Functional interplay between the Yersinia pseudotuberculosis YpsRI and YtbRI quorum sensing systems modulates swimming motility by controlling expression of flhDC and fliA
Summary
Quorum sensing (QS) in Yersinia pseudotuberculosis involves two pairs of LuxRI orthologues (YpsRI and YtbRI) and multiple N-acylhomoserine lactones (AHLs). In a ypsI/ytbI mutant, AHL synthesis was abolished, unaffected in a ypsR/ytbR double mutant and substantially reduced in a ypsI/ytbR mutant, indicating that neither YpsR nor YtbR is essential for AHL synthesis. To determine the interrelationship between YpsRI and YtbRI we constructed chromosomal lux–promoter fusions to ypsR, ypsI, ytbR and ytbI and examined their expression in each of the QS mutant backgrounds. The YpsRI system negatively autoregulates itself but positively regulates the expression of the ytbRI system whereas the ytbRI system is positively autoregulated but only at the level of ytbI expression. YtbRI does not control expression of ypsR or ypsI. This hierarchical QS system controls swimming motility via regulation of flhDC and fliA. The AHLs synthesized via YtbI play a dual role, activating flhDC, in conjunction with YpsR but repressing fliA in conjunction with YtbR and YpsR. In liquid and plate assays, the early onset of motility observed in ypsR and ypsI mutants was abolished in ytbI, ytbR ypsI/ytbI, ypsR/ytbR mutants, indicating that QS regulates motility both positively (via YtbRI) and negatively (via YpsRI).
Introduction
Many Gram-negative bacteria use a cell population density dependent regulatory system that generates and senses N-acylhomoserine lactone (AHL) quorum sensing (QS) signal molecules. This cell-to-cell communication system couples gene expression with growth phase and controls virulence, secondary metabolite production, biofilm maturation and swarming motility (Swift et al., 2001). AHLs are usually produced via members of the LuxI protein family and sensed by LuxR-type response regulators which become activated on binding the cognate AHL. Some AHL producers possess several pairs of LuxRI orthologues and produce multiple QS signal molecules which function interdependently to regulate target genes either positively or negatively (Fuqua and Greenberg, 2003; Lazdunski et al., 2004). Of these, the most intensively investigated have been Pseudomonas aeruginosa and Rhizobium leguminosarum where LasRI and CinRI, respectively, are located at the top of the hierarchical regulatory cascades (Latifi et al., 1996; Wisniewski-Dye et al., 2002). In P. aeruginosa, LasR activated by binding to its cognate AHL, N-(3-oxododecanoyl) homoserine lactone (3-oxo-C12-HSL) positively regulates the corresponding AHL synthase gene, lasI as well as a second luxRI locus termed rhlRI (Latifi et al., 1996). Similarly, in R. leguminosarum, CinR, activated by the CinI-synthesized AHL, N-(3-hydroxy-7-cis-tetradecenoyl)homoserine lactone, positively controls both cinI and raiRI expression (Wisniewski-Dye et al., 2002).
Yersinia pseudotuberculosis is a mammalian enteropathogen that causes a self-limiting gastroenteritis in man (Johnson, 1992). Symptoms range from fever, diarrhoea and abdominal pain (Butler, 1994) to chronic systemic infections and reactive arthritis, particularly in immunocompromised individuals (Cover and Aber, 1989). The two other members of the Yersinia which are pathogenic to mammals are Yersinia enterocolitica which gives rise to similar symptoms to those of Y. pseudotuberculosis and Yersinia pestis which presents as pneumonic, bubonic or septicaemic plague. At the genomic level, Y. pseudotuberculosis and Y. pestis share over 99% DNA homology and Y. pestis has been shown to be a highly uniform clone of Y. pseudotuberculosis which arose shortly before the first recorded pandemic which occurred between the 6th and the 8th century AD (Bercovier et al., 1980; Achtman et al., 1999).
All three human pathogenic species of Yersinia synthesize AHLs and both luxI and luxR orthologues have been identified (Throup et al., 1995; Atkinson et al., 1999; Swift et al., 1999). Y. pseudotuberculosis possess two LuxRI pairs termed YpsRI and YtbRI in which the corresponding I and R genes are convergently organized (Atkinson et al., 1999).
YpsI and YtbI are responsible for the production of at least 24 different AHLs, at least seven of which have been detected at levels considered to be physiologically significant (Ortori et al., 2006). Both YpsI and YtbI are capable of synthesizing N-hexanoylhomoserine lactone (C6-HSL), N-(3-oxohexanoyl)homoserine lactone (3-oxo-C6-HSL) and N-3-(oxo-septanoyl)homoserine lactone (3-oxo-C7-HSL) while YtbI is responsible for the synthesis of N-3-(hydroxy-octanonoyl)homoserine lactone (3-OH-C8-HSL), N-3-(oxo-octanoyl)homoserine lactone (3-oxo-C8-HSL), N-octanoylhomoserine lactone (C8-HSL) and N-3-(oxo-decanoyl)homoserine lactone (3-oxo-C10-HSL) (Ortori et al., 2006).
Previously we have shown that AHL-dependent QS in Y. pseudotuberculosis is involved in the control of cell–cell contact (aggregation) and flagellar-mediated motility (Atkinson et al., 1999). On soft-agar motility plate assays, both ypsR and ypsI mutants, but not the parent Y. pseudotuberculosis strain, are motile after 24 h when grown at 22°C but not 37°C. In liquid batch culture at 22°C, motility is induced after 6 h in both ypsI and ypsR mutants but delayed in excess of 15 h in the parent (Atkinson et al., 1999). These data indicate that the YpsRI QS system represses swimming motility at 22°C in Y. pseudotuberculosis.
The regulation of flagellar expression in Yersinia has been predicted to be similar to that of other members of the Enterobacteriaceae in that it is co-ordinated with ordered flagellar assembly proceeding in response to environmental cues (Young, 2004). This type of flagellar regulatory hierarchy consists of three major gene classes, I, II and III. Class I genes consisting of the master regulator operon, flhDC are expressed at the top of the hierarchy and are required for the expression of all other flagellar genes. Class II genes encode structural and accessory proteins required for the assembly of the basal body and hook components of the flagellum plus the flagellar specific sigma factor FliA and antisigma factor, FlgM which functions to limit FliA activity until the flagellar basal body assembly is ready for flagellin export (MacNab, 1996). Class III genes are transcribed from FliA-dependent promoters and encode proteins required for maturation of the flagellum into an external filamentous structure, and for expression of the motility-guiding, chemosensory system (MacNab, 1996). However, in contrast to Salmonella, flagella are not expressed at 37°C in Yersinia (Young, 2004) which becomes flagellate at or below 30°C. (Brubaker, 1991). In Y. enterocolitica, temperatures below 30°C induce co-ordinate transcription from fliA-dependent promoters of the three class III tandem flagellin genes fleA, fleB and fleC to promote flagellar synthesis (Kapatral and Minnich, 1995). Following a downshift in temperature from 37°C to 28°C flagellin transcription ensues after approximately two generations (Kapatral and Minnich, 1995; Kapatral et al., 1996). The Y. pseudotuberculosis flagellar external protein export system also contributes to virulence as an flhB mutant (FlhB is involved in export of external flagellar proteins such as filament and hook) is attenuated when administered orally to mice (Young, 2004). Virulence, temperature and motility therefore share tight relationships, congruent with a ‘swim to arrive, stay to infect’ model and illustrate the fitness requirement for motility in aquatic environments outside the host.
