Arabidopsis DRB4 protein in antiviral defense against Turnip yellow mosaic virus infection
Summary
RNA silencing is an important antiviral mechanism in diverse eukaryotic organisms. In Arabidopsis DICER-LIKE 4 (DCL4) is the primary antiviral Dicer, required for the production of viral small RNAs from positive-strand RNA viruses. Here, we showed that DCL4 and its interacting partner dsRNA-binding protein 4 (DRB4) participate in the antiviral response to Turnip yellow mosaic virus (TYMV), and that both proteins are required for TYMV-derived small RNA production. In addition, our results indicate that DRB4 has a negative effect on viral coat protein accumulation. Upon infection DRB4 expression was induced and DRB4 protein was recruited from the nucleus to the cytoplasm, where replication and translation of viral RNA occur. DRB4 was associated with viral RNA in vivo and directly interacted in vitro with a TYMV RNA translational enhancer, raising the possibility that DRB4 might repress viral RNA translation. In plants the role of RNA silencing in viral RNA degradation is well established, but its potential function in the regulation of viral protein levels has not yet been explored. We observed that severe infection symptoms are not necessarily correlated with enhanced viral RNA levels, but might be caused by elevated accumulation of viral proteins. Our findings suggest that the control of viral protein as well as RNA levels might be important for mounting an efficient antiviral response.
Introduction
RNA silencing is a generic term for RNA-guided regulatory mechanisms occurring in a wide range of eukaryotes. All of the silencing systems characterized to date share common components: a double-strand (ds)RNA trigger is processed into small (s)RNAs by a type-III endoribonuclease Dicer. dsRNA-binding proteins (DRBs) can interact with Dicers to facilitate sRNA biogenesis or subsequent loading of the effector complex, termed RNA-induced silencing complex (RISC). Once produced, small RNAs are recruited by an ARGONAUTE (AGO) protein (catalytic component of RISC), and they act as a specificity determinant for recognition of the target RNA or DNA. The possible outcomes are either post-transcriptional gene silencing, operating through RNA cleavage or translational arrest, or transcriptional gene silencing, involving DNA or chromatin modifications.
In plants, RNA silencing components have diversified to execute specialized although partially overlapping functions. The model plant Arabidopsis thaliana encodes four DICER-LIKE proteins (DCL1–4), 10 AGOs and five DRB proteins, involved in distinct endogenous small RNA pathways (Vazquez et al., 2010). DCL1 interacts with DRB1, and the interaction is important for the efficient and precise processing of microRNA precursors by DCL1 (Kurihara et al., 2006). DCL4 interacts with DRB4, which facilitates the biogenesis of trans-acting small RNA (tasiRNAs) generated by DCL4 (Adenot et al., 2006; Fukudome et al., 2011; Hiraguri et al., 2005; Nakazawa et al., 2007).
In addition to regulating cellular gene expression, RNA silencing functions as an important antiviral mechanism in plants and invertebrates (Csorba et al., 2009; Ding, 2010). Viral dsRNA is processed by Dicers into viral small (vs)RNAs. In Arabidopsis, DCL4 has been shown to be the primary producer of vsRNAs for several (+)RNA viruses, whereas DCL2 rescues the antiviral silencing when DCL4 is genetically inactivated or suppressed (Blevins et al., 2006; Bouche et al., 2006; Deleris et al., 2006; Diaz-Pendon et al., 2007; Fusaro et al., 2006; Garcia-Ruiz et al., 2010). Inactivation of both DCL4, generating 21-nt vsRNAs, and DCL2, producing 22-nt vsRNAs, is required to obtain the highest susceptibility to infection for the (+)RNA viruses tested.
The DCL-mediated cleavage of viral RNA alone is unlikely to be sufficient to impede virus replication (Aliyari and Ding, 2009; Csorba et al., 2009; Ding and Voinnet, 2007). An additional layer of antiviral defense comes from loading vsRNAs into antiviral RISC, which further targets viral RNA for degradation (Omarov et al., 2007; Pantaleo et al., 2007). AGO1 has been implicated as the catalytic component of antiviral RISC (Mi et al., 2008; Morel et al., 2002; Pantaleo et al., 2007; Zhang et al., 2006b), but other AGO proteins may also be involved in antiviral silencing (Takeda et al., 2008). Interestingly, AGO1 has been implicated in micro (mi)RNA-mediated translational repression of cellular mRNAs (Brodersen et al., 2008; Lanet et al., 2009), opening the possibility that vsRNA-programmed RISC might not only degrade viral RNA but might also repress the translation of viral proteins.
The antiviral function of DRB4, i.e. DCL4 interacting partner, has been investigated only in a few cases so far. DRB4 has been shown to be required for the production of 21-nt vsRNAs derived from Tomato spotted wilt virus, a (–)RNA virus (Curtin et al., 2008). However, the impact of drb4 mutation on viral RNA accumulation or infection symptoms has not been determined. The role of DRB4 in the antiviral response has been demonstrated for Turnip crinkle virus, a (+)RNA virus (Qu et al., 2008). In this study an increase of viral RNA levels was observed in drb4 mutant plants when compared with wild-type (WT) controls, and the analysis of vsRNAs has led to the hypothesis that DRB4 might function downstream of vsRNA biogenesis. Last but not least, DRB4 has been shown to physically interact with the P6 silencing suppressor of Cauliflower mosaic virus (Haas et al., 2008). P6 overexpression was genetically equivalent to DRB4 inactivation, supporting the hypothesis that P6 inhibits DRB4 and possibly interferes with its function in antiviral response.
To investigate DRB4 function in antiviral defense, we used Turnip yellow mosaic virus (TYMV) and A. thaliana as a model to study virus–host interactions. TYMV is a (+)RNA virus belonging to the Tymovirus genus. TYMV genomic (+)RNA directs the expression of two proteins: a 206-kDa replication protein (206K) and a 69-kDa silencing suppressor (69K), whereas the viral coat protein (CP) is produced from a subgenomic (+)RNA (Figure 1a). During the replication cycle the viral genomic (+)RNA first acts as a template for the synthesis of a complementary, i.e. negative-strand (–), RNA, which in turn directs the synthesis of progeny genomic (+)RNA and subgenomic (+)RNA.
