Phosphorylation and ubiquitination of dynamin-related proteins (AtDRP3A/3B) synergically regulate mitochondrial proliferation during mitosis
Summary
The balance between mitochondrial fission and fusion is disrupted during mitosis, but the mechanism governing this phenomenon in plant cells remains enigmatic. Here, we used mitochondrial matrix-localized Kaede protein (mt-Kaede) to analyze the dynamics of mitochondrial fission in BY-2 suspension cells. Analysis of the photoactivatable fluorescence of mt-Kaede suggested that the fission process is dominant during mitosis. This finding was confirmed by an electron microscopic analysis of the size distribution of mitochondria in BY-2 suspension cells at various stages. Cellular proteins interacting with Myc-tagged dynamin-related protein 3A/3B (AtDRP3A and AtDRP3B) were immunoprecipitated with anti-Myc antibody-conjugated beads and subsequently identified by microcapillary liquid chromatography–quadrupole time-of-flight mass spectrometry (CapLC Q-TOF) MS/MS. The identified proteins were broadly associated with cytoskeletal (microtubular), phosphorylation, or ubiquitination functions. Mitotic phosphorylation of AtDRP3A/AtDRP3B and mitochondrial fission at metaphase were inhibited by treatment of the cells with a CdkB/cyclin B inhibitor or a serine/threonine protein kinase inhibitor. The fate of AtDRP3A/3B during the cell cycle was followed by time-lapse imaging of the fluorescence of Dendra2-tagged AtDRP3A/3B after green-to-red photoconversion; this experiment showed that AtDRP3A/3B is partially degraded during interphase. Additionally, we found that microtubules are involved in mitochondrial fission during mitosis, and that mitochondria movement to daughter cell was limited as early as metaphase. Taken together, these findings suggest that mitotic phosphorylation of AtDRP3A/3B promotes mitochondrial fission during plant cell mitosis, and that AtDRP3A/3B is partially degraded at interphase, providing mechanistic insight into the mitochondrial morphological changes associated with cell-cycle transitions in BY-2 suspension cells.
Introduction
Mitochondria are dynamic entities that undergo frequent division and fusion and move three-dimensionally throughout the cell (Arimura et al., 2004a,b, Berman et al., 2008; Zheng et al., 2009a; Logan, 2010). Their morphology varies between organisms and differs with the developmental stage and physiological conditions of the cell (Okamoto and Shaw, 2005; Scott and Logan, 2008; Braschi and McBride, 2010). The mechanism of mitochondrial dynamics during mitosis, especially of yeast and mammalian cells, has received considerable attention (Tanaka et al., 1985; Gorsich and Shaw, 2004; Taguchi et al., 2007; Zunino et al., 2009; Horn et al., 2011). Three-dimensional electron microscopic (EM) analysis of the yeast mitochondrial network demonstrated that it fragments during mitosis and resumes a single giant form before cytokinesis (Tanaka et al., 1985; Gorsich and Shaw, 2004). Similarly, the long, tubular mitochondria of HeLa cells fragment early in mitosis, and the filamentous network structures subsequently re-form in the daughter cells (Taguchi et al., 2007; Zunino et al., 2009; Horn et al., 2011). In contrast, in the only published study on mitochondrial morphology in Arabidopsis shoot apical meristem and leaf primordium meristematic cells, punctate mitochondria fused to form a cage-like structure during mitosis; this structure divided into two independent tentacular mitochondria during cytokinesis and continued to divide into very small particles (Seguí-Simarro et al., 2008). However, these conclusions are undermined because ascertaining the specific stage of shoot apical meristem and leaf primordium meristematic cells is difficult. An understanding of how the various pleomorphic mitochondria function in the conserved process of mitosis requires further investigation, particularly in the area of regulation of plant mitochondrial morphology.
Mitochondrial morphology is regulated by the dynamic equilibrium between fission and fusion. In mammalian cells, mitochondria fusion is mediated by FZO1, OPA1 and mitofusin1/2 (Okamoto and Shaw, 2005; Chan, 2006), but no obvious homologs of these proteins have been identified in plants (Arimura et al., 2004a,b; Logan, 2006). Studies on mitochondrial fission in plants have been more fruitful, resulting in identification of two Arabidopsis homologs of the mitochondrial fission protein hFis1, AtBIGYIN1 (AtFIS1A) and AtBIGYIN2 (AtFIS1B) (Scott et al., 2006; Zhang and Hu, 2009), and two Arabidopsis homologs of dynamin-related protein 1 (DRP1), AtDRP3A and AtDRP3B (hereafter ‘AtDRP3A/3B’). These latter two proteins shuttle between the cytosol and the outer surface of mitochondrial fission sites (Arimura and Tsutsumi, 2002; Hong et al., 2003; Arimura et al., 2004a; Logan et al., 2004; Fujimoto et al., 2009). Another protein, ELONGATED MITOCHONDRIA 1 (ELM1), is localized on the outer mitochondrial membrane and is required for re-localization of DRP3A (and possibly also DRP3B) from the cytosol to mitochondrial fission sites (Arimura et al., 2008).
As an evolutionarily conserved large dynamin-like GTPase, DRP appears to be the major orchestrator protein for mitochondrial outer membrane fission and for responses to cellular signals, particularly in mammalian cells (Chang and Blackstone, 2007; Cribbs and Strack, 2007; Karbowski et al., 2007; Han et al., 2008; Cho et al., 2009; Zunino et al., 2009). However, no evidence exists to suggest that DRP3A/3B is involved in the regulation of mitochondrial fission in plants.
Recent studies on mammalian cells have demonstrated that post-translational modifications, such as phosphorylation, ubiquitination, nitrosylation and sumoylation [attachment of small ubiquitin-like modifier (SUMO) proteins], are involved in the control of mitochondrial fission in DRP-dependent ways (Santel and Frank, 2008; Chang and Blackstone, 2010). In mammalian cells, hDrp1 was phosphorylated by Cdk1/cyclin B and Aurora A, thereby mediating mitochondrial fission during mitosis (Taguchi et al., 2007; Kashatus et al., 2011; Yamano and Youle, 2011). Ubiquitination regulates protein degradation or quality control. As cells exit mitosis, the anaphase-promoting complex/cyclosome and its coactivator Cdh1 (APC/CCdh1) E3 ubiquitin ligase complex partially ubiquitinates Drp1, thereby driving reassembly of the mitochondrial network (Horn et al., 2011). Sumoylation usually alters the subcellular localization of substrates or protects them from ubiquitin-triggered destruction. Zunino et al. (2009) reported that the SUMO-specific protease SenP5 re-localizes from the nucleoli to the mitochondria at the G2/M phase transition, driving mitochondrial fragmentation by desumoylating Drp1. Whether similar post-translational modifications also regulate plant mitochondrial morphology during mitosis remains to be determined.
In the present study, we used mitochondrial matrix-localized Kaede protein (mt-Kaede), a photoconvertible green-to-red fluorescent protein, to qualitatively examine the dynamic imbalance between fission and fusion during mitosis of BY-2 Nicotiana tabacum cells in suspension. This imbalance was further investigated using transmission electron microscopy (TEM). Heterologously expressed AtDRP3A/3B was used to investigate the regulatory mechanisms underlying mitochondrial morphological changes, and proteins putatively interacting with AtDRP3A/3B were identified by immunoprecipitation and mass spectrometry (MS). Finally, the phosphorylation and ubiquitination of AtDRP3A/3B during mitosis were analyzed. Our findings support the hypothesis that post-translational modifications of DRP3A/3B in BY-2 suspension cells regulate the morphological dynamics of mitochondria during mitosis.