Although the YpsRI system represses the onset of motility, the contribution of the YtbRI system is not known, neither has the relationship between the two QS loci been established. Here we show that the YpsRI system negatively regulates itself (both ypsI and ypsR) and positively regulates ytbI and ytbR whereas the ytbRI system is positively autoregulated but only at the level of ytbI expression. The onset of swimming motility is determined by the actions of the two QS systems which modulate expression of the flagellar master operon, flhDC and the alternative sigma factor, fliA in a temperature dependent manner.
Results
Mutagenesis of ytbRI, the second AHL-dependent QS system in Y. pseudotuberculosis
To define roles for YtbI and YtbR, deletion insertion mutants were constructed by removing 298 and 321 bp from positions 154 and 215 of the ytbI and ytbR genes, respectively, followed by insertion of a chloramphenicol (Cm) cassette into engineered HindIII sites within the deleted region to give the ytbI and ytbR mutants. To aid clarification of the regulatory relationship between YpsRI and YtbRI, four double deletion insertion mutants were also constructed in each of the single (ypsR, ypsI, ytbR and ytbI) mutant backgrounds to provide the ypsI/ytbI, ypsR/ytbR, ypsR/ytbI and ypsI/ytbR mutants respectively. For each mutant, gene replacement in the chromosome was confirmed by polymerase chain reaction (PCR) and Southern blot analysis (data not shown). Despite many attempts we have been unable to construct a complete quadruple QS mutant.
Analysis of AHL synthesis by Y. pseudotuberculosis quorum sensing mutants
To investigate the relationship between the two QS systems and the production of AHLs, cell-free culture supernatants were prepared from the Y. pseudotuberculosis parent, ytbI, ytbR, ypsI/ytbI, ypsR/ytbR, ypsR/ytbI and ypsI/ytbR mutants grown at 22°C in LBmops for 24 h. Supernatants were extracted with dichloromethane and subjected to thin layer chromatography (TLC) as described before (McClean et al., 1997; Atkinson et al., 1999). AHLs were detected by overlaying each chromatogram with the AHL biosensor, Chromobacterium violaceum CV026 which responds to the major Yersinia short chain AHLs (Fig. 1). Extracts prepared from ytbR and ypsR/ytbR mutants showed that these mutants produced the same major AHLs as the parent strain, i.e. they produced 3-oxo-C6-HSL, C6-HSL and C8-HSL (Fig. 1A compare lanes 3, 6 and 7). Thus, neither of the Y. pseudotuberculosis LuxR proteins is required for the synthesis of these AHLs. Furthermore, the ytbI mutant was deficient in C8-HSL (Fig. 1 lane 4) and AHL production was completely abolished in the ypsI/ytbI mutant (Fig. 1A, lane 5). When the ypsI/ytbI mutant was complemented with pSA291, which carries functional copies of ypsI and ytbI, AHL synthesis was restored to levels in excess of those of the parent strain (Fig. S1). Using liquid-chromatography mass spectrometry (LC-MS), these data were confirmed and extended. All 24 AHLs which were previously detected in the parent strain by LC-MS were absent from the ypsI/ytbI mutant whereas the AHL profile of the ypsR/ytbR mutant was the same as the parent strain (Ortori et al., 2006 and data not shown).
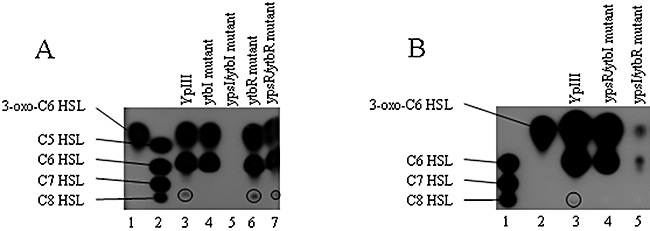
TLC chromatogram overlaid with the biosensor C. violaceum CV026 of the AHL profiles from extracts of Y. pseudotuberculosis parent and isogenic ypsRI and ytbRI single and double mutants grown in LBmops at 22°C.A. Lane 1, synthetic standard, 1.4 × 10−8 mol 3-oxo-C6-HSL, lane 2, synthetic standards (from top) 5.4 × 10−8 mol C5-HSL, 5.0 × 10−9 mol C6-HSL, 4.7 × 10−7 mol C7-HSL, 2.2 × 10−8 mol C8-HSL, lanes 3–7, AHLs extracted from parent, ytbI, ypsI/ytbI, ytbR and ypsR/ytbR mutants respectively.B. Lane 1, synthetic standards (from top) 5.0 × 10−9 mol C6-HSL, 4.7 × 10−7 mol C7-HSL, 2.2 × 10−8 mol C8-HSL, lane 2, synthetic standard, 1.4 × 10−8 mol 3-oxo-C6-HSL, lanes 3–5, AHLs extracted from parent, ypsR/ytbI and ypsI/ytbR mutants respectively. Because of the low levels of C8-HSL produced and the presence of a comigrating compound that inhibits CV026, the position of this AHL is circled.
The ypsI/ytbR and ypsR/ytbI mutants were also analysed by TLC using the CV026 AHL biosensor (Fig. 1B). When compared with the parent strain, the ypsR/ytbI mutant produces 3-oxo-C6-HSL and C6-HSL but is deficient for C8-HSL (Fig. 1B, lane 4) while the ypsI/ytbR mutant produces greatly reduced amounts of 3-oxo-C6-HSL and C6-HSL (Fig. 1B, lane 5). These data are consistent with YpsR functioning as a positive regulator of AHL synthesis.
Defining the regulatory relationships between the ypsR/I and ytbR/I loci
To determine how the ypsR/I and ytbR/I systems are regulated at the transcriptional level we constructed PypsI::luxCDABE, PypsR::luxCDABE, PytbI::luxCDABE and PytbR::luxCDABE promoter fusions which were introduced as single copies into the chromosome of the parent, ypsI, ypsR, ytbI and ytbR mutants. The expression of ypsI, ypsR, ytbI and ytbR was determined as a function of temperature and growth phase by recording bioluminescence throughout growth in liquid culture in LBmops at 22°C or 37°C. Where appropriate, promoter fusion expression was determined in the relevant QS mutant complemented with a plasmid borne copy of the wild-type gene (on plasmids pSA290-pSA295). Complementation data are presented in the supplementary material (Figs S2 and S4). Figure 2 reveals that in Y. pseudotuberculosis grown at 22°C or 37°C, ypsR expression is much higher than ypsI expression. Moreover, at 22°C ypsI expression peaks at ∼6 h before steadily falling (Fig. 2A), while at 37°C ypsI expression remains mainly constant throughout growth (Fig. 2C) as does ypsR expression at both temperatures (Fig. 2B and D). At 22°C mutation of ypsR alone results in an increase (approximately fourfold) in ypsI expression, which suggests that YpsR can negatively regulate ypsI (Fig. 2A). Figure 2B shows that at 22°C ypsR expression is negatively autoregulated by YpsR, possibly in conjunction with the AHLs associated with ypsI as when compared with the parent strain ypsR expression is approximately ninefold higher in the ypsR mutant and there is also an approximately threefold increase in ypsR expression in the ypsI mutant (for complementation data see Fig. S2). Similar profiles were obtained when the reporter strains were grown at 37°C although ypsR expression is sustained throughout growth in the ypsI mutant (Fig. 2C) rather than steadily falling after an initial peak. The expression of ypsI and ypsR are not appreciably altered in the ytbI or ytbR mutants at either 22°C or 37°C when compared with the parent, indicating that neither YtbR nor the AHLs associated with YtbI play a major role in the regulation of the ypsR/I system (Fig. 2A–D).
