The Candida albicans ELMO homologue functions together with Rac1 and Dck1, upstream of the MAP Kinase Cek1, in invasive filamentous growth
Summary
Regulation of Rho G-proteins is critical for cytoskeletal organization and cell morphology in all eukaryotes. In the human opportunistic pathogen Candida albicans, Rac1 and its activator Dck1, a member of the CED5, Dock180, myoblast city family of guanine nucleotide exchange factors, are required for the budding to filamentous transition during invasive growth. We show that Lmo1, a protein with similarity to human ELMO1, is necessary for invasive filamentous growth, similar to Rac1 and Dck1. Furthermore, Rac1, Dck1 and Lmo1 are required for cell wall integrity, as the deletion mutants are sensitive to cell wall perturbing agents, but not to oxidative or osmotic stresses. The region of Lmo1 encompassing the ELMO and PH-like domains is sufficient for its function. Both Rac1 and Dck1 can bind Lmo1. Overexpression of a number of protein kinases in the rac1, dck1 and lmo1 deletion mutants indicates that Rac1, Dck1 and Lmo1 function upstream of the mitogen-activated protein kinases Cek1 and Mkc1, linking invasive filamentous growth to cell wall integrity. We conclude that the requirement of ELMO/CED12 family members for Rac1 function is conserved from fungi to humans.
Introduction
The ability of eukaryotic cells to reorganize their actin cytoskeleton is essential for life, as it is required for cell shape change, cell division and cell motility. Cell shape changes are also necessary for a range of specialized functions and enable adaptation to new environments. In certain tumours, for instance, cells switch from an elongated mesenchymal morphology to a rounded amoeboid morphology, depending on matrix metallo-protease activity (Wolf et al., 2003; Symons and Segall, 2009). In fungi, cell shape changes are required for processes including mating and/or substrate invasion. In addition, in pathogenic fungi, morphology changes are associated with virulence (Berman, 2006; Sinha et al., 2007). Reorganization of the actin cytoskeleton, critical for these cell shape changes, is controlled by members of the highly conserved small Rho-GTPase family, which consists of 20 G-proteins in mammalian cells compared with only six in fungi (Hall, 2005; Park and Bi, 2007). The temporal and spatial regulation of these G-proteins is therefore crucial for cell morphology changes, which are critical for responses to the environment.
Rho-GTPases, such as Rac1 (Bosco et al., 2009), are molecular switches that cycle between an inactive GDP-bound state and an active GTP-bound state (Jaffe and Hall, 2005). They are activated by guanine nucleotide exchange factors (GEF) and inactivated by GTPase activating proteins (GAP), respectively, as well as regulated by guanine nucleotide dissociation inhibitors (GDIs) (DerMardirossian and Bokoch, 2005; Buchsbaum, 2007; Tcherkezian and Lamarche-Vane, 2007). Homologues of Rac1 are present in virtually all fungi, yet are not found in yeast such as Saccharomyces cerevisiae or Schizosaccharomyces pombe (Brugnera et al., 2002). This small Rho G-protein has been demonstrated to play an important role in the development and pathogenicity of different filamentous fungi, including Penicillium marneffei, Cryptococcus neoformans, Ustilago maydis, Aspergillus nidulans and Magnaporthe grisea (Boyce et al., 2003; Vallim et al., 2005; Virag et al., 2007; Mahlert et al., 2006; Chen et al., 2008). However, little is known about how Rac1 is regulated in vivo and about what determines its specific functions, in particular with respect to the GTPase Cdc42, which it has high overall sequence identity to. Furthermore, the pathways within which the Rac1 GTPase functions remain obscure. In mammalian cells, Rac1 can be activated by members of two GEF families (Cote and Vuori, 2007): those which include the proto-oncogene Dbl, with 69 identified members (Hall, 2005; Rossman et al., 2005) and the second more recently characterized family, defined by Caenorhabditis elegans CED5, Human Dock180, Drosophila melanogaster Myoblast city (the CDM family) (Hasegawa et al., 1996; Erickson et al., 1997; Wu and Horvitz, 1998), which includes 11 identified members (Brugnera et al., 2002; Cote and Vuori, 2002). GEFs from both the Dbl and CDM families are present in fungi, including in the budding yeast S. cerevisiae (Meller et al., 2005). In the human opportunistic pathogen Candida albicans, Rac1 and its specific activator Dck1, a Dock180 homologue, are required for invasive filamentous growth (Bassilana and Arkowitz, 2006; Hope et al., 2008).
In C. albicans, the switch from budding to filamentous growth (Sudbery et al., 2004) is important for the virulence of this organism (Gow et al., 2002; Saville et al., 2003) and is triggered by diverse stimuli (Berman, 2006; Cottier and Muhlschlegel, 2009), both in liquid and solid media (Kumamoto and Vinces, 2005; Kumamoto, 2008). Similar to S. cerevisiae invasive pseudohyphal growth (Park and Bi, 2007), the mitogen-activated protein (MAP) kinase and cAMP-dependent protein kinase A (PKA) pathways, in particular, are required for C. albicans filamentous growth in response to various stimuli (Biswas et al., 2007; Roman et al., 2007; Whiteway and Bachewich, 2007; Alonso-Monge et al., 2009; Hall et al., 2009). While Rac1 and Dck1 are not required for filamentous growth in liquid media, these two proteins are necessary for invasive filamentous growth in solid media and when embedded in a matrix (Bassilana and Arkowitz, 2006; Hope et al., 2008). However, little is known about how the Rac1/Dck1 module is regulated and in which pathway these two proteins function.
Rac1 activation by members of the CDM family and subsequent function, has been shown to require additional proteins such as those that belong to the engulfment and cell motility (ELMO/CED12) protein family (Cote and Vuori, 2007; Ravichandran and Lorenz, 2007). ELMO is required for Rac1 function in a range of different organisms and biological processes, such as phagocytosis of apoptotic cells and cell motility in C. elegans (Gumienny et al., 2001; Wu et al., 2001; Zhou et al., 2001) and Dictyostelium discoideum (Para et al., 2009), cell migration and myoblast fusion in D. melanogaster (Bianco et al., 2007; Geisbrecht et al., 2008), mammalian cell migration (Grimsley et al., 2004; Katoh et al., 2006) and human cell invasion (Jarzynka et al., 2007). In the present report, we identified a protein with similarity to human ELMO1 in C. albicans and investigate its importance in this organism. Our results show that Rac1, Dck1 and Lmo1 function together in C. albicans invasive filamentous growth and cell wall integrity, upstream of the Cek1 and Mkc1 MAP kinases.
Results
Identification of a protein with similarity to ELMO/CED-12 in C. albicans
In C. elegans, D. melanogaster and mammalian cells, Rac1 signalling mediated by Dock180 requires the presence of additional proteins such as ELMO/CED-12 (Lu and Ravichandran, 2006; Cote and Vuori, 2007; Kinchen and Ravichandran, 2007; Geisbrecht et al., 2008). Members of this conserved family of scaffold proteins contain an ELMO domain and a PH-like domain (Gumienny et al., 2001; Zhou et al., 2001; Debakker et al., 2004). We searched the C. albicans genome, using the BLAST algorithm, for proteins with similarity to the human ELMO1 protein and found one ORF (19.5147) with approximately 26% sequence similarity, which will hereafter be referred to as Lmo1. This ORF is predicted to contain two exons, with an intron from base pair 56–130 (Fig. 1A). In order to confirm that this intron was spliced, RT-PCR was carried out on cDNA and gDNA samples prepared from wild-type budding C. albicans, using different primer pairs that either spanned the intron or were in the exon 2 region (Fig. 1A). Figure 1B shows the respective products amplified from gDNA and cDNA samples using primer pairs 1/2 and 3/4, in addition to actin control primers. The size of product, amplified with primer pair 1/2, indicates that it contains the intron, only when gDNA was used as a template. The amount of PCR product, amplified when cDNA was used as a template, was less than the respective products using gDNA as a template, despite similar amounts of actin PCR product. This difference can be attributed to the spliced LMO1 mRNA product being of low abundance, compared with actin mRNA transcript. Therefore in C. albicans, LMO1 is comprised of two exons with a 75-base pair intron separating them. The intron in LMO1 appears to be fully spliced in budding cells, as RT-PCR only revealed spliced mRNA transcript. Nonetheless, it remains possible that LMO1 splicing plays a role in the regulation of LMO1 expression under specific growth conditions.
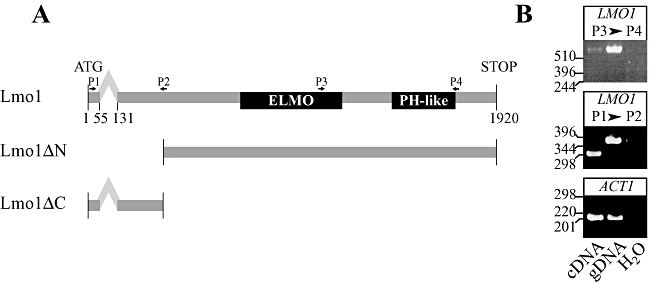
Structure of LMO1 gene.A. Schematic representation of LMO1 gene with intron and exons indicated. Numbers refer to base pair with 1 being the A of the start codon and predicted intron location from the Candida Genome Database (http://www.candidagenome.org). The location of the ELMO homology and PH-like domains are indicated. The functional N-terminal truncation (Lmo1ΔN) and the non-functional C-terminal truncation that results when the majority of exon 2 is removed in the lmo1Δ-2/lmo1Δ-2 strain are indicated. The location of PCR primers P1, P2, P3 and P4 (which corresponds to Calmo1TMp1, Calmo1TMm2, Calmo1TMp3 and Calmo1TMm4; Table 2) is indicated.B. PCR analyses of LMO1 confirm the processing of 5′ intron. cDNA and gDNA were prepared from wild-type C. albicans cells. Indicated primer pairs were used to amplify cDNA and gDNA samples. ACT1 controls reveal similar amounts of DNA in two samples. Similar results were obtained with different primer pairs.
Candida albicans LMO1 encodes a predicted protein of 614 amino acids which contains a region of 170 amino acids with similarity to the ELMO domain of human ELMO1 and a region of 101 amino acids with similarity to its PH-like domain, indicated in Fig. S1 by a black line and a grey line respectively. Candida albicans Lmo1 has 15% overall sequence identity with human ELMO1. Lmo1 has 17% sequence similarity between its ELMO domain and that of human ELMO1 and 42% sequence similarity between its PH-like domain and that of human ELMO1. We found proteins with similarity to ELMO1 in other fungal species and Fig. S1 shows an alignment of human, fly and worm CED-12/ELMO, together with those identified from C. neoformans, Aspergillus fumigatus, U. maydis and S. cerevisiae. In addition to the ELMO and PH-like domains, human, D. melanogaster and C. elegans ELMO/CED-12 proteins contain a carboxy-terminal PxxP motif which does not appear to be conserved in fungi, including C. albicans. Therefore, ELMO/CED-12 homologues appear to be present throughout the fungal kingdom, raising the possibility that their function may be conserved.