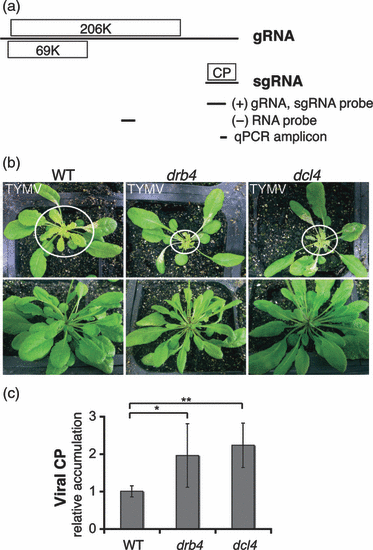
DRB4 and DCL4 contribute to antiviral response to TYMV. (a) TYMV genome structure and expression. TYMV genomic RNA (gRNA) directs the expression of a replication protein (206K) and an RNA silencing suppressor (69K). The subgenomic RNA (sgRNA), which is derived from the 3′ end of gRNA, directs the expression of the coat protein (CP). A schematic representation of the northern blot probes and quantitative PCR amplicon used for viral RNA detection is shown below. Genomic coordinates are the following: 5717–6124 nt for (+)gRNA and sgRNA detection, 3297–3558 nt for (–)RNA detection and 6004–6066 nt for qPCR amplicon. (b) TYMV infection symptoms in the wild type (WT), and in drb4 and dcl4 mutant plants. Images of infected plants (upper panel) and healthy controls (lower panel) were taken 12 days after infection. Systemically infected leaves are encircled. The enhanced infection symptoms in drb4 and dcl4 mutant plants were consistently observed in independent experiments (n ≥ 2). (c) Accumulation of the TYMV CP is enhanced in drb4 and dcl4 mutant plants. Extracts from systemically infected leaves of individual plants containing equal total protein levels were subjected to ELISA using CP-specific antibody. The data are presented as mean viral CP levels normalized by total protein content relative to the value obtained for WT plants. Error bars represent standard deviation (n = 15–19 plants per genotype). The statistical significance was tested using a two-tailed Mann–Whitney non-parametric test: *P = 10−3; **P < 10−5.
Although the contribution of RNA silencing to host defense against TYMV has not been investigated so far, TYMV is likely to be targeted by this antiviral mechanism because it encodes an RNA silencing suppressor (Chen et al., 2004). Moreover, AGO1, the catalytic component of RISC, has been shown to recruit TYMV-derived sRNAs (Zhang et al., 2006b).
Here, we provide evidence indicating that DCL4 and DRB4 indeed contribute to antiviral defense against TYMV, as evidenced by an enhanced severity of virus infection symptoms in Arabidopsis plants carrying genetic lesions in DCL4 and DRB4 loci. We have shown that both proteins are required for the efficient biogenesis of TYMV-derived sRNAs. However, DRB4 and DCL4 appear to have distinct functions in antiviral response. Whereas the impairment of DCL4 resulted in enhanced viral RNA accumulation, inactivation of DRB4 promoted the accumulation of viral CP, suggesting a role for RNA silencing machinery in the control of viral protein levels.
Results
DCL4 and DRB4 participate in antiviral response to TYMV
To investigate the role of DCL4 and DRB4 proteins during TYMV infection we used Arabidopsis mutant plants carrying genetic lesions in the corresponding genes. Upon TYMV infection, Arabidopsis drb4 and dcl4 mutant plants displayed similar enhanced developmental defects, characterized by the drastically reduced size of systemically infected leaves (Figure 1b).
Viral CP accumulation in systemically infected leaves of drb4 and dcl4 mutants and of WT plants was determined by ELISA. Consistent with the observed enhanced severity of TYMV infection symptoms, both mutants displayed a significant increase (P ≤ 0.001) in CP accumulation compared with WT plants (Figure 1c). The increased severity of infection symptoms and elevated levels of viral CP in the dcl4 and drb4 mutant plants support the hypothesis that DRB4 and DCL4 participate in the antiviral response to TYMV.
DCL4 and DRB4 are required for the biogenesis of vsRNAs
To gain insight into the molecular mechanisms of DRB4 and DCL4 function in antiviral defense, we investigated vsRNA accumulation in infected WT plants and in dcl4 and drb4 mutant plants using Illumina deep-sequencing technology.
The removal of sequence tags with no recognizable 3′ adapter sequence or resulting from adapter self-ligation yielded a total of 18 767 123, 21 756 915 and 23 210 343 sequences in the libraries prepared from infected WT, dcl4 and drb4 plants, respectively. Within the three libraries 85–90% of sequence tags were found to perfectly match either the TYMV or Arabidopsis genome (Figure 2a).
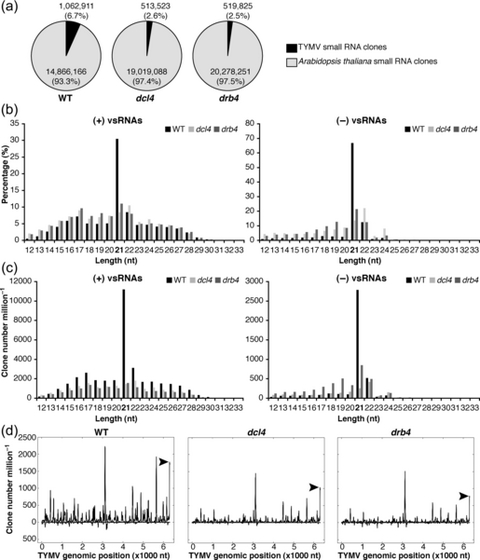
DCL4 and DRB4 are required for the biogenesis of viral small (vs)RNAs. RNA extracted from systemically infected leaves of wild-type (WT), dcl4 mutant and drb4 mutant plants were used to construct small RNA libraries for deep sequencing analyses. (a) Virus- and host-specific small RNAs in WT, dcl4 mutant and drb4 mutant plants. Sequence tags with a perfect match to the Arabidopsis genome are presented in light gray (14866166, 19019088 and 20278251 sRNA clones in the libraries obtained from WT, dcl4 and drb4 mutant plants, respectively). Sequence tags with a perfect match to the TYMV genome are presented in black (1062911, 513523 and 519825 sRNA clones in the libraries obtained from WT, dcl4 and drb4 mutant plants, respectively). sRNA clones with a perfect match to both Arabidopsis and TYMV genomes (0.25, 0.23 and 0.20% of mapped small RNAs in the libraries obtained from WT, dcl4 and drb4 mutant plants, respectively) are not presented. (b,c) Length distribution of vsRNAs in WT, dcl4 mutant and drb4 mutant plants. The size distribution of (+)vsRNAs (left panels) or (–)vsRNAs (right panels) is presented as the percentage of total (+)vsRNAs or (–)vsRNAs, respectively (b), and as the sum of library-size normalized sRNA counts (c) for each class. (d) TYMV genomic view of vsRNAs in WT, dcl4 mutant and drb4 mutant plants. (+)vsRNAs are presented above the x-axis. (–)vsRNAs are shown below the x-axis. The abundance of vsRNAs was calculated and plotted as the sum of library-size normalized sRNA counts in each single nucleotide sliding window along the TYMV genome. Peaks of vsRNAs derived from the tRNA-like structure at the 3′ end of the genome are indicated by arrowheads.