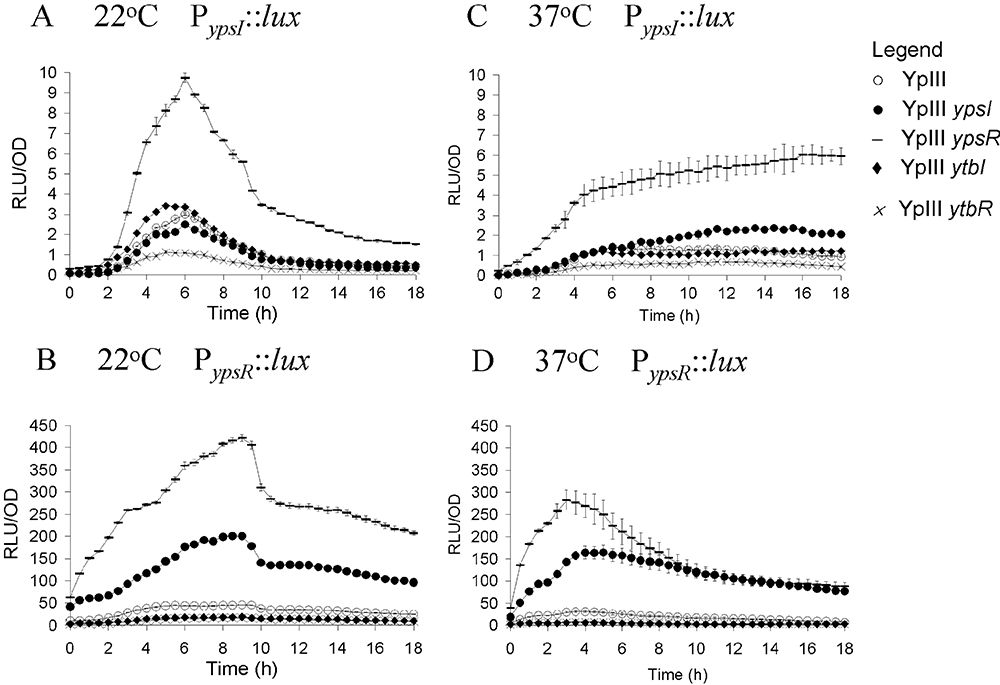
Expression of PypsI::lux (A and C) and PypsR::lux (B and D) in Y. pseudotuberculosis parent (○), ypsI mutant (●), ypsR mutant (–), ytbI mutant (◆), ytbR mutant (×) as a function of growth at 22°C (A and B) and 37°C (C and D).
Figure 3 shows that ytbR and ytbI expression levels are considerably higher at 22°C compared with 37°C. At 22°C the expression of both ytbR and ytbI peaks at ∼7 h, and then steadily rises to 18 h (Fig. 3A and B). At 22°C the expression of ytbI is approximately sevenfold lower in both the ypsR and ytbR mutants (Fig. 3A) and is also lower in the ypsI and ytbI mutants. When taken together these data suggest that both YpsR and YtbR can positively activate ytbI expression in the presence of AHLs (for complementation data see Fig. S3). Moreover, Fig. 3B shows that ytbR expression is approximately threefold lower in the ypsI, ytbI and ypsR mutants, indicating that YpsR in association with AHLs positively activates ytbR expression (for complementation data see Fig. S4). However, ytbR expression is almost identical to that of the parent in the ytbR mutant, suggesting that ytbR expression is not autoregulated. A similar pattern emerges at 37°C although ytbR expression is slightly lower in the ytbR mutant at this temperature.
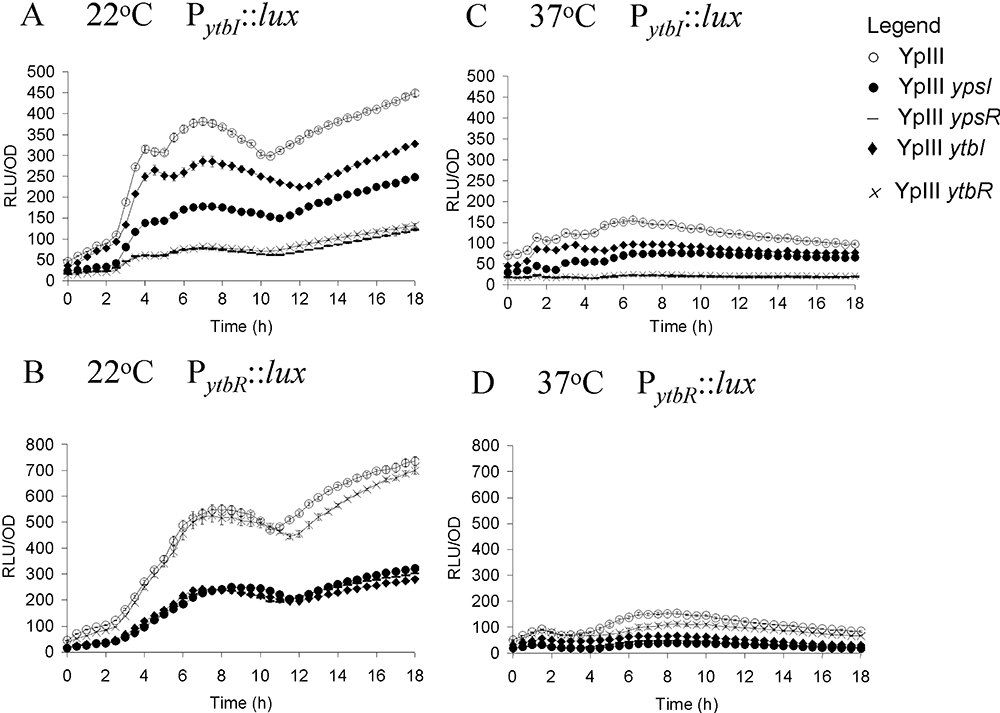
Expression of PytbI::lux (A and C) and PytbR::lux (B and D) in Y. pseudotuberculosis parent (○), ypsI mutant (●), ypsR mutant (–), ytbI mutant, ytbR mutant (×) as a function of growth at 22°C (A and B) and 37°C (C and D).
The promoter regions of Y. pseudotuberculosis quorum sensing and flagellar genes contain lux box like consensus sequences
The DNA sequence of the Vibrio fischeri LuxR binding site (lux box) has been determined (Devine et al., 1989; Egland and Greenberg, 1999; Stevens and Greenberg E.P., 1999). This DNA element has features that are conserved with the binding sites of LuxR-type proteins in many different bacteria and is commonly positioned at around −42.5 with respect to the transcriptional start site of the target genes (Devine et al., 1989; Fuqua et al., 1994; Gray et al., 1994). Analysis of the regions upstream of the translational start sites of ypsI, ypsR, ytbI and ytbR revealed the presence of lux box-like consensus sequences in the promoters (Fig. S5). Given that motility is also regulated by AHL-dependent QS in Y. pseudotuberculosis we cloned and sequenced the flhDC and fliA homologues from Y. pseudotuberculosis using primers designed to the homologous Y. enterocolitica sequences. Analysis of the potential promoter regions of the Y. pseudotuberculosis flhDC and fliA genes revealed the presence of sequences with significant homologies to lux boxes (Fig. S5). While none of these putative lux boxes are identical to each other they all match the lux box consensus at several positions (Fig. S5).
