Monitoring changes in alternative precursor messenger RNA splicing in multiple gene transcripts
Summary
Alternative splicing (AS) increases the proteomic and functional capacity of genomes through the generation of alternative mRNA transcripts from the same gene. AS is now estimated to occur in a third of Arabidopsis and rice genes, and includes genes involved in the control of growth and development, responses to stress and signalling. Regulation of AS reflects the interactions between positive and negative cis sequences in the precursor messenger RNA and a range of trans-acting factors. The levels and activities of these factors differ in different cells and growth conditions. To identify changes in AS in multiple genes simultaneously, we have established a reproducible RT-PCR panel that can analyse 96 alternative splicing events and accurately measure the ratio of alternatively spliced products. This procedure detected statistically significant changes in AS in different plant organs, in plants grown under different light and day-length conditions, and in plants overexpressing splicing factors. The system provides a convenient, medium-throughput means of monitoring changes in AS in multiple genes. It can readily be applied to much larger or targeted sets of gene transcripts to generate information on the significance and regulation of AS in plant growth and development, specific processes and responses to external stimuli.
Introduction
Precursor messenger RNA (pre-mRNA) splicing is the excision of intron sequences from mRNAs mediated by the spliceosome. The accuracy of splicing depends on the recognition of intron and exon signals by protein factors that determine spliceosome assembly. Alternative splicing (AS) produces more than one mRNA from a single gene through the selection and utilization of alternative splice sites in the pre-mRNA (Black, 2003; Blencowe, 2006; Graveley, 2001; Matlin et al., 2005). AS is widespread in higher eukaryotes, with up to 74% of human multi-exon genes containing one or more AS events (Brett et al., 2002; Johnson et al., 2003; Modrek and Lee, 2002). The main consequences of AS are changes in protein structure/function and regulation of gene expression. The inclusion or exclusion of whole or parts of exons or introns can alter protein domains, activity, localization, interactions with other proteins or substrates, and post-translational modification (Black, 2003; Graveley, 2001; Kriventseva et al., 2003; Lareau et al., 2004; Maniatis and Tasic, 2002; Stamm et al., 2005). As such, AS increases the protein complexity of an organism, and gene expression can be affected directly by AS of transcription and splicing factors that modulate DNA recognition and binding, transcriptional complex assembly, and pre-mRNA splicing. In addition, AS can affect mRNA stability and turnover, because many alternatively spliced transcripts contain premature termination codons (PTCs) that are potential substrates for non-sense-mediated decay (NMD), which recognizes PTCs and targets the mRNA for degradation (Maquat, 2004). Although around 35% of human alternatively spliced transcripts contain PTCs (Lewis et al., 2003), NMD may only be involved in regulating transcript levels of a small number of these transcripts (Pan et al., 2006).
The mechanisms of selection of alternative splice sites involve the recognition of cis-acting splicing signals by RNA binding factors to enhance or suppress the use of a particular splice site (Black, 2003; Maniatis and Tasic, 2002; Matlin et al., 2005; Smith and Valcárcel, 2000). Signal sequences are either classical intron splice site or polypyrimidine tract sequences, or sequences found in introns or exons called exonic/intronic enhancers or suppressors (ESE/ISE and ESS/ISS, respectively). Splice-site choice is determined by virtue of their position relative to competing splice sites, and/or through interactions or interference with other proteins or complexes. Factors involved in alternative splice site regulation are arginine/serine-rich (SR) proteins and heterogenous nuclear ribonucleoproteins (hnRNPs) or specific regulatory proteins, which affect the AS of particular gene transcripts or sets of transcripts. SR and hnRNP proteins are families of RNA-binding proteins that have multiple roles associated with splicing, mRNA transport and translation. SR proteins usually contain one or two RNA binding domains of the RNA recognition motif (RRM) type and a reversibly phosphorylated arginine- and serine-rich domain (Fu, 1995; Graveley, 2000). They generally recognize ESE/ISE sequences to promote selection of associated splice sites. On the other hand, hnRNP proteins also contain RNA-binding motifs (RRM and KH domains), but generally interact with splicing sequences to interfere and inhibit selection of associated splice sites, such that SR and hnRNP proteins often act antagonistically (Black, 2003; Maniatis and Tasic, 2002; Smith and Valcárcel, 2000). Therefore, the relative levels of alternatively spliced transcripts from a wide range of genes reflect the different levels of the interacting factors in different cells, tissues or under different conditions, and is referred to as combinatorial control (Smith and Valcárcel, 2000). A second level of regulation is exemplified by the control of AS by specific factors, the expression of which is often restricted to particular cells, tissues or developmental stages. Recent evidence suggests that such factors regulate the AS of genes with related functions, thereby coordinately controlling AS in multiple genes involved in the same biochemical or developmental pathway (Blencowe, 2006; Matlin et al., 2005; Ule et al., 2005).
Bioinformatic estimates of the number of plant genes that undergo AS have increased from 7% to 36.9% (Brett et al., 2002; Campbell et al., 2006; Chen et al., 2007; Iida et al., 2004; Wang and Brendel, 2006). An experimental approach that examined sequences encoding predicted genes on chromosome 2 of Arabidopsis by generating cDNA sequences showed a similar level, with at least 29% of genes showing AS (Xiao et al., 2005). Therefore, although AS appears to occur less frequently in plants than in animal systems, it is clearly significant and affects up to a third of plant genes.
Alternatively spliced genes are involved in a range of plant functions, such as growth and development, signal transduction, responses to biotic and abiotic stress, disease resistance, flowering time, the circadian clock, metabolism and physiology, with regulatory and stress-response genes being particularly well represented (Balasubramanian et al., 2006; Dinesh-Kumar and Baker, 2000; Egawa et al., 2006; Iida et al., 2004; Jia et al., 2004; Jordan et al., 2002; Kazan, 2003; Larkin and Park, 1999; Ner-Gaon et al., 2004; Zhou et al., 2003). Patterns of AS across the genome also change in response to environmental factors and stresses, and in particular temperature (Balasubramanian et al., 2006; Iida et al., 2004). The link with stress and development involves transcriptional, post-transcriptional (including AS) and post-translational effects on various splicing factors. For example, plant SR protein genes have differential transcription patterns, undergo AS and the proteins are phosphorylated (Gao et al., 2004; Gupta et al., 2005; Iida and Go, 2006; Isshiki et al., 2006; Kalyna and Barta, 2004; Kalyna et al., 2003, 2006; Lazar and Goodman, 2000; Lopato et al., 1999; Tanabe et al., 2007). The phosphorylation state of SR proteins in animals regulates their activity in modulating splicing patterns by affecting their ability to bind RNA or interact with other factors in response to external signals (Stamm, 2002). In plants, different stresses can affect the levels of SR protein transcripts and proteins, which in turn affect the AS of downstream genes (Fung et al., 2006; Lazar and Goodman, 2000; Tanabe et al., 2007). In addition, consistent with their influence over the AS of a wide range of genes, overexpression of SR proteins and lammer kinase (a kinase involved in phosphorylating SR proteins; Savaldi-Goldstein et al., 2003) induces many developmental and growth defects, and directly effects the altered AS of pre-mRNAs of particular genes (Kalyna et al., 2003; Lopato et al., 1999). Thus, environmental and developmental cues affect the transcriptional and AS regulation of these factors, which in turn modulate AS to control metabolic and developmental processes (Balasubramanian et al., 2006; Fang et al., 2004; Golovkin and Reddy, 1998; Iida et al., 2004; Kalyna et al., 2003; Lopato et al., 1999, 2002).