Results
Laser scanning confocal microscopic (LSCM) analysis of mitochondrial morphology in BY-2 suspension cells at various cell stages
LCSM analysis of BY-2 tobacco cells in various phases of the cell cycle showed that mitochondrial morphology varied greatly with cell phase. In most interphase cells, the nucleus was located to one side, near the plasma membrane, and large punctate mitochondria with strong mt-Kaede fluorescence signals were distributed in the cortex region. Smaller punctate mitochondria with a moderate mt-Kaede fluorescence signal were distributed randomly throughout the cell at midplane (Figure 1a,b). At prometaphase, the chromosomes moved toward the equatorial plate, and the mitotic apparatus was surrounded by small, round mitochondrial particles (Figure 1c,d). In metaphase cells, the chromosomes were aligned into a straight line, with smaller punctate mitochondria at the cortex region and weak mitochondrial mt-Kaede fluorescence observed at midplane (Figure 1e,f). At anaphase, two straight lines of chromosomes moved toward opposite poles, with small particles at the cortex region and weak mitochondrial fluorescence observed at midplane (Figure 1g,h). In early cytokinesis, prominent phragmoplasts formed between the daughter nuclei, and the mitochondrial fluorescence signal increased (Figure 1i,j). In late cytokinesis, a complete cell plate formed and two daughter nucleoli emerged. Punctate mitochondria were distributed throughout the region of the phragmoplast (Figure 1k,l).
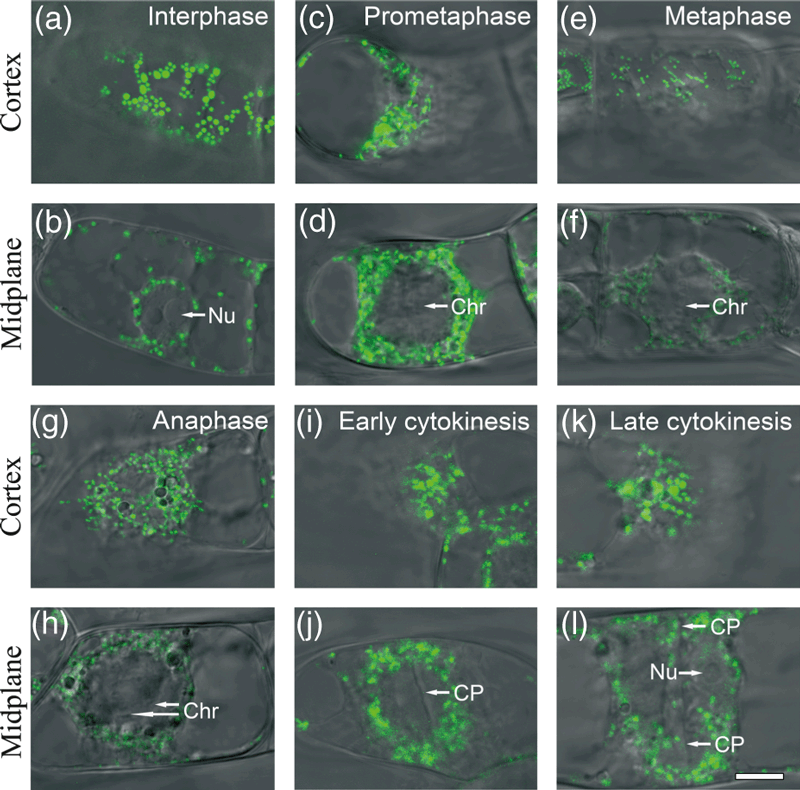
Mitochondrial distribution at various stages of the BY-2 cell cycle. BY-2 suspension cells expressing mitochondrial localized kaede (mt-Kaede) were imaged at the cortex (a, c, e, g, i, k) and midplane (b, d, f, h, j, l) regions by fluorescence microscopy (488 nm) and differential interference contrast (DIC) microscopy, and the fluorescence and bright-field images were merged. Green dots correspond to punctate mitochondria. (a, b) In the interphase cell, the nucleus is displaced to one side, almost touching the plasma membrane. (c, d) In prometaphase, the chromosomes are condensed and moving toward the equatorial plate. (e, f) In metaphase, the chromosomes are aligned at the equatorial plate. (g, h) In anaphase, the chromosomes are moving toward opposite spindle poles. (i, j) In early cytokinesis, a prominent phragmoplast is present between the daughter nuclei. (k, l) In late cytokinesis, formation of the cell plate is complete and two daughter nuclei are present. Scale bar = 10 μm. Chr, chromosome; CP, cell plate; Nu, nucleolus.
Dynamic analysis of mitochondrial fission and fusion revealed by photoconversion at various stages
To examine the balance between mitochondrial fission and fusion during mitosis, we followed the changes in partially photoconverted mt-Kaede protein in BY-2 cells in interphase (G1/S), prophase and metaphase (G2/M). The green and red mt-Kaede fluorescence signals were monitored for at least 2 h after exposure to ultraviolet light (UV) to determine the period required for full co-localization. The data were analyzed using Manders’ co-localization coefficients (Manders, Verbeek and Aten, 1993).
We first compared the co-localization periods of mitochondria at various stages. At interphase, granular mitochondria were distributed throughout the cytoplasm. After photoconversion, the green and red signals were completely co-localized within 2 h, such that almost all of the mitochondria were uniformly yellow (Figure 2a). When prophase cells were exposed to UV, the cells entered metaphase after 3 h, with weak and diffuse green and red fluorescence intermingled with each other, and only a few yellow signals (Figure 2b). When metaphase cells were exposed to UV, the cell went through anaphase, cytokinesis and re-entered interphase, and uniform mitochondrial fluorescence did not occur until 3 h after photoconversion (Figure 2c).

Mitochondrial fission/fusion dynamics at various stages of the cell cycle in BY-2 suspension cells. (a) Longitudinal photoconversion of mt-Kaede fluorescence at interphase (n = 4). (b) Transverse photoconversion at prometaphase (n = 3). The increase in green fluorescence over time is indicated by an asterisk. (c) Transverse photoconversion at metaphase (n = 4). (d) After longitudinal photoconversion at metaphase or early cytokinesis with the equatorial plate as the border, no fusion is detected between red and green mt-Kaede signals (n = 3). Scale bar = 20 μm. In each panel, column 1 (reading left to right) shows mitochondrial fluorescence due to mt-Kaede at the G1/S or G2/M stages, column 2 shows the corresponding time-lapse DIC images, column 3 shows the co-localization analysis with Manders’ co-localization coefficients, and column 4 is an enlargement of the boxed area in column 1.
Next, we examined the fate of mt-Kaede protein synthesized in the mitochondrial matrix during interphase and prophase (Figure 2a). At interphase, small green particles corresponding to newly synthesized mt-Kaede were attached to the yellow mitochondria (Figure S1, magnified version of Figure 2a, row 5, column 4), indicating that the newly synthesized protein was incorporated into existing mitochondria. When cells were photoconverted at prophase, the intensity of the green signal decreased after 1 h but significantly increased after 2 or 3 h (Figure 2b, row 3, column 3). The nascent green signal was evenly distributed and intermingled with the pre-existing red signal, without co-localization (Figure 2b, rows 5 and 6, column 4).
Finally, we longitudinally converted BY-2 cells in metaphase (or early cytokinesis), when the condensed chromosomes were arranged in straight lines (or in a newly formed cell plate). No merging between the red and green signals was observed, even after late cytokinesis (Figure 2d), suggesting that mitochondrial inheritance by daughter cells is determined before the cell wall serves as a physical barrier.
Cell stage-dependent analysis of mitochondrial size distribution in BY-2 suspension cells
Mitochondrial morphology in BY-2 cells at various stages was also examined using TEM to analyze the size and distribution of mitochondria in the cells. Wild-type cells stained with 4′,6-diamidino-2-phenylindole (DAPI) were sorted into various cell stages under an epifluorescence microscope and re-located before sectioning. At interphase, nucleoli with a complete nuclear envelope were located near one side of the plasma membrane and mitochondria were distributed throughout the cytoplasm (Figure 3a). At metaphase, chromosomes distributed in the nucleoplasm and mitochondria presented as small round particles (Figure 3b). At anaphase, the tubular vesicle network joined together at the equatorial plate (Figure 3ci,ii), and most of the observed mitochondria were small, both in the vicinity of the daughter nucleolus and in the vicinity of the newly forming cell plate (Figure 3c,d).