Influence of flhDC on fliA expression
The effect of Y. pseudotuberculosis FlhDC on fliA expression has not previously been investigated. We therefore fused the fliA promoter to luxCDABE and introduced the reporter gene fusion onto the chromosome of the Y. pseudotuberculosis parent and the flhDC mutant. YpIII flhDC PfliA::luxCDABE was also complemented with a functional copy of flhDC carried on pSA220. The expression of fliA was determined as a function of temperature by recording bioluminescence throughout growth in liquid culture in LBmops. During these and subsequent experiments the motility of the reporter strains was examined by phase contrast microscopy and compared with the parent strain to confirm that introduction of the lux fusions onto the Y. pseudotuberculosis chromosome did not affect motility.
Figure 4 shows that at 22°C fliA expression peaks after 8.5 h of batch culture and, as predicted from the flagellar regulatory hierarchy in other enterobacteria, fliA expression was completely abolished in a flhDC mutant demonstrating that fliA is dependent on flhDC in Y. pseudotuberculosis. When flhDC was introduced into YpIII flhDC PfliA::luxCDABE on pSA220, fliA expression was only partially restored presumably because of a multicopy effect (Fig. 4). However, the functionality of the plasmid-borne flhDC genes was confirmed on plate assays, as pSA220 restored swimming motility (Fig. S6). When grown at 37°C, the expression profiles of both flhDC and fliA were similar to those observed at 22°C, indicating that expression of both genes is independent of growth temperature (data not shown).
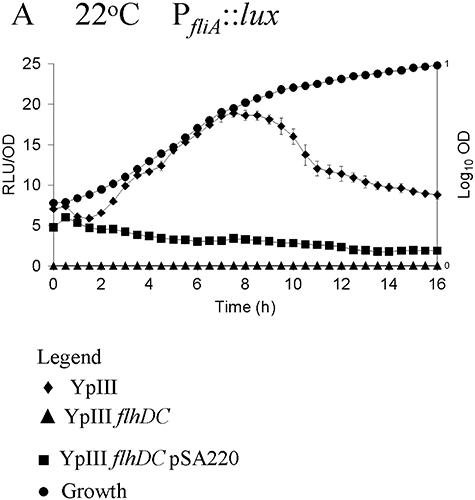
Expression of PfliA::lux as a function of growth phase and temperature. fliA expression was examined at 22°C in the Y. pseudotuberculosis parent (◆), flhDC mutant (▴) and flhDC mutant complemented with pSA220 (▪). Growth (●) is shown as OD600. fliA expression is abolished in the flhDC mutant but is partially restored in the presence of pSA220 carrying the intact flhDC genes.
flhDC and fliA are regulated by YpsRI and YtbRI
To investigate the impact of QS on flhDC and fliA expression, the corresponding luxCDABE promoter fusions were introduced onto the chromosomes of each of the single and double ypsI, ypsR, ytbI and ytbR mutants respectively. Bioluminescence was recorded throughout growth and the data obtained for each mutant compared with that of the parent strain. For all of the mutants, flhDC expression was delayed and reduced during growth at both 22°C and 37°C compared with the parent strain (Fig. 5A and B) where expression was induced during the log phase and sustained into early stationary phase (Fig. 5A). Mutation of ytbI, or ypsI and ytbI together resulted in an approximately fourfold reduction in flhDC expression levels, indicating that at both temperatures the AHLs produced via YtbI are required for positive regulation of flhDC expression. Mutation of ypsR or ypsR and ytbR together resulted in an approximately eightfold or fourfold reduction in flhDC expression levels, respectively, indicating YpsR is involved in activating flhDC expression, probably in conjunction with YtbI AHLs (Fig. 5A and B; see Fig. S7 for complementation data). The influence of QS on fliA regulation is shown in Fig. 6. In contrast to the parent strain, the ypsI/ytbI mutant exhibits a marked de-repression of fliA expression (∼20-fold increase in RLU) at 22°C (Fig. 6A), which peaks at about 9 h in the mid-log phase of growth and then falls back towards, but remains higher (∼10-fold) than parental expression levels. Similar results were obtained with ytbI although there was only an ∼10-fold difference when compared with the parent, suggesting that the AHLs produced via YtbI are required for fliA repression (Fig. 6A). The expression of fliA was at least fivefold higher in the ypsR/ytbR mutant, and as fliA expression in both the ypsR and ytbR mutants is similar to the parent, this suggests that YpsR and YtbR are both required to repress fliA expression at 22°C (see Fig. S8 for complementation data).
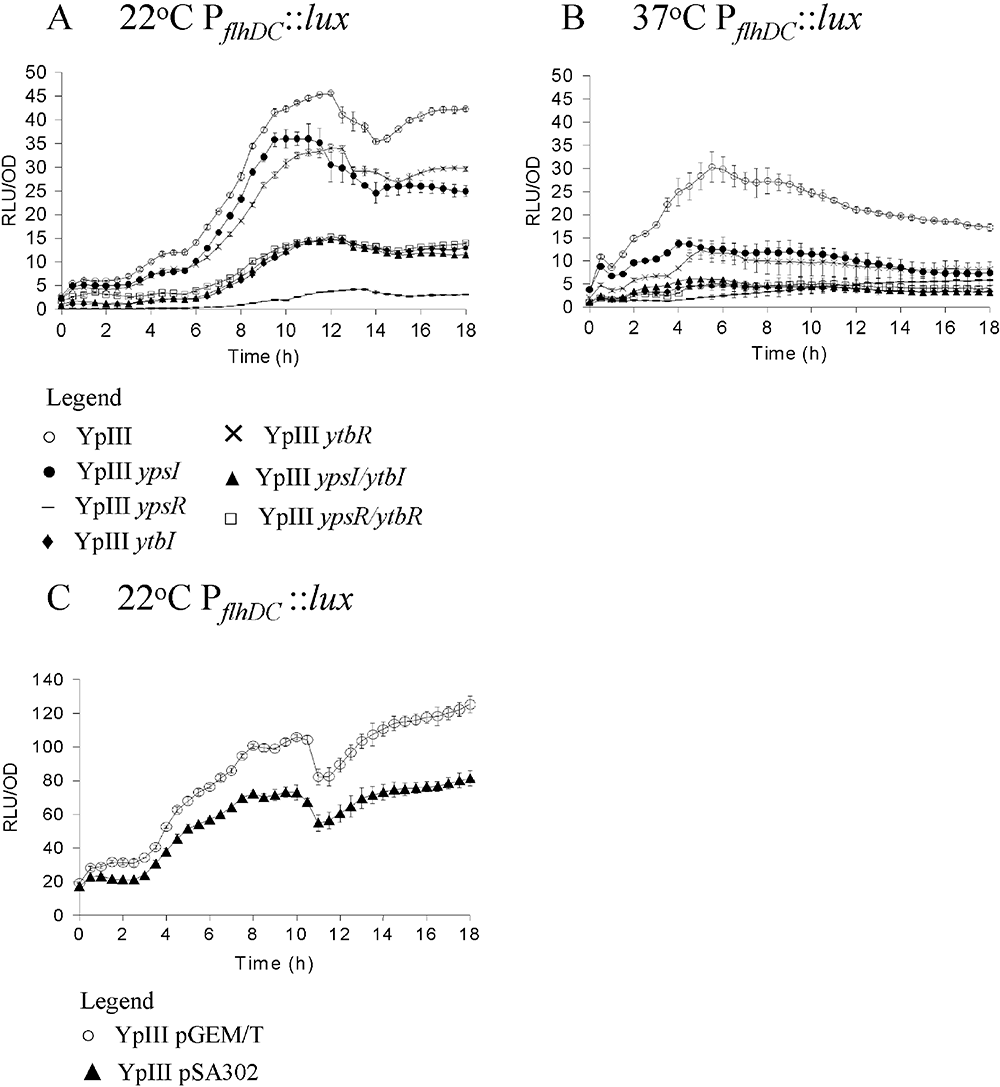
Expression of flhDC using promoter fusions to luxCDABE in Y. pseudotuberculosis YpIII and the isogenic quorum sensing mutants during growth at 22°C (A) and 37°C (B) respectively. Key: parent (○), ypsI mutant (●), ypsR mutant (–), ytbI mutant (◆), ytbR mutant (×), ypsI/ytbI mutant (▴), ypsR/ytbR mutant (□). Expression of flhDC at 22°C in Y. pseudotuberculosis YpIII with and without pSA302 which carries the AHL lactonase aiiA (C). Key: parent pGEM/T (○), YpIII pSA302 (▴).
