Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana
Summary
Gravitropism aligns plant growth with gravity. It involves gravity perception and the asymmetric distribution of the phytohormone auxin. Here we provide insights into the mechanism for hypocotyl gravitropic growth. We show that the Arabidopsis thaliana PIN3 auxin transporter is required for the asymmetric auxin distribution for the gravitropic response. Gravistimulation polarizes PIN3 to the bottom side of hypocotyl endodermal cells, which correlates with an increased auxin response at the lower hypocotyl side. Both PIN3 polarization and hypocotyl bending require the activity of the trafficking regulator GNOM and the protein kinase PINOID. Our data suggest that gravity-induced PIN3 polarization diverts the auxin flow to mediate the asymmetric distribution of auxin for gravitropic shoot bending.
Introduction
Plants display remarkable abilities to adapt their physiology and development in response to environmental stimuli. Directional growth responses (tropisms) belong to the visually most obvious adaptation responses. Phototropism and gravitropism allow both shoots and roots to direct their growth in response to light and gravity, respectively (Estelle, 1996; Holland et al., 2009). These adaptive growth adjustments allow optimization of organ positioning for better light perception in the case of shoots, and for better water or nutrient acquisition in the case of roots. Therefore, shoots show a negative gravitropism and grow upwards, whereas roots show a positive gravitropism manifested by their downward growth. Accepted models for tropisms are based on the classical Cholodny–Went theory, in which the differential distribution of the plant signalling molecule auxin underlies the unequal growth at the two sides of a bending organ (Went, 1974). Accordingly, an asymmetry in auxin distribution and auxin response has been detected in gravistimulated organs of various plant species. This asymmetric auxin distribution during tropisms is triggered by directional, intercellular auxin transport (Briggs, 1963; Young et al., 1990; Epel et al., 1992; Luschnig et al., 1998; Rashotte et al., 2000; Friml et al., 2002a) that is mediated by transporters of the AUX/LAX (Bennett et al., 1996; Yang et al., 2006), PGP (Geisler and Murphy, 2006) and PIN (Petrášek et al., 2006) families (Yang and Murphy, 2009). The directionality of polar auxin transport is determined by a polar, subcellular, localization of PIN auxin efflux carriers (Wiśniewska et al., 2006). However, how the auxin transport is regulated by gravity to generate the asymmetry in the auxin distribution remains unclear.
In plants, gravity is perceived by sedimentation of specialized starch-containing organelles (statoliths) in cells of the root cap and shoot endodermis (Morita and Tasaka, 2004; Morita, 2010). In the gravity-sensing root cap cells, PIN3 is localized symmetrically, but after the gravity-induced statolith sedimentation, it polarizes and accumulates predominantly at the lower side of cells (Friml et al., 2002a; Harrison and Masson, 2008). This asymmetric PIN3 localization suggests that the flow of auxin is redirected towards the lower side of the root, where auxin is known to accumulate in response to gravistimulation (Perrin et al., 2005). Nonetheless, the mechanism of the PIN3 relocation, and its possible requirement for asymmetric auxin distribution, remains unclear.
Here, we analyse the role of PIN3-dependent auxin transport during the gravitropic response of Arabidopsis thaliana hypocotyls. We show that PIN3 in the endodermal cells of hypocotyls is needed for auxin redistribution, and that it is polarized in response to gravity. Furthermore, we demonstrate that the established vesicle trafficking and cell polarity regulators are necessary for both PIN3 polarization and hypocotyl gravitropism.
Results
PIN3 is required for asymmetric auxin distribution and hypocotyl gravitropism
Multiple genetic and pharmacological experiments have demonstrated that the inhibition of auxin transport interferes with tropisms (Perrin et al., 2005). Specifically, pin3 mutants had reduced hypocotyl gravitropic responses (Friml et al., 2002a). First, we tested the contribution of PIN3 to the hypocotyl gravitropic response by detailed kinematic analyses of hypocotyl bending. As expected, the bending in pin3 loss-of-function seedlings was much less pronounced than that in the wild type (Figure 1a,b,d). In contrast, mutants of the closest homologues pin4 and pin7, and the pin4 pin7 combination, did not show any defects in hypocotyl gravitropism (Figure 1c,e). Nonetheless, multiple mutant combinations of pin3 with pin4 and pin7, including pin3 pin4 pin7, did show increasingly stronger defects in hypocotyl gravitropism (Figure 1e), suggesting the redundant action of different PIN auxin transporters in hypocotyl gravitropism. Thus, unlike the situation in root gravitropism (Kleine-Vehn et al., 2010), PIN3 seems to be the major factor in the hypocotyl because the closest homologues PIN4 and PIN7 do not seem to play an important role in hypocotyl gravitropism, despite the fact that they redundantly contribute to some extent in the absence of the PIN3 function. The strong developmental defects and even lethality of higher order pin mutants (Friml et al., 2003; Blilou et al., 2005) do not allow us to test whether PIN proteins are the sole auxin transporters required for hypocotyl gravitropism; however, the strong effects of auxin transport inhibitors (Jensen et al., 1998; Friml et al., 2002a) demonstrate an essential role of auxin transport. Additionally, the auxin transporters from the PGP family that functionally co-operate with the PIN-dependent transport (Blakeslee et al., 2007; Mravec et al., 2008) contribute to the gravitropic response (Rojas-Pierce et al., 2007). Overall, these results revealed that PIN3 is the major contributor for PIN-dependent auxin transport during the hypocotyl gravitropic response, with redundant contributions from other related PIN transporters.