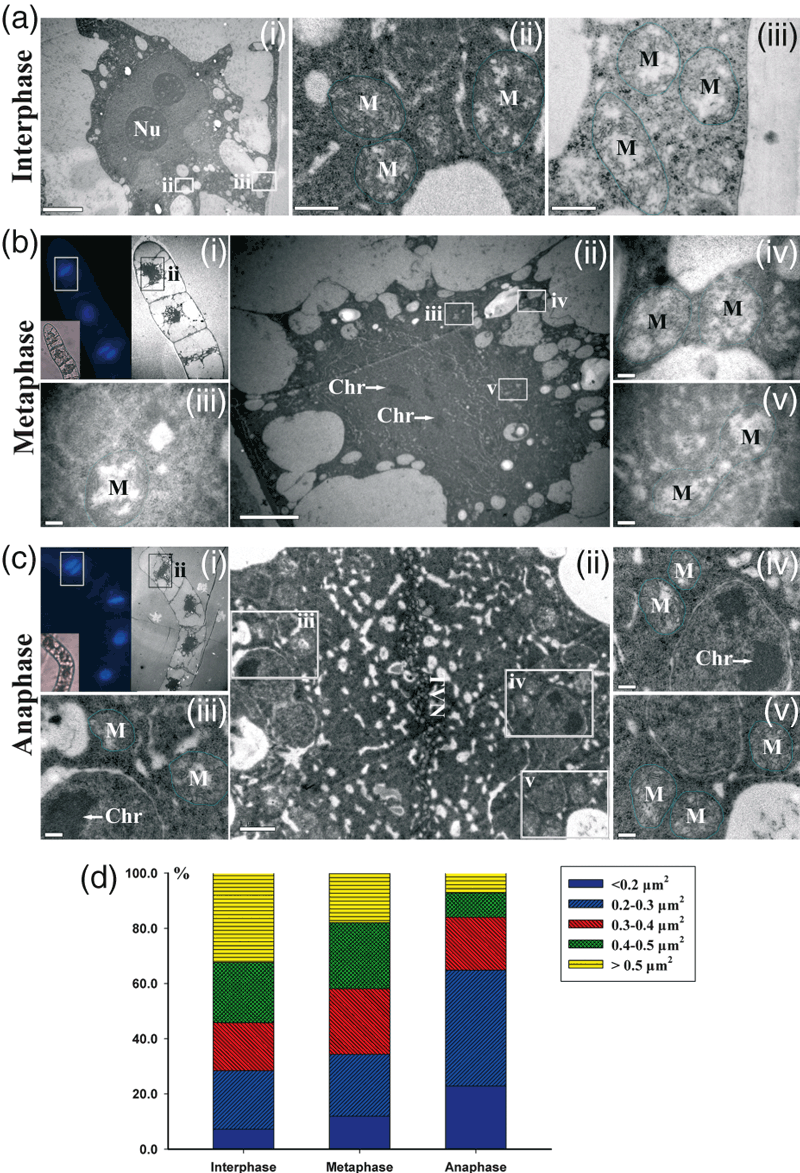
Electron microscopy and statistical analysis of mitochondrial morphology in BY-2 suspension cells at various stages. (a) The cell is in interphase, as shown by the complete nuclear membrane and nucleus in (i) (scale bar = 5 μm). Images (ii) and (iii) are enlargements of the boxed areas in (i) (scale bars = 0.5 μm). (b) The cell is in metaphase, as determined by chromosome condensation and DAPI staining in (i). Image (ii) is an enlargement of the boxed area in (i) (scale bar = 5 μm). Images (iii–v) are enlargements of the boxed areas in (ii) (scale bars = 0.2 μm). (c) The cell is in anaphase, as determined by DAPI staining of the aligned condensed chromosomes and the developing cell plate in (i). Image (ii) is an enlargement of the boxed area in (i) (scale bar = 2 μm). Images (iii–v) are enlargements of the boxed areas in (ii) (scale bars = 0.2 μm). (d) Size distribution of mitochondrial area in cells at various stages. The areas of at least 100 randomly selected mitochondria were measured in each cell, and three or four cells were analyzed for each stage. Chr, chromosome; M, mitochondria; Nu, nucleolus; TVN, tubulovesicular network.
The size distributions of randomly selected mitochondria in cells at various stages are shown in the histogram in Figure 3(d). Small mitochondria (area <0.3 μm2) constituted 34.3% of the mitochondria in metaphase cells and 64.8% of those in anaphase cells, but only 28.2% of those in interphase cells. In contrast, 32.4% of the mitochondria in interphase cells were large (area >0.5 μm2), whereas only 7.2% of those in anaphase cells were large. Statistical analysis of the EM data revealed that the proportion of mitochondria with areas <0.3 μm2 increased significantly during mitosis.
Heterologously expressed AtDRP3A/3B functions in mitochondrial fission in BY-2 suspension cells
No homologs of known mitochondrial fusion proteins have yet been identified in plants. A BLAST search of the National Center for Biotechnology Information database using the AtDRP3A/3B sequence yielded two contigs (BP136295 and DV160432) from N. tabacum ESTs. These sequences were identified as belonging to a single NtDRP3 candidate (Hamada et al., 2006). Pairwise alignment using the EMBOSS program (http://www.ebi.ac.uk/Tools/psa/) showed that NtDRP3 is quite similar to sequence to AtDRP3A (59.9% identity, 69.1% similarity) and AtDRP3B (59.8% identity, 70.8% similarity) (Figure S2).
When AtDRP3A/3B constructs with Dendra2 fused to their C-termini were expressed in BY-2 suspension sells, counterstaining of the mitochondria showed that the constructs promoted mitochondrial fission (Figure S3). Consistent with a previous report that over-expression of ΔDRP3A/3B containing a defective GTPase domain in BY-2 suspension cells induces the formation of tubular mitochondria (Arimura et al., 2004a), our finding indicates that heterogeneously expressed AtDRP3A and AtDRP3B are functional in mitochondrial fission in BY-2 suspension cells.
Identification of proteins putatively interacting with Myc-tagged AtDRP3A/3B by immunoprecipitation and MS
To explore the molecular mechanisms that govern mitochondrial fission in plant cells, we transformed BY-2 suspension cells with a vector expressing Myc-tagged AtDRP3A, Myc- tagged AtDRP3B or Myc. After 3 days of growth, total cellular protein was extracted from each culture and mixed with anti-Myc antibody-conjugated agarose beads. Electrophoretic separation and staining of the resulting immunoprecipitated protein showed three main bands. These bands were excised from the gel and analyzed by microcapillary liquid chromatography quadrupole time-of-flight (CapLC Q-TOF) MS/MS. Two main protein bands of approximately 130 and 100 kDa were identified as Myc-AtDRP3A or Myc-AtDRP3B. Comparisons of data from wild-type and Myc-expressing cells allowed exclusion of some proteins interacting non-specifically with Myc-DRP3A and Myc-DRP3B. The remaining proteins were assigned to one of three categories (Table 1). As most components of the cytoskeleton have been reported to interact with dynamin in vitro (Shpetner and Vallee, 1989; Praefcke and McMahon, 2004), the high-confidence identification of tubulin as protein interacting with AtDRP3A/3B suggested that our immunoprecipitation/CapLC Q-TOF MS/MS procedure was effective for identifying AtDRP3A/3B-interacting proteins.