LMO1 exon 2 is necessary and sufficient for embedded filamentous growth
To investigate LMO1 function we deleted both copies of this gene. These deletion mutants were viable and grew similar to a wild-type strain (data not shown). We previously showed that Rac1 (Bassilana and Arkowitz, 2006) and Dck1 (Hope et al., 2008) are required for invasive filamentous growth, hence we investigated whether lmo1 deletion mutants were able to form filamentous colonies, when embedded in an agar matrix. Figure 2 shows that, similar to rac1 and dck1 deletion mutants (8, 10), the lmo1Δ/lmo1Δ mutant strain formed colonies that did not filament when embedded in agar. This defect in embedded filamentous growth was observed when either the entire LMO1 ORF was removed (lmo1Δ-1/lmo1Δ-1; Fig. 10A) or the majority of exon 2 (lmo1Δ-2/lmo1Δ-2; essentially the ELMO and PH-like domains) was removed (Fig. 2A), indicating that the ELMO and PH-like domains are critical for filamentous growth under embedded conditions. Conversely, we examined the function of an N-terminally truncated protein lacking its amino-terminal 93 amino acids. The embedded filamentous growth defect of the lmo1Δ-2/lmo1Δ-2 strain was fully complemented by overexpression of this truncated version of LMO1, lmo1ΔN. In contrast, this defect was only partially complemented by reintroduction of a copy of LMO1 behind its own promoter, compared with the wild-type strain, with colonies exhibiting shorter filaments (Fig. 2A). Analyses of mRNA transcript levels by RT-PCR show that the level of LMO1 expression in lmo1Δ-2/lmo1Δ-2 PLMO1-LMO1 was lower than in wild-type cells (Fig. 2B), suggesting that the partial complementation may be due to insufficient Lmo1 levels. Given that reintroduction of LMO1 behind its own promoter only partially complemented the embedded invasive growth defect of this lmo1Δ strain, we anticipated that the LMO1 heterozygote should exhibit a similar defect. Surprisingly, a heterozygote mutant of LMO1 formed filamentous colonies when embedded in an agar matrix, similar to the wild-type strain (Fig. S2A). Determination of LMO1 mRNA transcript levels qualitatively by RT-PCR and quantitatively by real-time qRT-PCR revealed that two independent heterozygote mutants (in which one copy of LMO1 was replaced by either URA3 or HIS1) had levels of LMO1 mRNA transcripts comparable with that of wild-type cells, suggesting that the LMO1 gene is auto-regulated (Fig. S2B and C). Together, these results indicate that LMO1 is required for invasive filamentous growth in an agar matrix and that the region of Lmo1 encoded by exon 2, which contains the ELMO and PH-like domains, is necessary and sufficient for this function.
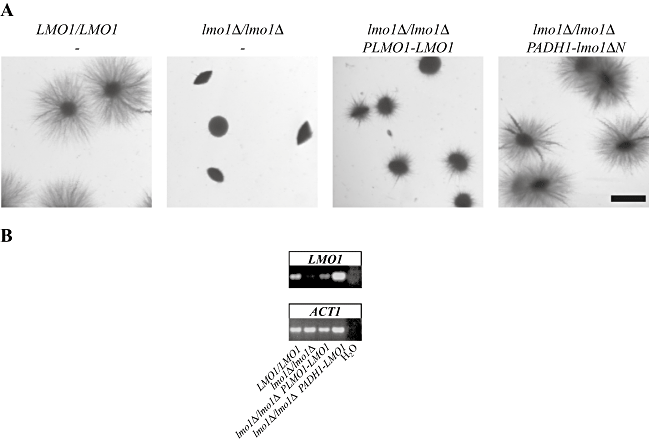
Lmo1 is required for filamentous growth in an agar matrix.A. Strains deleted from LMO1 are defective in matrix-induced filamentous growth. Cells from wild-type (PY1005), lmo1Δ-2/lmo1Δ-2 (PY1009), lmo1Δ-2/lmo1Δ-2 PLMO1-LMO1 (PY1012) and lmo1Δ-2/lmo1Δ-2 PADH1-lmo1ΔN (PY1018) strains were embedded in YEPS as described (Brown et al., 1999). The lmo1Δ-2 deletion strains all have the majority of exon 2 removed. After 5 days at 25°C, images of colonies were taken. Similar results were observed in four independent experiments. Bar, 1 mm.B. LMO1 mRNA levels in different strains analysed by RT-PCR. mRNA and cDNA were prepared from C. albicans cells with the indicated genotypes. PCR was carried out with primers CaElmo1TMpB and CaElmo1TMmB.
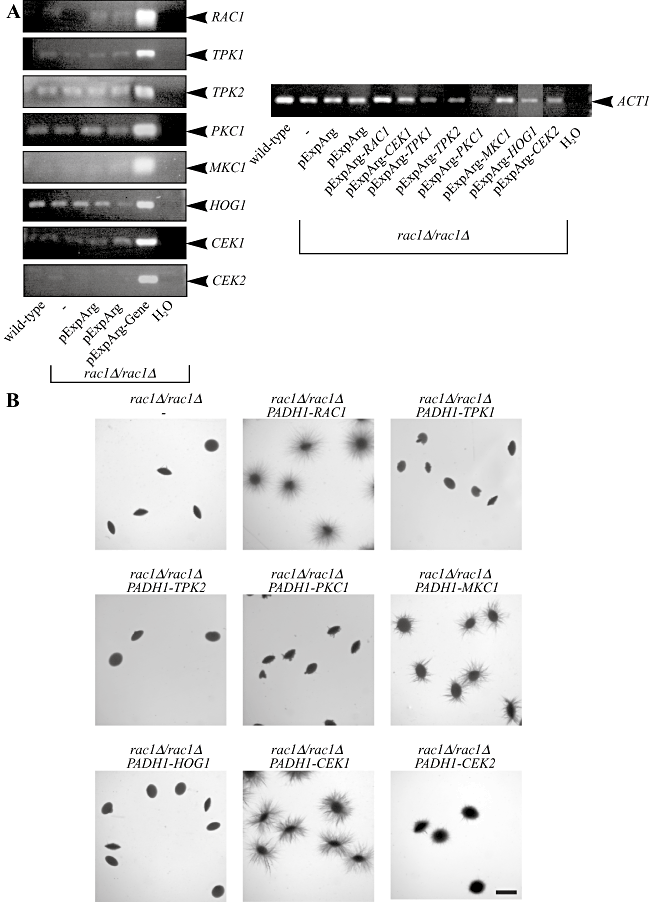
Overexpression of the Cek1 MAP kinase restores filamentous growth in the rac1 mutant.A. The different kinases are overexpressed in rac1Δ/rac1Δ cells. mRNA transcript levels in the different strains were analysed by RT-PCR. mRNA and cDNA were prepared from indicated C. albicans strains (BWP17, PY189, PY1338, PY1343, PY1348, PY1350, PY1357, PY1360, PY1361, PY1362, PY1413 and PY1446) and PCR was carried out with indicated primers (Table 2). Similar results were observed in an additional independent transformant.B. Overexpression of the MAP kinase Cek1 and to a lesser extent Mkc1 restore embedded filamentous growth in rac1Δ/rac1Δ cells. Cells from PY189 strain overexpressing the indicated genes (PY1338, PY1343, PY1348, PY1350, PY1357, PY1360, PY1361, PY1413 and PY1446) were embedded in an agar matrix as described in Fig. 2A and incubated for 5 days at 25°C. Identical results were obtained with two independent transformants.
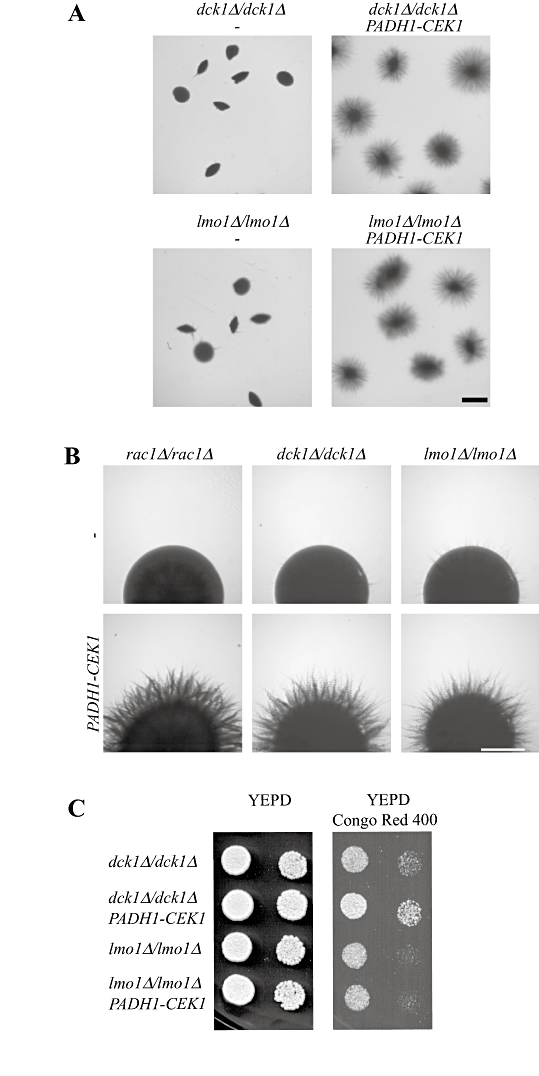
Overexpression of the Cek1 MAP kinase restores invasive filamentous growth in dck1 and lmo1 mutants.A. Overexpression of the Cek1 MAP kinase restores embedded filamentous growth in dck1 and lmo1 mutants. Cells from dck1Δ/dck1Δ (PY704) and lmo1Δ-1/lmo1Δ-1 (PY1112) strains overexpressing CEK1 (PY1386, PY1400, PY1404 and PY1408) were embedded in an agar matrix as described in Fig. 2A and incubated for 5 days at 25°C. Identical results were obtained with two independent transformants. Bar, 1 mm.B. Overexpression of the Cek1 MAP kinase restores invasive filamentous growth on solid Spider media in rac1, dck1 and lmo1 mutants. Strains indicated above were spotted on solid Spider media and images were taken after 4 days. Similar results were observed in two independent experiments. Bar, 1 mm.C. Overexpression of the Cek1 MAP kinase restores growth in the presence of Congo red in dck1 but not lmo1 mutants. Strains indicated above were spotted on to YEPD media containing Congo red (400 µg ml−1). Images were taken after 2 days' incubation at 30°C and similar results were observed in two independent experiments.