Strand-specific analysis of vsRNA populations revealed that (+)vsRNAs were more abundant that (–)vsRNAs, corresponding to 90% of the total vsRNAs in WT and dcl4 mutant plants, and to 78% of the total recovered vsRNAs in drb4 mutant plants.
Previous work demonstrated that 21- and 22-nt vsRNA are generated by DCL4 and DCL2, respectively, and both proteins are involved in defense against the (+)RNA viruses investigated so far (Csorba et al., 2009). Consistently, we found that in WT plants 21-nt vsRNAs were the most abundant and constituted 30% of (+)vsRNA and 67% of (–)vsRNA populations (Figure 2b). The second most abundant class was the 22-nt vsRNAs, corresponding to 8% of (+)vsRNAs and 12% of (–)vsRNAs (Figure 2b). These results suggest that DCL4 and DCL2 participate in the biogenesis of TYMV-derived sRNAs.
Comparative analyses of TYMV-derived sRNA populations showed a substantial reduction in vsRNA abundance in dcl4 and drb4 mutant plants with respect to the WT control (Figure 2a,c). The levels of 21-nt vsRNAs were most severely reduced in mutant plants (Figure 2c), indicating that DCL4 and DRB4 are required to generate these sRNA species. The abundance of (–)vsRNAs of other lengths was either unaltered or slightly enhanced in the mutant plants (Figure 2c). In contrast, the levels of (+)vsRNAs of all lengths were reduced in the mutant plants, with the most substantial decrease in the accumulation of 21-nt sRNAs. A possible explanation of these results is that a proportion of (+)vsRNAs is generated by antiviral RISC programmed with complementary (–)vsRNAs. The reduction of (–)vsRNAs in the mutant plants might therefore impair RISC-dependent cleavage, resulting in the reduced accumulation of RNA fragments generated by an AGO protein. Consistent with this hypothesis is the observation that in WT plants, 21- and 22-nt vsRNAs, which can be generated by DCL4 and DCL2, respectively, constituted 39% of total (+)vsRNAs, which was in clear contrast to (–)vsRNAs, where 21- and 22-nt vsRNAs represented 80% of (–)vsRNAs. These results suggest that a substantial proportion of (+)vsRNAs might not be generated by direct DCL processing.
Mapping of vsRNAs of all lengths to the TYMV genome revealed a non-uniform pattern characterized by the presence of vsRNA ‘hot spots’ distributed along the viral genome (Figure 2d), a finding consistent with the results obtained for other (+)RNA viruses (Donaire et al., 2009; Garcia-Ruiz et al., 2010; Molnar et al., 2005; Qi et al., 2009). The same analysis performed with vsRNAs detected in drb4 and dcl4 mutant plants confirmed the overall reduction in the abundance of vsRNA species (Figure 2d). The analysis of the genomic distribution of 21-nt vsRNAs revealed a similar pattern characterized by a more substantial reduction of 21-nt vsRNA abundance in drb4 and dcl4 mutant plants (Figure S1a).
Altogether, the deep-sequencing analyses of vsRNA populations in infected WT plants, and in dcl4 and drb4 mutant plants indicate that DCL4 and DRB4 are required for an efficient biogenesis of vsRNAs, in particular the production of 21-nt vsRNAs.
Inactivation of DRB4 and DCL4 has distinct effects on the accumulation of viral RNA
We analyzed viral RNA accumulation in infected plants by northern blots (Figure 3a). Mutant plants of dcl4 accumulated increased levels of (+)RNA, sgRNA in particular, but approximately WT levels of (–)RNA. These results suggest that DCL4 participates in the degradation of viral (+)RNAs, but does not notably affect the stability of viral (–) RNA.
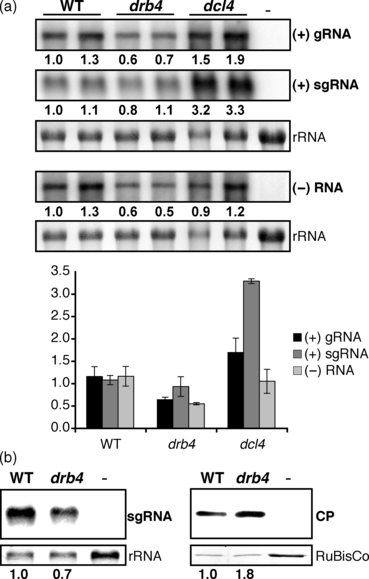
Accumulation of viral products in wild-type (WT), drb4 mutant and dcl4 plants. (a) Accumulation of viral RNA in WT, drb4 mutant and dcl4 mutant plants. RNA extracted from systemically infected leaves of WT and drb4 and dcl4 mutant plants were assayed by northern blots using TYMV (+)RNA- and (–)RNA-specific probes. RNA extracted from healthy plants was used as a negative control (–). The relative viral RNA levels are indicated below each panel and the average ± standard deviation values calculated from biological replicates are presented in the graph below. Methylene blue staining of 28S rRNA is shown as a loading control. (b) Accumulation of viral coat protein (CP) and subgenomic (sg)RNA in WT and drb4 mutant plants. Systemically infected leaves and healthy controls were subjected to RNA (left panels) and protein (right panels) analyses. sgRNA was detected using a strand-specific RNA probe. RNA extracted from healthy leaves was used as a negative control (–). Methylene blue staining of 28S rRNA is shown as a loading control. For viral CP analysis total protein content was measured and equal total protein levels were analyzed by western blot using CP-specific antibody. Brilliant blue Commassie staining of the large subunit of ribulose-1,5-biphosphate carboxylase (RuBisCo) is shown as a loading control. Extract from healthy leaves was used as a negative control (–). CP quantification was performed using serial dilutions of protein extracts and quantifying CP signal by imagej. The relative levels of sgRNA and CP are indicated below each panel.