Our understanding of AS in plants is not as well developed as in animal systems, because there are far fewer publicly available expressed sequence tags (ESTs), compared with human and mouse, and a lower depth of full-length sequencing of cDNAs, such that many events have not yet been detected and have incomplete annotation (Xiao et al., 2005). For the vast majority of the reported alternatively spliced plant genes there is little or no information on functional differences among the proteins produced, or any mechanistic understanding of how alternative splice sites are selected. In addition, nothing is known about higher order combinatorial control or coordination of the AS of sets of genes. The advances made in understanding AS in human and mouse are based not only on genomic and bioinformatic analyses of the large numbers of EST and cDNA data, but also on extensive RT-PCR and high-throughput exon microarrays. To begin to address some of the principles in plant AS, we have established a new AS-RT-PCR-based panel that directly measures changes in 90 AS events simultaneously. The utility of this approach was demonstrated by detecting significant changes in splicing in seedlings grown under different conditions and in transgenic lines overexpressing plant SR splicing factors.
Results
Alternative splicing events represented on an RT-PCR panel
For the 96-well format, 90 AS events from 89 genes were selected, along with six control genes that either do not undergo AS or undergo well-documented AS. Events were selected from published reports and by screening databases at the Riken Genomic Science Centre (http://rarge.gsc.riken.jp/a_splicing/index.pl), the Institute for Genomic Research (TIGR; http://www.tigr.org/tdb/e2k1/ath1/splicing_anomalies.html), the Hangzhou Genomics Institute (Zhou et al., 2003) and, latterly, the Alternative Splicing in Plants (ASIP) database (http://www.plantgdb.org/ASIP). To demonstrate the utility of the system, a range of 5′ and 3′ AS events were selected that had a minimum of 11 nt between the alternative splice sites, to increase the probability that alternative splice site usage was regulated. Of the 90 events, 31 used alternative 5′ splice sites and 59 used alternative 3′ splice sites (Table S1). The majority of events involved AS in the coding region, but events involving 5′ and 3′ untranslated region (UTR) sequences were also included (Table S1). The events were not selected on the basis of the biological function of the genes themselves. As controls, RT-PCR was carried out over intronless regions of transcripts of ubiquitin 3, ribosomal protein L12C and RSp31, constitutively spliced introns of actin 11 and FY, and the U12-dependent intron of LUMINDEPENDENS (Lewandowska et al., 2004). AS controls were rubisco activase, which is expressed constitutively and alternatively spliced with a ratio of approximately 1:1 in all tissues studied so far, and SR protein genes, the AS of which is well documented (Kalyna et al., 2006). These were included both as controls and to monitor changes in their AS in response to growth conditions.
Optimization of AS RT-PCR panel
The procedure for measuring AS changes was optimized by assessing different RNA extraction and clean-up methods, starting RNA concentrations, PCR clean-up methods and developing efficient data extraction for statistical analyses. Briefly, total RNA was isolated from 50–100 mg of ground Arabidopsis seedlings and plant organs. Following DNase treatment, RNA was purified on Qiagen columns. An RT reaction was performed using oligo dT primers, and then aliquoted into a 96-well plate. Each well contained gene-specific primers, one of which was fluorescently labelled. Primers were designed to generate PCR products of between 60 and 700 nt, and to amplify across a region undergoing AS to directly detect different AS products in the same reaction. PCR was carried out for 24 cycles (within the linear amplification range), giving an estimate of transcript levels (see below). One hundredth of the reaction mix was separated on an automated capillary DNA sequencer (ABI3730; resolution ± 0.5 nt), and the detected bands were analysed using GeneMapper software (designed for fragment/genotype analysis). RT-PCR product size and peak area were assembled in a database, and the expected alternatively spliced products were extracted. Quantification of each alternatively spliced PCR product allowed the determination of the ratio of alternatively spliced products in each reaction, and this value was used to compare treatments to identify changes in AS. At least three biological repetitions were carried out, which allowed statistical analyses to be performed.
Optimal PCR cycle numbers were determined by analysing the ratios of AS products for 24 different gene transcripts using 20–26 PCR cycles. With different cycle numbers, the ratio of alternatively spliced products was constant for highly expressed genes from 20–24 cycles (Figure 1a,b), whereas for genes with low levels of expression, products were difficult to detect with 20 cycles, but amplification ratios were constant in the 22–26-cycle range (Figure 1c), such that, overall, 22–24 cycles was optimal. The maximum standard error (SE) of the mean in these experiments was < ±5%, with the majority (22 out of 24 samples) having an SE of < ±3%. Experimental variation was determined by RT-PCR at 24 cycles of the 24 AS events, and comparing the ratios of products obtained with the same RNA in three separate experiments. The maximum standard error was ± 1.5%, with half of the AS events having a standard error of < ±0.5% (data not shown). Thus, the system is quantifiable and highly reproducible. Experiments with the full RT-PCR panel were therefore carried out at 24 cycles, and used three or four biological repetitions. The significance of detected changes in AS was determined statistically for each series of experiments.
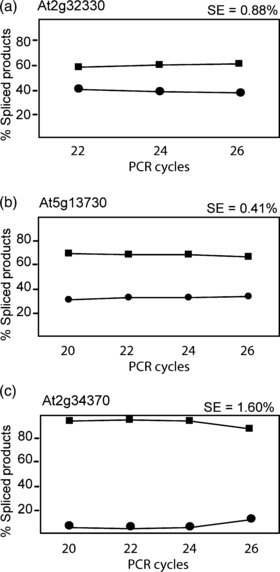
Quantification of the relative levels of alternatively spliced products with different PCR cycle regimes.Representative graphs of PCR products for different primer pairs are shown for genes with low (a) and higher (b and c) expression levels over 22–26 cycles (a) or 20–26 cycles (b and c). Circles and squares represent the percentage of the total spliced products of the two different alternatively spliced forms. The standard error (SE) between the different cycles for three technical samples is shown.