Lmo1 is required for invasive filamentous growth, similar to Rac1 and Dck1
We have previously shown that Rac1 and Dck1 are both required for invasive filamentous growth in response to different filamentous growth inducers, such as N-acetyl glucosamine (GlcNAc) and Spider containing solid media, but not in the respective liquid media (Hope et al., 2008). Hence, we examined the growth of the lmo1 deletion mutant in response to decreased nutrients levels or the presence of serum both in liquid and solid media. We observed that the lmo1Δ-2/lmo1Δ-2 mutant, similar to rac1 and dck1 deletion mutants, form hyphal filaments in liquid media in response to either GlcNAc, serum or Spider media (Fig. 3A). Furthermore little to no invasive filamentous growth defect was observed on solid media containing serum, similar to what was observed with rac1 and dck1 mutants (Hope et al., 2008). In contrast, this lmo1Δ mutant is defective in invasive filamentous growth in Spider solid media (Fig. 3B). The invasive filamentous growth defect of the lmo1Δ-2/lmo1Δ-2 mutant was restored by lmo1ΔN overexpression (Fig. 3B). The invasive filamentous growth defect of this lmo1 mutant on solid media was also similar, yet somewhat less pronounced, to that observed with rac1 and dck1 deletion mutants (Fig. 3B and Hope et al., 2008). However, little to no invasive filamentous growth defect was observed on solid media containing GlcNAc, in contrast to rac1 and dck1 mutants (Hope et al., 2008). In addition, we examined whether overexpression of a constitutively active form of Rac1 (Rac1[Q61L]) restored embedded filamentous growth in lmo1 mutants, as was previously observed in a dck1 mutant (Hope et al., 2008); this overexpression of Rac1[Q61L] resulted in little to no invasive filamentous growth of lmo1Δ mutant cells (data not shown). These results are consistent with Lmo1 not being an activator, or at least not the only activator, of Rac1 or having additional functions necessary for embedded filamentous growth that are independent of Rac1 activation. As Lmo1 is not required for filamentous growth in response to serum, we consider it unlikely that Lmo1 plays a significant role in Cdc42 activation, which is required for hyphae formation in response to serum (Bassilana et al., 2003). To investigate whether Lmo1 functions in the Czf1 invasive growth pathway, we examined if overexpression of Lmo1 could restore embedded filamentous growth in a czf1 deletion mutant and conversely if overexpression of Czf1 could restore embedded filamentous growth in the lmo1 deletion mutant. Figure S3 shows that neither overexpression of Lmo1 nor Czf1 could restore embedded filamentous growth in the czf1Δ/czf1Δ or lmo1Δ-1/lmo1Δ-1 mutants respectively. Similarly, we observed that overexpression of Czf1 could not restore embedded filamentous growth in the rac1 deletion mutant (Hope et al., 2008). Taken together these results suggest Rac1, Dck1 and Lmo1 function in a common pathway required for efficient invasive filamentous growth, different from that of Czf1.
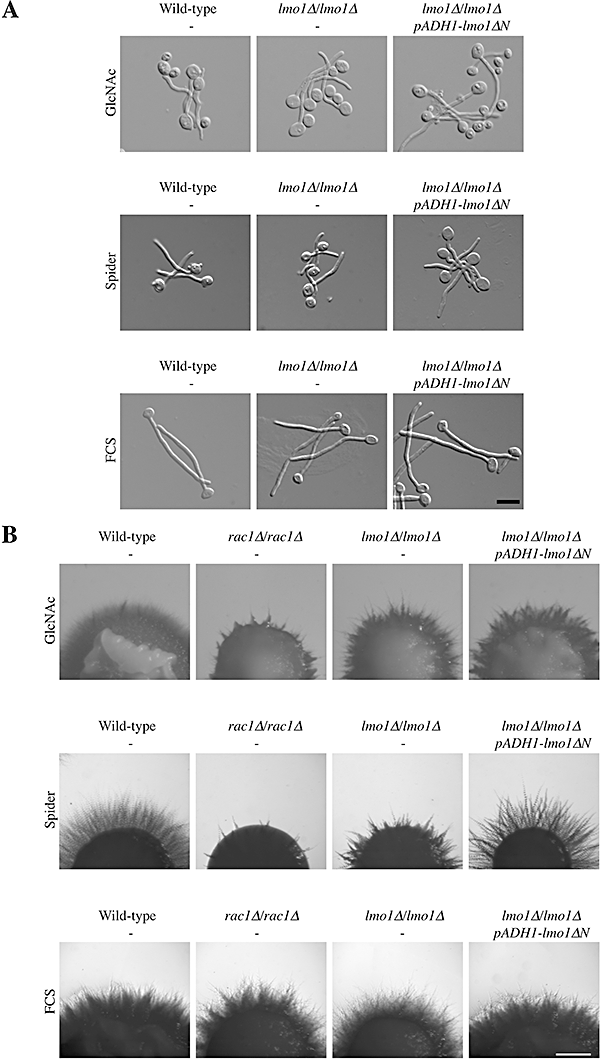
Lmo1 is required for efficient invasive filamentous growth on solid media.A. Lmo1 is not necessary for filamentous growth in liquid GlcNAc containing media, Spider media and FCS containing media. Cells from wild-type (PY1005), lmo1Δ-2/lmo1Δ-2 (PY1009) and lmo1Δ-2/lmo1Δ-2 PADH1-lmo1ΔN (PY1018) strains were starved for 1 h in water, suspended in GlcNAc media and incubated for 4 h at 37°C. For Spider liquid media induction, overnight cultures were suspended in Spider media and incubated for 2 h at 37°C. For FCS liquid media induction, logarithmically growing cells were suspended in a final concentration of 50% FCS and incubated for 2 h at 37°C. Cells were fixed and images taken. Similar results were observed in two independent experiments. Bar, 10 µm.B. Lmo1 is important for invasive filamentous growth on Spider solid media. Indicated strains (PY1005, PY191, PY1009 and PY1018) were spotted on GlcNAc media, Spider media and YEPD FCS containing agar and images were taken after 5 days. Similar results were observed in three independent experiments. Bar, 1 mm.
Lmo1 interacts with Dck1 and Rac1
Studies in mammalian cells, flies and worms indicate that Rac1, Dock180/myoblast city/CED-5 and ELMO/CED-12 function together as a complex (Gumienny et al., 2001; Brugnera et al., 2002; Cote and Vuori, 2002; Lu et al., 2004; Komander et al., 2008). Given the phenotypic similarities between lmo1, dck1 and rac1 mutants, we investigated whether these three proteins interact in vivo. We initially examined whether Dck1 and Rac1 from budding yeast cell extracts could bind GST-Lmo1 purified from E. coli. Lmo1 cDNA was fused behind the gene encoding for GST and the fusion protein was purified from bacteria; Fig. 4A illustrates GST-Lmo1 (lane 1) and GST (lane 2), analysed on a Coomassie blue stained SDS-PAGE. Given that Rac1 migrates at essentially the same size as GST, we used extracts from yeast cells in their budding form expressing GFP-Rac1 and Dck1-GFP. Immobilized GST-Lmo1 was incubated with clarified yeast extracts containing Dck1-GFP and GFP-Rac1 and bound proteins were analysed by SDS-PAGE followed by immuno-blotting. Figure 4B shows the results of one such binding experiment in which anti-GFP sera was used to visualize Dck1-GFP and GFP-Rac1. Both Dck1-GFP and GFP-Rac1 were observed to bind to GST-Lmo1 (lane 3), whereas little to no binding was observed with GST alone (lane 2). These results demonstrate that Lmo1 can bind both Rac1 and Dck1 and are consistent with these three proteins functioning together in vivo. In mammalian cells it has been shown that ELMO1 binds directly to Dock180 independent of Rac1, whereas Dock180 is required for Rac1 to bind to ELMO1 (Brugnera et al., 2002; Lu et al., 2004; Lu et al., 2005). Hence, we examined whether the observed interaction between Lmo1, Rac1 and Dck1 required the presence of Dck1 or Rac1 by using cell lysates from a strain expressing GFP-Rac1 in a dck1 deletion background or a strain expressing Dck1-GFP in a rac1 deletion background. Figure 4B shows that Dck1-GFP binds GST-Lmo1 in the absence of Rac1 (lane 8) and conversely GFP-Rac1 binds GST-Lmo1 in the absence of Dck1 (lane 7). Furthermore, Fig. 4C shows that GST-Lmo1 binds Rac1, the constitutive active form of Rac1 (Rac1[G12V]) and the constitutive inactive form of Rac1 (Rac1[T17N]) to the same extent, suggesting that Rac1 binding to Lmo1 is independent of its guanine nucleotide state. These results, along with the similar phenotypes of the rac1, dck1 and lmo1 deletion mutants, suggest that Rac1, Dck1 and Lmo1 function together during invasive filamentous growth.