In contrast to dcl4 mutant plants, drb4 mutant plants did not accumulate elevated levels of viral RNA (Figure 3a). Unexpectedly, despite the enhanced severity of virus infection symptoms (Figure 1b) and a significantly increased accumulation of viral CP (Figure 1c), drb4 mutant plants displayed a reduced accumulation of viral (+)gRNA and (–)RNA, suggesting that DCL4 and DRB4 may have distinct functions in antiviral defense.
The accumulation of viral CP is enhanced in drb4 mutant plants
Interestingly, in drb4 mutant plants the increase in CP levels was not correlated with an enhanced accumulation of viral sgRNA that directs CP expression (1, 3). To confirm these results we analyzed the accumulation of viral sgRNA and CP in WT and drb4 mutant plants using the same infected materials. In drb4 mutant plants sgRNA levels were slightly reduced, whereas viral CP levels were increased approximately twofold (Figure 3b). These results show that CP accumulation is enhanced in the absence of DRB4, suggesting that DRB4 might regulate viral protein expression or stability. The potential involvement of an RNA silencing component DRB4 in the regulation of viral protein levels is hitherto unknown in plants, leading us to further investigate DRB4 function in viral infection.
DRB4 protein expression and subcellular localization are altered in infected plants
To characterize the DRB4 expression pattern in infected plants, we generated two independent Arabidopsis transgenic lines PDRB4:DRB4-YFP-FLAG, expressing DRB4-YFP-FLAG fusion under the control of the native promoter. In healthy seedlings DRB4 was expressed in all organs with a stronger accumulation in root and shoot meristems and in leaf vascular tissues (Figure 4a). Consistent with a previous report where DRB4 localization was investigated in a heterologous plants expression system (Hiraguri et al., 2005), DRB4 was found to localize mainly in the nucleus in Arabidopsis.
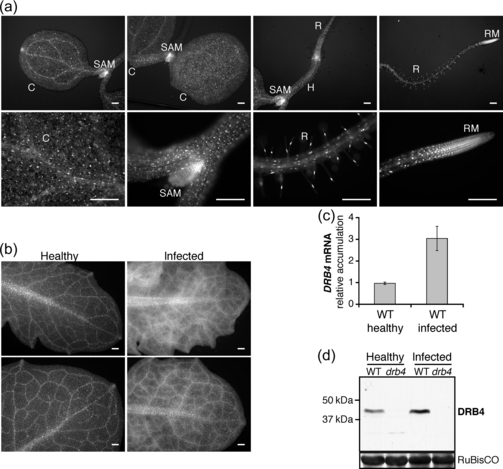
DRB4 expression in healthy and infected plants. (a) DRB4 expression pattern in healthy seedlings. The expression pattern of DRB4-YFP-FLAG fusion protein expressed under the control of the DRB4 native promoter in 4-day-old seedlings of two independent transgenic lines (PDRB4:DRB4-YFP-FLAG) was analyzed by epifluorescence microscopy, and representative images are shown. C, cotyledon; H, hypocotyl; R, root; RM, root meristem; SAM, shoot apical meristem. Scale bar: 200 μm. (b) DRB4 expression pattern in healthy and systemically infected leaves. The same lines as in (a) were infected with TYMV. Healthy and systemically infected leaves were observed by epifluorescence microscopy 10 days after infection, and representative images of DRB4 expression patterns are shown. Scale bar: 200 μm. (c) DRB4 transcript accumulation is enhanced in infected plants. Endogenous DRB4 transcript accumulation was analyzed by qRT-PCR in healthy and systemically infected leaves of WT plants. mRNA levels were normalized to 18S rRNA. The results are presented as the average ± standard deviation calculated from two biological replicates. (d) DRB4 protein accumulation is enhanced in infected plants. Total protein content in extracts from healthy and systemically infected leaves of WT and drb4 mutant plants was measured and equal total protein levels were analyzed by western blot using DRB4-specific antibody. The positions of DRB4 and molecular mass markers are indicated. Ponceau red staining of the large subunit of ribulose-1,5-biphosphate carboxylase (RuBisCo) is shown as a loading control.
Upon TYMV infection the DRB4 expression pattern in systemically infected leaves was changed. DRB4 accumulation appeared to be enhanced, particularly in the vasculature and surrounding tissues (Figure 4b). Given that the systemic movement of the virus occurs through veins, from where it spreads to the surrounding tissues, we anticipated that the modified DRB4 expression pattern might result from enhanced DRB4 expression in infected cells. Reverse transcription quantitative PCR (RT-qPCR)-PCR analysis of the endogenous DRB4 transcript showed that DRB4 expression was indeed induced in infected WT plants (Figure 4c). Furthermore, using a DRB4-specific antibody we confirmed that the elevated transcript levels were correlated with an increased accumulation of endogenous DRB4 protein (Figure 4d).
To further characterize the role of DRB4 during virus infection, we investigated DRB4 localization at the subcellular level. To this end we obtained protoplasts from healthy and systemically infected leaves of PDRB4:DRB4-YFP-FLAG plants. Consistent with the results obtained with transgenic seedlings (Figure 4a), in cells isolated from healthy leaves DRB4 was mainly localized in the nucleus with a diffuse distribution in the cytoplasm (Figure 5a). In a small number of cells (5% cells, n > 50), DRB4 was concentrated in cytoplasmic speckles in healthy cells (data not shown). By contrast, a variable number of cytoplasmic speckles was observed in the majority (96%, n > 50) of protoplasts isolated from infected leaves (Figure 5b–d). Furthermore, in approximately 40% of cells the nuclear signal was lost and DRB4 was detected exclusively in the cytoplasm (Figure 5c,d). We have not observed virus-induced changes in the localization of a nuclear protein LHP1 in protoplasts obtained from 35S:LHP1-GFP plants (Figure 5e,f). LHP1 was localized exclusively in the nucleus in cells isolated from healthy and infected leaves, indicating virus-induced changes in subcellular localization do not affect all nuclear proteins. Altogether, these results indicate that DRB4 protein is recruited from the nucleus to the cytoplasm upon infection.
