central carbon metabolism and stress-responsive proteins show altered expression in the higher-heterosis hybrids
Several enzymes that are components of glycolysis, the tricarboxylic acid (TCA) cycle, and the electron transport chain (ETC.) were differentially expressed in the higher-heterosis hybrids. Expression changes for proteins in these pathways suggest that heterotic plants may require optimized levels of such enzymes to produce sufficient energy for increased growth. Our findings are consistent with the hypothesis that multigenic heterosis involves energy-use efficiency (Goff, 2011).
The levels of three cytosolic glycolytic enzymes (FRK, GAPDH and PGK) were increased in the B73 × NC350 high-heterosis hybrids in the total protein analysis, and GAPDH levels were also increased in the other high-heterosis hybrid (B73 × Mo17). Increased levels of a number of glycolytic enzymes presumably increase the overall pool of pyruvate. Correspondingly, analysis of mitochondrial proteomes showed that several proteins in the oxidative phosphorylation (OXPHOS) pathway and the TCA cycle, such as complex I subunits, SDH1, fumarate hydratase 1, MDH, DLDH and FDH, differentially accumulated in the higher-heterosis plants.
Mitochondrial FDH transcripts were found to be induced in response to various stresses in potato leaves, and the FDH/SHMT expression ratio was also changed (Hourton-Cabassa et al., 1998). Therefore, it is possible that the increase in an FDH isoform (and relative decrease in SHMT) that we observed with higher levels of heterosis represents ‘priming’ of the field-grown plants for stress, resulting in increased vigor.
Additionally, several other stress-responsive proteins were differentially expressed in the higher-heterosis plants, including GSTIIIA and B, ascorbate peroxidase, mitochondrial ALDH, β-glucosidase, ADF3, VDAC proteins, GRBP7, r40c1-like protein, a salt tolerance protein, TCP1, JIP and a lipase domain-containing hypothetical protein. These proteins have been identified as stress-responsive in a number of studies (El-Hafid et al., 1989; Ouellet et al., 2001; Wang and Pesacreta, 2004; Dooki et al., 2006; Kim et al., 2008; Lee et al., 2009; Gill and Tuteja, 2010; Pechanova et al., 2010). GAPDH transcripts were also shown to be induced under environmental stress (Laxalt et al., 1996).
An abundant ‘uncharacterized’ protein was present in both total and mitochondrial proteomes, and was changed similarly in both of the high-heterosis hybrids. BLASTP analysis of this protein sequence demonstrated significant homology to an r40c1-like protein. It contains ricin B lectin domains and was also identified in the rachis proteome of maize (Pechanova et al., 2010). A similar protein was identified in the mitochondrial proteome of rice (Wei et al., 2010), and also in rice as a drought-responsive phosphoprotein (Ke et al., 2009). In addition, our analysis of publically available transcript data (using genevestigator, www.genevestigator.com) showed that RNAs corresponding to the maize r40c1-like protein are increased under stress. Our studies suggest that isoform modulation of the maize r40c1-like protein is correlated with heterosis, which may point to a priming of the plants for stress.
Plant productivity is related to the ability of plants to respond and adapt to environmental stresses (Sachs and Ho, 1986). Heterotic plants are fitter than their corresponding inbred lines, which may be due to increased plasticity of these hybrids towards diverse environmental variations. Identification of various stress-responsive proteins as differentially expressed in higher-heterosis plants indicates that these stress-responsive proteins may contribute to better adaptation of the higher-heterosis plants to various stresses.
Is heterosis related to the choice of a ‘better’ isoform?
In both total and mitochondria-enriched protein gels, several differentially expressed proteins were identified by multiple spots. These include β-glucosidase (two spots) and the r40c1-like protein (two spots) in total protein samples. The r40c1-like protein was also identified as three spots in the mitochondrial proteome. Additionally, multiple spots were observed for the following proteins in the mitochondrial proteome: SDH1 (four spots), DLDH (two spots), mMDH (two spots), the complex I 24 kDa subunit (two spots), VDAC (two spots) and GRBP7 (two spots). Most of them represent isoelectric isoforms of these proteins.
The collection of spots identified as SDH1 (as well as the r40c1-like protein discussed above) is particularly interesting, as some electrophoretic isoforms were decreased whereas others were increased. SDH1 is a core subunit of complex II, which couples the oxidation of succinate and the reduction of quinone, and therefore plays key roles in both the TCA cycle and the ETC. There are two genes encoding SDH1 in Arabidopsis, but only the product of the SDH1-1 gene has been found in complex II (Huang et al., 2010). Studies have revealed that disruption of the SDH1-1 gene results in defective gametophyte development, pollen abortion and reduced seed set in Arabidopsis (Leon et al., 2007). SDH1 gene redundancy has also been reported for maize (Eprintsev et al., 2010). SDH1 is phosphorylated in both potato and Arabidopsis and appears as multiple isoforms in 2D gels (Bykova et al., 2003). It is interesting that isoform modulation of SDH1 appears to be correlated with differences in heterosis levels in maize.