Initial experiments analysed RNA from seedlings grown under light and dark conditions, and demonstrated the ability of the RT-PCR system to detect a number of different splicing phenotypes (Figure 2). Firstly, alternatively spliced transcripts were detected for three-quarters of the AS events in the conditions used (70/89 events). Secondly, significant changes in AS were detected between light- and dark-grown seedlings for 10 of the 89 events (see below). Thirdly, even within the relatively limited number of genes in the panel and the size of regions amplified, some primer pairs showed major, unexpected RT-PCR products. Twenty of the primer pairs detected products in only one treatment, which may reflect differential transcription in light and dark. For a small number of genes either no transcripts (8/89) or only a single transcript (11/89) was detected (Figure 2). Lack of detection was not because of inefficient priming, because the primers amplified products from DNA. Lack of detection therefore reflects low overall expression levels, or expression in particular cells or tissues relative to the plant material used to extract RNA (all of the genes where transcripts were not detected have low expression, as judged by MPSS; http://mpss.udel.edu/at). Similarly, the lack of detection of an alternatively spliced product may reflect the low abundance of the alternatively spliced transcript in the particular conditions used, its occurence in a small number of cells relative to the plant material used for RNA extraction, or an AS event has been annotared by virtue of a rare EST. Finally, in two cases, the amplified products were not of the expected sizes. Manual inspection of the gene sequences identified 5′ and 3′ splice sites, which would generate the new products and would probably reflect errors in annotation.
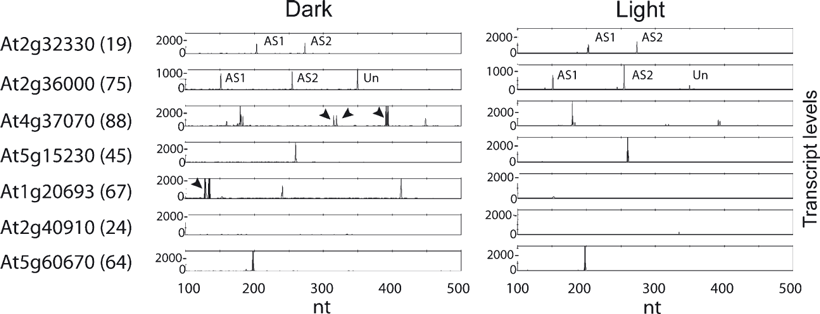
Electropherograms of RT-PCR products of various genes illustrating the range of splicing phenotypes.Transcript analysis of seven different genes in dark- and light-grown plants showing different splicing phenotypes: no change in levels of alternative splicing (AS) transcripts (At2g32330, primer pair 19); change in ratios of AS transcripts (At2g36000, 75); change in ratios of AS transcripts plus unexpected products (arrowheads) (At4g37070, 88); no AS detected (At5g15230, 45); detection of transcripts in one treatment only (At1g20693, 67); no transcripts detectable (At2g40910, 24); and actin control (At5g60670, 64). Key: AS1 and AS2, RT-PCR product representing alternatively spliced mRNAs; Un, unspliced product; arrowheads, novel products. Numbers on the x-axis represent the size markers in nt; numbers on the y-axis represent relative fluorescence, reflecting transcript abundance.
Alternative splicing in light- and dark-grown seedlings
The AS RT-PCR panel was used to monitor AS in RNAs extracted from four biological repetitions of 10-day-old seedlings grown in 16-h light/8-h dark conditions, and etiolated seedlings grown in the dark. Although the AS events on the RT-PCR panel were not selected on the basis of biological function, nine genes showed statistically significant changes (p < 0.01) in the ratios of AS transcripts between light and dark treatments (Table 1; Figure 3). Eight of the nine events showed an increased use of the distal site in the light-grown material. The consequences of the AS events showing significant changes were altered N-terminal or C-terminal sequences, the addition/loss of amino acids or the generation of premature termination codons, which potentially lead to either production of truncated proteins or NMD (Table 1; Figure 3). In some cases, AS occurred in the 5′ or 3′ UTR, such that the coding sequence of the mRNA appeared to be unaffected. These changes could potentially affect the stability or translatability of the mRNAs.
| Gene identifier | Alternative splicing events | Dark (%)a | Light (%)a | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Event | Position | Dist. (nt) | Consequence | Distal | Proximal | Distal | Proximal | ||
| At5g13730 | Alt 3′ss | IVS2 | 14 | Truncated protein – PTC | 58 | 42 | 69 | 31 | 0.001 |
| At1g71696 | Alt 3′ssbAlt3′ss | IVS3IVS11/Ex12 | 23 31 | Change in N-terminal sequence – signal peptideTruncated protein – PTC | 49 0 | 51100 | 70 0 | 30100 | 0.026 |
| At2g37340 | Alt 3′ss | IVS2 | 218 | Truncated protein – PTC | 83 | 17 | 94 | 6 | 0.033 |
| At2g43160 | Alt 3′ss | Ex2 | 18 | Alternative 5′ UTR sequences | 6 | 94 | 9 | 91 | 0.038 |
| At1g10590 | Alt 3′ss | IVS1 | 45 | Alternative ATG adds 14 aa to N-terminal – putative signal peptide | 91 | 9 | 97 | 3 | 0.049 |
| At3g57520 | Alt 3′ss | Ex14 | 34 | Alternative stop codon – changes and shortens C-terminal end | 0.5 | 99.5 | 2 | 98 | 0.053 |
| At2g36000 | Alt 5′ss | Ex1/3′ UTR | 104 | Change in C-terminal end | 44 | 56 | 38 | 62 | 0.058 |
| At3g03890 | Alt 5′ss | IVS11 | 20 | Change in C-terminal end | 98 | 2 | 99 | 1 | 0.061 |
| At2g21620 | Alt 5′ss | IVS 1 | 18 | In frame ± 6aa | 90 | 10 | 97 | 3 | 0.100 |
- Genes: At5g13730 – plastid-encoded RNA polymerase sigma subunit D; At1g71696 – carboxypeptidase; At2g37340 – RSZ33; At2g43160 – clathrin-binding protein (Epsin); At1g10590 - tyrosyl-tRNA synthetase isolog; At3g57520 – imbibition protein homolog; At2g36000 – mitochondrial transcription termination factor-related; At3g03890 – pyridoxamine 5′ phosphate oxidase-related, FMN-binding; At2g21620 – universal stress protein RD2 (auxin regulated). Abbreviations: aa, amino acids; Ex, exon; IVS, intervening sequence or intron; PTC, alternative splicing event generates a premature termination codon truncating the protein.
- aThe results are the means from four different experiments using biological repetitions. For AS events that generate truncated proteins, the figures in bold represent the percentage splicing value for the splicing event that generates the full-length protein as annotated (TAIR).
- bThe alternative 3′ splice site occurs in an alternative intron, which is spliced or unspliced in different transcripts to include or exclude a signal peptide sequence.
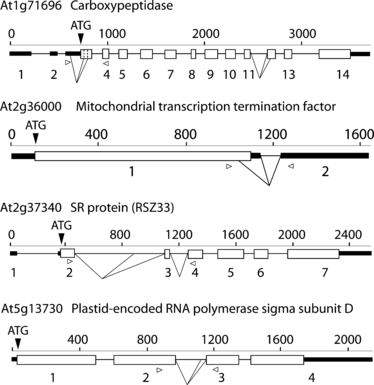
Gene structures and alternative splicing events of selected genes, with significant changes in alternative splicing (AS) between light- and dark-grown seedlings.The size of the gene, number of exons and translation start site (ATG) are indicated. The analysed AS events are indicated by lines below the gene, and the positions of the primers used are shown by open arrowheads. Key: white boxes, coding exons; black boxes, non-coding, untranslated region (UTR) exons; lines, introns.