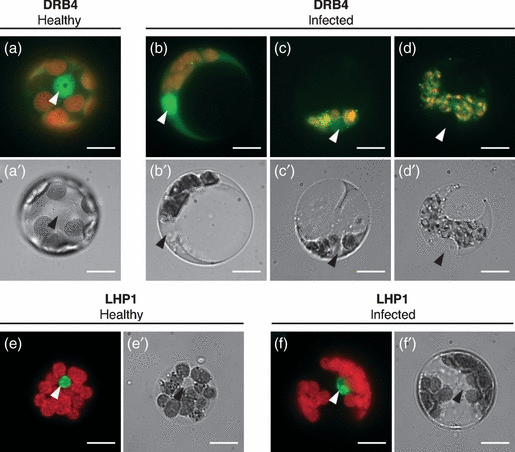
DRB4 is recruited to the cytoplasm upon TYMV infection. Protoplasts were isolated from healthy (a,e) and systemically infected (b–d,f) leaves of PDRB4:DRB4-YFP-FLAG plants (a–d) or 35S:LHP1-GFP plants (e,f) harvested 16 days after infection and observed by epifluorescence microscopy. YFP or GFP signal (green) was superimposed onto the chlorophyll autofluorescence (red) (a–f). The corresponding bright-field images are shown in (a′–f′). LHP1-GFP protein was detectable exclusively in the nucleus in all observed cells isolated from healthy (n > 50) and infected (n > 50) plants. Nuclei are indicated by arrowheads. Scale bar: 10 μm.
DRB4 binds to viral RNA in vivo
The recruitment of DRB4 to the cytoplasm, where viral RNA replication occurs, prompted us to test whether DRB4 interacts with viral RNA in vivo. To this end we performed FLAG-specific immunoprecipitations using extracts from TYMV-infected 35S:DRB4-FLAG transgenic plants, as well as from WT plants and 35S:FLAG-JMJ24 transgenic plants as controls. RNA isolated from total extracts and from immunoprecipitates was subjected to strand-specific RT-qPCR in order to detect TYMV RNAs of positive and negative polarity (Figure 6a). In DRB4-FLAG immunoprecipitates we detected a 12-fold enrichment of viral (+)RNA and an 80-fold enrichment of viral (–)RNA with respect to the WT control. No substantial enrichment in viral RNAs was detected in immunoprecipitates obtained from FLAG-JMJ24 extracts. In comparison, 18S rRNA, the abundance of which is of the same order of magnitude as viral RNA levels, was enriched only 2.6 times in DRB4-FLAG immunoprecipitates. These results suggest that DRB4 specifically interacts with viral RNA in vivo, with a higher affinity for viral (–)RNA than for (+)RNA.
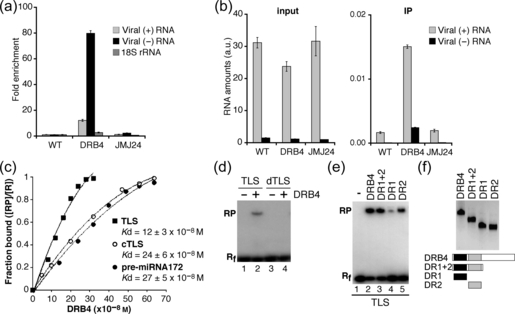
DRB4 interacts with viral RNA. (a,b) DRB4 is associated with viral (+)RNA and (–)RNA in vivo. Immunoprecipitations were performed using FLAG-specific antibodies and extracts from leaves of TYMV-infected WT plants (WT), 35S:DRB4-FLAG plants (DRB4) and 35S:FLAG-JMJ24 plants (JMJ24). Total RNA was extracted from an aliquot of each extract. The RNA levels in total RNA (input) and immunoprecipitates (IPs) were assessed by strand-specific RT-qPCR using a serially diluted cDNA fragment for each target to generate standard curves. The RNA levels were normalized by luciferase transcript levels, which had been added to each sample before RNA purification in order to monitor the efficiency of RNA recovery. (a) The fold enrichment of viral (+)RNA and (–)RNA and 18S rRNA was calculated by dividing luciferase-normalized RNA levels in the immunoprecipitates by the luciferase-normalized RNA level in the input, and setting the value obtained for WT plants to 1. (b) The luciferase-normalized viral RNA levels are presented in arbitrary units (a.u.) corresponding to viral RNA levels divided by the luciferase levels. Error bars represent standard deviations from results of three technical replicates. The control reactions without reverse transcriptase prepared and analyzed in parallel were negative. A higher enrichment of viral (–)RNA with respect to (+)RNA and a higher enrichment for both viral RNAs with respect to 18S rRNA in immunoprecipitates obtained from DRB4-FLAG plants was observed in at least two immunoprecipitation experiments. (c–f) DRB4 interacts with structured RNAs in vitro. (c) Determination of apparent dissociation constant (Kd) for DRB4 binding to TYMV TLS, TLS complementary RNA (cTLS) and pre-miRNA172 by gel mobility shift assays. Labeled RNA probes were incubated with increasing concentrations of full-length DRB4 protein (0–320 nm for TLS and 0–640 nm for cTLS and pre-miRNA172). The results presented are the average of three experiments for each substrate. (d) Gel shift mobility assays were performed using full-length DRB4 protein (lanes 2, 4) and labeled TLS (lane 2) or denatured TLS (dTLS) that had been heat treated prior to the assay (lane 4). The migration of free RNA probes is shown in lanes 1 and 3. (e) Gel shift mobility assays were performed with labeled TYMV TLS and full-length DRB4 protein (lane 2), or with its deletion derivatives containing DR1 (lane 4) and DR2 (lane 5), or with both motifs (lane 3). The migration of free RNA probe is shown in lane 1. (f) Western blot analysis (upper panel) and schematic representation (lower panel) of recombinant proteins used for gel mobility shift assays shown in (e). DR1, dsRNA binding motif 1 (black boxes); DR2, dsRNA binding motif 2 (grey boxes). Rf, free RNA; RP, protein-bound RNA; R = Rf + RP.