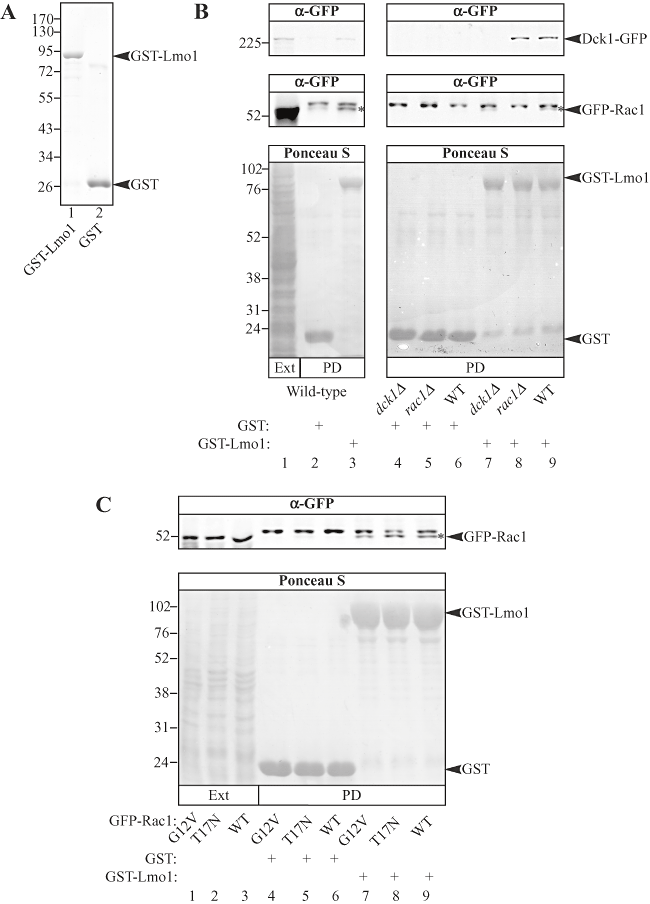
Rac1 and Dck1 bind Lmo1.A. Lmo1 fusion protein used for binding assays with yeast extracts. GST fusion proteins purified from E. coli (described in Experimental procedures) were analysed by SDS-PAGE and stained with Coomassie Blue. Approximately 5 µg of each of purified GST-Lmo1 (lane 1) and GST (lane 2) were loaded.B. Dck1-GFP and GFP-Rac1 bind GST-Lmo1. Either GST-Lmo1 (lanes 3, 7, 8 and 9) or GST (lanes 2, 4, 5 and 6) bound to GSH-agarose was incubated with the 5000 g clarified yeast extracts from dck1Δ/dck1Δ cells expressing Dck1-GFP and GFP-Rac1 (PY1278; lanes 1–3, 6 and 9), or GFP-Rac1 alone (PY1265; lanes 4 and 7) or from rac1Δ/rac1Δ cells expressing Dck1-GFP alone (PY1382; lanes 5 and 8) and followed by SDS-PAGE analysis. Right and left panels are from different experiments. Immuno-blots were probed with anti-GFP sera (top). All proteins were visualized with Ponceau S staining (bottom). Lane 1 has 0.25% of the total input of cell extract used in the binding experiment. The star indicates GFP-Rac1 and the band above is observed irrespective of the strain or resin used, hence is non-specific. Similar results were observed in three independent experiments.C. GFP-Rac1 binds GST-Lmo1 irrespective of its guanine nucleotide state. Binding experiments as described above were carried out with either GST-Lmo1 (lanes 7, 8 and 9) or GST (lanes 4, 5 and 6) and clarified yeast extracts from rac1Δ/rac1Δ budding form cells expressing GFP-Rac1[G12V] (PY209; lanes 1, 4 and 7), GFP-Rac1[T17N] (PY212; lanes 2, 5 and 8) or GFP-Rac1 (PY205; lanes 3, 6 and 9). Lanes 1, 2 and 3 have 0.1% of the total input of cell extract used in the binding experiment.
As Rac1 is localized to the plasma membrane we next examined whether Lmo1 and Dck1 were also localized to this cellular membrane. Previously, we had been unable to observe a discrete fluorescence signal within strains expressing functional Dck1 GFP fusion proteins (Hope et al., 2008). To examine Lmo1 localization we used a strain overexpressing GFP-Lmo1 fusion protein, which complemented the invasive filamentous growth defect of the lmo1Δ-2/lmo1Δ-2 strain (Fig. 5A). Similar to Dck1, we did not observe a fluorescence signal localized to a distinct site with GFP-Lmo1 (Fig. 5B), although the signal was not uniformly distributed within the cell. We showed by sub-cellular fractionation (Hope et al., 2008) that Rac1 and Dck1 were found in the same cellular fraction, hence we investigated whether Lmo1 was also present in this fraction. We used a strain expressing functional Dck1 (Hope et al., 2008) and Lmo1 GFP fusions. These Dck1-GFP and GFP-Lmo1 fusions were integrated at the ADH1 and RP10 loci, respectively, and the resulting strain was used for fractionation studies. Figure 5C shows that Lmo1 is predominantly found in the 10 000 g pellet fraction (lane 2), with a small but reproducible signal in the 100 000 g pellet fraction (lane 4), similar to Rac1 and Dck1. Thus these three proteins all are associated with the membrane fractions.
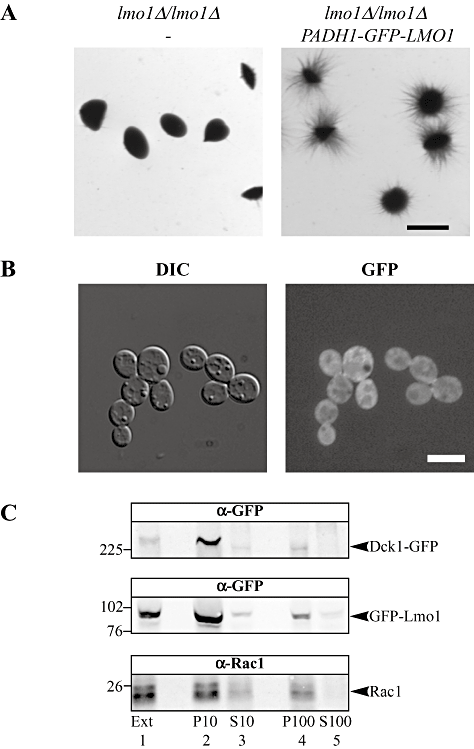
Lmo1 is present in the same membrane fraction as Rac1 and Dck1.A. GFP-Lmo1 fusion is functional. Cells from lmo1Δ-2/lmo1Δ-2 (PY1009) and lmo1Δ-2/lmo1Δ-2 PADH1-GFPLMO1 (PY1099) strains were embedded in an agar matrix as described in Fig. 2A and incubated for 5 days at 25°C. Bar, 1 mm.B. Localization of GFP-Lmo1. DIC and fluorescence images of lmo1Δ-2/lmo1Δ-2 PADH1-GFPLMO1 (PY1099) cells are shown. Bar, 5 µm.C. Lmo1, Rac1 and Dck1 co-fractionate. Extracts (Ext; lane 1) from exponentially growing dck1Δ/dck1ΔPADH1-DCK1GFP PADH1-GFPLMO1 (PY1278) (∼1 × 108 cells) were centrifuged at 10 000 g, resulting in the P10 pellet (lane 2) and S10 (lane 3) supernatant fractions. The S10 fraction was then centrifuged at 100 000 g resulting in the P100 pellet (lane 4) and the S100 supernatant (lane 5) fractions. Fractions were analysed by SDS-PAGE followed by immunoblotting and probing with anti-GFP and anti-Rac1 sera. Relative to the extract, 2.5-times S10, 1.5-times P10 and 5-times both P100 and S100 were loaded on the SDS-PAGE. Ponceau S staining revealed proteins in all lanes, including the S100 fraction. Similar results were observed in three different experiments.
If Lmo1 were to function as a scaffold protein, binding both Dck1 and Rac1, it is possible that Rac1 localization or dynamics (movement within the cell) may be affected by this protein. We compared the cellular distribution of GFP-Rac1 in wild-type, rac1Δ/rac1Δ, dck1Δ/dck1Δ and lmo1Δ-1/lmo1Δ-1 cells. Figure 6A shows that GFP-Rac1 is localized uniformly to the plasma membrane in all four strains, indicating that neither Dck1 nor Lmo1 are required for Rac1 plasma membrane localization. We also examined whether GFP-Rac1 dynamics at the plasma membrane were affected by absence of Dck1 or Lmo1 using fluorescence recovery after photobleaching (FRAP) (Fig. 6B). The FRAP t½ for GFP-Rac1 rac1Δ/rac1Δ cells was 1.15 ± 0.23 s (n = 17), identical to what we previously observed (Bassilana and Arkowitz, 2006). In the presence of two wild-type copies of Rac1, the FRAP t½ for GFP-Rac1 increased to 1.64 ± 0.34 s (n = 16), and increased further by 50% in the absence of either Dck1 (2.44 ± 1.07 s; n = 18) or Lmo1 (2.52 ± 0.92 s; n = 23). These results suggest that in the absence of Dck1 or Lmo1, the mobility of Rac1 is reduced.
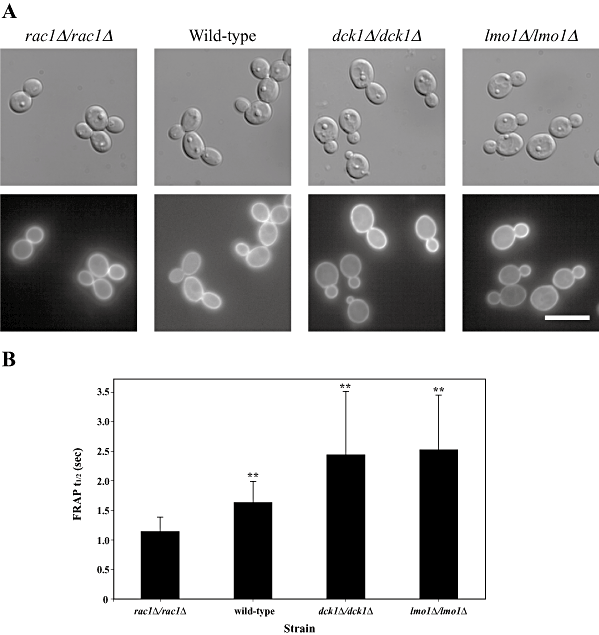
Lmo1 and Dck1 are not required for Rac1 localization but alter its dynamics.A. GFP-Rac1 localizes to the plasma membrane similarly in wild-type, rac1Δ/rac1Δ, dck1Δ/dck1Δ and lmo1Δ-1/lmo1Δ-1 cells. DIC and fluorescence images of indicated strains (PY201, PY205, PY1195 and PY1197, respectively) are shown. Bar, 10 µm.B. GFP-Rac1 FRAP dynamics is altered in dck1Δ/dck1Δ and lmo1Δ/lmo1Δ strains. FRAP t½'s (values are the means ± standard deviation) for GFP-Rac1 were determined from single-phase exponential curve fits of fluorescence recovery after photobleaching intensities in rac1Δ/rac1Δ cells (n = 17), wild-type cells (n = 16), dck1Δ/dck1Δ cells (n = 18) and lmo1Δ-1/lmo1Δ-1 cells (n = 23). The two-tailed P-values of the indicated mean FRAP t½'s (**) are less than 0.0001 compared with the FRAP t½ in rac1Δ/rac1Δ cells. The two-tailed P-value for the mean FRAP t½ compared with the wild-type is 0.007 for the FRAP t½ of GFP-Rac1 in dck1Δ/dck1Δ cells and 0.0007 for the FRAP t½ of GFP-Rac1 in lmo1Δ-1/lmo1Δ-1 cells.