Two of the AS events led to differential inclusion of putative signal peptides. In carboxypeptidase (At1g71696) transcripts, the alternative intron in exon 3 (Figure 3) removed the authentic translation start site, and altered the N-terminal sequence of the protein, thereby including a putative signal peptide. This gene is alternatively spliced in two different regions (Figure 3), but only the AS of exon 3 responded to the dark/light conditions. Similarly, AS of the tyrosyl tRNA synthetase (At1g10590) transcripts generates a new translation start codon, producing a putative signal peptide. Interestingly, two of the genes that showed significant changes were SR proteins, which are modulators of AS (Figure 3).
Alternative splicing in different organs of plants grown under long- and short-day conditions
Significant changes in AS were observed among different organs (root, leaf and whole flowers) in plants grown under different day length regimes using three biological repetitions. The results are presented in terms of significant changes in AS between organs (Table 2) and between long and short days (Table 3). Seventeen AS events showed significant changes between different organs, of which three also showed changes in both long- and short-day treatments. Increased use of both distal and proximal 5′ and 3′ splice sites was observed among the AS events. The genes showing significant changes in AS included AS factors (SR proteins), transcription factors, and a putative nucleic acid binding protein. Some of the largest changes were increased use of the distal 3′ splice site of At5g13730 in leaf, and of At1g09140 in leaf and flower; whereas in At4g35450 there was an increased use of the distal 5′ splice site in root (Table 2). The carboxypeptidase gene (At1g71696) showed significant changes in splice site usage among all three organs tested under long-day conditions. In short days, the transcriptional regulator, Sir2 (At5g09230) and At4g12790 had greatly increased usage of the distal 3′ splice site in leaf and flower/root respectively (Table 2). Use of the alternative splice site in exon 2 of At4g12790 either introduces premature stop codons into the authentic open reading frame (ORF) or, by selection of a second reading frame, generates part of the authentic ORF with a shorter, different N-terminal sequence that encodes a putative signal peptide. Six genes showed significant differences in AS between short- and long-day grown plants in particular organs (Table 3). Of these, three (At4g16845, At2g36000 and At2g37340) also showed significant differences among different organs (Table 2).
| Gene identifier | Event | Position | Dist. (nt) | Consequence | Leaf (%)a | Flower (%)a | Root (%)a | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Distal | Prox. | Distal | Prox. | Distal | Prox. | ||||||
| Long days | |||||||||||
| At1g71696 | Alt 3′ssb | IVS3 | 23 | Change in N-terminal sequence – signal peptide | 59 | 41 | 70 | 30 | 44 | 56 | 0.000 |
| At5g13730 | Alt 3′ss | IVS2 | 14 | Truncated protein – PTC | 77 | 23 | 60 | 40 | nd | nd | 0.001 |
| At4g16845 | Alt 5′ss | IVS5 | 22 | Truncated protein – PTC | 96 | 4 | 97 | 3 | 91 | 9 | 0.002 |
| At2g37340 | Alt 3′ss | IVS2 | 218 | Truncated protein – PTC | 92 | 8 | 94 | 6 | 97 | 3 | 0.006 |
| At5g65050 | Alt 3′ss | Ex 4 | 33 | In frame ± 11 aas | 9 | 91 | 10 | 90 | 17 | 83 | 0.008 |
| At1g09140 | Alt 3′ss | IVS10 | 338 | Truncated protein – PTC towards C-terminal | 71 | 29 | 66 | 34 | 48 | 52 | 0.039 |
| At4g35450 | Alt 5′ss | IVS1 | 44 | Alternative 5′ UTR sequences | 69 | 31 | 68 | 32 | 80 | 20 | 0.047 |
| At3g10770 | Alt 5′ss | Ex2 | 24 | In frame ± 8 aas | 6 | 94 | 8 | 92 | 5 | 95 | 0.055 |
| At3g14230 | Alt 5′ss | Ex1 | 12 | In frame ± 4 aas | 98 | 2 | 96 | 4 | 98 | 2 | 0.060 |
| At1g23970 | Alt 5′ss | IVS3 | 15 | In frame ± 5 aas | 15 | 85 | 20 | 80 | 21 | 79 | 0.076 |
| At2g36000 | Alt 5′ss | Ex1/3′ UTR | 104 | Change in C-terminal end | 36 | 64 | 38 | 62 | 49 | 51 | 0.082 |
| Short days | |||||||||||
| At1g78810 | Alt 5′ss | IVS3 | 56 | Small change in C-terminal | 74 | 26 | 80 | 20 | 72 | 28 | 0.001 |
| At2g37340 | Alt 3′ss | IVS2 | 218 | Truncated protein – PTC | 96 | 4 | 94 | 6 | 99 | 1 | 0.004 |
| At4g12790 | Alt 3′ss | 5′ UTR | 126 | uORF | 33 | 67 | 47 | 53 | 47 | 53 | 0.013 |
| At2g39730 | Alt 5′ss | IVS6 | 11 | Alternative stop codon – changes and shortens in C-terminal end | 50 | 50 | 54 | 46 | 52 | 48 | 0.049 |
| At1g52500 | Alt 3′ss | Ex8 | 158 | Alternative stop codon – changes and shortens in C-terminal end | 86 | 14 | 77 | 23 | 92 | 8 | 0.052 |
| At5g09230 | Alt 3′ss | Ex2 | 39 | Alternative 5′ UTR sequences | 41 | 59 | 29 | 71 | 28 | 72 | 0.054 |
| At3g53270 | Alt 3′ss | IVS1 | 96 | Alternative 5′ UTR sequences | 63 | 37 | 73 | 27 | 58 | 42 | 0.072 |
| At3g10770 | Alt 5′ss | Ex2 | 24 | In frame ± 8 aas | 8 | 92 | 11 | 89 | 5 | 95 | 0.076 |
| At3g57520 | Alt 3′ss | Ex14 | 34 | Alternative stop codon – changes and shortens C-terminal end | 1.5 | 98.5 | 0.7 | 99.3 | 0.5 | 99.5 | 0.081 |
| At5g65050 | Alt 3′ss | Ex4 | 33 | In frame ± 11 aas | 13 | 83 | 11 | 89 | 16 | 84 | 0.084 |
- Genes: At1g71696, carboxypeptidase; At5g13730, RNA polymerase sigma subunit D (Sig D); At4g16845, vernalization 2 protein (VRN2); At2g37340, RSZ33; At5g65050, MADS-box protein AGL31 FLM; At1g09140, SF2/ASF-like (SRp30); At4g35450, ankyrin repeat-containing protein; At3g10770, unknown function, single-stranded nucleic acid binding R3H domain containing; At3g14230, transcription factor EREBP like; At1g23970, unknown protein (Auxin regulated); At2g36000, mitochondrial transcription termination factor-related; At1g78810, expressed protein; At4g12790, hypothetical ATP binding protein; At2g39730, rubisco activase; At1g52500, formamidopyrimidine–DNA glycosylase; At5g09230, transcriptional regulator (Sir2); At3g53270, unknown; At3g57520, imbibition-protein homolog. Abbreviations: aa, amino acids; Ex, exon; IVS, intervening sequence or intron; PTC, alternative splicing event generates a premature termination codon truncating the protein; IVS, intervening sequence or intron.