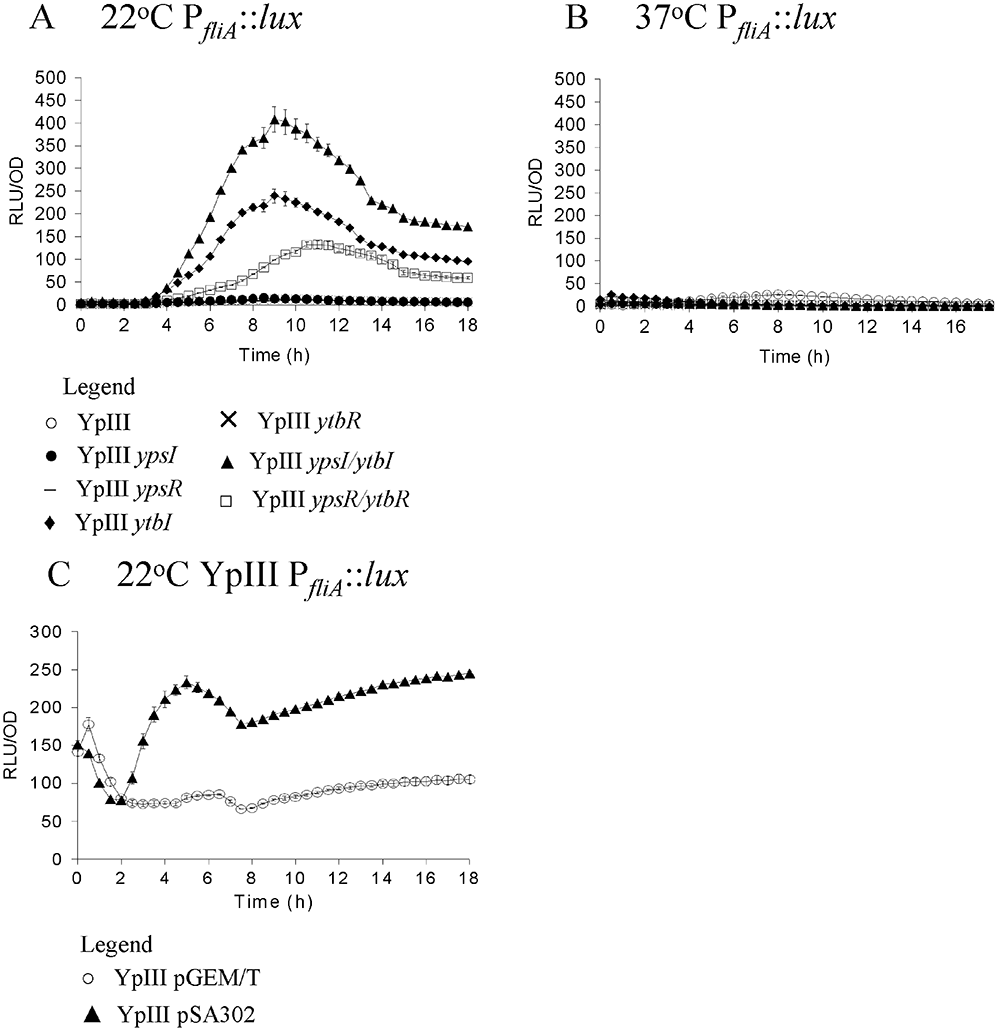
Expression of fliA using promoter fusions to luxCDABE in Y. pseudotuberculosis YpIII and isogenic quorum sensing mutants during growth at 22°C (A and C) and 37°C (B and D). Key: parent (○), ypsI mutant (●), ypsR mutant (–), ytbI mutant (◆), ytbR mutant (×), ypsI/ytbI mutant (▴), ypsR/ytbR mutant (□). Expression of fliA at 22°C in Y. pseudotuberculosis YpIII with and without pSA302 which carries the AHL lactonase aiiA (C). Key: parent pGEM/T (○), YpIII pSA302 (▴).
To confirm that the function of YpsI and YtbI in regulating flhDC and fliA expression was via AHL synthesis, we introduced the AHL-degrading enzyme, AiiA (Dong et al., 2000) on a recombinant plasmid (pSA302) into both YpIII PflhDC::luxCDABE and YpIII PfliA::luxCDABE. AiiA renders Y. pseudotuberculosis AHL-negative as determined by TLC (data not shown). Figure 5 shows that at 22°C flhDC expression is reduced in the presence of AiiA (compare Fig. 5A with Fig. 5C), whereas fliA expression is increased (compare Fig. 6A with Fig. 6C), consistent with the behaviour of the ypsI ytbI double mutant. These data unequivocally confirm the role of AHLs in regulating flhDC and fliA expression.
Exogenous AHLs do not modulate fliA or flhDC expression
In most AHL-dependent QS systems, the phenotype of an AHL synthase mutant can be restored by the provision of exogenous AHLs. Consequently, we synthesized a series of the major (C6-HSL, 3-oxo-C6-HSL, C8-HSL and 3-oxo-C8-HSL) and minor (3-oxo-C10-HSL, 3-oxo-C12-HSL and 3-oxo-C14-HSL) AHLs produced by Y. pseudotuberculosis. When provided exogenously to the parent or AHL synthase mutants carrying the PflhDC::luxCDABE fusion, light output was unaffected whether the synthetic compounds were added singly or in combination at a range of different concentrations from 0.1 to 10 μM (data not shown). A lack of response was also noted when the fusions were provided with a range of dilutions of an organic extract of supernatant prepared from stationary phase cultures containing the AHL mixture produced by the parent strain (data not shown). The experiments were also repeated with PfliA::luxCDABE but again no response to the exogenously provided AHLs was observed.
To determine whether the inability of exogenous AHLs to modulate flhDC or fliA expression was a consequence of the inability of the AHLs to gain intracellular access, we transformed the ypsI/ytbI mutant with the AHL biosensor plasmid, pSB401. If AHLs accumulate intracellularly in Y. pseudotuberculosis, then this strain which does not bioluminesce should respond by emitting light. The biosensor was activated by both short-chain (C6-HSL) and long-chain (3-oxo-C12-HSL) AHLs, indicating that they are capable of entering Y. pseudotuberculosis and activating LuxR (Fig. 7A). These data were confirmed by incubation of both the parent and ypsI/ytbI mutant with tritiated 3-oxo-C12-HSL. Figure 7B shows that similar levels of 3-oxo-C12-HSL accumulated in both strains. Consequently, the inability of exogenous AHLs to influence flhDC or fliA expression is not because of a failure to gain intracellular access.