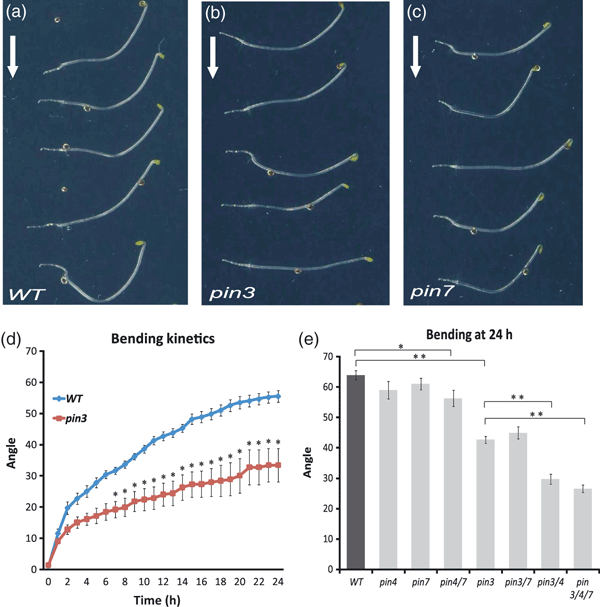
Hypocotyl gravitropic response of pin mutants.(a–c) Bending of wild-type (a), pin3 (b) and pin7 (c) hypocotyls after 24 h of gravistimulation. Arrows mark gravity direction.(d) Hypocotyl bending kinetics of the wild type and pin3 mutants. Hypocotyl curvatures were measured every hour and average curvatures were calculated. Values are the average of three biological replicates (n > 10 per time point on each replicate). Error bars are SEs (Student’s t-test, *P < 0.05).(e) Hypocotyl bending of the wild type, pin4, pin7, pin4 pin7, pin3, pin3 pin7, pin3 pin4 and pin3 pin4 pin7 mutants. Hypocotyl curvatures were measured after 24 h and average curvatures were calculated. Values are the average of three biological replicates (n > 10 per time point on each replicate). Error bars are SEs (Student’s t-test between wild type and pin4 pin7, *P < 0.02; Student’s t-test between wild type and pin3, pin3 pin7, pin3 pin4 and pin3 pin4 pin7 mutants, **P < 0.0001).
Next, we investigated whether the PIN3 function is required for generating the asymmetric auxin distribution following gravistimulation. We used the auxin-responsive DR5rev::GFP line, for which the spatial activity pattern has been shown to correlate with auxin distribution, as inferred from indole-3-acetic acid (IAA) immunedetection in embryos and lateral organs (Benkováet al., 2003; Friml et al., 2003), and in gravistimulated roots (Ottenschläger et al., 2003). Consistent with previous reports (Friml et al., 2002a), we detected an asymmetric DR5 activity across the gravistimulated hypocotyls, with higher relative fluorescence intensity at the lower side (Figure 2a,c,d). Notably, when the PIN3 activity was compromised in pin3 mutants, the asymmetry in DR5rev::GFP activity was significantly less pronounced in gravistimulated hypocotyls as compared with the wild type (Figure 2b,e,f,g). These observations confirm a role for PIN3 in hypocotyl gravitropism, and show that PIN3 activity is required for the generation of the lateral auxin gradient underlying the hypocotyl gravitropic response.
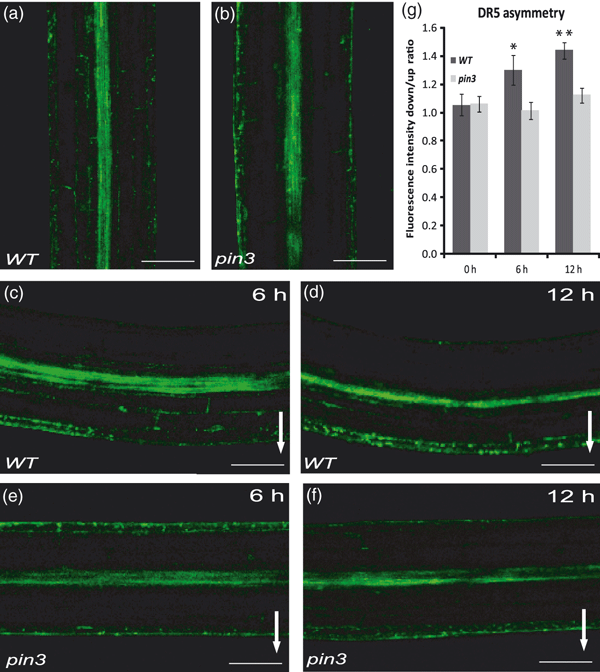
Asymmetric distribution of the auxin responses during hypocotyl gravitropism.(a–f) Expression of the DR5rev::GFP auxin response reporter in wild type (a, c, d) and pin3 (b, e, f) hypocotyls. Controls without stimulation (a, b), and stimulation for 6 h (c, e) or 12 h (d, f). More pronounced asymmetry in the DR5 activity between the upper and lower sides of wild-type hypocotyls after gravistimulation (c, d), as compared with the pin3 mutant (e, f). Arrows mark direction of gravity. Scale bar: 100 μm.(g) Quantification of DR5rev::GFP fluorescence intensity at the lower side of hypocotyls in the wild type and pin3 mutant after different times of gravistimulation. Values are the average of three biological replicates (n > 10 per time point on each replicate). Error bars are SEs (Student’s t-test, *P < 0.1, **P < 0.001).