- aThe results are the means from three different experiments using biological repetitions. For AS events that generate truncated proteins, the figures in bold represent the percentage splicing value for the splicing event that generates the full-length protein, as annotated (TAIR).
- bThe alternative 3′ splice site occurs in an alternative intron that is spliced or unspliced in different transcripts to include or exclude a signal peptide sequence.
| Gene identifier | Event | Position | Dist. (nt) | Consequence | Long (%)a | Short (%)a | Organ | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Distal | Prox. | Distal | Prox. | |||||||
| At4g16845 | Alt 5′ss | IVS5 | 22 | Truncated protein – PTC | 91 | 9 | 95 | 5 | Root | 0.007 |
| At1g20693 | Alt 5′ss | IVS3 | 104 | Truncated protein – PTC | 98 | 2 | 95 | 5 | Root | 0.007 |
| At5g53300 | Alt 3′ss | IVS1 | 27 | Alternative 5′ UTR sequences | 99 | 1 | 92 | 8 | Leaf | 0.016 |
| At2g36000 | Alt 5′ss | Ex1/3′ UTR | 104 | Change in C-terminal end | 38 | 62 | 44 | 56 | Flower | 0.017 |
| At2g37340 | Alt 3′ss | IVS2 | 218 | Truncated protein – PTC | 92 | 8 | 96 | 4 | Leaf | 0.030 |
| At2g37340 | Alt 3′ss | IVS2 | 218 | Truncated protein -–PTC | 97 | 3 | 99 | 1 | Root | 0.038 |
| At5g43910 | Alt 3′ss | IVS9/Ex10 | 31 | Truncated protein – PTC | 39 | 61 | 42 | 38 | Flower | 0.089 |
- Genes: At4g16845, vernalization 2 protein (VRN2); At1g20693, HMG box domain containing; At5g53300, ubiquitin-conjugating enzyme; At2g36000, mitochondrial transcription termination factor-related; At2g37340, RSZ33; At5g43910, Ribokinase. Abbreviations: aa, amino acids; Ex, exon; IVS, intervening sequence or intron; PTC, alternative splicing event generates a premature termination codon truncating the protein.
- aThe results are the means from three different experiments using biological repetitions. For AS events that generate truncated proteins, the figures in bold represent the percentage splicing value for the splicing event that generates the full-length protein, as annotated (TAIR).
Characterization of novel alternative splicing events
Twenty-three primer pairs amplified major RT-PCR products in addition to the expected products. To show that these products arose from the gene in question and represented novel AS events, we cloned products from 20 primer pairs and sequenced around 300 clones. Although not all RT-PCR products were recovered, we identified many of the new products as bona fide AS events. For example, analysis of a MADS box protein AGL31 FLM (At5g65050) identified a novel, major 328-bp product (AS3, Figure 4a). This new alternatively spliced product retained intron 3, whereas introns 2 and 4 were removed. Sequencing also identified minor products, showing utilization of a novel 5′ splice site in the upstream intron (†, Figure 4a) and a novel 3′ splice site 4 nt downstream of the authentic 3′ splice site in intron 4 (*, Figure 4a). The sizes of some of the novel RT-PCR products identified AS transcripts, which are now annotated in TAIR. For example, AS3 of the transcriptional regulator Sir2 family protein (At5g09230) represents a novel exon skipping event, which leads to the production of differently sized proteins through the presence and absence of different translational start codons (Figure 4b). Finally, in a few cases, AS was highly complex with many of the RT-PCR products being identified by either sequencing or being represented in TAIR. For example, the gene encoding an unknown protein (At3g53270) generates eight different 5′ UTR sequences, five of which were annotated in TAIR, and three of which were characterized here by cloning and sequencing (Figure 4c). These AS events included the use of alternative 5′ and 3′ splice sites, intron retention and exon skipping (Figure 4c). For the purpose of demonstrating the utility of the AS RT-PCR panel, only the results for the original selected AS event are presented.
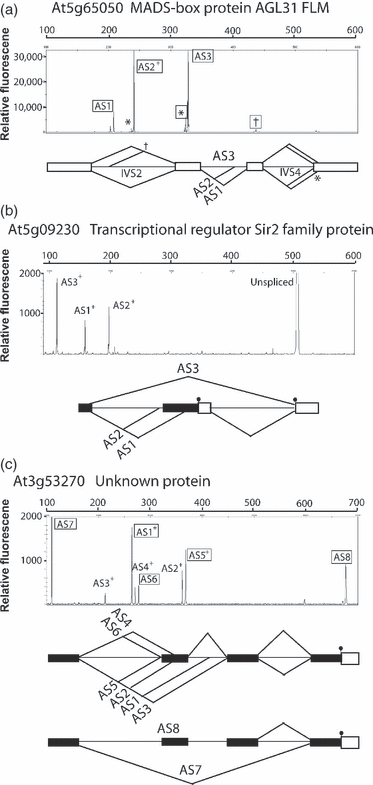
Characterization of novel alternative splicing events.Unexpected RT-PCR products were characterized by cloning and sequencing and/or comparison with TAIR (version 7). Transcript analysis of (a) At5g65050, (b) At5g090230 and (c) At3g53270. Electropherograms and schematic transcript structure of major RT-PCR products are labelled from AS1 to AS8. †A minor product using a novel alternative 5′ splice site is labelled in (a); *two products using a novel alternative 3′ splice site are labelled in (a), and were identified by sequencing. +AS events that have a TAIR model. Boxed AS labels, * and † are AS events established by cloning and sequencing, and include novel splicing events. Transcript abundance is measured in relative fluorescence units. Splicing events are shown by lines above and below the gene structures. Open boxes represent protein coding exons, black boxes represent untranslated region (UTR) exons and lines are introns. Small black discs indicate translation starts.
Alternative splicing in transgenic plants overexpressing SR proteins
SR proteins are important splicing factors in both constitutive splicing and AS. We have shown previously that in lines overexpressing SR proteins, the AS of specific transcripts is affected (Kalyna et al., 2003; Lopato et al., 1999). We examined changes in AS in transgenic lines overexpressing the plant SR proteins RSZ33 and SRp30 using three biological repetitions in each case. RSZ33 is a plant-specific SR protein, whereas SRp30 is a plant orthologue of animal SF2/ASF. The SR protein genes are themselves alternatively spliced, with the AS mainly confined to the long intron within each of the genes, and the major AS isoforms contain premature stop codons (Figure 5). Overexpression of the SR proteins caused significant changes in the AS patterns of some of the AS events on the panel, consistent with the role of SR proteins in regulating AS (Table 4; 5, 6). Overexpression of SRp30 affected the splicing pattern of its own pre-mRNAs (Figure 5), consistent with previously reported changes (Lopato et al., 1999) demonstrating the accuracy and reliability of the system. Besides the autoregulation of SRp30, overexpression of SRp30 and RSZ33 induced significant changes in the transcripts of sixteen genes on the RT-PCR panel (Table 4; Figure 6). Firstly, overexpression of RSZ33 significantly affected the AS of another SR protein, RSp31 (as previously detected; Kalyna et al., 2006), and RNA-interacting proteins, including an hnRNP-like protein (At5g66010). The reduction in use of the distal 3′ splice site in RSp31 (At3g61860) could potentially reduce the levels of this protein substantially, as this AS product encodes the protein (Table 4; Figure 6). As with the growth-condition treatments and different organs, overexpression of SR proteins caused use of either distal or proximal splice sites, leading to changes in amino acid sequence or the inclusion of premature termination codons. For example, overexpression of SRp30 led to increased the use of the distal 3′ splice site in At5g41150 (RAD1), but decreased the use of the distal 3′ splice site in At4g12790.