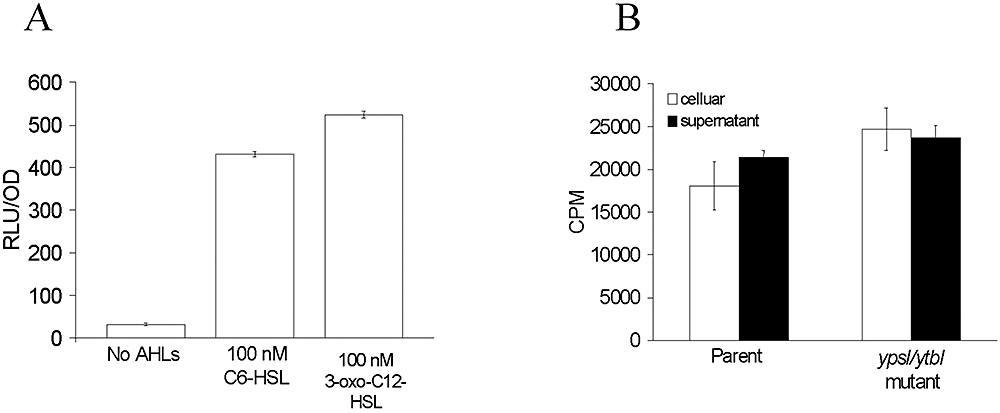
Intracellular accumulation of AHLs in Y. pseudotuberculosis.A. Maximum induction of bioluminescence in the AHL-negative Y. pseudotuberculosis ypsI/ytbI mutant transformed with the AHL biosensor pSB401 in the absence or presence of C6-HSL or 3-oxo-C6-HSL (100 nM).B. Uptake of tritiated 3-oxo-C12-HSL by the Y. pseudotuberculosis parent and ypsI/ytbI mutant.
YtbRI is required for motility on swim plates but not in liquid media
As the ypsR/I locus negatively regulates swimming motility (Atkinson et al., 1999), the ytbI, ytbR, ypsI/ytbI and ypsR/ytbR mutants were assayed for their ability to swim on motility agar plates at 22°C and 37°C respectively. As previously observed (Atkinson et al., 1999) the ypsI and ypsR mutants were swimming within 5 h at 22°C (Fig. 8A compare parent with ypsI and ypsR mutants respectively). The ytbR, ytbI, ypsI/ytbI, ypsR/ytbR mutants were not motile on swim plates at any temperature after 24 h (Fig. 8A) and up to 48 h (data not shown). Thus, in the absence of ytbR or ytbI, mutation of ypsI or ypsR does not result in the induction of motility. These data indicate that the ytbRI locus is required for the early onset of swimming in these plate assays. However, as the parent strain does not swim under these conditions, it is not clear whether the ytbRI system is an absolute requirement for motility in Y. pseudotuberculosis. Consequently, we examined the motility of the parent, ypsR, ypsI, ytbR, ytbI, ypsI/ytbI and ypsR/ytbR mutants at four different time points in liquid media. Figure 8B shows that the ytbI and ytbR mutants are both motile in liquid media with a similar onset to that of the parent strain. These data demonstrate that the ytbRI system may not be essential for the later induction of motility in liquid media that is observed in the parent. Furthermore, the introduction of ytbI and ytbR mutations into the ypsI and ypsR mutants, respectively, both abolish the early onset of motility observed in the single ypsI and ypsR mutants, consistent with the swim plate assays and indicating that the ytbRI system positively influences the onset of motility.
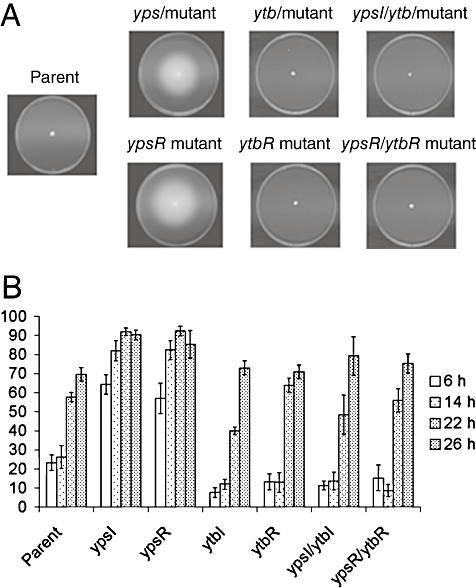
Swim plate motility assays of Y. pseudotuberculosis parent, and the ypsI, ypsR, ytbI, ytbR, ypsI/ytbI, ypsR/ytbR mutants after 24 h (A). Swimming motility in liquid media in the parent and mutants after 6, 14, 22 and 26 h incubation (B). For both experiments, bacteria were incubated at 22°C.
Discussion
In Y. pseudotuberculosis, AHL-dependent QS exclusively uses the ypsRI and ytbRI loci as YpsR and YtbR are the only LuxR homologues present in the genome (Chain et al., 2004), and mutation of both ypsI and ytbI results in the abolition of AHL synthesis which can be complemented when functional copies of ypsI and ytbI are introduced into the ypsI/ytbI double mutant.
Here we show that expression of the two pairs of LuxRI orthologues in Y. pseudotuberculosis are linked. The YpsRI and YtbRI systems of Y. pseudotuberculosis, in common with those of bacteria such as P. aeruginosa and R. leguminosarum form a regulatory hierarchy. Using lux-based chromosomal fusions to the ypsR, ypsI, ytbR and ytbI promoters, we have established that the ypsR/I system is negatively autoregulated while the ytbR/I system is positively autoregulated. While YpsR negatively regulates the expression of ypsI, ypsR expression is negatively regulated by YpsR and the AHLs synthesized via YpsI (i.e. primarily C6-HSL and 3-oxo-C6-HSL; Ortori et al., 2006). Furthermore, YtbR positively regulates ytbI expression but does not autoregulate itself. Both ytbR and ytbI are also positively regulated by YpsR. However, there does not appear to be any regulation of the ypsRI system by the YtbRI system which confirms that the link between ypsR/I and ytbR/I is one of hierarchical positive regulation driven by the YpsRI system. These findings are summarized in Fig. 9.
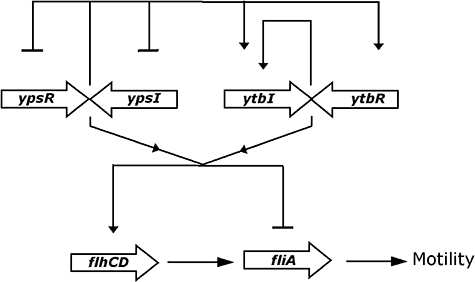
A simplified model for the regulatory cascade linking the ypsRI and ytbRI QS systems to flhDC, fliA and swimming motility in Y. pseudotuberculosis. Lines ending with ‘▾’ indicates activation, those ending in ‘–’ indicate repression. YpsR/I negatively autoregulates its own expression and positively regulates the expression of ytbR/I while YtbRI positively autoregulates ytbI expression. Both flhDC and fliA expressions are under the duel control of the YpsRI and YtbRI systems with YpsR being responsible for the activation of flhDC while YpsR and YtbR negatively regulate fliA expression. Both systems require the AHLs associated with YtbI.