Rac1, Dck1 and Lmo1 are required for cell wall integrity
Different inducers of filamentous growth trigger a range of cellular stresses. Given that rac1, dck1 and lmo1 mutants are defective in invasive filamentous growth, we examined whether these genes were important for responses to cell wall stress. Indeed, one explanation for the invasive growth defect of the rac1, dck1 and lmo1 mutants is that these mutants have weakened cell walls, and hence are unable to invade a semi-solid matrix.
Calcofluor white and Congo red are anionic dyes that bind to and interfere with the cell wall. Altered susceptibility to these compounds has been used to identify S. cerevisiae and C. albicans mutants with cell wall defects (Lagorce et al., 2003; Ram and Klis, 2006). We examined the sensitivity of rac1, dck1 and lmo1 deletion mutants to calcofluor white and Congo red. Figure 7A shows that these mutants exhibit increased sensitivity to such cell wall perturbing agents, whereas reintroduction of a copy of the respective gene partially complemented these defects. These results suggest that Rac1, Dck1 and Lmo1 are important for cell wall integrity, either playing a role in cell wall remodelling and/or in the response to cell wall stress.
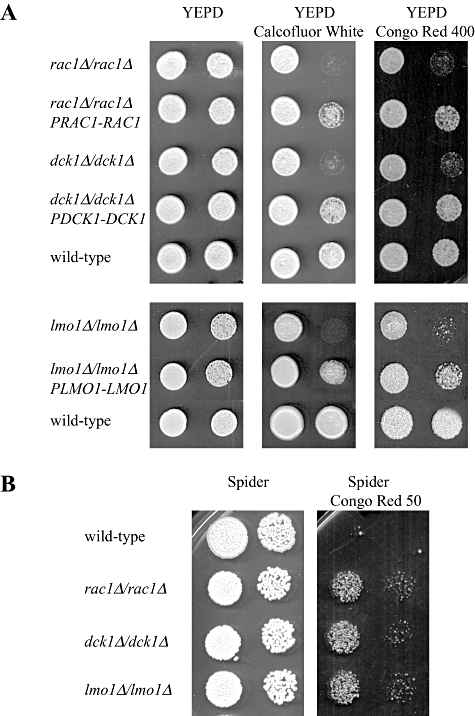
Rac1, Dck1 and Lmo1 are required for cell wall integrity.A. Rac1, dck1 and lmo1 mutants exhibit increased sensitivity to calcofluor white and Congo red. Serial dilutions of cells from wild-type (PY1005), rac1Δ/rac1Δ (PY191), rac1Δ/rac1ΔPRAC1-RAC1 (PY275), dck1Δ/dck1Δ (PY706), dck1Δ/dck1ΔPDCK1-DCK1 (PY710), lmo1Δ-2/lmo1Δ-2 (PY1009) and lmo1Δ-2/lmo1Δ-2 PLMO1-LMO1 (PY1012) strains were spotted on to YEPD solid media containing calcofluor white (25 µg ml−1) or Congo red (400 µg ml−1). Images were taken after 2–3 days incubation at 30°C and similar results were observed in three independent experiments.B. Wild-type cells are more sensitive to Congo red on invasive filamentous growth inducing solid media compared with rac1, dck1 and lmo1 mutants. Serial dilutions of cells from wild-type (PY1005), rac1Δ/rac1Δ (PY191), dck1Δ/dck1Δ (PY706) and lmo1Δ-2/lmo1Δ-2 (PY1009) strains were spotted on to solid Spider media containing Congo red (50 µg ml−1). Images were taken after 3 days' incubation at 30°C.
To determine the specificity of this cell wall stress hypersensitivity, we examined whether rac1 and dck1 mutants, which exhibit somewhat stronger invasive growth defects than the lmo1 mutant, were affected by oxidative or osmotic stresses. Figure S4A shows that growth of both of these mutants is unaffected by oxidative stress, induced in the presence of diethylmaleate (DEM), paraquat or hydrogen peroxide. Growth of these mutants was also unaffected by osmotic stress, i.e. a high concentration of NaCl or by perturbation of the plasma membrane by SDS, compared with the wild-type strain (Fig. S4A). As an additional stress, we used the microtubule depolymerizing drug thiabendazole. Figure S4B shows that growth of wild-type strain and rac1 and dck1 mutants are reduced similarly on media containing TBZ. Together, these results indicate that Rac1, Dck1 and Lmo1 play a role in cell wall integrity and raise the possibility that cell wall defects may contribute to the invasive filamentous growth defect observed in these mutants.
We next examined whether during invasive filamentous growth, the different strains exhibited an increased sensitivity to cell wall perturbants. Wild-type and rac1, dck1 and lmo1 mutant cells were spotted on solid Spider media either in the presence or absence of Congo Red. While wild-type cells grew on YEPD media containing 400 µg ml−1 Congo red, no growth was observed on Spider media with the same concentration of Congo red. Figure 7B shows that while the wild-type strain grew on Spider media, there was a substantial growth defect in the presence of Congo red even at 50 µg ml−1, suggesting that under invasive filamentous growth inducing conditions, cells exhibit increased sensitivity to cell wall perturbation. Rac1, dck1 and lmo1 mutants are substantially defective in invasive filamentous growth on Spider media (Fig. 3B and Hope et al., 2008) and exhibit less sensitivity to Congo red than the wild-type strain in this media (Fig. 7B).
Rac1, Dck1 and Lmo1 function upstream of the Cek1 MAP kinase
Filamentous growth is likely to involve a number of different pathways, for example a recent study indicated that the cell wall integrity pathway contributes to filamentous growth in the budding yeast S. cerevisiae (Birkaya et al., 2009). In order to determine in which signalling pathway Rac1, Dck1 and Lmo1 function in, we overexpressed critical components from the Cek1 MAP kinase pathway, the cAMP-dependent PKA pathway, the Hog1 MAP kinase pathway and the protein kinase C – Mkc1 MAPK pathway in rac1, dck1 and lmo1 mutants and examined growth in an agar matrix. The Cek1 MAP kinase pathway has been shown to be involved in C. albicans morphogenesis and cell wall formation via Cek1 (Csank et al., 1998; Roman et al., 2005; Eisman et al., 2006; Roman et al., 2009) and the mating process via Cek2 (Chen et al., 2002). cAMP-dependent PKA catalytic subunits Tpk1 and Tpk2 are critical for solid and liquid media induced stress responses, respectively (Bockmuhl et al., 2001) and Hog1 is critical for responses to a range of stresses including oxidative stress (Alonso-Monge et al., 2003). Finally, Pkc1 and Mkc1 function together in cell wall integrity pathway (Paravicini et al., 1996; Navarro-garcia et al., 1998; Navarro-Garcia et al., 2001). Initially, we examined overexpression of CEK1, CEK2, HOG1, MKC1, along with TPK1 and TPK2 and PKC1 in a rac1 deletion mutant, and all of these genes were overexpressed relative to both a wild-type strain and an internal actin control, using RT-PCR (Fig. 8A). Strains overexpressing these different kinases were examined for invasive filamentous growth when embedded in an agar matrix. Figure 8B shows that overexpression of either TPK1, TPK2, PKC1 and HOG1 did not restore embedded filamentous growth in the rac1 mutant, compared with a control strain overexpressing RAC1. In contrast, overexpression of the Cek1 MAP kinase and to a lesser extent the Mkc1 MAP kinase restored embedded filamentous growth in this mutant. Overexpression of the Cek2 MAP kinase in the rac1 deletion mutant resulted in limited filamentous growth. Given the similarity between Cek1 and Cek2, we attribute this limited restoration to the functional overlap between these two kinases. These results indicate that Rac1 may function in the Cek1 MAP kinase pathway during invasive filamentous growth and furthermore that this MAP kinase is likely to be downstream of Rac1.
We subsequently examined whether overexpression of other members of this MAP kinase cascade could restore the invasive filamentous growth defect of the rac1 mutant, including the PAK kinase Ste20 homologue Cst20, the MAPKKK Ste11 and the MAPKK Hst7. RT-PCR analyses of mRNA transcript levels (Fig. 9A) revealed that each of the kinase genes was substantially overexpressed, yet overexpression of these protein kinases had little to no effect in restoring invasive filamentous growth in the rac1 mutant (Fig. 9B). These results do not favour the notion that Rac1 functions upstream of the entire Cek1 MAP kinase pathway and rather raise the possibility that Rac1 functions upstream of the Cek1 MAP kinase alone. Therefore we examined whether overexpression of Cek1, but not Cst20, could restore normal cell wall integrity to the rac1 mutant. Figure 9C shows that overexpression of Cek1, but not Cst20, suppresses the observed growth defect in the presence of Congo red. Indeed, overexpression of Cst20 appears to increase the sensitivity to Congo red in the rac1 mutant. The embedded filamentous growth results, together with the restoration of growth in the presence of Congo red, suggest that Cek1 functions independent of its upstream kinases.
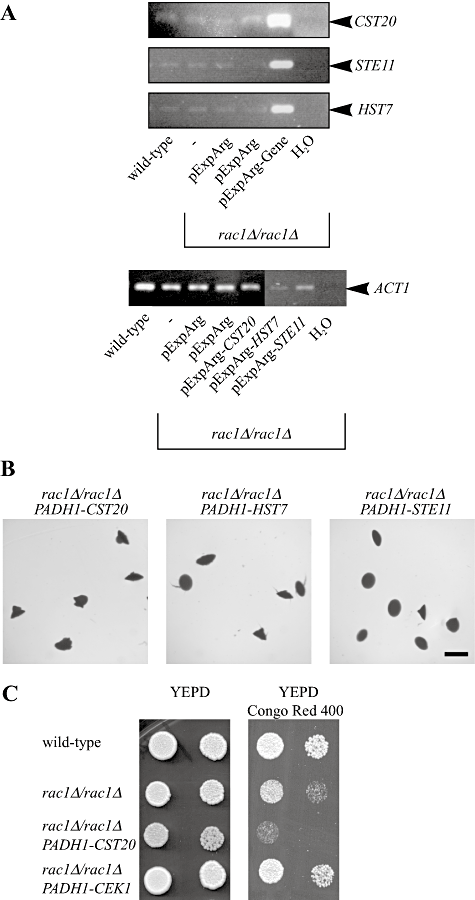
Overexpression of components upstream of the MAP kinase Cek1 does not restore filamentous growth in the rac1 mutant.A. Cst20, Ste11 and Hst7 kinases are overexpressed in rac1Δ/rac1Δ cells. mRNA transcript levels in different strains were analysed by RT-PCR. mRNA and cDNA were prepared from indicated C. albicans strains (BWP17, PY1332, PY1420, PY1439, PY1440 and PY1444) and PCR was carried out with indicated primers (Table 2).B. Overexpression either of the PAK kinase Cst20, the MAP kinase kinase kinase Ste11 or the MAP kinase kinase Hst7 does not restore embedded filamentous growth in rac1Δ/rac1Δ cells. Cells from PY189 strain overexpressing the indicated genes (PY1332, PY1420 and PY1439) were embedded in an agar matrix as described in Fig. 2A and incubated for 5 days at 25°C. Identical results were obtained with two independent transformants.C. Overexpression of the MAP kinase Cek1 but not the PAK kinase Cst20 restores growth in the presence of Congo red in rac1Δ/rac1Δ cells. Serial dilutions of cells from wild-type (PY1005), rac1Δ/rac1Δ (PY191), rac1Δ/rac1ΔPADH1-CST20 (PY1332) and rac1Δ/rac1ΔPADH1-CEK1 (PY1338) strains were spotted on to YEPD media containing Congo red (400 µg ml−1). Images were taken after 2 days' incubation at 30°C. Similar results were observed in two independent experiments.