Gravity induces rearrangements of PIN3 localization in hypocotyl endodermis cells
Next, we studied the mechanism by which gravity triggers the redirection of the PIN3-dependent auxin flow to the lower side of hypocotyls. Gravistimulation has been shown to induce changes in the polar PIN3 localization in roots, where PIN3 relocates towards the bottom side of columella cells following gravistimulation (Friml et al., 2002a; Harrison and Masson, 2008; Kleine-Vehn et al., 2010). Therefore, we analysed the effect of gravistimulation on the localization of the functional PIN3-GFP (Žadníkováet al., 2010) in hypocotyls. As PIN3 is strongly expressed in the hypocotyl endodermis (starch sheath) (Friml et al., 2002a), a strong PIN3-GFP signal was detected at both the inner and outer lateral sides of endodermal cells, and weaker expression was found in cortical and epidermal cells (Ding et al., 2011; Figure 3a). We performed the experiments in the dark to avoid any influence of light on PIN3 localization (Ding et al., 2011). After gravistimulation by placing the hypocotyl horizontally, an asymmetry in the PIN3 localization gradually developed in endodermal cells: a strong PIN3-GFP signal persisted at the outer sides of endodermal cells at the lower hypocotyl side, whereas at the outer cell sides at the upper hypocotyl side the signal gradually became weaker (Figure 3a–c,g–j). This generation of asymmetry in PIN3 localization following gravistimulation, hereafter termed ‘gravity-induced PIN3 polarization’, could be visualized mainly in the outer sides of endodermal cells because the inner sides of endodermal cells were largely obscured by the strong GFP signal in the middle vascular cylinder. Therefore, measurements were performed comparing the PIN3 signal in the outer sides of endodermal cells at the upper versus lower side of endodermal cells. Notably, these changes of PIN3 localization following gravistimulation were not observed along the whole hypocotyl, but were restricted to the gravity-responding, more apical part (Figure 3k). Genetic data pointed to PIN4 as an additional regulator of the gravitropic response in the absence of PIN3. However, PIN4 expression level in the hypocotyls was very weak and only present in epidermal cells (data not shown). The other closer homolog of PIN3, PIN7, which does not play a major role in hypocotyl gravitropism (Figure 1c,e) was present in endodermal cells. However, in contrast to PIN3-GFP, PIN7-GFP did not show any visible changes in polarity (Figure 3d–f, j).
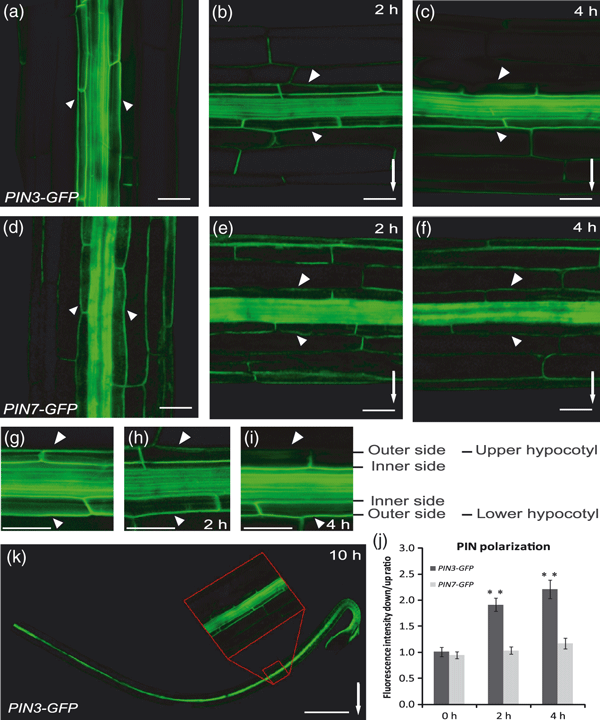
Gravity-mediated PIN3 polarization.(a–c) PIN3-GFP in the upper, gravity-responsive part of the hypocotyl. The GFP signal was detected at the outer and inner sides of endodermal cells (a). Following gravistimulation, the PIN3-GFP localization disappeared from the upper side of endodermal cells of the upper hypocotyl half, but was difficult to visualize in the lower hypocotyl half because of the strong GFP fluorescence in the inner vascular cylinder cells (b, c).(d–f) PIN7-GFP in the upper, responsive hypocotyl part. The GFP signal was visible at the outer and inner membranes of endodermal cells (d). Following gravistimulation, no pronounced change in the PIN7-GFP localization was seen (e, f). Scale bar: 50 μm.(g–i) Details of gravity-induced PIN3-GFP polarization. Gradual disappearance of the GFP signal at the outer sides of the endodermis cells in the upper hypocotyl halves (h, i). Non-stimulated control (g). Scale bar: 50 μm.(j) Ratio of PIN3-GFP and PIN7-GFP fluorescence intensities at the outer side of endodermis cells at the lower versus upper hypocotyl sides after different times of gravistimulation. Values are the average of three biological replicates (n > 10 per time point on each replicate). Error bars are SEs. (Student’s t-test, **P < 0.0001). (k) Approximate position at the hypocotyl where gravity-induced PIN3-GFP polarization was observed. Arrows and arrowheads indicate gravity direction and the GFP localization at the outer sides of endodermal cells, respectively. Scale bar: 0.8 mm.