There are several reasons for the occurrence of multiple spots in 2D gels. First, post-translational modification of the same gene product may result in multiple forms of a protein. These modifications often alter the properties of proteins and may be affected by physiological conditions (Seo and Lee, 2004). Acquisition of such altered functional properties may be important for heterosis. Second, different versions of the same gene (allelic variants) may encode proteins with variations in amino acid sequence and expression patterns. Finally, duplicate genes may also encode similar proteins with altered activities or expression patterns. Maize is known to have functional duplications for many enzyme-coding loci because it is a segmental allotetraploid (Gaut and Doebley, 1997).
Allele-specific expression at the RNA level has been reported in hybrid rice and maize (Stupar et al., 2007, 2008; Guo et al., 2008). In addition, allelic interaction at some loci causes gene expression in the hybrid to deviate from simple additive allelic expression patterns of the parents (Birchler et al., 2003; Gibson and Weir, 2005).
Multimeric enzyme complexes are thought to be important in heterotic responses. A number of mitochondrial proteins found to be changed in the current study are members of multiprotein complexes, including SDH1 of the mitochondrial ETC complex II and the 24 kDa subunit of complex I. Expression of specific isoforms of these proteins and others is clearly evident from our data. The presence of a specific isoform in a complex may confer altered activity or stability on the entire complex (Veitia and Vaiman, 2011).
Several of the proteins for which isoforms were seen on 2D gels were examined for overall abundance using a label-free mass spectrometry method (geLC-MS). Interestingly, the overall abundance of these proteins was not significantly changed (Figure 6a,b). From a technical viewpoint, observation of these hybrid-specific isoform expression patterns would be impossible without 2D gel-based experiments. Many of the proteins reported herein would be missed using traditional quantitative methods such as Western blotting and geLC-MS, which measure gross changes in protein abundance. Our results suggest that it is not simply the case that higher-heterosis hybrids have more of a certain protein, but that specific isoforms confer changes in protein properties that contribute to increased vigor. Three singleton proteins (JIP, the lipase domain-containing uncharacterized protein, and another uncharacterized protein) showed clear differences in amounts both in 2D DIGE and geLC-MS. It is interesting that two of the three proteins that differ quantitatively by the two methods employed are currently uncharacterized proteins. These singletons, as well as those isoforms that are altered similarly in different heterotic hybrids, may provide clues as to the basis of heterosis, as well as serving biomarkers of heterosis, and are the subject of ongoing experiments in our laboratory.
Conclusions
Heterosis remains a complex and poorly explained phenomenon despite multiple attempts at the genetic, genomic, transcriptomic and proteomics levels to understand the fundamental principle(s) driving increased vigor. To date, no specific biochemical pathways have been established that reveal a direct connection to multigenic heterosis. However, based on our data, we propose that efficient energy metabolism and stress response mechanisms are important factors in heterosis, as some components of these pathways showed consistently different expression in higher-heterosis hybrids compared to the low-heterosis hybrid. A broad pool of energy metabolism proteins involved in glycolysis, the TCA cycle, photorespiration and the ETC. were all affected in higher-heterosis hybrids, showing their importance in heterosis. The differential abundance of various stress-related and detoxifying proteins indicates the role of better adaptation in higher-heterosis hybrids. The abundance of a few hypothetical proteins of as yet unknown function is also correlated with heterosis, and these are worthy of further study to determine their functions and amounts in a number of different heterotic hybrids. Moreover, identification of similar sets of proteins that consistently changed with similar trends in the two higher-heterosis hybrids suggests that these proteins may be correlated with the phenomenon of heterosis. The fact that the higher-heterosis hybrids studied herein have distantly related male parental lines also suggests that these differentially accumulated proteins have the potential to be used as ‘biomarkers’ for heterosis. Such biomarkers could be used as a basis for assessing ‘heterotic potential’ at very early stages of development, which may speed progress in maize crop improvement.
Experimental procedures
Chemicals
All chemicals were from Fisher Scientific (http://www.fishersci.com) unless stated otherwise.