DRB4 has been reported to bind perfect dsRNA but not single-stranded (ss)RNA or structured RNA in vitro (Hiraguri et al., 2005; Pouch-Pelissier et al., 2008), suggesting that DRB4 might be preferentially associated with viral ds(+)/(–) RNA duplexes. If this is the case, DRB4-bound RNA should contain equimolar levels of viral (+) and (–)RNA. However, we detected an excess of (+)RNA with respect to (–)RNA in DRB4-FLAG immunoprecipitates (Figure 6b). A possible interpretation of these results is that DRB4 interacts with high affinity with ds(+)/(–)RNA duplexes, and with a lower affinity with viral ss(+)RNA. As viral ss(+)RNA is present in large excess in infected cells (Figure 6b), it may be recovered with a lower efficiency but in higher quantities than (–) RNA, which is potentially engaged in dsRNA duplexes.
DRB4 interacts with single-stranded structured RNAs in vitro
Our results show that (+)vsRNAs are more abundant than (–) vsRNAs (Figure 2), and that DRB4 might interact with viral (+)ssRNA in vivo (Figure 6). These findings, together with the previous report showing that DRB4 is required for DCL4-dependent processing (Fukudome et al., 2011), open the possibility that DRB4 might contribute to the cleavage of ss(+)RNA, possibly adopting secondary structures. The 3′ region of TYMV (+)RNA forms a well-characterized tRNA-like structure (TLS) that has been shown to act as a translational enhancer in vivo, and to be essential for virus replication as the initiation site for the viral (–)RNA synthesis (Dreher, 2009). We observed that this region was one of the ‘hot-spots’ of (+)vsRNA production (Figure 2d). We therefore tested whether recombinant DRB4 was able to interact with TYMV TLS using electrophoretic mobility shift assays. DRB4 formed a complex with TLS in vitro with an apparent dissociation constant estimated at 0.12 ± 0.03 μM (Figures 6c and S2a). The identity of the DRB4/RNA complex was confirmed by the addition of an unlabelled competitor TLS (Figure S2b). As controls, we chose RNA templates of comparable length, predicted to form secondary structures of similar folding energy (Figure S3) (Zuker, 2003). We tested RNA complementary to TLS (cTLS), predicted to be folded into short hairpin structures, and an miRNA precursor (pre-miRNA172), adopting a single stem-loop structure (Figure S3). We found that DRB4 was able to interact with these RNAs with a modestly lower affinity than with TLS, with apparent dissociation constants approximately twofold higher with respect to TLS (Figures 6c and S2a). By contrast, DRB4 interaction with TLS was severely impaired when the RNA substrate had been denatured by heat treatment prior to the interaction assay (Figure 6d), indicating that DRB4 affinity for structured RNA is higher than for denatured RNA (i.e. a less structured substrate).
Using truncated DRB4 derivatives we determined that dsRNA binding motif 2 (DR2) was important for TLS binding in vitro, whereas dsRNA binding motif 1 (DR1) displayed a weaker binding affinity (Figure 6e,f). However, full binding was restored in the presence of both motifs, suggesting that DR1 and DR2 may function cooperatively for substrate recognition.
These results demonstrate that DRB4 can interact with structured RNAs in vitro, and appears to have a higher affinity for structured RNA than for a denatured substrate. Furthermore, they open the possibility that TYMV TLS might be among viral (+)ssRNA regions that may be targeted by DRB4.
TLS generates abundant sRNAs in vivo
The association of DRB4 with TLS prompted us to further analyze TLS-derived sRNAs. We observed that this region was enriched in (+)vsRNAs with respect to (–)vsRNAs (Figure 2d). The most abundant vsRNAs derived from the TLS complementary strand were 21-nt long (Figure S1b). By contrast, the most abundant TLS-derived sRNAs were 16- and 17-nt long, indicating that the TLS cleavage gives rise to sRNAs of atypical lengths (Figure S1b). Further analyses showed that the majority of TLS-derived vsRNAs (77% in WT plants) mapped exactly to the 3′ end of TLS, with the most abundant vsRNA being 17-nt long. In WT plants the levels of this single sRNA species constituted 44% of TLS-derived sRNAs and 2.6% of total (+)vsRNAs, corresponding to the most abundant vsRNA among the 74 726 unique sequences mapped to the viral genome. As this vsRNA is derived from the 3′ end of TLS, which is from the 3′ end of the viral genome, it was generated by a single cleavage within the TLS acceptor arm, which might account for its atypical length. Consistently, the analysis of genomic distribution of all 17-nt long vsRNAs revealed that most of them mapped to a single genomic location at the 3′ end of the genome (Figure S1c). The enrichment of TLS-derived (+)vsRNAs with respect to (–)vsRNAs, and production of vsRNAs of different lengths from both strands, support the hypothesis that TLS secondary structure, rather than the paired (+)/(–)RNA duplex, is processed in vivo.
Comparative analyses of TLS-derived sRNAs revealed that their abundance was reduced in drb4 and dcl4 mutant plants with respect to WT controls (Figure S1b,c). These findings suggest that DCL4 and DRB4 may participate in TLS processing, although their contribution to the biogenesis of TLS-derived sRNAs appears less crucial than for the production of 21-nt vsRNAs (Figure S1a,c).
Recombinant DRB4 protein does not inhibit the translation in vitro
The interaction of DRB4 with viral RNA might interfere with the translation of the latter, and therefore account for enhanced viral CP levels in drb4 mutant plants. To test this hypothesis we performed in vitro translation assays using viral RNA or a CP-encoding transcript mimicking viral subgenomic RNA in the presence of purified recombinant DRB4 protein. We did not detect an inhibitory effect of DRB4 on the translation of viral proteins in this system (data not shown). These results suggest that DRB4 association with viral RNA is not sufficient to affect the translation in vitro. Other host factors not included in the in vitro translation assays might be required for the inhibition of viral RNA translation. Furthermore, it should be noted that although TYMV TLS has been shown to promote the translation of reporter transcripts in vivo (Matsuda and Dreher, 2004), it is not likely to retain this function in vitro (Matsuda and Dreher, 2007). Therefore, the in vitro experimental system does not allow us to address the issue of whether DRB4 interferes with TLS function as a translational enhancer. Alternatively, DRB4 might affect viral CP levels in vivo independently of translation. Further studies are therefore required to characterize the precise role of DRB4 protein in the regulation of viral CP levels.
Discussion
Previous genetic studies have implicated DCL, AGO and RDR proteins in RNA silencing-based antiviral defense, indicating that dicing and slicing functions as well as amplification of vsRNAs by RDRs are required for an efficient antiviral response. DCL4 has been identified as a primary antiviral DCL protein for the plant (+)RNA viruses investigated so far. Here, we show that DCL4 and its interacting partner DRB4 contribute to antiviral defense during TYMV infection.