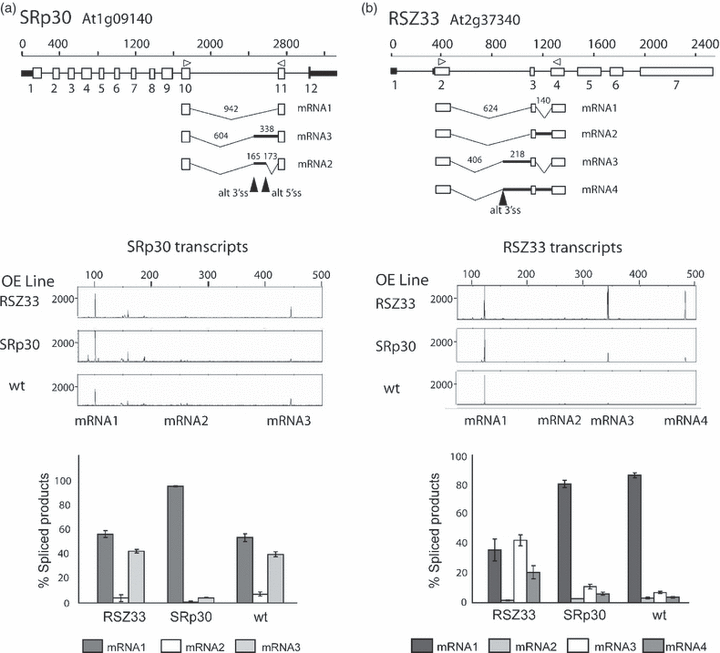
Gene structure and alternative splicing (AS) of SRp30 and RSZ33 in plants overexpressing SRp30 and RSZ33.AS events detected by RT-PCR are shown below the gene structures for SRp30 (a) and RSZ33 (b). Electropherograms and histograms demonstrate significant changes in the ratios of alternatively spliced transcripts in transgenic lines overexpressing RSZ33 and SRp30, compared with wild-type (wt) seedlings.
| Gene identifier | Alternative splicing events | Wild type (%)a | OE RSZ33 (%)a | OE SRp30 (%)a | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Position | Distance | Consequence | Distal | Prox. | Distal | Prox. | Distal | Prox. | ||
| At1g09140 | Alt 3′ss | IVS10 | 338 | Truncated protein – PTC towards C-terminal | 57 | 43 | 57 | 43 | 96 | 4 | 0.000 |
| At3g61860 | Alt 3′ss | IVS2 | 398 | Truncated protein – PTC | 97 | 3 | 42 | 58 | 97 | 3 | 0.000 |
| At5g41150 | Alt 3′ss | IVS5 | 53 | Truncated protein – PTC | 82 | 18 | 80 | 20 | 90 | 10 | 0.000 |
| At2g37340 | Alt 3′ss | IVS2 | 218 | Truncated protein – PTC | 93 | 7 | 45 | 55 | 88 | 12 | 0.000 |
| At4g12790 | Alt 3′ss | 5′ UTR | 126 | uORF | 51 | 49 | 50 | 50 | 29 | 61 | 0.000 |
| At1g04400 | Alt 3′ss | IVS 1 | 67 | Alternative 5′ UTR | 98 | 2 | 98 | 2 | 93 | 7 | 0.000 |
| At2g32330 | Alt 3′ss | Ex2 | 71 | Truncated protein – PTC | 37 | 63 | 33 | 67 | 43 | 57 | 0.001 |
| At5g04430 | Alt 3′ss | IVS5 | 63 | In frame ± 21 aas | 67 | 33 | 66 | 34 | 62 | 38 | 0.002 |
| At5g65050 | Alt 3′ss | IVS3 | 33 | In frame ± 11 aas | 16 | 84 | 18 | 82 | 15 | 85 | 0.003 |
| At5g66010 | Alt 3′ss | Ex3 | 77 | Truncated protein – PTC | 36 | 64 | 34 | 66 | 29 | 61 | 0.011 |
| At3g54790 | Alt 3′ss | Ex2 | 237 | Removes annotated ATG – alternative ATG but also uORF | 8 | 92 | 11 | 89 | 13 | 87 | 0.016 |
| At3g53270 | Alt 3′ss | IVS1 | 96 | Alternative 5′ UTR sequences | 78 | 22 | 75 | 25 | 79 | 21 | 0.034 |
| At3g55630 | Alt 5′ss | IVS5 | 66 | In frame ± 22 aas | 7 | 93 | 8 | 92 | 6 | 94 | 0.044 |
| At3g57520 | Alt 3′ss | Ex14 | 34 | Alternative stop codon – changes and shortens C-terminal end | 0.7 | 99.3 | 0.5 | 99.5 | 0.3 | 99.7 | 0.069 |
| At4g30480 | Alt 3′ss | Ex4 | 45 | Alternative stop codon – changes and shortens C-terminal end | 99.3 | 0.7 | 98.8 | 1.2 | 99.1 | 0.9 | 0.078 |
| At2g36000 | Alt 5′ss | Ex1/3′ UTR | 104 | Change in C-terminal end | 37 | 63 | 36 | 64 | 41 | 59 | 0.089 |
- Genes: At1g09140, SF2/ASF-like splicing modulator (SRp30); At3g61860, RSp31 RS-rich splicing factor; At5g41150, repair endonuclease (RAD1); At2g37340, RSZ33; At4g12790, hypothetical ATP binding protein; At1g04400, crytochrome 2 apoprotein (CRY2)(PHH1); At2g32330, tRNA His guanylyltransferase; At5g04430, putative RNA binding protein; At5g65050, MADS-box protein AGL31 FLM; At5g66010, putative RNA binding protein, hnRNP-like; At3g54790, putative armadillo repeat, U-box containing; At3g53270, unknown protein; At3g55630, tetrahydrofolylpolyglutamate synthase; At3g57520, imbibition protein homolog; At4g30480, tetratricopeptide repeat (TPR); At2g36000, mitochondrial transcription termination factor-related. Abbreviations: aa, amino acids; Ex, exon; IVS, intervening sequence or intron; OE, overexpression line; PTC, alternative splicing event generates a premature termination codon truncating the protein.