To test specifically the role of endodermal PIN3 in hypocotyl gravitropism, and also better visualize PIN3 polarity changes at the inner sides of endodermis cells, we placed the PIN3 coding sequence under the control of the SCARECROW (SCR) promoter that drives expression exclusively in endodermis (Wysocka-Diller et al., 2000). The expression level of SCR::PIN3-YFP in the endodermis of pin3 mutant was visibly lower but showed a normal, apolar signal similar to that observed in PIN3::PIN3-GFP hypocotyls (Figure 4a). Following gravistimulation, we measured the PIN3-YFP fluorescence intensity and observed the progress of the asymmetry in PIN3-YFP localization, reflected by the gradual disappearance of the signal from the upper sides of endodermal cells (outer side in the upper half and inner side in the lower half). Importantly, the absence of the strong fluorescence signal in the vascular cylinder of SCR::PIN3-YFP also allowed us to observe the gravity-induced changes in PIN3 localization at the inner sides of endodermal cells (Figure 4b,c). Notably, the SCR::PIN3-YFP rescued to a significant extent the gravitropic defect of pin3 mutant hypocotyls (Figure 4d–h), strongly suggesting that the PIN3 action specifically in endodermis cells is sufficient to mediate the hypocotyl gravitropic response. The incomplete rescue might result from the much weaker expression of SCR::PIN3-YFP, as compared with PIN3::PIN3-GFP.
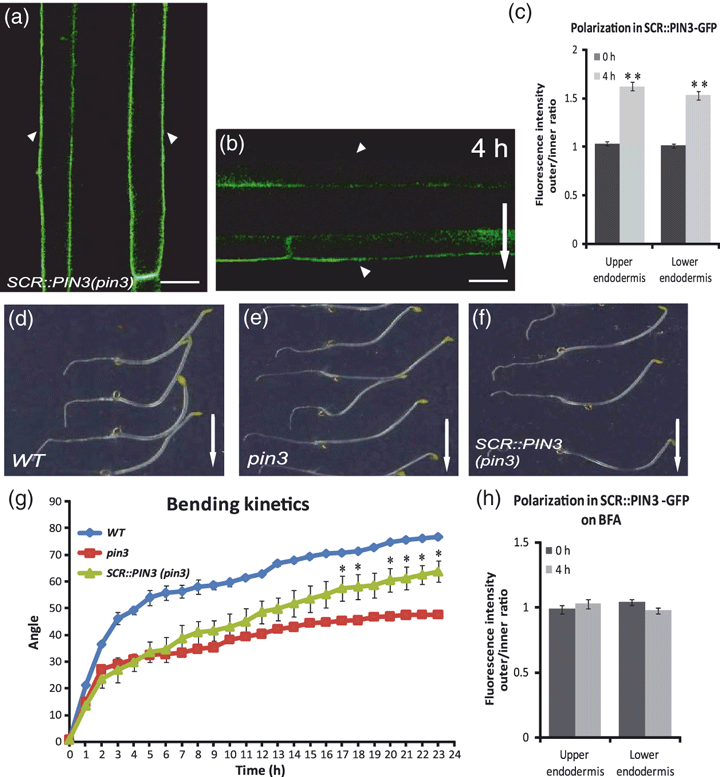
Gravity-induced polarization in the endodermis of SCR::PIN3-YFP.(a, b) pin3 mutant expressing PIN3-YFP under the control of the SCR promoter. PIN3-YFP localizes exclusively in the endodermal cells of the seedling. Image of the gravity-responsive hypocotyl part of the pin3 mutant carrying SCR::PIN3-YFP showing a symmetric signal between both sides (a). Following gravistimulation, PIN3-YFP disappeared from the upper sides of endodermal cells in both upper and lower hypocotyl halves (b). Arrowheads indicate the YFP localization at the outer side of endodermal cells. Scale bar: 20 μm.(c) Ratio of PIN3-GFP fluorescence intensities at the outer and inner sides of upper and lower endodermis cell sides after different times of gravistimulation. Values are the average of three biological replicates with at least 10 seedlings measured for each time point. Error bars are SEs (Student’s t-test: **P < 0.0001).(d–g) SCR::PIN3-YFP significantly rescued the hypocotyl gravitropism defect of pin3 after 24 h of gravistimulation (d–f). Arrows indicate gravity direction. Bending kinetics of wild-type, pin3 and SCR::PIN3-YFP in pin3 background (g). Values are the average of one biological experiment (n > 10 per time point on each replicate). Error bars are SDs (Student’s t-test: *P < 0.03). Similar results where obtained with other SCR::PIN3-YFP lines.(h) Ratio of PIN3-GFP fluorescence intensities at the outer and inner sides of upper and lower endodermis cells after different times of gravistimulation on brefeldin A (BFA). Values are the average of three biological replicates (n > 10 per time point on each replicate). Student’s t-tests show no significant differences.