Plant materials
Maize (Zea mays L.) seeds (fully mature ear kernels) from hybrids B73 × N192, B73 × Mo17 and B73 × NC350 obtained from S. Flint-Garcia (Division of Plant Sciences, University of Missouri, and USDA/ARS, Columbia, MO, USA) were grown during the summers of 2010 and 2011 on the Genetics Farm of the University of Missouri. These hybrids were representative of the most consistent hybrids in a large heterosis phenotyping study, showing similar expression for a number of heterotic traits across a variety of environments (Flint-Garcia et al., 2009). The common female inbred line was the sequenced line B73 (Schnable et al., 2009), a stiff stalk line. The inbred line N192, another stiff stalk line (Flint-Garcia et al., 2005), was used as the male parent to generate a hybrid exhibiting very little heterosis. The inbred line Mo17 (a non-stiff stalk, NSS variety) gives a vigorous, high-yielding hybrid with B73 that has often been used as the ‘standard’ for studies of heterosis. Crosses of B73 with NC350, a tropical germplasm (and a founder line in nested association mapping populations), gave even greater increases in plant height compared with the mean of both its parents. Thus, in the present study, the B73 × Mo17 and the B73 × NC350 F1 plants are considered to be ‘high-heterosis’ hybrids. They were compared with the B73 × N192 ‘low-heterosis’ hybrid.
All samples were collected from the field in the morning (9–10 am). The husk leaves and silks were removed from the ear shoots prior to protein extraction. Three ear shoots were combined to make one biological replicate in order to reduce any individual plant variation and to increase the accuracy of the results. Each member of a pooled sample was taken from different plants in the same family, i.e. plants grown from kernels of the same ear. Three biological replicates of each hybrid (B73 × N192, B73 × Mo17 and B73 × NC350) were analyzed. In total, at least nine ear shoots from the three hybrids comprised the three biological replicates.
Total protein extraction
Ear shoots were harvested within 2 days of the first appearance of silks, frozen immediately in liquid nitrogen and transported from the field on dry ice. Three ear shoots (per biological replicate) were ground to a fine powder with a mortar and pestle using liquid nitrogen, and 1 g of the powder was used for protein extractions. The extraction was performed using a Tris-buffered phenol/methanol/ammonium acetate method (Mooney et al., 2006) modified from the original protocol (Hurkman and Tanaka, 1986). The resulting protein pellet was stored in acetone/dithiothreitol at −80°C until further analysis.
Mitochondrial isolation
Mitochondrial isolations from fresh ear shoots were begun within 2 h of harvesting. Approximately 60 g of starting material combining three to four ear shoots were used. A differential centrifugation and sucrose gradient method was used (Stern and Newton, 1985). The mitochondria recovered from the 35–47% sucrose interface after ultracentrifugation were diluted, pelleted, washed and stored at −80°C as previously described (Cooper et al., 1990).
Two-dimensional difference gel electrophoresis (2D DIGE)
Both the total protein and mitochondrial protein pellets were dissolved in DIGE lysis buffer (7 m urea, 2 m thiourea, 4% w/v CHAPS and 20 mm Tris, pH 8.5), and, following 1 h solubilization, were centrifuged at 16 000 g at 4°C for 15 min to remove insoluble particles. Protein concentration was estimated using an EZQ protein quantification kit according to the manufacturer’s instructions (Molecular Probes, http://www.invitrogen.com/site/us/en/home/brands/Molecular-Probes.html). An FLA5000 laser scanner and Multigauge software version 2.0 (Fujifilm, http://www.fujifilm.com/products/life_science_systems/science_imaging/multigauge/) were used for imaging and analysis of EZQ data. Protein concentration was adjusted to 5 μg μl−1 with DIGE lysis buffer. Each replicate from the low-heterosis hybrids was labeled with Cy3. Those from two high-heterosis hybrids were labeled with Cy5. Labeling with cyanine dyes and 2D DIGE were performed as described previously (Mooney et al., 2006) with the following modifications: a Cy2-labeled internal standard consisting of equal amounts of pooled protein from each control and treated sample (i.e. 5.6 μg from all nine samples) was used to facilitate normalization of quantification across replicates, linear pH 3–10 IPG strips were used, and the second dimension SDS–PAGE gels were isocratic (12% acrylamide).
Gel imaging and spot analysis
Gels were scanned inside the glass plates using a FLA5000 laser scanner (Fujifilm) with default settings. Image analysis was performed using the ‘Difference In-gel Analysis’ program of the DeCyder software suite (GE Healthcare, http://www.gelifesciences.com/). Spot detection, background subtraction, normalization and matching were performed on raw TIFF images using default settings. Initial spot detection settings were for 1300 total spots, followed by an exclude filter for spots with slope >2 and area <150. Only spots showing differences in all three replicates with a t-test P value ≤ 0.05, and ≥1.5-fold differences in their relative abundance were considered as differentially expressed and selected for identification.
In-gel trypsin digestion of protein spots
One of the three replicate gels from each comparison was loaded with 750 μg unlabeled protein and was stained overnight with colloidal Coomassie blue (20% ethanol, 1.6% phosphoric acid, 8% ammonium sulfate and 0.08% Coomassie brilliant blue G-250). The positions of differentially expressed protein spots revealed by DeCyder software were manually correlated with Coomassie-stained spots based on the local spot pattern, and excised using a 1.5 mm spot picker (The Gel Company, http://www.gelcompany.com). Excised spots were digested with trypsin as described previously (Mooney et al., 2006). Peptide extracts were lyophilized and re-suspended in 3 μl 50% acetonitrile and 1% formic acid with ddH2O.