- aThe results are the means from three different experiments using biological repetitions. For AS events that generate truncated proteins, the figures in bold represent the percentage splicing value for the splicing event that generates the full-length protein, as annotated (TAIR).
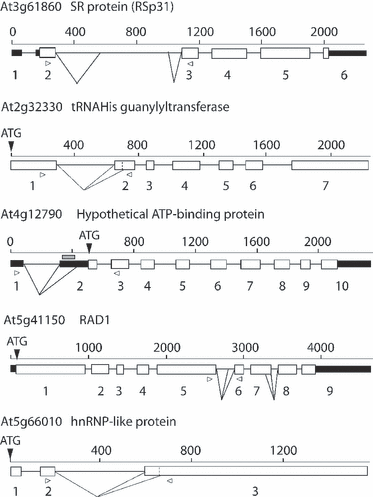
Gene structures and alternative splicing (AS) events of selected genes with significant changes in AS in plants overexpressing RSZ33 or SRp30.The size of the gene, number of exons and translation start site (ATG) are indicated. The analysed AS events are indicated by lines below the gene structures, and the positions of the primers used are shown by open arrowheads. Key: white boxes, coding exons; black boxes, non-coding, untranslated region (UTR) exons; grey box, upstream open reading frame; lines, introns.
Discussion
Recent analyses have demonstrated the widespread occurrence of AS in plants, and the range of essential functions in which it is involved (Campbell et al., 2006; Chen et al., 2007; Kazan, 2003; Wang and Brendel, 2006; Xiao et al., 2005;). The function of AS in the regulation of expression is well established in animal systems, and the growing number of characterized examples in plants demonstrates its importance. Major issues concerning AS in plants are the mechanisms of alternative splice site selection, the functions of different proteins produced from the same gene, other consequences of AS on expression, such as NMD or translational blocks, and combinatorial and coordinated control of AS. A prerequisite for many of these areas is the ability to study multiple AS events at the same time, to identify changes in patterns of AS in different cells, tissues and organs, and in plants grown under different conditions. Here, we demonstrate that the AS RT-PCR system allows the analysis of multiple AS events simultaneously, and detects significant changes in AS in different organs, in seedlings grown under light/dark and long-/short-day conditions, and following overexpression of SR proteins. The advantages of this system are that it allows direct visualization of RT-PCR products, clearly identifies product sizes, distinguishes different AS products, quantifies products, thereby allowing the determination of the ratio of products (differential splice site use) within the same reaction, and detects novel products within the amplification range. In addition, although we have concentrated on alternative 5′ and 3′ splice site usage, and have only reported on the original defined events, the system is clearly capable of detecting exon skipping and intron-retention events within a detectable size range, and quantifying multiple RT-PCR products.
Of the 89 events included on the AS RT-PCR panel, 36 showed a significant change in AS in at least one of the different treatments, organs or overexpressing lines with some transcripts, such as the SR protein, RSZ33 and the mitochondrial transcription termination factor (At 2g36000), showing significant changes in all four experiments. In a number of cases, the degree of change in AS, although significant, appeared relatively small. However, the experiments were conducted on whole organs, seedlings or plants, and thereby reflect the average level of alternatively spliced transcripts across many different cell types, such that small changes in AS may have significant biological effects in particular cells. In animal systems, tissue- or cell type-specific AS is an important mechanism for modulating function, in a highly regulated manner, in different cells or at specific developmental changes. Similarly, some genes have different levels of overall transcription under different conditions, such that the absolute levels of AS transcripts can vary greatly. The biological relevance of the AS events in this situation will require the integration of transcriptional data from microarrays with changes in AS ratios, and knowledge of the functions of the resultant proteins.
The changes in protein sequence resulting from AS ranged from the addition or loss of a small number of amino acids to major changes in N- and C-terminal sequences of proteins. Three examples involved the inclusion of putative signal peptides, which will clearly affect the localization of the protein. For well-characterized genes, such as those encoding SR proteins, the effects of AS on the domain structure of resultant proteins can be determined. However, in the majority of cases no protein domain information was available to predict changes in protein function. One of the more common outcomes was to alter the frame of the coding region generating premature termination codons in alternatively spliced isoforms. If actively turned over by NMD, the observed level of the PTC-containing transcript would be reduced. More than half of the genes where AS generated a PTC had levels of the PTC-containing transcripts lower than 20% of the total. This may suggest degradation by NMD but remains to be demonstrated, particularly as some plant PTC-containing mRNAs appear to be long-lived, including those of SR proteins (Kalyna and Barta, 2006). In addition, recent work in animal systems suggests that the number of genes regulated by NMD may be relatively small (Pan et al., 2006). Finally, some of the significant changes involved AS in 5′ and 3′ UTRs. Around 20% of plant AS occurs in UTRs (Wang and Brendel, 2006). In animal systems, AS in 5′ UTRs is often coupled to the use of differentially regulated promoters and transcription start sites affecting the N-terminal exon sequence (Mironov et al., 1999). Here, AS in 5′ UTRs either altered the N-terminal sequence of proteins or altered upstream open reading frames (uORFs), which can potentially interfere with or stimulate the translatability of the mRNA (Meijer and Thomas, 2002). Likewise, AS in the 3′ UTR can generate different 3′ exons, and can be associated with the use of differential polyadenylation sites.
Despite the increasing number of ESTs, many AS events in plants remain to be discovered (Xiao et al., 2005). The overall number of AS events makes it highly unlikely that each event is regulated by specific factors, but is instead regulated by the relative levels of general splicing and RNA binding factors in cells. Orthologues of SR proteins, splicing factors and hnRNP proteins have been identified in plants (Lorkovic and Barta, 2002; Wang and Brendel, 2004), but a direct effect on AS of pre-mRNAs of other genes has only been demonstrated for a small number of SR proteins (Isshiki et al., 2006; Kalyna et al., 2003; Lopato et al., 1999). Transgenic plants overexpressing SR proteins show major pleiotropic effects on growth and development (e.g. embryo development, bilateral symmetry, retarded growth of meristems and seedlings, and stomatal development) (Kalyna et al., 2003; Lopato et al., 1999). It is likely that such effects are the result of the inappropriate AS of many genes. We have shown here, even within the limited number of AS events analysed, that plants overexpressing SR proteins, and light/dark and short-/long-day conditions alter the splicing of transcripts of other SR proteins, RNA-interacting proteins and transcription factors, all of which can affect gene expression patterns. Thus, developmental stage, cell type, or external stimuli or stress can alter the relative levels of RNA-interacting proteins (including SR and hnRNP proteins), both transcriptionally and post-transcriptionally to modulate the splicing patterns of downstream, target genes. One of the challenges is to understand how the relative levels of such regulatory proteins bring about the combinatorial control to determine the splicing of subsets of pre-mRNAs.