| Function | Protein name | Accession number | Peptides | Score |
|---|---|---|---|---|
| Putative interacting protein of Myc-DRP3A | ||||
| Structural constituent of cytoskeleton | α-tubulin | gi|11967906 | 3 | 180 |
| TUBA2/4 | 3 | 94 | ||
| TUBB1 | 2 | 57 | ||
| Ubiquitination | E3 SINA-like 2 | At1g66620 | 1 | 30 |
| E3 ubiquitin-protein ligase | gi|42567657 | 1 | 29 | |
| 26S protease regulatory subunit homolog A/B | gi|15217431 | 2/2 | 36 | |
| AAA-type ATPase family protein | AT5G16930.1 | 1 | 27 | |
| Protein kinase | Protein kinase family protein | gi|76869647| | 2 | 31 |
| MAP kinase kinase | gi|32873530 | 1 | 28 | |
| Putative interacting protein of Myc-DRP3B | ||||
| Structural constituent of cytoskeleton | α-tubulin | gi|76868587 | 2 | 49 |
| TUBB8 | 7 | 273 | ||
| TUBB1/5/6 | 2 | 116 | ||
| TUB7 | AT2G29550.1 | 4 | 137 | |
| TUA4 | AT1G04820.1 | 2 | 84 | |
| ACT8 | AT1G49240.1 | 2 | 71 | |
| TUA3 | AT5G19770.1 | 1 | 80 | |
| Ubiquitination | Probable E3, ARI3 | gi|30688899 | 2 | 25 |
| AAA-ATPase subunit RPT2a | gi|83417482 | 2 | 105 | |
| 26S proteasome subunit RPN6a | gi|92029427 | 2 | 80 | |
| 26S proteasome regulatory subunit-like | gi|92037048 | 2 | 66 | |
| Phosphatase/protein kinase | PP2A | gi|1568511 | 2 | 73 |
| MAP kinase kinase | gi|32873530 | 2 | 39 | |
| Serine/threonine-protein phosphatase 2A | gi|543715 | 3 | 45 | |
| Protein kinase family protein | AT1G67580.1 | 1 | 31 | |
| Ribose-phosphate pyrophosphokinase 3 | gi|94328346 | 2 | 83 | |
- Peptides, number of distinct peptides identified for the protein; No. of peptides, number of peptides identified in the MS analysis; Score, the score retrieved by the mascot program. The identified proteins are listed according to score. A score of 25 or greater indicates an identification event of at least 95% confidence.
Among the other proteins identified by this method were protein phosphatases 2A and 2C; these members of the mitogen-activated protein kinase kinase family were classified as candidates involved in (de)phosphorylation. Also identified were E3 ubiquitin protein ligase, the 26S proteasome AAA-ATPase and regulatory subunits; these proteins were classified as candidates involved in ubiquitination. However, the confidence scores for the identified phosphorylation- and ubiquitination-related proteins were low, probably because these proteins, which are involved in signal transduction, are expressed at very low levels. In a Western blot analysis using a polyclonal antibody against BIGYIN, the Arabidopsis ortholog of yeast Fis1p, NtBIGYIN, appeared in the column input and flow-through fractions, but not in the anti-Myc antibody immunoprecipitation fraction (Figure S4a), serving as negative control for indirect interaction with DRP3A/3B, which is also confirmed by our co-immunoprecipitation experiment (Figure S4b).
Oryzalin treatment of BY-2 suspension cells enhances mitochondrial fission
To investigate the role of microtubules in mitochondrial fission, we examined the effects of two microtubule inhibitors, oryzalin and taxol, on interphase BY-2 suspension cells expressing mt-Kaede (Figure 4). The mean mitochondrial area decreased by approximately half (51%) after 10 min of exposure to 10 μm oryzalin, but it increased by approximately one-third (38.0%) after 10 min of exposure to 5 μm taxol, and the phenotype induced by oryzalin was reversed by taxol when both inhibitors were simultaneously applied (Figure 4b,c).

Changes in mitochondrial morphology induced by oryzalin and/or taxol treatment of interphase BY-2 suspension cells. (a) Mitochondrial morphology in cells treated with DMSO (control), 10 μm oryzalin, 5 μm taxol, or 10 μm oryzalin +5 μm taxol for 10 min. mt-Kaede fluorescence merged with DIC bright-field images are shown (scale bars = 20 μm). Images shown are representative of results obtained for cells treated with DMSO (n = 4), oryzalin (n = 6), taxol (n = 5) and oryzalin/taxol (n = 5). (b) Quantitative analysis of mitochondrial size (μm2) in cells treated as in (a). Differences in the mitochondrial mean area for cells treated with DMSO versus oryzalin, DMSO versus taxol, or oryzalin versus taxol are statistically significant by Student’s t-test (P < 0 .01). However, the difference in mitochondrial mean area for cells treated with taxol versus oryzalin + taxol is not significant by Student’s t-test (P > 0.05). (c) Images showing progress of mitochondrial morphology changes over time after treatment with oryzalin and/or taxol as in (a) (scale bars = 20 μm).
Using photoconversion, we also examined the effects of these inhibitors on the balance between mitochondrial fission and fusion in interphase BY-2 suspension cells. After a lengthy incubation (30 min, 1 hour or 2 hours) with oryzalin, smaller mitochondria with weak mt-Kaede fluorescence were observed (similar to the pattern seen at metaphase), and almost no co-localization of the green and red signals was detected (Figure 4c). In contrast, incubation with taxol or double treatment (oryzalin + taxol) did not enhance mitochondrial fission (Figure 4c).
Mitotic phosphorylation of Myc-AtDRP3A/3B is important in mitochondrial fission during mitosis
We next investigated whether Myc-DRP3A/3B is phosphorylated during mitosis using G2/M-synchronized Myc-AtDRP3A- and Myc-AtDRP3B-expressing BY-2 suspension cells. After enrichment of the fraction of mitotic cells by a sequential aphidicolin and propyzamide treatment, flow cytometric analyses showed that approximately 30–50% of the cells were mitotic. Non-synchronized cells were used as interphase (G1/S) cells. After synchronization, total cellular protein was extracted, subjected to immunoprecipitation with anti-Myc antibody-conjugated agarose beads, and then to electrophoresis. Phosphorylation was visualized using Pro-Q Diamond phosphoprotein gel staining, and the amount of protein was monitored using Coomassie brilliant blue staining. The specificity of Pro-Q Diamond staining was validated by calf intestinal phosphatase treatment (Figure S5). The ratios of the phosphorylated Myc-DRP3A/3B signals (Pro-Q Diamond staining of 130 kDa) to their respective Myc-DRP3A/3B signals (amount of 130 kDa protein) at G1/S or G2/M stages suggest that Myc-DRP3A/3B is phosphorylated during mitosis (Figure 5a,b).