DRB4 is recruited to the cytoplasm in infected cells
The spatial regulation of RNA silencing proteins involved in antiviral immunity remains poorly understood. DCL4 and its interacting partner DRB4 have been shown to localize in the nucleus in heterologous plant expression systems (Hiraguri et al., 2005; Kumakura et al., 2009). The nuclear localization is consistent with the role of DCL4 and DRB4 in the production of endogenous tasiRNAs, but not in the biogenesis of vsRNAs derived from (+)RNA viruses that replicate exclusively in the cytoplasm (Salonen et al., 2005; Sanfaçon, 2005). Here, we show that upon infection with TYMV, DRB4 expression is induced (Figure 4), and that it is relocated from the nucleus to the cytoplasm (Figure 5). Further studies are required to determine whether the subcellular redistribution of RNA silencing components, DRB4 or other proteins, in infected cells is a general feature of virus infections.
DCL4 contributes to the degradation of viral (+)RNA
Deep-sequencing analyses of vsRNA populations in infected plants revealed that DCL4 is required for an efficient production of vsRNAs, in particular 21-nt vsRNAs (Figure 2), which is consistent with the results obtained for other (+)RNA viruses (Blevins et al., 2006; Bouche et al., 2006; Deleris et al., 2006; Diaz-Pendon et al., 2007; Fusaro et al., 2006; Garcia-Ruiz et al., 2010).
The impairment of vsRNA biogenesis in dcl4 mutant plants correlated with an increased accumulation of viral (+)RNA, with a greater effect on (+)sgRNA than on (+)gRNA, but it did not substantially alter (–)RNA levels (Figure 3a). These results suggest that DCL4 contributes to the degradation of viral (+)RNA, but does not notably affect the stability of viral full-length (–)RNA. In this respect it should be noted that (–)RNA and (+)gRNA are engaged in viral replication complexes located within virus-induced membranous compartments (Jakubiec et al., 2004; Prod’homme et al., 2003, 2001), which might limit the access of the degradation machinery to replicating RNAs.
Our results here raise the issue of the nature of dsRNA substrate for DCL4. As no increase in full-length (–)RNA accumulation was detected in the dcl4 mutant plants (Figure 3a), it seems unlikely that DCL4 directly cleaves secondary structures adopted by (–)RNA or by dsRNA composed of (–)RNA and (+)RNA. The deep sequencing analyses of vsRNA populations revealed that (+)vsRNAs were more abundant than (–)vsRNAs (Figure 2c), which is consistent with the hypothesis that DCL4 may directly process secondary structures adopted by viral (+)RNA. (–)vsRNAs might be generated from (+)RNA converted into dsRNA by cellular RDRs. Last but not least, DCL4 may contribute to the degradation of viral (+)RNA indirectly through the production of (–)vsRNAs that can be loaded into antiviral RISC to target complementary (+)RNA for destruction.
DRB4 and DCL4 have distinct modes of action in antiviral response
The impairment of DRB4 function resulted in reduced levels of vsRNAs with respect to WT plants, with the strongest effect on the levels of 21-nt vsRNAs. As DRB4 has been shown to be required for DCL4 activity in vitro (Fukudome et al., 2011), we conclude that DRB4 facilitates the processing of viral RNA by DCL4. DRB4 might be involved in DCL4 recruitment to viral RNA, e.g. by assisting DCL4 relocation from the nucleus to the cytoplasm and/or facilitating DCL4/RNA interactions and subsequent cleavage.
Surprisingly, we did not observe an enhanced accumulation of viral RNA in drb4 mutant plants, a result expected from impaired processing into vsRNAs. We therefore conclude that in addition to vsRNA biogenesis, DRB4 may have yet uncharacterized functions, the impairment of which results in a reduced accumulation of viral RNA in drb4 mutant plants.
DRB4 function in regulation of viral CP levels
We observed that drb4 mutant plants accumulated enhanced levels of viral CP without a concomitant increase in the levels of sgRNA that directs CP expression (Figure 3b). These findings indicate that DRB4 inhibits the accumulation of viral CP, possibly by reducing its translation or stability. The effect of DRB4 inactivation on the accumulation of viral 69K and 206K proteins, expressed from viral gRNA (Figure 1a), remains to be characterized. The analysis of the accumulation of viral silencing suppressor 69K would be of particular interest, because 69K expression has been shown to induce pleiotropic developmental defects in Arabidopsis (Chen et al., 2004). The potential enhanced expression of 69K might therefore account for the increased severity of infection symptoms observed in drb4 mutant plants.
DRB4 interacts with viral RNA
We showed that DRB4 was associated with viral RNA in vivo (Figure 6a,b). An excess of (+)RNA with respect to (–)RNA was recovered from DRB4/RNA complexes, suggesting that DRB4 is able to interact with ss(+)RNA. Consistently we found that DRB4 could interact with single-stranded structured RNA substrates in vitro. DRB4 displayed a modestly higher affinity for TLS than for the complementary sequence (cTLS) and a miRNA precursor, which raises the possibility that TLS might be among viral structured regions of positive polarity targeted by DRB4.
Consistent with this hypothesis is the finding that TLS is one of the ‘hot spots’ of the (+)vsRNA production (Figure 2d). The most abundant TLS-derived sRNA was 17-nt long, and its production might be partly dependent on the presence of DRB4 and DCL4 proteins (Figure S1c). It would be interesting to determine whether 3′ ends of other viral genomes also generate abundant vsRNAs that may have been missed in deep sequencing experiments because of their non-canonical lengths. Such sRNA species might compete with full-length viral templates for the replication complex, thus reducing their replication rate. For TYMV, short TLS-derived RNAs have been shown to inhibit (–)RNA synthesis in vitro (Gargouri-Bouzid et al., 1991), and the expression of TLS in rapeseed has been reported to confer partial resistance to TYMV infection (Zaccomer et al., 1993).