Both ypsRI and ytbRI are convergent to and overlapping with each other rather than arranged in tandem as are lasRI, rhlRI, cinRI and raiRI. Although most of the LuxR orthologues so far studied are positive regulators, such as reported here for YtbR, EsaR from Pantoea (Erwinia) stewartii (Minogue et al., 2002) (whose gene is also convergent and overlapping with esaI) and SpnR from Serratia marcescens (Horng et al., 2002) both function as negative regulators in the presence of their cognate AHLs. However, while neither of these LuxR orthologues influences AHL production, spnR expression is positively regulated by SpnR and is further enhanced by the provision of SpnI-generated AHLs (Horng et al., 2002). In contrast, EsaR regulates its own expression by AHL-independent repression and AHL-dependent de-repression (Minogue et al., 2002). De-repression of ypsI in the ypsR mutant (Fig. 2) is analogous to EsaR in that ypsR expression is not affected by the absence of YpsI, suggesting that repression of ypsI expression is AHL-independent. The negative autoregulatory role of YpsR at its own promoter differs from EsaR such that AHLs in conjunction with YpsR contribute to ypsR repression as mutation of ypsI results in de-repression of ypsR expression given the increased light output observed from the PypsR::luxDCABE fusion in a ypsI mutant background (2, 9). The expression of ytbR is not autoregulated but the fall in expression levels of the ytbI promoter when compared with the parent, in either the ypsI, ytbI or ytbR mutants (Fig. 3), is consistent with positive regulation seen in the SpnR-AHL dependent system. The absence of complete de-repression or activation may be a consequence of the continued production of a common core of AHLs (C6-HSL and 3-oxo-C6-HSL) which can be synthesized by both YtbI and YpsI (Ortori et al., 2006).
The promoter regions of ypsR, ytbR, ypsI and ytbI contain a sequence which is similar to the lux box consensus (Fig. S5) and is consistent with the QS-dependent regulation of ypsR, ytbR, ypsI and ytbI. Previously, we have shown that mutation of either ypsR or ypsI influences the onset of motility in Y. pseudotuberculosis (Atkinson et al., 1999). In addition to ypsR, ytbR, ypsl and ytbI, the upstream regions of the class I and class II motility regulator genes, flhDC and fliA also contain lux box-like consensus sequences (flux box; Fig. S5). These data suggested that QS may regulate motility in Y. pseudotuberculosis via the control of fliA and/or flhDC expression. Although the expression of these major flagellar regulatory genes has been investigated extensively in other members of the Enterobacteriaceae such as Escherichia coli and Salmonella (MacNab, 1996) which lack AHL-dependent QS systems, there is comparatively little information on their regulation in Yersinia. LuxR/AHL-dependent regulation of flagellar-mediated motility has however, been observed in Y. enterocolitica (Atkinson et al., 2006a) and in Burkholderia glumae (Kim et al., 2007), while Sinorhizobium meliloti possesses two regulatory genes with similarity to LuxR-type proteins. These regulate genes involved in flagellar assembly, chemotaxis and motility (Sourjik et al., 2000). A microarray analysis of Vibrio fischeri QS mutants also revealed that a number of motility genes were repressed by the ain AHL-dependent QS system and that ain mutants display altered motility behaviour in vitro on minimal but not on nutrient rich agar plates (Lupp and Ruby, 2005).
Here we have shown that flhDC is required for motility in Y. pseudotuberculosis and that, as reported for other Enterobacteriaceae, fliA expression is flhDC-dependent. flhDC transcription precedes fliA expression commensurate with their respective positions within the flagellar regulatory hierarchy. (Fig. 9). QS does however, impact on flhDC and fliA expression in a temperature-dependent manner. Compared with the parent strain, at 22°C, flhDC expression is delayed (in all eight QS mutants examined) or delayed and reduced (ytbI, ypsR and ypsI/ytbI, ypsR/ytbR mutants), suggesting that QS positively influences flhDC transcription at this temperature (Fig. 9). These data are also consistent with the fact that YpsR positively regulates ytbR and ytbI expression and it is the AHLs which are associated with YtbI which positively regulate flhDC expression. This accounts for the 10-fold decrease in flhDC expression in the ypsR mutant (Fig. 5A). In contrast, fliA expression is most strongly de-repressed in the ypsI/ytbI mutant at 22°C followed by the ytbI and ypsR/ytbR mutants respectively. This implies that AHLs from both synthases contribute to the negative regulation of fliA although the YtbI AHLs play the major role. fliA expression remains comparable with the parent in both the ypsR and ytbR mutants but is substantially lower in the ypsR/ytbR mutant. This suggests that both YpsR and YtbR synergistically repress fliA at this temperature (Fig. 9). To confirm the role of AHL-dependent QS in flhDC and fliA expression we introduced the lactonase gene aiiA into both the flhDC and fliA promoter fusions. These strains are AHL-negative and have an AHL phenotype which is analogous to the ypsI/ytbI double mutant. The expression profiles of both flhDC and fliA were similar to those of the ypsI/ytbI double mutant which unequivocally confirms that flhDC and fliA expression are regulated by AHL-mediated QS. Taken together these data show that temperature and QS exhibit differential effects on flhDC and fliA expression but the QS system is dominant.
flhDC and fliA expression peaks in the parent at ∼10 h whereas the expression of the four QS genes peaks at ∼8 h (compare 2, 3 with 5, 6). The earlier onset of QS gene expression is to be expected given that the QS system is regulating the expression of flhDC and fliA. Although there is an ∼2 h delay in the expression of flhDC and fliA when compared with the expression of ypsI, ypsR, ytbI and ytbR, the profiles of the curves for ypsI/R and fliA and of ytbI/R and flhDC are similar, emphasizing the fact that the regulatory relationships between these systems are synchronized.
At 22°C but not 37°C, the parent Y. pseudotuberculosis strain is motile, with motility beginning to develop in 22°C liquid cultures after ∼6 h while flhDC expression profiles remain the same in the parent strain and are not affected by temperature. The temperature-independent expression of flhDC reflects the global regulatory role of FlhDC which, in Y. enterocolitica, has been shown to regulate numerous genes which are unrelated to the flagellar cascade, many of which appear to be involved in nitrogen metabolism (Kapatral et al., 2004) as well as contributing to the regulation of yop expression (Bleves et al., 2002). These other flhDC controlled genes will be required at 37°C for the invasion of the mammalian host, thus downregulating flhDC expression at 37°C to control motility would preclude such roles. However, at 37°C the pool of FlhDC could activate fliA and an additional level of control is therefore provided by the QS system that prevents motility by repressing fliA expression. Whether FliA controls yersinia genes other than those involved in flagellar biogenesis and chemotaxis remains to be established, but our data suggest that fliA expression is a major checkpoint in the regulation of motility and is dependent not only on QS but temperature as, at 22°C, expression is ∼25-fold higher than at 37°C and could explain why the organism is unable to initiate motility in liquid culture at this temperature. We must also consider that FliA activity is dependent on the presence of an antisigma factor FlgM. Extensive mutation and transcriptional studies will therefore be required to define the QS-FliA-FlgM relationship but these are beyond the scope of this study.
In the context of swimming motility in semi-solid agar plate assays, mutation of ypsR or ypsI results in the early onset of motility at 22°C. Peak flhDC expression in liquid culture in these mutants is virtually unchanged in the ypsI mutant but severely reduced in the ypsR mutant, although early flhDC expression is reduced not abolished and fliA expression is not affected. This is presumably because sufficient FlhDC is being made to induce fliA even in these mutants. It is important to note however, that YpsR is negatively regulating the expression of its own locus and positively regulating the YtbRI locus and it is the AHLs associated with YtbI which regulate the transcription of both flhDC and fliA in conjunction with YpsR either alone or in combination with YtbR. This could indicate that the impact of the YpsRI system on motility is not directly via the transcriptional control of flhDC upon fliA as if this were the case, mutation of the QS system might result in the early induction of flhDC and/or fliA.