Overall, the gravity-induced rearrangements in the PIN3 localizations resulted in the polarization of PIN3 towards the lower side of endodermal cells of hypocotyls perpendicular to the gravity vector. Here, PIN3 is perfectly positioned to redirect auxin flow from the basipetal stream in the centre of the hypocotyl towards its lower side, where auxin accumulates to drive growth for gravitropic bending.
Gravity induces the translocation of PIN3 in hypocotyl endodermal cells
Next, we addressed the cellular mechanism underlying the PIN3 polarization in response to gravity. Conceptually, the rearrangements of the PIN3 localization can result from de novo protein synthesis, degradation and translocation, or a combination of these processes.
First, we examined whether protein synthesis is involved in PIN3 polarization during the hypocotyl gravitropic response. We blocked protein biosynthesis with cycloheximide (CHX) and observed normal PIN3-GFP polarization following gravistimulation (Figure S1f). For longer CHX treatments, as expected, no hypocotyl growth and bending occurred (Figures S1c,g and S2d). Next, we tested the effect of proteolytic protein degradation using MG132 treatment that also affects the differential degradation of PIN2 during root gravitropism (Abas et al., 2006). MG132 had no visible effect on gravity-induced PIN3 polarization (Figure S1d), or on hypocotyl growth or gravitropic bending (Figures S1b,e and S2c). Accordingly, the snx1 mutant defective in PIN2 degradation (Jaillais et al., 2006; Kleine-Vehn et al., 2008) showed normal hypocotyl gravitropism (Figure S1h,i). These results show that de novo protein synthesis or proteolytic protein degradation are not the major mechanisms underlying gravity-induced PIN3 polarization. This suggests that similar to gravity-induced PIN3 polarization in the root cap (Kleine-Vehn et al., 2010), PIN3 polarization in the hypocotyl is also achieved by a translocation of PIN3 protein from the pre-existing protein pool towards the lower side of endodermal cells.
GNOM ARF GEF is required for gravity-induced PIN3 translocation
The PIN proteins are recycled constitutively between the plasma membrane and the endosomes (Geldner et al., 2001; Dhonukshe et al., 2007). An endosomal membrane-associated guanosine-nucleotide exchange factor on the ADP-ribosylation factors (ARF GEF), GNOM, mediates PIN trafficking to the plasma membrane and is inhibited by the fungal toxin brefeldin A (BFA) (Geldner et al., 2003). BFA treatment interfered with PIN3-GFP polarization in response to gravistimulation, as manifested by the persistent PIN3::PIN3-GFP and SCR::PIN3-YFP localizations at both the outer and inner sides of endodermal cells (Figure 5a–c; compare with 3, 4). Accordingly, BFA also affected the establishment of asymmetry in DR5-monitored auxin responses and hypocotyl bending after gravitropic stimulation (Figure 5g–i). These observations revealed that both PIN3 polarization in response to gravity as well as the downstream events require the action of BFA-sensitive ARF GEFs.