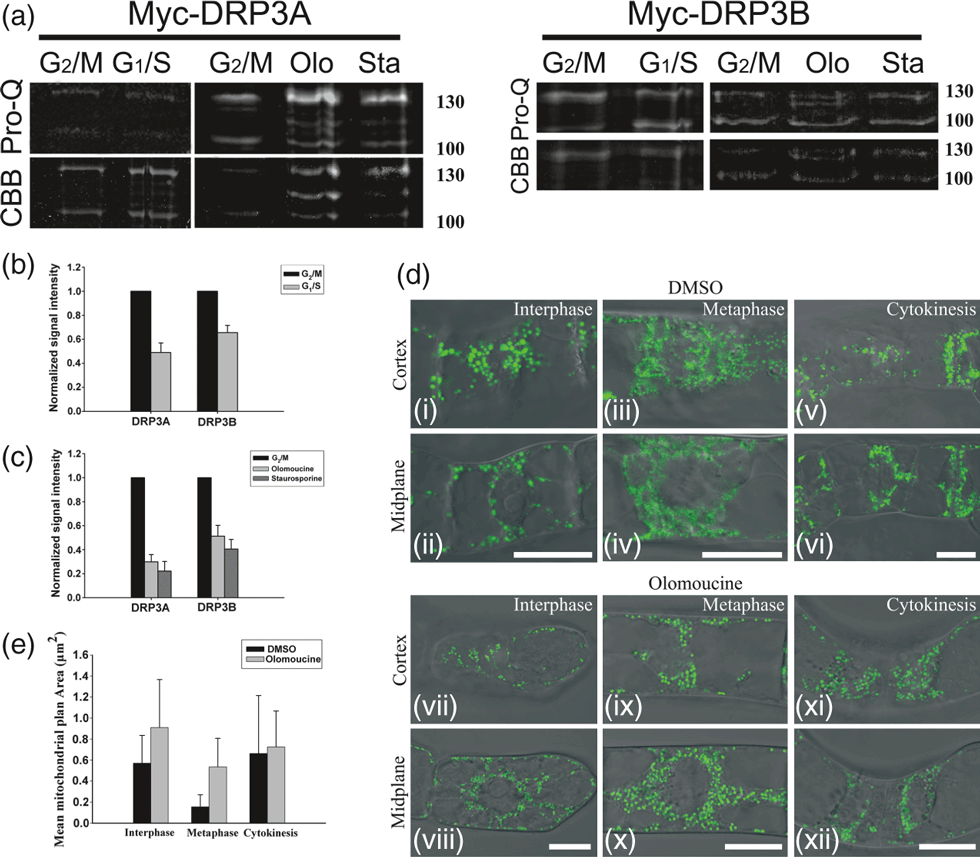
Mitotic phosphorylation of DRP3A/3B is involved in mitochondrial fission during mitosis. (a) Phosphoprotein (Pro-Q staining) and total protein amount (Coomassie brilliant blue, CBB staining) immunoprecipitated from Myc-DRP3A/3B-expressing BY-2 cells using anti-Myc antibody-conjugated agarose beads at the G1/S or G2/M stages, and for G2/M samples treated with olomoucine (Olo) or staurosporine (Sta). (b) Signal ratio plot showing the relative phosphorylation levels of DRP3A/3B (approximately 130 kDa) at the G1/S and G2/M stages, respectively. (c) Signal ratio plot showing the relative phosphorylation levels of DRP3A/3B (approximately 130 kDa) at G2/M stages (untreated) or treated with olomoucine (olo) or staurosporine (sta). (d) Mitochondrial morphology at various stages upon olomoucine treatment (scale bar = 20 μm). BY-2 suspension cells expressing mitochondrial-localized kaede were imaged at the cortex (i, iii, v, vii, ix, xi) and midplane (ii, iv, vi, viii, x, xii) regions by fluorescence microscopy (488 nm) and differential interference contrast (DIC) microscopy; the fluorescence and bright-field images were merged. (i–vi) Cells were treated with DMSO and imaged at the indicated stages. (vii–xii) Cells were treated with olomoucine and imaged at the indicated stages. (e) Quantitative analysis of mitochondrial size (μm2) in cells treated with DMSO or olomoucine. Differences in the mitochondrial mean area at interphase and at metaphase for cells treated with DMSO versus olomoucine are statistically significant by Student’s t-test (P < 0.01). However, the difference in mitochondrial mean area at cytokinesis treated for cells with DMSO versus olomoucine is not significant by Student’s t-test (P > 0.05).
Protein phosphorylation during mitosis is predominantly driven by cyclin-dependent kinases. To determine whether CdkB/cyclin B is involved in the mitotic phosphorylation of DRP3A/3B, we investigated the effects of kinase inhibitors on the phosphorylation level of DRP3A/3B and mitochondrial morphology. When synchronized Myc-DRP3A/3B-expressing BY-2 suspension cells were treated with olomoucine, a potent, selective, ATP-competitive inhibitor of CdkB/cyclin B and related kinases, the phosphorylation level of Myc-DRP3A/3B (approximately 130 kDa) at G2/M stages was inhibited. A similar phenomenon was observed when cells were treated with staurosporine, a serine/threonine protein kinase inhibitor (Figure 5a,c). When mt-Kaede-expressing BY-2 suspension cells were treated with olomoucine, mitochondrial fission at metaphase was impaired, and granular mitochondria appeared instead (Figure 5d,e). When the cells were treated with staurosporine, punctate mitochondria appeared in both interphase and metaphase cells, but almost no cells undergoing cytokinesis were detected (Figure S6).
DRP3A/3B is partially degraded at interphase
To investigate DRP3A/3B protein degradation during the cell cycle, we fused the photoconvertible fluorescent protein Dendra2 to the C-terminus of AtDRP3A/3B and expressed the fusion proteins in BY-2 suspension cells. By exposing the cells to UV, we were able to activate the AtDRP3A/3B–Dendra2 protein, marking it with red fluorescence, and exclude any newly synthesized (green) AtDRP3A/3B–Dendra2 protein from the analysis. We followed the decay of the red fluorescence intensity as a direct marker of the degradation of AtDRP3A/3B–Dendra2 (Zhang et al., 2007; Gerbin and Landgraf, 2010).
Using this method, we were able to monitor protein degradation at the single-cell level, and we used 3 days of culture to monitor both interphase and mitotic trajectories. As shown in Figure 6(a,b), analysis of red DRP3A–Dendra2 fluorescence showed that, during the first 2 h after UV exposure, the cell went through metaphase and entered into cytokinesis, with a concomitant 20% decrease in red fluorescence. During the next 4 h, the cell finished cytokinesis and re-entered the G1/S stages, with a concomitant 30% decrease in red fluorescence. The red DRP3A–Dendra2 fluorescence of interphase cells decreased 35% during the first 2 h, and 55% during the whole 6 h (Figure 6a,c).
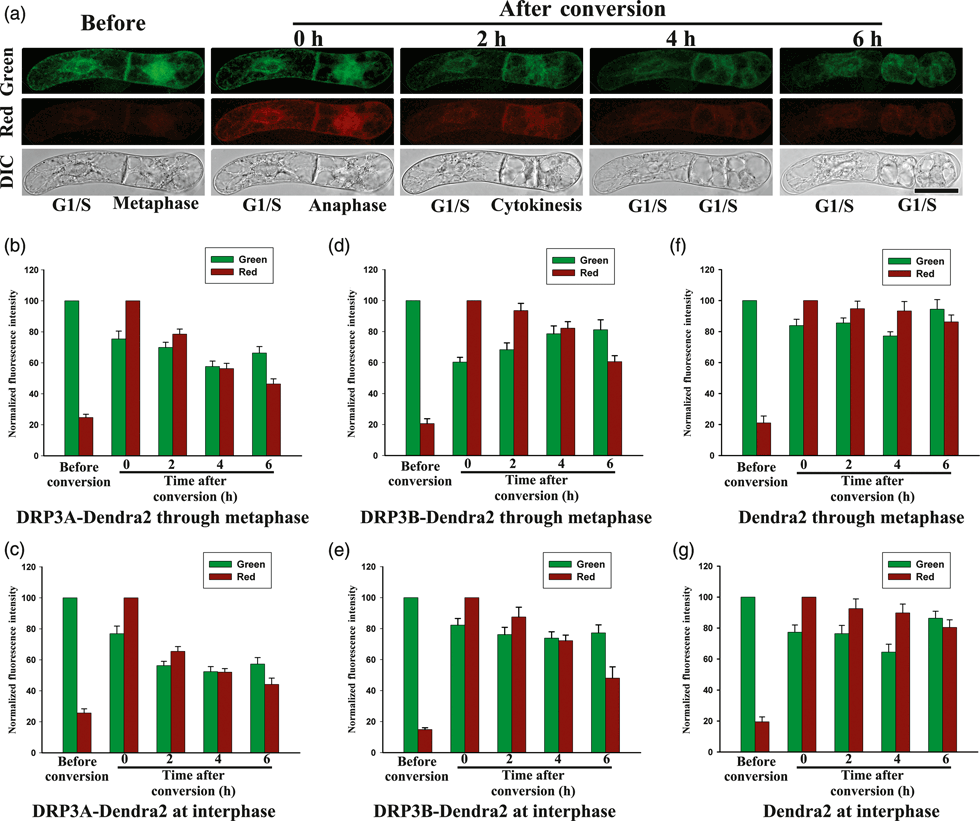
Fluorescence decay analysis of DRP3A/3B–Dendra2 degradation in BY-2 suspension cells during mitosis. (a) DIC and fluorescence images of cells expressing DRP3A–Dendra2. Images were recorded before and after photoconversion for cells at the G1/S stage and cells that have gone through metaphase (scale bar = 50 μm). (b, c) Normalized red and green fluorescence intensities for DRP3A–Dendra2 in cells photoconverted at metaphase or interphase, respectively. (d, e) Normalized red and green fluorescence intensities for DRP3B–Dendra2 in cells photoconverted at metaphase or interphase, respectively. (f, g) Normalized red and green fluorescence intensities due to Dendra2 (control) in cells photoconverted at metaphase or interphase, respectively.