Matrix-assisted laser desorption and ionization time of flight (MALDI-TOF/TOF) analysis
Peptides extracted from mitochondrial proteome spots were analyzed by MALDI-TOF/TOF MS. Tryptic peptides (0.5 μl) were spotted onto the MALDI plate and topped with the same volume of α-cyano-4-hydroxy-cinnamic acid (Fluka, http://www.flukka.com.br/) matrix (5 μg μl−1 in 50% acetonitrile, 0.3% trifluoroacetic acid and 10 mm ammonium phosphate). Peptide calibrant mixture (0.5 μl) was spotted in six outer wells of the target plate and used for calibration in MS and MS/MS modes.
Both MS and MS/MS spectra (of the eight most-abundant peptide ions) were acquired for each peptide digest on an ABSciex (http://www.absciex.com) 4700 MALDI-TOF/TOF MS. Database searches were performed using GPS explorer software version 3.6 (ABSciex) using the ‘combined MS and MS/MS’ function and integrated MASCOT search engine version 2.2 (http://www.matrixscience.com) against the NCBInr Viridiplantae database (http://www.ncbi.nlm.nih.gov/protein/?term=viridiplantae, 2011/09/06; 827 830 protein sequences). The search included the following parameters: one trypsin mis-cleavage, carbamidomethyl cysteines fixed modification, oxidation of methionine variable modification, MS and MS/MS mass tolerances of 100 ppm and 0.25 Da, respectively. Protein identification was considered significant based on the confidence interval percentage and protein scores reported by GPS explorer. For MS/MS data, only peptide scores exceeding a 95% confidence level reported by MASCOT were accepted.
Liquid chromatography quadrupole time of flight (LC-Q-TOF) analysis
Mitochondrial sample spots not identified or identified with low confidence by MALDI-TOF/TOF were re-analyzed by LC-Q-TOF, and all total protein sample spots were analyzed with Q-TOF. Peptide extracts were lyophilized and dissolved in 5 μl of 5% acetonitrile and 1% formic acid, and 3 μl were loaded onto a 43 mm C18 CHIP column (Agilent, http://www.chem.agilent.com/en-US/Pages/Homepage.aspx, catalog number G4240-62005) and analyzed using a short gradient from 0 to 40% B (solvent A, 0.1% formic acid in water; solvent B, 99.9% acetonitrile/0.1% formic acid) over 10 min. Data were acquired as follows: each cycle (3.2 s) consisted of an MS scan followed by MS/MS scans of the five most abundant peptides. Data for a total of 22 min elution were collected, and MASCOT generic format (MGF) files were extracted from the data using Agilent qualitative analysis software. MGF files were then searched against the NCBInr Viridiplantae database using MASCOT.
One-dimensional SDS–PAGE coupled with HPLC-MS (geLC-MS) analysis
One-dimensional SDS–PAGE fractionation of 12 samples from the total protein fraction and nine samples from the mitochondria-enriched fraction [three hybrid groups (one low, two high) each containing four biological replicates in total protein and three biological replicates in mitochondrial protein] was performed as described previously (Yao et al., 2011). Bands were excised from the gels and trypsin-digested. The resulting peptides were separated by reversed-phase nanoHPLC followed by MS analysis with LTQ Orbitrap XL (Thermo Scientific, http://www.thermoscientific.com), and database searches and spectral counting-based quantification were performed as described previously (Yao et al., 2011). A custom database was created as follows: redundant entries in the working set of B73 maize protein sequences (136 770 sequences downloaded from http://www.maizesequence.org) were removed using CD-hit (http://weizhong-lab.ucsd.edu/cd-hit, 120 290 sequences remained) and concatenated with, their randomized (decoy) sequences, and the common repository of adventitious proteins (cRAP, http://www.thegpm.org/crap/index.html, 112 sequences). Peptide spectral count data were examined for the top five differentially expressed proteins from the total protein 2D map and the top ten proteins from the mitochondria-enriched 2D map.
Acknowledgements
The authors would like to thank Roy J. Lowery (Charles W. Gehrke Proteomics Center, University of Missouri, Columbia, MO, USA) for assistance with the 2D gels. We thank Sherry Flint-Garcia (Division of Plant Sciences, University of Missouri, and USDA/ARS, Columbia, MO, USA) for her invaluable advice in hybrid selection and the gift of seeds used in this study. This work is supported by National Science Foundation, Division of Biological Infrastructure (NSF-DBI) grant 0922769 to K.N. and B.P.M.