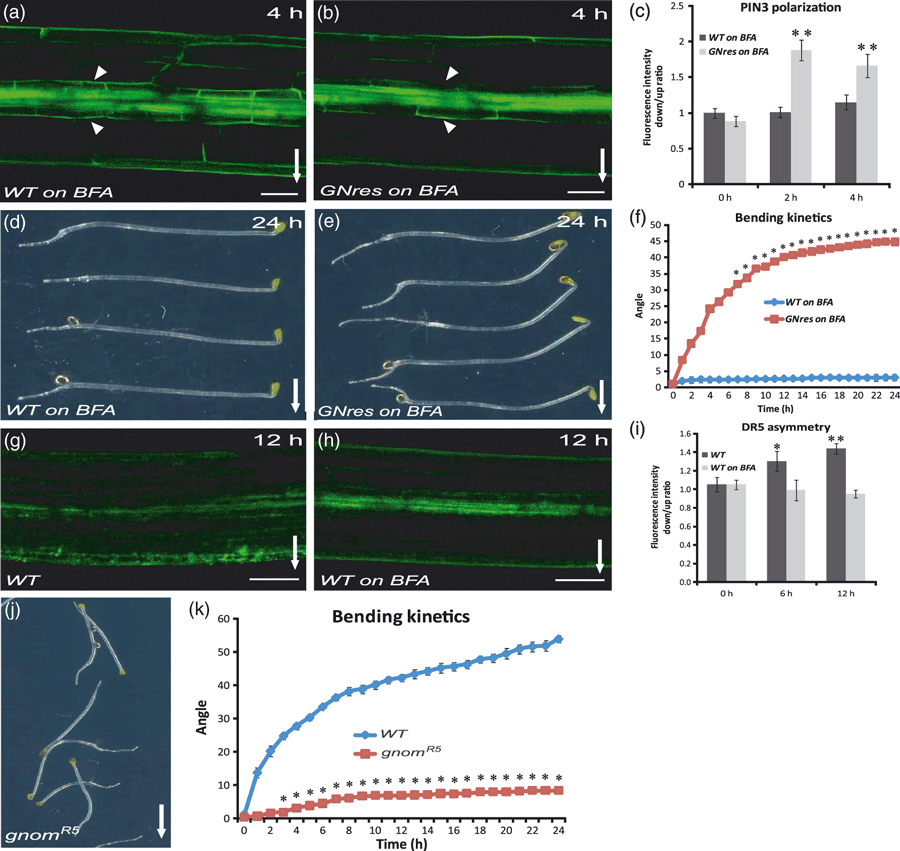
ARF GEF GNOM action in gravity-induced PIN3 polarization.(a–c) Brefeldin A (BFA) effects on PIN3-GFP polarization in hypocotyl endodermal cells after 4 h of gravistimulation. BFA treatment (50 μm) interfered with the PIN3 polarization in the wild type (a), but not in the BFA-resistant GNOMM696L line (b). Scale bar: 100 μm. Ratio of PIN3-GFP fluorescence intensity at the outer side of endodermis cells at the lower versus upper hypocotyl side after different times of gravistimulation on BFA (c). Values are the average of three biological replicates (n > 10 per time point on each replicate). Error bars are SEs (Student’s t-test, **P < 0.0001).(d–f) BFA treatment interferes with the hypocotyl bending of the wild type (d), but not of the BFA-resistant GNOMM696L line (e) after 24 h of gravistimulation. Bending kinetics of wild-type and BFA-resistant GNOMM696L hypocotyls on BFA (f). Values are the average of three biological replicates (n > 10 per time point on each replicate). Error bars are SEs (Student’s t-test, 1–5 h, P < 0.03; 6–24 h, *P < 0.0001).(g–i) BFA treatment interferes with the establishment of asymmetric DR5rev::GFP activity after 12 h of gravistimulation (h), as compared with the untreated control (g). Scale bar: 50 μm. Ratio of DR5rev::GFP fluorescence intensity at lower and upper hypocotyl sides after different times of gravistimulation (i). Values are the average of three biological replicates with at least 10 seedlings measured for each time point. Error bars are SEs (Student’s t-test, *P < 0.1, **P < 0.0004). Arrows and arrowheads indicate gravity direction and the GFP localization at the outer sides of endodermal cells, respectively.(j, k) Bending of wild-type and gnomR5 hypocotyls. (k) Hypocotyl bending kinetics of the wild type and gnomR5 mutant. Hypocotyl curvatures were measured every hour and average curvatures were calculated. Values are the averages of two biological replicates (n > 10 per time point on each replicate). Error bars are SEs (Student’s t-test, *P < 0.001).
To test whether GNOM is the specific ARF-GEF component that is needed for the BFA-sensitive PIN3 polarization, we used the GNOMM696L line carrying the engineered BFA-resistant version of GNOM (Geldner et al., 2003). In GNOMM696L hypocotyls, the gravity-induced polarization of PIN3 (Figure 5b,c) and the gravitropic bending proceeded largely as normal, even after treatment with BFA (Figure 5e,f), demonstrating that the inhibition of GNOM specifically causes the strong effect of BFA on the PIN3 polarization, and hence on the gravitropic response. As a control, we confirmed that BFA did not cause a defect in hypocotyl growth in this line (Figure S2b). Additionally, the role of GNOM was confirmed using the weak gnomR5 loss-of-function allele (Geldner et al., 2004) that shows strongly reduced root (Kleine-Vehn et al., 2010) and hypocotyl (Figure 5j,k) gravity responses.
Thus, our observations imply that the gravity-dependent PIN3 polarization, the downstream asymmetric auxin distribution and gravitropic response, both in root (Kleine-Vehn et al., 2010) and hypocotyls, require BFA-sensitive, GNOM-dependent trafficking. Notably, these observations further correlate the PIN3 polarization with functional gravitropic responses.