For (+)RNA viruses viral genome is a multifunctional molecule serving as a template for translation, replication and encapsidation. Replication and translation are thought to be mutually antagonistic (Ahlquist, 2002), because ribosomes and viral polymerase move in opposite directions, and their action on a single substrate was shown to be incompatible (Gamarnik and Andino, 1998). For TYMV TLS has been shown to be important for the replication and translation of viral RNA and has been implicated in the regulation of the transition between these processes (Dreher, 2009). It is therefore conceivable that independent of its function vsRNA biogenesis DRB4 interaction with viral (+)RNA and with TLS in particular might have an impact on translation, replication and/or encapsidation, or the balance between these processes, which might account for the enhanced protein expression (1, 3) and reduced viral RNA levels (Figure 3a) observed in drb4 mutant plants.
For plant viruses the role of RNA silencing-based mechanisms in the degradation of viral RNA is well established, but their potential function in the regulation of viral protein levels has not been investigated so far. As evidenced by the drb4 mutant phenotype, severe infection symptoms are not necessarily correlated with elevated viral RNA levels, and might be caused by an enhanced accumulation of viral proteins, suggesting that the control of viral protein as well as RNA levels might be important for establishing an efficient antiviral response.
Experimental Procedures
Plant material
The drb4-1 mutant (Adenot et al., 2006) was obtained from the SALK collection (SALK_000736). The absence of DRB4 transcript and protein in homozygous lines was confirmed by RT-PCR and western blots, respectively. The dcl4-1 allele had been previously characterized (Gasciolli et al., 2005). The 35S:LHP1-GFP overexpressor line has also been previously described (Exner et al., 2009).
Arabidopsis thaliana (Col-0) plants were transformed by the floral-dip method (Zhang et al., 2006a). The PDRB4:DRB4-YFP-FLAG lines were obtained by transformation with the binary vector pBA002a-PDRB4-DRB4-YFP-FLAG, driving the expression of DRB4-YFP-FLAG fusion under the control of the DRB4 native promoter. The 35S:DRB4-FLAG line was generated by transformation with pH7-DRB4-FLAG-2, in which a 35S promoter was used to express DRB4-FLAG fusion protein. The 35S:FLAG-JMJ24 line expresses FLAG-JMJ24 protein under the control of the 35S promoter (S. Deng and N.-H. Chua, unpublished data).
Plant growth and TYMV infection
For virus infections, ∼5-week-old Arabidopsis plants grown under 8-h light/16-h dark were sap-inoculated with TYMV maintained on Brassica rapa (Chinese cabbage). Systemically infected leaves were harvested 12 days after infection (unless indicated otherwise) and analyzed.
RNA analysis
RNA was extracted with TRIZOL reagent (Invitrogen, http://www.invitrogen.com) from healthy rosette leaves or from systemically infected leaves (at least 24 plants per sample). Random primed cDNAs were subjuected to quantitative PCR (qPCR) using DRB4f7 and DRB4r1 primers (for DRB4 RNA detection) and 18Sf and 18Sr (for 18S rRNA detection). The 18S rRNA signal was used to normalize the RNA content. Each sample was analyzed in triplicate.
Probes for TYMV northern blots were generated using T7 Maxiscript kit (Ambion, http://www.appliedbiosystems.com) by in vitro transcription of TYMV cDNA fragments amplified with the following primers: TYMV_6127R_T7 and TYMV_5717F for (+)gRNA and sgRNA detection; T7_TYMV_3295F and TYMV_3557R for (–)RNA detection. Radioactive signals were acquired using phosphorimaging and quantified with imagequant.
Preparation and analysis of small RNA libraries
Small (s)RNA libraries were prepared from the same RNA samples that were used for northern blot analyses following the protocol in illumina 1.5. The libraries obtained from infected WT, drb4 and dcl4 mutant plants were sequenced on a Solexa-Illumina Genome Analyzer at the Rockefeller University Genomics Resource Center. Data were analyzed using Python scripts adapted from Qi et al. (2009). Sequence tags with a recognizable 3′ adapter were searched for sRNAs perfectly matching the TYMV RNA of positive and negative polarities. Sequence tags presenting a perfect match to the A. thaliana genome were identified using bowtie (Langmead et al., 2009).
Protein analysis
Frozen leaves were homogenized in ice-cold extraction buffer (10.5 mm KH2PO4, 30 mm Na2HPO4, 1.54 m NaCl, supplemented with protease inhibitor cocktail for plant cell and tissue extracts; Sigma-Aldrich, http://www.sigmaaldrich.com). The total protein content of cleared extracts was measured and adjusted to the same concentration with extraction buffer. Extracts containing equal total protein levels were analyzed by western blotting using anti-TYMV CP antibody (LOEWE, http://www.loewe-info.com) or anti-DRB4 antibodies.
Viral CP levels in individual plants were measured in duplicate by indirect enzyme-linked immunosorbent assay (ELISA) using anti-TYMV CP antibody (LOEWE), alkaline phosphatase-conjugated goat anti-rabbit IgG (Invitrogen) and SIGMAFAST p-nitrophenyl phosphate substrate (Sigma-Aldrich).
Analysis of DRB4 localization by epifluorescence microscopy
Whole seedlings, and healthy and systemically infected leaves of PDRB4:DRB4-YFP plants were mounted on slides in MS media. Protoplasts were prepared from healthy and systemically infected leaves of PDRB4:DRB4-YFP plants, as described by Yoo et al. (2007). Images were acquired using a wide-field fluorescence microscope and Hamamatsu Orca ER B/W digital camera, driven by metavue acquisition software (Universal Imaging Co., now Molecular Devices, http://www/moleculardevices.com). The sequence of primers and supplementary experimental procedures are presented in Table S1 and Appendix S1, respectively.
Acknowledgements
We thank Herve Vaucheret for seeds of dcl4-1 mutant plants, Shou-Wei Ding for providing TYMV cDNA, Lars Hennig for the 35S:LHP1-GFP overexpressor line and Shulin Deng for 35S:FLAG-JMJ24 seeds. We are grateful to Scott Dewell and Christina Caserio of the Rockefeller University Genomics Resource Center for performing the sequencing of sRNA libraries, and to Isabelle Jupin for providing TYMV RNA and for critical comments on this article. We thank Lucia Bernad for helpful discussions and comments on the article and Mengdai Xu for technical assistance. This work was supported by NIH grant GM44640 to N-HC and by the UNIK synthetic biology program to SWY. AJ was supported in part by a Rockefeller University Women and Science Fellowship.
References
Accession Numbers
DRB4 AT3G62800 seed stock: drb4-1 (SALK_000736). DCL4AT5G20320 seed stock: dcl4-1 (FLAG_330A04).