To address whether Rac1 functions together with Dck1 and Lmo1 upstream of the Cek1 MAP kinase, we examined if overexpression of this MAP kinase restored invasive filamentous growth and resistance to the cell wall perturbant Congo red in the dck1 and lmo1 mutants. Figure 10A shows that overexpression of this MAP kinase similarly restored embedded invasive filamentous growth in these two mutants. Furthermore, overexpression of Cek1 restored invasive filamentous growth on solid Spider media in rac1, dck1 and lmo1 mutants (Fig. 10B). Figure 10C shows that overexpression of the Cek1 MAP kinase decreased Congo red sensitivity of the dck1 mutant, similar to that observed with the rac1 mutant. However, overexpression of this MAP kinase did not decrease the Congo red sensitivity of the lmo1 mutant, suggesting that Lmo1 may have additional functions independent of Rac1 and Dck1. Overexpression of the Cek1 MAP kinase did not alter cell morphology in rac1, dck1 or lmo1 cells (Fig. S5). Our results suggest that Rac1, Dck1 and Lmo1 function together upstream of the Cek1 and Mkc1 MAP kinases during invasive filamentous growth.
Discussion
We identified and characterized Lmo1, a protein with similarity to human ELMO1 in the pathogenic yeast C. albicans. We showed that Lmo1 is necessary for invasive filamentous growth, similar to Rac1 and its activator Dck1. In addition, Rac1, Dck1 and Lmo1 are required for cell wall integrity, but not for osmotic or oxidative stress responses. Lmo1 can bind both Rac1 and Dck1 and all three proteins are found in a membrane fraction. While Lmo1 is not necessary for the plasma membrane localization of Rac1, both Lmo1 and Dck1 alter the mobility of Rac1 at the plasma membrane. The region of Lmo1, encompassing the ELMO and PH-like domains, is sufficient for its function. We conclude that the requirement for ELMO/CED12 proteins in Rac1 function is conserved from fungi to humans. Furthermore, our data suggest that Rac1, Dck1 and Lmo1 function upstream of the MAP kinase Cek1, during C. albicans invasive filamentous growth.
Proteins from the ELMO/CED12 family, together with proteins from the CDM family of GEFs, are necessary for Rac1 function (Cote and Vuori, 2007; Ravichandran and Lorenz, 2007; Isik et al., 2008). No ELMO homologues have been described in fungi, yet proteins with similarity to ELMO1 have been uncovered by genome sequencing, including in the yeast C. albicans. Intriguingly, the yeast S. cerevisiae and the filamentous fungus A. gossypii, which do not have identifiable Rac1 homologues, have homologues of the CDM proteins (Meller et al., 2005) and proteins with similarity to ELMO. In C. albicans, Rac1 and Dck1 are required for invasive filamentous growth (Bassilana and Arkowitz, 2006; Hope et al., 2008). Here we show that Lmo1 is also required for such invasive filamentous growth in an agar matrix. Interestingly, it was shown in a chemotaxis assay that D. discoideum elmoA mutant cells moved less well under agar compared with wild-type cells even in the absence of a cAMP gradient, suggesting that these mutants could not generate enough co-ordinated force to move in such conditions (Isik et al., 2008). Together these results demonstrate that ELMO/CED12 proteins are found in a range of species, from fungi to humans, and suggest a common role in Rac1 function.
How do Rac1, Dock180 and ELMO proteins interact and how does this complex function? It was proposed that ELMO1 is part of a bipartite GEF that activates Rac1 (Brugnera et al., 2002; Lu et al., 2004), but other studies are consistent with an alternate mechanism for ELMO1 function, in which this protein is not required for Rac1 activation (Cote and Vuori, 2002; Cote et al., 2005; Komander et al., 2008). The ELMO1 carboxyl-terminal 550 amino acids, which contain an atypical PH-like domain, appear to be the main determinant for DOCK180 binding in mammalian cells (Komander et al., 2008). The amino-terminal region of ELMO1 has been also shown to be important for function, as it is required for association with Rho G (Katoh et al., 2006) and ERM proteins (Grimsley et al., 2006). Our results demonstrate that an activated form of Rac1 cannot bypass the requirement for Lmo1 in vivo, while this form of Rac1 can bypass the Dck1 requirement (Hope et al., 2008). Furthermore, a Lmo1 carboxyl-terminal region, which contains the ELMO and PH-like domains, is necessary and sufficient for function, while the amino terminal region does not appear necessary for function in vivo. Together, these results highlight a conserved requirement for the ELMO carboxyl-terminal region in its function. Our data also indicate that Lmo1 does not activate Rac1, consistent with the notion that ELMO proteins would have additional functions.
As several studies suggest that ELMO proteins function as scaffolds, bringing together Rac1, Dock180 and effector proteins (Wolf et al., 2003; Santy et al., 2005; Katoh et al., 2006; Komander et al., 2008), it is critical to determine where and when this Rac1/Dock180/ELMO1 complex is localized and to identify the interacting components necessary for its function. In mammals, a number of upstream components, critical for ELMO protein localization, have been identified. For example, ELMO1 and Dock180, which are found predominantly in the cytoplasm, can be recruited to the plasma membrane via Rho G (Katoh and Negishi, 2003; Debakker et al., 2004), or Arf6 (Santy et al., 2005). ELMO1 and Dock180 have been observed at actin rich membrane ruffles (Grimsley et al., 2004). In contrast, in D. discoideum ElmoA was associated with the myosin II heavy chain, which interacts with actin filaments outside of the leading-edge (Isik et al., 2008). We found that in C. albicans Rac1, Dck1 and Lmo1 are predominantly associated with membrane fractions. We have been unable to observe a discrete localization of Dck1 or Lmo1 and both of these proteins appear to be intracellular by fluorescence microscopy. However, GFP-Rac1 is localized to the plasma membrane (Bassilana and Arkowitz, 2006) irrespective of the presence of Lmo1 or Dck1. In the absence of Lmo1 or Dck1 the dynamics of GFP-Rac1, determined by FRAP t½, is reduced suggesting that both Lmo1 and Dck1 facilitate Rac1 movement in membrane or association/dissociation with the membrane. Further studies will be necessary to distinguish between these two possibilities.
Signalling components downstream of the Rac1/Dock180/ELMO1 complex remain largely unknown in mammalian cells. To identify such components in C. albicans, we overexpressed a number of protein kinases, from a range of different signalling pathways (Biswas et al., 2007; Roman et al., 2007; Whiteway and Bachewich, 2007; Alonso-Monge et al., 2009; Hall et al., 2009) in a rac1 deletion mutant. Of these kinases, only overexpression of the Cek1 MAP kinase, and to a lesser extent the Mkc1 MAP kinase, substantially restored invasive filamentous growth. Similarly, overexpression of Cek1 in dck1 and lmo1 deletion mutants restored invasive growth. Together these results suggest that Rac1, Dck1 and Lmo1 function upstream of Cek1 and Mkc1. Cek1, the homologue of S. cerevisiae Kss1 (Bennett and Johnson, 2005), and Mkc1, the homologue of Slt2, are required for C. albicans invasive growth (Csank et al., 1998; Navarro-Garcia et al., 1998; Kumamoto and Vinces, 2005). Both Cek1 and Mkc1 are also required for cell wall integrity (Navarro-Garcia et al., 1998; Roman et al., 2005; Eisman et al., 2006). In particular, cek1 mutants are sensitive to the cell wall perturbing agents, but not to osmotic or oxidative stresses, similar to rac1, dck1 and lmo1 mutants. These results are also consistent with Rac1, Dck1 and Lmo1 functioning in the same pathway as Cek1. Roman et al. (2005) showed that specific Cek1 phosphorylation is unaffected in cst20 PAK kinase mutants, indicating that Cek1 can be activated independent of Cst20. Intriguingly, overexpression of Cst20, the MAPKKK Ste11 or the MAPKK Hst7 did not restore invasive growth in the rac1 deletion mutant. While one must be cautious in interpreting these results, they raise the possibility that Rac1/Dck1/Lmo1 might only function upstream of the MAP kinase Cek1. Similarly, overexpression of Pkc1, which functions upstream of Mkc1, did not restore invasive growth in the rac1 mutant. Further investigations will be necessary to elucidate the linkage between this conserved Rho G-protein module and these two MAP kinases. Irrespective of the specifics of this connection, our results indicate that the Rac1/Dck1/Lmo1 protein module signals to two MAP kinases, critical for invasive growth and cell wall integrity. It is attractive to speculate that invasive filamentous growth imposes specific constraints on the cell wall, and that the conserved Rho G-protein/GEF/scaffold module would co-ordinate responses from these two pathways. Further studies on this invasive growth pathway will be necessary to shed light on the stimuli that cells detect and how this pathway leads to specific cell shape changes necessary for invasion into a solid surface or tissue.
Experimental procedures
Growth conditions
Yeast extract-peptone dextrose (YEPD) or synthetic complete (SC) medium was used, and strains were grown at 30°C, unless indicated otherwise. To select nourseothricin resistant transformants, 200 µg ml−1 of nourseothricin (Werner Bioagents, Jena, Germany) was added to YEPD agar. Filamentous growth induction was carried out in liquid or solid media as described previously either in 50% serum (Bassilana et al., 2003), Spider medium (Calera et al., 2000), or N-acetyl glucosamine (Castilla et al., 1998). For filamentous growth induction on solid media, 2% agar was included. Filamentous growth induction in embedded media was carried out in YEP containing 2% sucrose, as previously described (Brown et al., 1999).