PID kinase is involved in PIN3 polarization and hypocotyl gravitropism
An important component of the PIN polarity regulation is the serine/threonine protein kinase PINOID (PID; Christensen et al., 2000; Benjamins et al., 2001; Friml et al., 2004; Sukumar et al., 2009; Ding et al., 2011). PID phosphorylates PIN proteins (Michniewicz et al., 2007), and PIN phosphorylation is sufficient to change the polarity of PIN localization (Huang et al., 2010; Zhang et al., 2010). Thus, the levels of PID-dependent phosporylation within cells largely contribute to the decision on the polar targeting of PIN proteins (Friml et al., 2004; Michniewicz et al., 2007). To test whether the PID-based mechanism is involved in the PIN3 polarization during gravitropic responses, we analysed the 35S::PID seedlings that strongly and constitutively express PID (Benjamins et al., 2001). Gravity-dependent PIN3 polarization did not occur in the 35S::PID plants (Figure 6a–d). Accordingly, a time-lapse analysis revealed that 35S::PID hypocotyls have a strongly disrupted gravitropic response (Figure 6e,f), but normal hypocotyl growth (Figure S2a).
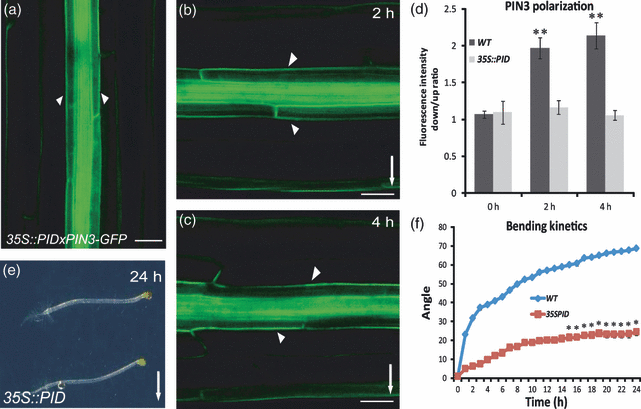
PINOID action in gravity-induced PIN3 polarization.(a) Non-stimulated 35S::PID control.(b, c) No gravity-induced PIN3-GFP polarization in the 35S::PID hypocotyls after 2 and 4 h of gravistimulation, respectively. Arrowheads indicate the GFP localization at the outer sides of endodermal cells. Arrows indicate gravity direction. Scale bar: 50 μm.(d). Ratio of PIN3-GFP fluorescence intensity at the outer side of endodermis cells at the lower versus upper hypocotyl side after different times of gravistimulation. Values are the average of three biological replicates, with at least 10 seedlings measured for each time point. Error bars are SEs (Student’s t-test, **P < 0.0001).(e) Reduced hypocotyl gravitropic response in the 35S::PID line.(f) Hypocotyl bending kinetics of the wild type and the 35S::PID line following gravistimulation. Values are the average of three biological replicates (n > 10 per time point on each replicate). Error bars are SEs (Student’s t-test, 1–14 h, P < 0.001; 16–24 h, *P < 0.0001).
Next, we studied the hypocotyl gravitropism of pid loss-of-function seedlings. We used wag1 wag2 pid triple-mutant lines lacking activity of PID and the closest homologues WAG1 and WAG2 (Cheng et al., 2008; Dhonukshe et al., 2010). In contrast to the gain-of-function allele, the wag1 wag2 pid mutant hypocotyls showed gravity-induced PIN3 polarization (Figure S3d–f) and hypergravitropic hypocotyl growth (Figure S3a–c). These observations suggest that the gravity-dependent PIN3 polarization and downstream gravitropic response involve the PID-dependent regulation of the PIN polar targeting.
Discussion
Reorientation of plant growth according to gravity anchors plants in the soil, helps roots exploit nutrient-containing soils and orients shoots to maximize their exposure to the sun. The underlying mechanism involves a cascade of signalling events, starting with gravity perception, and including the asymmetric distribution of the signalling molecule auxin that mediates differential growth rates, ultimately leading to the bending of the stimulated organ. However, the connections between these events are still not well understood (Perrin et al., 2005; Esmon et al., 2006; Muday and Rahman, 2007).
Here we provide insight into the mechanism underlying hypocotyl gravitropism, in particular, on how the gravity signal is translated into the asymmetrical auxin accumulation, thus aligning the directions of the gravity vector and shoot growth. In hypocotyls, gravity is perceived by the sedimentation of starch-containing amyloplasts in endodermal cells (Morita, 2010). In the same cells the originally symmetrically localized PIN3 auxin transporter polarizes to the lower side of the endodermis in response to gravistimulation. Such localization presumably diverts auxin flow to the lower side of hypocotyls, where auxin accumulates and stimulates growth to promote the gravitropic bending. This PIN3 polarization in the hypocotyl requires a known component of PIN trafficking, GNOM ARF GEF, and involves the regulator of PIN polarity, the protein kinase PID.