BY-2 cells transformed with DRP3B–Dendra2 or Dendra2 at metaphase or interphase were also UV-exposed and analyzed (Figure 6d,g). By 6 h after UV exposure, the red DRP3B–Dendra2 fluorescence signals decreased 40 and 52%, respectively, from their metaphase and interphase values (Figure 6d,e). However, the red Dendra2 fluorescence decreased by 14 and 20%, respectively, from the metaphase and interphase values (Figure 6f,g). The red fluorescence of DRP3A/3B–Dendra2 cells decreased dramatically during G1/S stages, and had a relative slow downward trend when the cell was UV-exposed at metaphase.
To investigate the ubiquitination of Myc-AtDRP3A/3B, we used BY-2 cells grown in suspension for 7 days as an interphase sample. Because of the relatively low synchronization efficiency, mitotic samples intermingled with interphase cells may interfere with the protein expression pattern, therefore samples at G2/M stages were not analyzed. Two main protein bands of approximately 130 and 100 kDa were detected in interphase cells, and the intensity of both bands increased when stationary cells transferred to fresh medium were used (without any treatments) (Figure 7a). When the cells were treated with dimethyl sulfoxide (DMSO), a trend similar to independent turnover of Myc-DRP3A/3B was observed (Figures 7b,c and S7). When the Myc-DRP3A/3B cells were treated only with the protein synthesis inhibitor cycloheximide (CHX), the intensity of the lower band (approximately 100 kDa, both Myc-DRP3A and Myc-DRP3B) increased, whereas that of the upper band (approximately 130 kDa) of Myc-DRP3A remained at a similar level, and the intensity of the upper band (approximately 130 kDa) of Myc-DRP3B decreased (Figures 7b,c and S7) over time. When Myc-DRP3A/3B cells were treated with both MG132, an inhibitor of the chymotrypsin-like activity of the proteasome, and CHX, the intensity of both the upper band and the lower band remained almost constant compared with samples treated with DMSO.
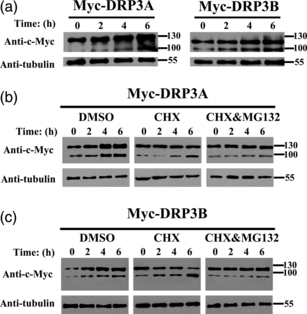
Western blot analysis of Myc-DRP3A/3B degradation in BY-2 suspension cells at interphase. (a) Interphase Myc-DRP3A/3B-expressing cells were collected at the indicated times after transfer to fresh medium and analyzed for the amount of Myc-DRP3A/3B over time. (b) Cells were treated with DMSO (control), CHX or CHX/MG132 at interphase and analyzed for the amount of Myc-DRP3A over time. (c) Cells were treated with DMSO (control), CHX or CHX/MG132 at interphase and analyzed for the amount of Myc-DRP3B over time. Tubulin is shown as a loading control.
Discussion
Networked or tubular mitochondria in yeast or mammalian cells fragment early in mitosis (Taguchi et al., 2007; Zunino et al., 2009). However, the punctate nature of plant mitochondria hinders direct quantification of the mitochondrial fission and fusion processes (Arimura et al., 2004a,b; Berman et al., 2008), and the morphological dynamics of mitochondria during mitosis in de-differentiated plant cells in suspension or in differentiated plant cells have not yet been reported. In the present study, we used mt-Kaede to evaluate mitochondrial fission and fusion during mitosis.
In our dynamics analysis, we found that mitochondria at the cortex or midplane regions of BY-2 suspension cells decrease in size at metaphase and anaphase. Photoconversion/co-localization experiments also demonstrated that the period required for complete mitochondrial fusion is longer during G2/M stages than in G1/S stages (3 h versus 2 h, respectively). Moreover, high-resolution EM observations showed that the fraction of small mitochondria (<0.3 μm2) increases significantly at anaphase. In addition, we noted an increase in green fluorescence corresponding to non-photoconverted mt-Kaede during time-lapse imaging in interphase and prophase, indicating that new protein is synthesized and incorporated into pre-existing mitochondria during these stages. Based on our findings, we conclude that mitochondrial matrix proteins are continuously synthesized and transported into pre-existing mitochondria during G1/S stages, and that mitochondrial fission dominates at G2/M stages, ensuring mitochondrial proliferation during mitosis.
Our findings are in agreement with previous reports that networked tubular mitochondria in fission yeast and HeLa cells fragment during mitosis (Tanaka et al., 1985; Taguchi et al., 2007; Zunino et al., 2009; Horn et al., 2011). A recent study, however, reported that small mitochondria in Arabidopsis shoot apical meristem and leaf primordium meristematic cells fuse into a single large mitochondrion, and that this structure is eventually reorganized into a cage-like structure encompassing the mitotic spindle (Seguí-Simarro et al., 2008). This apparent discrepancy may have occurred because only static observations were used in that report. However, we cannot rule out the possibility that the difference is a result of using different tissues.
Actin cytoskeletons play a vital role in cellular organization, organelle movement and inheritance, cytokinesis and signal transduction. Actin bundles have been reported to provide the main track for mitochondrial movement in fission yeast and plant cells (Schauss and McBride, 2007; Zheng et al., 2009b), and, furthermore, the actin-depleted zone observed at metaphase has been reported to correspond exactly to the site of cell-plate formation during cytokinesis (Sheahan et al., 2004; Sano et al., 2005). However, in our photoconversion experiments, we found that longitudinal photoconversion of metaphase cells (UV exposure of half the cell, with the equatorial plate as the border) resulted in no detectable fusion between red and green mitochondria, similar to our observations of cytokinesis after longitudinal photoconversion with the newly formed cell plate as the border. We speculate that this discrepancy in findings arose, at least in part, because the actin-depleted zone at the metaphase plate limits mitochondrial movement between the daughter cells. Our finding that mitochondrial inheritance by daughter cells is determined as early as metaphase provides strong support for the hypothesis that mitochondrial movement is limited by the distribution of the actin cytoskeleton, and that the cell plate serves as a physical barrier for mitochondrial inheritance in BY-2 suspension cells.
Microtubules have also been reported to play important roles in mitochondrial positioning and fission (Yaffe et al., 2003; Jourdain et al., 2009). Using the dnm1Δ fission yeast strain, Jourdain et al. (2009) showed that Dnm1p (dynamin-related protein 1) fragmented mitochondria in a microtubule-dependent manner. In our immunoprecipitation experiments, we identified microtubular proteins as principal candidates for BY-2 proteins interacting with DRP. Furthermore, oryzalin treatment caused the BY-2 mitochondria to dramatically decrease in size, and gradually but significantly reduced their mt-Kaede fluorescence. These findings are consistent with the previously reported conclusion that dynamin associates with microtubules in vitro (Shpetner and Vallee, 1989; Obar et al., 1990), and provide evidence that mitochondrial morphology in vivo is affected by microtubule inhibitors. Given the link between microtubule dynamics (Vos et al., 2004) and mitochondrial morphological changes during mitosis, we propose that microtubule depolymerization is involved in mitochondrial fission during the cell cycle.