Growth in the presence of calcofluor white, Congo red, hydrogen peroxide (H2O2), 1,1′-dimethyl-4,4′-bipyridinium (paraquat), diethylmaleate (DEM), sodium chloride, SDS and thiabendazole (TBZ) (all from Sigma-Aldrich, Saint Quentin Fallavier, France, except for SDS, which was from Calbiochem, Fontenay-sous-bois, France) on YEPD plates was examined using serially diluted 10 µl drops of exponential cultures grown in YEPD media and incubated for 2–5 days. All solutions were prepared in water except for DEM, which was solubilized in 100% ethanol and calcofluor white, which was solubilized in 0.5% potassium hydroxide, 83% glycerol (Ram and Klis, 2006). Thiabendazole was solubilized in dimethylformamide (Merck, Fontenay-sous-bois, France). Calcofluor white and Congo red plates were buffered with 50 mM PIPES pH 5.5, as described (Ram and Klis, 2006).
Strains
Strains and primers used in this study are listed in Tables 1 and 2 respectively. lmo1Δ-1/lmo1Δ-1 mutants were obtained by initially transforming BWP17 with a lmo1Δ-1::URA3 PCR product generated from pGemUra (Wilson et al., 1999), using LMO1.P1 and LMO1.P2 primers, yielding the heterozygote strain PY1111. This deletion removed essentially all of the LMO1 ORF, with only 21 bp 3′ of the ATG and 51 bp 5′ of the stop codon remaining. Subsequently, a lmo1Δ-1::HIS1 PCR product was generated using the same primers and pGemHis1 (Wilson et al., 1999), transformed into PY1111 and URA+ HIS+ prototrophs were selected, yielding PY1112. The lmo1Δ-2/lmo1Δ-2 mutants were obtained by transforming BWP17 with a lmo1Δ-2::HIS1 PCR product generated from pGemHis1 using LMO1.P11 and LMO1.P2 primers, to yield the heterozygote strain PY1006. This deletion removed the majority of Exon 2, with 357 bp 3′ of the ATG and 51 bp 5′ of the stop codon remaining. Subsequently, a lmo1Δ-2::URA3 PCR product was generated using the same primers and pGemUra, transformed into PY1006 and URA+ HIS+ prototrophs were selected, yielding PY1007. lmo1Δ-2 homozygous deletion mutants are referred to as lmo1Δ/lmo1Δ mutants and are used unless otherwise indicated. Homozygous deletion mutants were confirmed by PCR for correct insertion of URA3 and HIS1 into the LMO1 locus, and the absence of the gene. To generate overexpression strains, the pExpArg derived plasmids were digested with StuI, and targeted to the RP10 locus, and the pNIMpADH derived plasmids were digested with SacII and ApaI, the relevant fragment purified and targeted to the ADH1 locus. Two independent clones of each strain were generated.
| Yeast strain | Relevant genotype | Reference |
|---|---|---|
| BWP17 | ura3Δ::λimm434/ura3Δ::λimm434 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG | Wilson et al., 1999 |
| PY82 | Same as BWP17 with ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/HIS1::his1::hisG arg4::hisG/URA3::ARG4::arg4::hisG | Bassilana et al., 2003 |
| PY189 | Same as BWP17 with rac1Δ::URA3/rac1Δ::HIS1 arg4Δ::hisG/arg4Δ::hisG | Bassilana and Arkowitz, 2006 |
| PY191 | Same as PY189 with RP10::ARG4 | Bassilana and Arkowitz, 2006 |
| PY201 | Same as BWP17 with RP10::ARG4-PADH1-GFPRAC1 | This study |
| PY205 | Same as PY189 with RP10::ARG4-PADH1-GFPRAC1 | Bassilana and Arkowitz, 2006 |
| PY209 | Same as PY189 with RP10::ARG4-PADH1-GFPrac1[G12V] | Bassilana and Arkowitz, 2006 |
| PY212 | Same as PY189 with RP10::ARG4-PADH1-GFPrac1[T17N] | Bassilana and Arkowitz, 2006 |
| PY275 | Same as PY189 with RP10::ARG4-PRAC1-RAC1 | Bassilana and Arkowitz, 2006 |
| PY704 | Same as BWP17 with dck1Δ::HIS1/dck1Δ::URA3 arg4::hisG/arg4::hisG | Hope et al., 2008 |
| PY706 | Same as PY704 with RP10::ARG4 | Hope et al., 2008 |
| PY710 | Same as PY704 with RP10::ARG4-PDCK1-DCK1 | Hope et al., 2008 |
| PY897 | czf1::HIS1/czf1::URA3 arg4::hisG/arg4::hisG | Hope et al., 2008 |
| PY901 | Same as PY897 with RP10::ARG4 | Hope et al., 2008 |
| PY1006 | Same as BWP17 with lmo1Δ-2::HIS1/LMO1 ura3Δ::λimm434/ura3Δ::λimm434 arg4Δ::hisG/arg4Δ::hisG | This study |
| PY1007 | Same as BWP17 with lmo1Δ-2::HIS1/lmo1Δ-2::URA3 arg4Δ::hisG/arg4Δ::hisG | This study |
| PY1009 | Same as PY1007 with RP10::ARG4 | This study |
| PY1012 | Same as PY1007 with RP10::ARG4-PLMO1-LMO1 | This study |
| PY1018 | Same as PY1007 with RP10::ARG4-PADH1-lmo1ΔN | This study |
| PY1099 | Same as PY1007 with RP10::ARG4-PADH1-GFPLMO1 | This study |
| PY1111 | Same as BWP17 with lmo1Δ-1::URA3/LMO1 his1Δ::hisG/his1::hisG arg4Δ::hisG/arg4Δ::hisG | This study |
| PY1112 | Same as BWP17 with lmo1Δ-1::URA3/lmo1Δ-1::HIS1 arg4Δ::hisG/arg4Δ::hisG | This study |
| PY1116 | Same as PY1112 with RP10::ARG4 | This study |
| PY1118 | Same as PY1112 with RP10::ARG4-PLMO1-LMO1 | This study |
| PY1120 | Same as PY1112 with RP10::ARG4-PADH1-LMO1 | This study |
| PY1195 | Same as PY704 with RP10::ARG4-PADH1-GFPRAC1 | This study |
| PY1197 | Same as PY1112 with RP10::ARG4-PADH1-GFPRAC1 | This study |
| PY1199 | Same as PY704 with ADH1::SAT1 | This study |
| PY1204 | Same as PY704 with ADH1::SAT1-PADH1-DCK1GFP | This study |
| PY1265 | Same as PY1199 with RP10::ARG4-PADH1-GFPRAC1 | This study |
| PY1278 | Same as PY1204 with RP10::ARG4-PADH1-GFPLMO1 | This study |
| PY1332 | Same as PY189 with RP10::ARG4-PADH1-CST20 | This study |
| PY1338 | Same as PY189 with RP10::ARG4-PADH1-CEK1 | This study |
| PY1343 | Same as PY189 with RP10::ARG4-PADH1-TPK1 | This study |
| PY1348 | Same as PY189 with RP10::ARG4-PADH1-TPK2 | This study |
| PY1350 | Same as PY189 with RP10::ARG4-PADH1-PKC1 | This study |
| PY1357 | Same as PY189 with RP10::ARG4-PADH1-MKC1 | This study |
| PY1360 | Same as PY189 with RP10::ARG4-PADH1-RAC1 | This study |
| PY1361 | Same as PY189 with RP10::ARG4 | This study |
| PY1362 | Same as PY189 with RP10::ARG4 | This study |
| PY1382 | Same as PY189 with RP10::ARG4-PADH1-DCK1GFP | This study |
| PY1386 | Same as PY704 with RP10::ARG4-PADH1-CEK1 | This study |
| PY1400 | Same as PY1112 with RP10::ARG4-PADH1-CEK1 | This study |
| PY1404 | Same as PY704 with RP10::ARG4 | This study |
| PY1408 | Same as PY1112 with RP10::ARG4 | This study |
| PY1413 | Same as PY189 with RP10::ARG4-PADH1-HOG1 | This study |
| PY1420 | Same as PY189 with RP10::ARG4-PADH1-HST7 | This study |
| PY1439 | Same as PY189 with RP10::ARG4-PADH1-STE11 | This study |
| PY1440 | Same as PY189 with RP10::ARG4-PADH1-RAC1 | This study |
| PY1444 | Same as PY189 with RP10::ARG4 | This study |
| PY1446 | Same as PY189 with RP10::ARG4-PADH1-CEK2 | This study |
| PY1486 | Same as PY1112 with RP10::ARG4-PADH1-CZF1 | This study |
| PY1491 | Same as PY897 with RP10::ARG4-PADH1-lmo1ΔN | This study |
| Primer | Sequence |
|---|---|
| LMO1.P1 | GACACTTGTTTTTTTTTTGACAACAACATCTATTCCTCTTTTCAATAGACAATATCATGTCGGAATCATCAAGAAGTGTGGAATTGTGAGCGGATA |
| LMO1.P2 | GTGTCCTCATTTCTATTTATAGTAGAAATCTTCATTTATCACTTCTAACAAATCTTCAAGAACTTTCCCAGTCACGACGTT |
| LMO1.P3 | TGATAGGCGGCCGCAAGTGATGATGTAGGTCTTG |
| LMO1.P4 | CCATTCAAAACAATTTAGATCTCGAGATG |
| LMO1.P5 | ATACGGACCGTGATGTCGGAATCATCAAGAAG |
| LMO1.P6 | GTGATAACGCGTAATAAGTGTCCCCATTTC |
| LMO1.P7 | TATACGCGTACAATATTGGTTAGACAACC |
| LMO1.P8 | CGTATACGGACCGTGATGTCATCGGAAACTTATAAGG |
| LMO1.P9 | CGAGGATCCATGTCGGAATCATCAAGAAGTATC |
| LMO1.P10 | TCTGCGGCCGCCTATTTATAGTAGAAATCTTC |
| LMO1.P11 | CGAATTTGTTCATATTTTGGACCACACATTATTCCAAACATTGTTTTCCATAGTCAGTGCCAATATGTGTGGAATTGTGAGCGGATA |
| VECTOR.P1 | GTAAGAATTGTAGTAGTTATTTCCGTCGACCGTCAAAACTAGAGAATAATAAAGAAAACGATC |
| VECTOR.P2 | GATCGTTTTCTTTATTATTCTCTAGTTTTGACGGTCGACGGAAATAACTACTACAATTCTTAC |
| VECTOR.P3 | CTCTATCACTGATAGGGAGTGGTAAAGTCGACAAGGTGCTGAACCAAACTGTGG |
| VECTOR.P4 | CCACAGTTTGGTTCAGCACCTTGTCGACTTTACCACTCCCTATCAGTGATAGAG |
| VECTOR.P5 | CCCATTCATTCCATCATAAAATGTCTCGAGGACCACCTTTGATTGTAAATAG |
| VECTOR.P6 | CTATTTACAATCAAAGGTGGTCCTCGAGACATTTTATGATGGAATGAATGGG |
| CaActETMpa | ATGTTCCCAGGTATTGCTGA |
| CaActETMma | ACATTTGTGGTGAACAATGG |
| CaLmo1TMp1a | CATCAAGAAGTATCAATC |
| CaLmo1TMm2a | GATTGCCTTATAAGTTTCCG |
| CaLmo1TMp3a | GGCAATTTTGAGTTGGGATTTAA |
| CaLmo1TMm4a | CTGAACCTTTCCCAAGCATCA |
| CaElmo1TMpBa | TGTTGGTGGTGCTACTGGTGTT |
| CaElmo1TMmBa | CAACCCATCAAACGCTGATTGG |
| CaCST20TM1pa | ACAACAGGAGACTGGGCAAAA |
| CaCST20TM1ma | AAGCTGCTTGTTTGGCATCA |
| CaCEK1TM1pa | GCTAACGCTGCAGCTACTACTTCTT |
| CaCEK1TM1ma | GGCTGAACAAACTATTCCATATGCT |
| CaTPK1TM1pa | GGTAATTTACAAGGTGGTTCTGATGAT |
| CaTPK1TM1ma | ACCTGAAGTTATAGGTGGTTCATATGG |
| CaPKC1TM1pa | GGATCCATCGCAGCGTTT |
| CaPKC1TM1ma | ATGTATGGTGCCGGAATTCG |
| CaAct1EpTMda | ATGTTCCCAGGTATTGCTGA |
| CaAct1EmTMda | ACATTTGTGGTGAACAATGG |
| CaTPK2TM1pa | TTGGGCTATCAAAGCTGCTTGT |
| CaTPK2TM1ma | AAAAACCGCCTTCTGGATCTG |
| CaMKC1TM1pa | CTTGTCGCTGTTCTTCACGTTT |
| CaMKC1TM1ma | TGAGGAACAGCAGCGAATACA |
| CaRac1pTMa | CCAGGGAAACAAGTTGGCTAGA |
| CaRac1mTMa | TTGAGTAGCAGCAGAACATTCCA |
| CaSte11TM1pa | ACTGCATTGCATAGGATTTCTATATCTC |
| CaSte11TM1ma | TCCGGCATAGTCAGAAATACCA |
| CaHst7TM1pa | TCATCTCCGTTGGAACCACAA |
| CaHst7TM1ma | AGCACCGATCCTGAGTTTCCT |
| CaHog1TM1pa | TTATCGAGCACCAGAAATCATGTTAA |
| CaHog1TM1ma | TGGAGAATTGATGCACGTGATC |
- a. Primers used for RT-PCR.