This process is largely analogous to PIN3 polarization in gravity-sensing root cap cells (Friml et al., 2002a; Harrison and Masson, 2008), but the root gravitropic response seems to be mediated by the redundant action of several PIN proteins (Kleine-Vehn et al., 2010), whereas PIN3 seems to be the major factor in the hypocotyls. Thus, the gravity-induced polarization of PIN proteins is the common signal transduction mechanism for both the positive gravitropic growth of roots and the negative gravitropism of shoots. In hypocotyls, PIN3 is mainly expressed in the endodermis and responds to gravity, whereas its closer homologues PIN4 and PIN7 are not highly expressed there or don’t respond to gravity, respectively. Hence, in a wild-type situation, PIN3 is the major factor, but in its absence, PIN4 can take part of the function. Notably, both PIN3 and PIN4 are expressed in the epidermis, where they might also contribute to the gravitropic response, but these epidermal roles remain unclear.
It has been recently shown that PIN3 polarization is also involved in the hypocotyl phototropic response. The light-induced PIN3 polarization in the endodermal cells appears to be at the heart of this process, and also depends on the PIN trafficking regulators GNOM and PID (Ding et al., 2011). However, whereas overexpression of PID negatively affects PIN3 relocation in both the phototropic and gravitropic responses, the opposite effects of the pid triple mutant between the two different tropic responses points to a more complex role of PID and related kinases in gravitropism. Whereas in the phototropic response, light-mediated transcriptional repression of PID links light perception and PIN3 polarization (Ding et al., 2011), in gravitropism, PID activity seems to be required to fine-tune the extent of hypocotyl bending and prevent a hyper-response to gravity.
The major open questions for future investigations concern the mechanism by which the initial event in gravity perception, the statolith sedimentation, is translated into PIN3 polarization, and how PINOID-mediated phosphorylation and ARF-GEF-mediated trafficking are exactly involved in this mechanism.
Experimental procedures
Plant material
The published transgenic and mutant lines were: DR5rev::GFP (Friml et al., 2003); PIN3::PIN3-GFP (Žadníkováet al., 2010); PIN7::PIN7-GFP (Blilou et al., 2005); GNOMM696L (Geldner et al., 2003); gnomR5 (Geldner et al., 2004); pin3-4 (SALK_005544); pin4-3 (Friml et al., 2002b); pin7-1 (Friml et al., 2003); 35S::PID (Benjamins et al., 2001); wag1 wag2 pid (Dhonukshe et al., 2010); and snx1 (Jaillais et al., 2006). The SCR::PIN3-YFP construct was cloned using Gateway technology. The PIN3-YFP genomic coding sequence (Žadníkováet al., 2010) and the SCR 2500-bp promoter region were cloned into donor vectors pDONR221 and pDONRP4P1R, respectively. The expression clone was generated by recombining both fragments into the expression vector pB7m24GW, and was then transformed to Arabidopsis (Col-0). Mutant combinations with DR5rev::GFP and PIN3::PIN3-GFP were generated through genetic crosses.
Growth conditions
Seeds were sown on plates with Arabidopsis medium and stratified at 4°C for 4 days. Germination was induced by placing the plates in light for 5–6 h, which were then transferred to darkness and kept at 19°C for 4 days. For gravitropic stimulation, plates with 4-day-old seedlings were turned 90°. For confocal microscopy, an LSM 510 or Exciter confocal scanning microscope (Zeiss, http://www.zeiss.com) was used. To monitor the gravitropic response, plates were scanned 24 h after gravistimulation. Images were processed in Adobe photoshop. Each experiment was performed at least three times.
Real-time analysis of hypocotyl gravitropism
Bending of seedlings after gravitropic stimulation was recorded at 1-h intervals for 1 day at 19°C. The experimental set-up consisted of an infrared light source (880-nm LED; Velleman, http://www.velleman.eu) and a spectrum-enhanced camera (EOS035 Rebel Xti, 400DH; Canon, http://www.canon.com) with a built-in clear wideband multicoated filter and standard accessories (Canon), and operated by the EOS utility software. Angles and fluorescence intensity were measured by imagej (National Institutes of Health; http://rsb.info.nih.gov/ij). The rate of the fluorescence intensity of PIN3-GFP was compared between the external plasma membrane sides of endodermal cells. The rate of the fluorescence of DR5rev::GFP was compared between the lower and upper side of the hypocotyl in the responsive part. Three replicates of at least 15 seedlings with a synchronized germination start were processed.
Pharmacological treatments
Wild-type seedlings were germinated and grown on mock medium. BFA, MG132 and CHX treatments in the dark were performed by transfer and incubation of 4-day-old etiolated seedlings on solid medium supplemented with BFA (50 μm), MG132 (25 μm) or CHX (50 μm). Seedlings treated for 1 h on plates containing BFA, MG132 or CHX were subsequently gravistimulated for bending-angle measurements or for imaging. For all comparisons, at least three independent experiments were performed with the same significant results.
Acknowledgements
We thank: Thierry Gaude, Gerd Jürgens and Remko Offringa for sharing published materials; the Nottingham Arabidopsis Stock Centre (NASC) for seed stocks; and Martine De Cock for help in preparing the manuscript. This work was supported by grants from the Research Foundation-Flanders (Odysseus) and GA ASCR (IAA601630703) to JF, and the ERC junior research award to EB. MV is a postdoctoral fellow of the Research Foundation-Flanders (FWO).