AtDRP3A/3B is phosphorylated and recruited to mediate mitochondrial and peroxisomal fission. In mammalian cells, hDrp1 is phosphorylated by several kinases; it is phosphorylated by Cdk1/cyclin B at Ser585 during mitosis, by cAMP-dependent protein kinase at Ser656, and by Ca2+/calmodulin-dependent protein kinase Iα at Ser600 (Cribbs and Strack, 2007; Taguchi et al., 2007; Han et al., 2008). However, the only data concerning putative post-translational modification of AtDRP3A/3B in plant cells are in the PhosPhate database of the Arabidopsis Information Resource (http://phosphat.mpimp-golm.mpg.de/phosphat.html), which shows that Ser575 (SR(pS)FLGR) of DRP3A and Ser560 (TR(pS)FLGR) of DRP3B are confirmed phosphorylation sites. Our experiments using phosphoprotein staining showed that mitotic phosphorylation of AtDRP3A/3B also occurs in BY-2 suspension cells, in agreement with a previous report stating that mitotic phosphorylation of hDrp1 is important in mitochondrial fission in HeLa cells (Taguchi et al., 2007). We also detected a low level of basal phosphorylation. Treatment of BY-2 cells with olomoucine, an inhibitor of CdkB/cyclin B and related kinases, impaired both mitotic phosphorylation of Myc-AtDRP3A/3B and mitochondrial fission at metaphase. Treatment of the cells with staurosporine, a Ser/Thr protein kinase inhibitor, had a similar effect on mitotic phosphorylation of Myc-AtDRP3A/3B and mitochondrial fission at metaphase, as well as severe physiological effects. These results are consistent with those of recent studies showing that the Cdk1/cyclin B complex and the mitotic Ser/Thr kinase Aurora A cooperate with the small Ras-like GTPase RalA and its effector, RalA-binding protein 1, to promote DRP1 phosphorylation and mitochondrial fission during mitosis (Kashatus et al., 2011; Yamano and Youle, 2011). From these results, we infer that AtDRP3A/3B has more than one phosphorylation site, and that CdkB/cyclin B may be an upstream regulator of AtDRP3A/3B mitotic phosphorylation.
The Dendra2-based fluorescence decay assay is a powerful and effective technique that not only allows spectroscopic monitoring of protein degradation at the single-cell level (Zhang et al., 2007; Gerbin and Landgraf, 2010), but also is also useful for correlating protein degradation with cell-cycle stages. The technique circumvents the low synchronization efficiency associated with the CHX chase technique. Although Drp1 ubiquitination has been studied extensively in mammalian cells (Karbowski et al., 2007; Horn et al., 2011; Wang et al., 2011), the post-translational modifications of AtDRP3A/3B in plant cells remain to be further investigated. Our immunoprecipitation and MS analyses identified a series of ubiquitin pathway-related proteins, including the AAA-ATPase and regulatory subunits of the 26S proteasome and ubiquitin protein ligase. In live cells, red Dendra2 fluorescence from AtDRP3A/3B–Dendra2 dramatically decreased at interphase. This finding was confirmed by the time-dependent effects of treatment with CHX and MG132. Thus, we speculate that the smaller Myc-AtDRP3A/3B electrophoretic band was the product of protein degradation by the 26S proteasome. These findings are in accordance with a more recent report that partial ubiquitination of Drp1 drives the re-formation of tubular mitochondria in mammalian cells as the cells exit mitosis (Horn et al., 2011). Therefore, the partial degradation of DRP3A/3B–Dendra2 seen at interphase suggests that AtDRP3A/3B is partially degraded at interphase to ensure that it remains at a relatively low level. However, our results on protein degradation by CHX chase or fluorescence decay with Dendra2 are not consistent. This discrepancy may have occurred because different interphase samples were used (7 days culture for CHX chase versus 3 days culture for the fluorescence decay assay). Another possible reason is fluorescence bleaching and the time lag of fluorophore maturation during the fluorescence decay assay (Zhang et al., 2007; Gerbin and Landgraf, 2010).
Our investigations into DRP3A/3B regulation at various stages of the plant cell cycle have provided new perspectives concerning the morphological dynamics of mitochondria during mitosis (Figure 8). Our three principal findings are that the dominance of mitochondrial fission at metaphase and of mitochondrial growth at the G1/S stages provides a foundation for mitochondrial proliferation during the cell cycle, that AtDRP3A/3B undergoes mitotic phosphorylation and partial degradation at interphase, and that microtubule depolymerization and the distribution of the actin cytoskeleton are involved in mitochondrial fission and inheritance during mitosis. Taken together, our findings expand the understanding of the molecular mechanism underlying the morphological control of mitochondria during mitosis in BY-2 suspension cells.
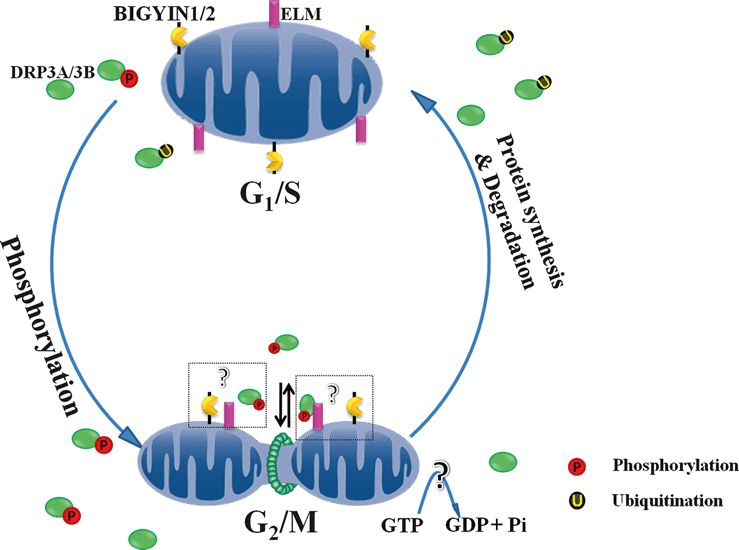
Schematic model for phosphorylation and ubiquitination of DRP3A/3B during mitosis and the subsequent induction of mitochondrial morphological changes during the cell cycle. During the G2/M stages, mitotic phosphorylation of AtDRP3A/3B ensures their high activity. Subsequently, GTP hydrolysis and AtDRP3A/3B dephosphorylation induce mitochondrial fission. During the G1/S stages, ubiquitination and partial protein degradation maintain AtDRP3A/3B activity at a relatively low level, allowing a dynamic balance between the fission and fusion processes.
Experimental Procedures
Plasmid construction
The coding region of the transient expression vector mt-Kaede (Arimura et al., 2004b), was amplified by PCR using primerSTAR™ (Takara), sub-cloned using SmaI and XbaI into a stable transformation vector to create pCM2300-mt-Kaede (conferring kanamycin-resistance in both bacteria and plants) for mitochondrial morphology visualization.
For immunoprecipitation and Western blotting experiments, the cDNAs of Arabidopsis AtDRP3A and AtDRP3B (mitochondrial fission complex member) were cloned into stable transformation vector pCM1307-6 × Myc using SpeI/SalI or SpeI/KpnI to give pCM1307-Myc-AtDRP3A and pCM1307-Myc-AtDRP3B, respectively. For protein degradation analysis, Dendra2 (kindly provided by Dr Ralf Landgraf, Department of Biochemistry and Molecular Biology, University of Miami), with/without a stop codon, was inserted into the plasmid pCM1307-6 × Myc using SalI and KpnI to give pCM1307-Myc-Dendra2, then AtDRP3A or AtDRP3B were inserted at at the N-terminus of pCM1307-Myc-Dendra2, to give pCM1307-Myc-AtDrp3A/3B-Dendra2 (DRP3A–Dendra2/DRP3B–Dendra2). All primers used in this study were listed in Table S1.
Plant materials, maintenance and transformation
Maintenance and transformation of BY-2 cells were performed as previously described (Nebenfuhr et al., 2000; Nagata et al., 2006) (http://mrg.psc.riken.go.jp/strc/index.htm), and maintained both in liquid culture and on solid plates via sub-culturing (once per week for suspension cultures and twice per month for calli on agar plates).
Using Agrobacterium-mediated transformation, pCM2300-mt-Kaede, pCM1307-Myc-DRP3A/3B, pCM1307-Myc-Drp3A/3B-Dendra2 and the corresponding empty vectors pCM1307-Myc and pCM1307-Myc-Dendra2 (with stop codon) were introduced into BY-2 suspension cells. The transgenic BY-2 cell lines were screened using kanamycin (pCM2300-mt-Kaede) or hygromycin (pCM1307-related vectors) and carbenicillin resistance, and incubated at room temperature for 3–4 weeks until transformed colonies were visible. Resistant cell colonies (50–100 colonies for each construct) were subjected to preliminary screening by PCR identification or fluorescence detection. Selected transgenic cell lines (5–10 per construct) were further transferred into MS liquid medium containing antibiotics to initiate suspension culture and used for subsequent analysis.