Plasmids
The LMO1 ORF, with 985 bp 5′ of the ATG and 752 bp 3′ of the stop codon (∼3.7 kb) was amplified by PCR from genomic DNA, using the primers LMO1.P3 (with a unique NotI site at the 5′ end) and LMO1.P4 (containing a unique XhoI site at the 3′ end). The amplified PCR product was digested with NotI/XhoI and cloned into the respective sites in pExpArg (derived from pExp-PADHGFPRAC1) (Bassilana and Arkowitz, 2006), to generate pExp-PLMO1LMO1. To generate pExp-PADHLMO1 and pExp-PADHGFPLMO1 plasmids, the primers LMO1.P5 (containing a unique RsrII site at the 5′ end) and LMO1.P6 (containing a unique MluI site at the 3′ end) were used to amplify by PCR the LMO1 ORF, with 2 bp 5′ of the ATG and 17 bp 3′ of the stop codon, from genomic DNA. The resulting PCR product was digested with RsrII/MluI and cloned into the respective sites in the plasmids pExp-PADH-R-GFPRAC1 and pExp-PADHGFP-R-RAC1 (Hope et al., 2008), yielding pExp-PADHLMO1 and pExp-PADHGFPLMO1. The lmo1ΔN mutant consisted of a truncation of the N-terminal region of the protein. Primers LMO1.P7 (containing a unique MluI site at the 3′ end) and LMO1.P8 (containing a unique RsrII site at the 5′ end) were used to amplify the region 355 bp 3′ of the ATG of LMO1 and 137 bp 3′ of the stop codon, an ATG codon is present 2 bp into the cloned sequence. This fragment was cloned into pCR2.1 TA (Invitrogen, Cergy Pontoise, France), yielding pCR-lmo1ΔN. pCR-lmo1ΔN was digested by RsrII and MluI to release the lmo1ΔN fragment, which was cloned into the respective sites in pExp-PADH-R-GFPRAC1, yielding pExp-PADHlmo1ΔN. For the overexpression of the different protein kinases, CST20, HST7, STE11, CEK1, CEK2, HOG1, PKC1, MKC1, TPK1 and TPK2, were amplified by PCR from genomic DNA using gene-specific primers, one with a unique RsrII site of the ATG codon and the other with a unique MluI site 3′ of the stop codon respectively. The resulting PCR products were digested by RsrII and MluI, and cloned into the respective sites in pExp-PADH-R-LMO1, yielding pExp-PADH-KINASE. To generate pNIM-pADHDCK1GFP, the two primer pairs VECTOR.P1 and VECTOR.P2, VECTOR.P3 and VECTOR.P4 were used to introduce SalI sites into the plasmid pNIM6 (Ramirez-zavala et al., 2008) 3′ of the Tetracycline promoter and 5′ of the Tetracycline terminator sequence using site-directed mutagenesis. The Tetracycline promoter-terminator sequence was then removed by SalI digestion, and the plasmid was subsequently religated. The primers VECTOR.P5 and VECTOR.P6 were used to introduce a XhoI site 3′ of the ACT1 terminator region, to generate the plasmid pNIM-PADH. The plasmid pExp-PADHDCK1GFP was digested with PstI and XhoI and the resulting DCK1GFP fragment was cloned into the respective sites in pNIM-PADH, yielding pNIM-PADHDCK1GFP. To generate the GST-LMO1 construct for protein pull-down experiments, LMO1 was amplified from cDNA using the primers pair LMO1.P9 and LMO1.P10, which introduced a unique BamHI site 5′ of the ATG and a NotI site 3′ of the stop codon. The BamHI/NotI LMO1 coding sequence was then cloned into pGEX 6P-2 (Amersham, Orsay, France), resulting in pGEX-LMO1. The sequences of all cloned PCR products and site-directed mutagenesis products were confirmed (Eurofins MWG Operon, Ebersberg Germany and Seqlab – Sequence Laboratories, Göttingen, Germany).
General techniques and microscopy
Real-time quantitative reverse transcription-PCR (qRT-PCR) and RT-PCR analyses were carried out as described previously (Bassilana et al., 2005), and the primers used are listed in Table 2. For analyses of colony morphology, plates were incubated for 3–6 days and images of colonies were taken with a Roper Scientific Micromax CCD camera on a Leica MZ6 dissection scope at a magnification of 10× or 20×. For cell morphology studies, cells were imaged with a CCD camera on a Leica DMR epifluorescence microscope with a numerical aperture 1.35 63× objective by differential interference contrast (DIC) optics, as described (Bassilana et al., 2003). FRAP analysis was performed essentially as described previously (Bassilana and Arkowitz, 2006) using a Zeiss LSM510 Meta confocal microscope with a Plan-Apo 63× (numerical aperture, 1.4) objective. For the plasma membrane FRAP experiments, images were captured every 0.2–0.4 s at 2–5% maximum laser intensity and 10 × 0.25–0.5 ms photobleaching scans at 100% laser intensity were performed on a circular area of 1 µm2 at the plasma membrane. Data analysis was carried out as described (Bassilana and Arkowitz, 2006).
Protein pull-downs and cell fractionation
GST-Lmo1 fusion proteins were prepared as described previously in Buffer A (PBS, 1 mM EDTA, 1 mM PMSF and 0.1% Triton-X100) (Barale et al., 2006) and pull-down experiments were carried out essentially as described (Hope et al., 2008). Briefly, logarithmically growing cells expressing Dck1-GFP in the presence of Rac1 (PY1278), in the absence of Rac1 (PY1382), or expressing GFP-Rac1 in the absence of Dck1 (PY1265) and in the presence of Dck1-GFP (PY1278) were resuspended in GPL buffer [20 mM Tris pH 7.4, 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 5 mM β-Glycerol Phosphate, 1 mM DTT and protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany)]. Cells were broken using glass beads in a Ribolyser (MP Biomedicals, Illkirch, France) and 10 000 g cell lysate supernatants were incubated with the GST-Lmo1 bound to glutathione (GSH)-Sepharose 4B (Amersham, Orsay, France) for 2 h at 4°C. After four washes with GPL Buffer, proteins were eluted in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer, analysed by 10% SDS-PAGE, followed by immunoblotting. For cell fractionation experiments, logarithmically growing cells expressing Dck1-GFP and GFP-Lmo1 (PY1278) were used and fractionations were carried out as described previously (Hope et al., 2008). Fractions were analysed by 8% (for Dck1-GFP and GFP-Lmo1) and 12% SDS-PAGE (for Rac1), followed by immunoblot analyses. Proteins on nitrocellulose membranes were initially visualized by Ponceau S staining. Blots were then probed with either a rabbit polyclonal serum against GFP (1:5000) (Nern and Arkowitz, 2000) or C. albicans Rac1 (1:1000) (Hope et al., 2008), then visualized by enhanced chemiluminescence (luminol-coumaric acid) on a Fuji-Las3000 (Clichy, France).
Acknowledgements
We thank R. Vauchelles for carrying out the FRAP experiments, A. Chessel for expert technical assistance and J. Morschhäuser (Universität Würzburg) for the pNim6 plasmid. This work was supported by the Centre National de la Recherche Scientifique and Fondation pour la Recherche Médicale-BNP-Paribas. H. H. was supported by EU Marie Curie Early Stage Training Fellowship (International PhD Program in Developmental and Cellular Decisions), Fondation de la Recherche Medicale and L'Oréal-UNESCO. C. S. was supported by Agence Nationale de la Recherche (ANR-06-PATHO-012-01).