Exon arrays have been used in animal systems to quantify the levels of thousands of exons and alternative exons in different cells, tissues and growth conditions (Pan et al., 2004, 2006). Exon arrays provide an important means of analysing AS globally, and are very appropriate to animals where the most common type of AS is the inclusion or skipping of whole exons. In plants, the prevalence of exon skipping is much lower, with intron retention being the most common form of AS, followed by selection of alternative 5′ and 3′ splice sites (Ner-Gaon et al., 2004; Wang and Brendel, 2006). This most likely reflects different gene structures, particularly with respect to the smaller intron size of plants compared with animals. In plants, whole genome tiling arrays offer another possibility to analyse aspects of AS, but will require further optimization combined with validation (Mockler and Ecker, 2005). Such arrays have been used to analyse intron retention in Arabidopsis, and although able to readily detect retained introns, the detection of exon skipping was unreliable, and many 5′ and 3′ AS events, spanning relatively short distances, would not be detected (Ner-Gaon and Fluhr, 2006).
Given our current level of knowledge of AS in plants and the number of potentially undescribed AS events, the RT-PCR system presented here provides a robust means to monitor significant changes in AS. However, it is important to note that discovery of AS events in plants is essential to improve our understanding of AS. We have shown here that many new AS events exist, even in the limited regions amplified in these experiments. In addition, by cloning and sequencing RT-PCR products across the whole-gene regions of a number of Arabidopsis genes, we have also detected novel, unannotated events (CS and JB; unpublished data). This approach or high-throughput sequencing approaches will be required to systematically identify AS events in genes and pathways. To fully understand the principles of AS in plants and, in particular, to address combinatorial control and co-ordinated regulation of AS in plants, the analysis of large numbers of AS events simultaneously is required. The system here lends itself to expansion to analyse several hundred events in a wide range of conditions, to create specific panels for genes expressed at low levels (allowing higher PCR cycles), and to create pathway- or process-specific AS RT-PCR panels.
Experimental procedures
Plant material and growth conditions
Arabidopsis thaliana (wild-type Columbia and overexpressing SR lines) seeds were surface sterilized by incubating in 30% v/v sodium hypochlorite, 0.02% v/v Triton X-100 for 5 min, followed by three washes in sterile 0.02% v/v Triton X-100. Sterilized seeds were resuspended in sterile 0.1% agar solution and plated out on MS10 media in Petri dishes. Plates were sealed with micropore tape, wrapped in foil and placed at 4°C for 72 h. After stratification, plates were placed in the growth room under light conditions (16-h day, 8-h night) at 23°C. For dark-grown seedlings, plates were exposed to light for 6 h before wrapping in foil, and were left in the growth room as for the light-treatment plates. Seedlings were harvested after 10 days, just prior to the development of true leaves, and were then flash frozen in liquid nitrogen and stored at −80°C. For long- and short-day-grown plantlets, seed was sown directly onto compost and incubated at 4°C for 72 h before transfer to a Snijders Microclima 1000 growth cabinet, maintained at 20°C and 70% humidity. Long-day plants were grown under 16 h of light (37 500 lx) and 8 h of dark, whereas short-day plants were grown under 10 h of light and 14 h of dark. Root, leaf and whole flower tissue was isolated from flowering plants. Transgenic lines overexpressing RSZ33 (Kalyna et al., 2003) and SRp30 (Lopato et al., 1999) (SRp30, cDNA; RSZ33, genomic) were grown as above.
RNA extraction and RT-PCR
RNA was extracted from 50–100 mg of ground tissue, as described for the RNeasy Plant Mini RNA isolation kit (Qiagen, http://www.qiagen.com). First-strand cDNA synthesis was performed on 5 μg of extracted RNA using ‘Ready-to-go you-prime’ first-strand beads (Amersham, http://www.amersham.com). The RNA was incubated for 10 min at 65°C, followed by chilling on ice for 2 min, and was then mixed with 2 μm oligo d(T)18 and beads containing all the components for reverse transcription. The reaction mix was incubated for 1 min at room temperature (21°C), mixed gently and further incubated at 37°C for 1 h. The reverse transcription reaction was diluted to a final volume of 100 μl, and 1 μl was aliquoted into a 96-well reaction plate along with 1 × PCR buffer (10 mm Tris-HCl, pH 8.3, 50 mm KCl and 3 mm MgCl2), 0.2 mm of dATP, dCTP, dGTP and dTTP (Promega, http://www.promega.com), 1.5 μm of each of the AS gene-specific primers and Taq DNA Polymerase (Roche, http://www.roche.com). The PCR reaction was incubated for 30 min at 48°C prior to performing the standard PCR reaction: 94°C for 2 min, followed by 24 cycles of 94°C for 15 sec, 50°C for 30 sec, 70°C for 1 min and completed with 10 min at 72°C (Perkin Elmer 9700, http://www.perkinelmer.com). All the reaction components apart from the primers and the cDNA were mixed prior to adding to each of the 96 PCR reactions. Specific alternative splice oligonucleotides (MWG) were selected to identify the expected alternatively spliced products, and gave products that were measurable between 60 and 700 bp. The forward primer was labelled with 6-carboxyfluoresceine (6-FAM) to visualize the RT-PCR products. A list of the primer sequences is shown in Table S1.
Splicing analysis
A 25-μl volume of the labelled RT-PCR products from the 96 simultaneous RT-PCR reactions was purified using the minElute 96-well UF PCR purification process (Qiagen). Purified RT-PCR product (1 μl) from each of the 96 reactions was suspended in 10 μl Hi Di Formamide (Applied Biosystems, http://www.appliedbiosystems.com) with 0.05 μl of GeneScan −500 LIZ internal size standard. RT-PCR fragments were separated on a 3730 DNA Analyzer (Applied Biosystems), and were then collected and analysed using Genemapper software (Applied Biosystems). RT-PCR products were accurately identified with the ± 0.5 nt resolution of the ABI 3730. The relative fluorescent peak areas for RT-PCR products with expected sizes for the alternatively spliced products were extracted, and a ratio for the AS events was calculated by dividing the value for the spliced product by the sum of the values for the alternatively spliced products. For an accurate measure of AS, the above procedure was replicated with RNA from three technical samples, for the optimization experiments described in Figure 1, and three or four biological samples for all other experiments. Mean AS efficiencies with standard errors were calculated. Means were compared by analysis of variance for the overexpression lines, light/dark and long-/short-day treatments; results were compared by analysis of variance and P values were generated. AS events with significant variation (P < 0.10) were selected.
Cloning and sequencing of RT-PCR products
RT-PCR products were cloned either from individual bands separated by polyacrylamide gel electrophoresis or by cloning of the RT-PCR reaction into pGEM-T Easy (Promega). Clones were grown in 96-well format, and plasmid DNA was extracted by Millipore’s Multi-screen Plasmid Mini-preparation protocol (Millipore, http://www.millipore.com). Sequencing was performed on plasmid templates using 1/16th reactions of ABI Big Dye v3.1, and using M13 forward and reverse primers. Samples were run through a capillary ABI3730 DNA sequencer, and the resulting sequence was analysed on Sequencher v4.5 software (GeneCodes, http://www.genecodes.com).
Acknowledgements
The work was supported by a grant-in-aid from the Scottish Government, and by the European Alternative Splicing Network of Excellence (EURASNET).