Confocal imaging, and photoconversion of Kaede protein and Dendra2
Unsynchronized BY-2 suspension cells were used for mitotic process observation 3 days after transfer to fresh medium. The samples were transferred into glass-bottomed dishes (MatTek Corp., http://www.mattek.com/pages/), and observed with an SP5 inverted laser scanning confocal microscope (Leica, http://www.leica.com/). First, differential interference contrast microscopy was used to find cells undergoing mitosis, then mitochondrial morphology was visualized with mt-Kaede excited at 488 nm (green Kaede), and emission spectra were collected between 500 and 550 nm. For Kaede protein photoconversion, a wavelength of 405 nm (operated at full power at an intensity of 30%, illumination of selected ROIs only, three pulses with imaging speed 1024 × 1024 pixels at 100 Hz) were used, followed by imaging at 488 nm for green Kaede and 543 nm for red Kaede (Arimura et al., 2004b). For Dendra2 photoconversion, a wavelength of 488 nm (strong blue light, 100% intensity of full power, illumination of the whole cell, three pulses with imaging speed 1024 × 1024 pixels at 10 Hz) was used. Photoconverted cells were incubated for 6 h and imaged every 2 h. The fluorescence decay of the red Dendra2 was recorded for protein degradation analysis. All images were captured using a 63 × 1.40 numerical aperture oil objective lens (Leica). Image analysis was performed using Adobe Photoshop CS2 (Adobe Systems, http://www.adobe.com/) and ImageJ 1.42e (http://rsbweb.nih.gov/ij/).
Electron microscopy
Synchronized wild-type BY-2 suspension cells were collected and fixed with 2.5% glutaraldehyde and 4% paraformaldehyde, washed twice with PBS (15 min each time). After staining with DAPI, the cell pellet was mixed with 2% low-melting-point agarose. After mounting the cell slush on slides, the cells were gently pressed with a cover glass in order to make the agarose layer as thin as possible, and cut into small pieces. Then small BY-2 agarose pieces were observed under the epifluorescence microscope using the DAPI channel, cells at different stages were chosen, washed twice with PBS, and fixed with 1% osmium tetraxide, followed by routine EM procedures. The agarose pieces were embedded on slides and thermally cured at 60°C for 24 h. BY-2 cells at specific stages were re-located with semi-ultrathin sections. Comparing with former images captured under epifluorescence microscope, BY-2 cells at specific stages were re-located and trimmed with semi-ultrathin section. Ultrathin sections were prepared at a thickness of 70 nm, post-stained with uranylacetate and lead citrate, and examined at 80 kV under a Hitachi H-600 (http://www.hitachi.com/) transmission electron microscope.
Protein extraction and immunoprecipitation
For immunoprecipitation experiments, suspension cells of Myc, Myc-DRP3A and Myc-DRP3B were collected and ground with liquid N2. The crude extract was suspended in homogenization buffer [25 mm Tris, 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40, 5% glycerol, 1 mm phenylmethylsulfonyl fluoride (PMSF), 20 mm NaF, 10 mm sodium orthovanadate, protease inhibitor and Phosstop cocktail tablets (Roche Diagnostics, http://www.roche.com/diagnostics/)]. After ultracentrifugation at 15 000 g for 20 min at 4°C, the supernatant was incubated with protein A–agarose beads to reduce non-specific binding. Then the supernatant was incubated with anti-Myc agarose beads (Sigma-Aldrich, http://www.sigmaaldrich.com/) for 2 h with end-over-end rotation at 4°C. Precipitates were recovered by centrifugation (2 min at 2000 g) and washed five times in homogenization buffer, and the beads were transferred into a fresh tube after the fifth wash to minimize carry-over of proteins from the lysate. Proteins were eluted from the beads by boiling in SDS sample buffer, and resolved on 8% polyacrylamide gels. The gel was cut out for identification by CapLC Q-TOF MS/MS, or stained with Pro-Q (Invitrogen, http://www.invitrogen.com/site/us/en/home.html) Diamond for phosphorylation detection.
MS identification and data analysis
To identify putative Myc-DRP3A- and Myc-DRP3B-interacting proteins. Immunoprecipitation samples were excised from gel stained with Coomassie brilliant blue after electrophoresis. In-gel digestion and CapLC Q-TOF MS/MS were performed as described previously (Chen et al., 2006).
taxonomy, Viridiplantae (green plants); one missed cleavage allowed; peptide tolerance 0.6; MS/MS tolerance 0.5; enzyme, trypsin; fixed modifications (carbamidomethyl) and variable modifications (oxidation, Phospho (S and T) and Phospho(Y)). For protein identification, the MASCOT search engine (http://www.matrixscience.com/search_form_select.html) was used with the following parameters: database, NCBInr; taxonomy, Viridiplantae (green plants); one missed cleavage allowed; peptide tolerance 0.6; MS/MS tolerance 0.5; enzyme, trypsin; fixed modifications (carbamidomethyl) and variable modifications (oxidation, Phospho (S and T) and Phospho(Y)).
Pharmaceutical treatments
For microtubule cytoskeleton analysis, stock concentrations of 1 mm taxol were made up in DMSO, and used at a final concentration of 10 μm. Oryzalin stocks were prepared at 20 mm in 100% ethanol, and used at a final concentration of 5 μm (Zheng et al., 2009b). Both taxol and oryzalin were used to examine the microtubule disorder effect on mitochondrial morphology.
For phosphorylation analysis, transgenic BY-2 cell lines expressing Myc-DRP3A/DRP3B or mt-Kaede were synchronized as described previously (Samuels et al., 1998). For cell stage phosphorylation analysis, synchronized Myc-DRP3A/DRP3B cell cultures at G1/S or G2/M were used for immunoprecipitation and Pro-Q Diamond staining. To determine the putative protein kinase involved in AtDRP3A/3B phosphorylation, 10 μm staurosporine (a serine/threonine protein kinase inhibitor) or 10 μm olomoucine (a CdkB/cyclin B inhibitor) were added to Myc-AtDRP3A/3B or mt-Kaede cell cultures at G2/M stage after release from propyzamide treatment. After treatment with these kinase inhibitors for 2 h, samples were used to investigate the phosphorylation level or mitochondrial morphology changes.
For protein degradation analysis, 20 μm CHX was used to inhibit protein synthesis (Gerbin and Landgraf, 2010), and 50 μm MG132 was used to detect proteasome interactions. After incubation for various periods, samples were harvested for Western blotting detection. After transferring proteins to nitrocellulose membranes, blots were detected using anti-Myc monoclonal antibody (eluted at 1:5000) and developed using horseradish peroxidase-linked secondary antibodies and the enhanced chemiluminescence detection system (Applygen, http://www.applygen.com.cn/).
Acknowledgments
We are very grateful to Professor Liwen Jiang (Department of Biology, Chinese University of Hong Kong) for providing the wild-type BY-2 suspension cells. We thank Dr Ralf Landgraf (Department of Biochemistry and Molecular Biology, University of Miami, FL) for providing the pET41b-Dendra2 vector. We thank Jingquan Li (Institute of Botany, Chinese Academy of Sciences) and Cheng Yuan (Institute of Botany, Chinese Academy of Sciences) for their expert support in LSCM. This work is supported by the National Basic Research Program of China (973 Program 2011CB809103), the Chinese Academy of Sciences/State Administration of Foreign Experts Affairs (SAFEA) International Partnership Program for Creative Research Teams (20090491019), the National Natural Science Foundation of China (30730009 and 30821007), the Knowledge Innovation Program of the Chinese Academy of Sciences (KJCX2-YW-L08 and KSCX2-EW-J-1), and the Ministry of Agriculture of China (2009ZX08009-011B and 2009ZX08009-095B). The authors declare that there is no conflict of interest.