For Y. pseudotuberculosis QS, flhDC and fliA mutants, genetic complementation partially restored expression levels to those observed in the parent (Figs S7 and S8). Partial rather than full restoration of the phenotype in some of the strains may be attributed to the presence of multiple plasmid-borne copies of the complementing gene. In contrast to most AHL-dependent QS systems, the exogenous provision of AHLs to the Y. pseudotuberculosis AHL synthase mutants did not restore the parental phenotype. Using tritiated 3-oxo-C12-HSL and a lux-based AHL reporter fusion, we have demonstrated that the lack of response is not due to the inability of exogenously supplied AHLs to gain intracellular access. Similar observations have been made for both Y. enterocolitica (Atkinson et al., 1999) and C. violaceum (McClean et al., 1997). With respect to the latter, the AHL-induced expression of the purple pigment, violacein in a cviI mutant was only achieved following a second round of mutagenesis in which a putative repressor gene was identified (McClean et al., 1997). Whether a similar repression mechanism exists in Y. pseudotuberculosis remains to be established.
Experimental procedures
Bacterial strains, plasmids and culture conditions
The bacterial strains and plasmids used in this study are listed in Table S1. Except where stated, bacterial cultures were routinely grown with shaking at 200 r.p.m. or static in L broth Lennox or on agar plates (Lennox, 1955) buffered to pH 6.8 with Mops (3-N-morpholino) propanesulphonic acid (LBmops) to ensure the supernatant did not rise above pH 7.2 and promote homoserine lactone ring opening (Yates et al., 2002). Antibiotics and other media supplements were used at the following concentrations: Cm at 30 μg ml−1, kanamycin at 50 μg ml−1, streptomycin at 50 μg ml−1, ampicillin at 50 μg ml−1, naladixic acid at 15 μg ml−1, isopropyl β-d-thiogalactopyranoside (IPTG) at 40 μg ml−1 and 5-Bromo-4-chloro-3-indoyl β-d-galactopyranoside (Xgal) at 40 μg ml−1. Minimal swim motility plates were prepared as previously described (Atkinson et al., 1999).
AHL synthesis and analysis
N-acylhomoserine lactones were synthesized as previously described (Chhabra et al., 2003). Tritiated 3-oxo-C12-HSL [N-(3-oxo-[6,7–3H2]-dodecanoyl)-l-homoserine lactone] was custom synthesized by Amersham Biosciences UK by titration of the unsaturated precursor, N-[(E)-3-oxo-6-dodecenoyl]-l-homoserine lactone by the procedure described previously (Atkinson et al., 2006b). AHLs were extracted from appropriate volumes of cell-free culture supernatants and TLC analysis was performed in 45% methanol in water on reverse phase (RP) C18 TLC plates as previously described (McClean et al., 1997; Atkinson et al., 1999).
DNA preparation and manipulation
Chromosomal DNA was prepared from 100 ml overnight cultures of Y. pseudotuberculosis using the cetyltrimethyl ammonium bromide method (Ausubel et al., 1989). Plasmid DNA, electro-competent cells, electroporation, agarose gel electrophoresis, DNA extraction from agarose gel slices and restriction modification enzymes were described previously (Atkinson et al., 1999).
Polymerase chain reaction was performed according to Ausubel et al. (1989) using primers synthesized by Genosys Biotechnologies (Europe), Cambridge. dNTPs (dinucleotide triphosphates) were supplied by Promega and reaction conditions have been described previously (Atkinson et al., 1999). In order to PCR-amplify flhDC and fliA from Y. pseudotuberculosis using primers designed to analogous sequences from Y. enterocolitica proof reading elongase polymerase was used following the manufacturers instructions (Invitrogen). Sequencing of DNA was carried out at the BSAU in the Queen's Medical Centre, Nottingham, UK using an Applied Biosystems 373A automated sequencer. Analysis of sequence was performed using the Lasergene DNAstar package.
Cloning of the complete ytbRI locus
We previously reported the cloning of a truncated ytbR/ytbI locus (Atkinson et al., 1999). We therefore cloned the complete ytbRI locus using heterologous complementation of an AHL-dependent bioluminescent reporter assay (Swift et al., 1996) as follows. HindIII digested chromosomal DNA of the Y. pseudotuberculosis ypsI mutant (defective in one system of AHL synthesis) was cloned into similarly digested pBluescript SK+ (Stratagene) and transformed into E. coli JM109[pSB401] (Winson et al., 1998a). Two clones which both carried an identical HindIII fragment were found to be highly bioluminescent, indicative of an insert capable of producing AHLs. Initial sequencing of one clone (pSA184) revealed two open reading frames of 687 (ytbI) and 744 (ytbR) base pairs which, when translated, exhibited significant homology to the LuxI and LuxR family of proteins respectively. These sequences were submitted to GenBank as AF079136.
Mutant construction and complementation
For ytbR and ytbI PCR primers (P1, P2 and P3, P4 respectively; Table S2) were designed to engineer XbaI-HindIII and HindIII-SalI restriction sites into the 5′ and 3′ regions of ytbR and ytbI or BglII-XhoI and XhoI-SpeI restriction sites into the 5′ and 3′ regions of flhC (primers FlhC-A and FlhC-S; Table S2) and flhD respectively (primers FlhD-A and FlhD-S; Table S2). PCR fragments were cloned stepwise into pBluescript SK+ (Stratagene) and a Cm cassette from pLysS (Novagen) inserted into the unique HindIII site of either ytbI or ytbR. For flhC the PCR product was cloned into pGEMT/Easy. flhD was then inserted into pGEM::flhC as a SpeI–XhoI fragment and a tetracycline cassette (amplified from pBR322 using primers Tet1 and Tet2 (Table S2) designed to introduce XhoI restriction sites 5′ and 3′ to the tetracycline cassette) was cloned into the newly generated unique XhoI site in pGEM::flhDC. For ytbI and ytbR the XbaI–SalI deletion–insertion fragment was excised from pBluescript and cloned into pKNG101 (Kaniga et al., 1991) to give pSA234 and pSA235, respectively, which was then transformed into E. coli CC118 λ-pir. For flhDC the SpeI–BglII deletion–insertion fragment was excised from pGEM::flhDC and cloned into pDM4 (OToole et al., 1996) to give pSA288 before being transformed into E. coli CC118 λ-pir.
The protocol for obtaining a mutant through the stable integration of deletion/insertion constructs of ytbI, ytbR, ypsI/ytbI, ypsR/ytbR and flhDC into the parent chromosome was carried out as previously described (Kaniga et al., 1991; Atkinson et al., 1999) using appropriate antibiotic selections. To construct ytbI/ypsI and ytbR/ypsR mutants the existing ypsI and ypsR mutant constructs in pKNG101 (Atkinson et al., 1999) were mated with the newly generated ytbI and ytbR mutants respectively. To construct ypsR/ytbI and ypsI/ytbR mutants the newly generated ytbI or ytbR mutants were mated with the ypsR or ypsI mutants respectively.
To demonstrate the role of AHLs in regulating fliA and flhDC we devised a complementary strategy to the mutagenesis of ypsI and ytbI. In these experiments, the gene coding for the AHL lactonase, aiiA (Dong et al., 2000), was amplified by PCR from Bacillus subtilis using primers AiiA-F and AiiA-R and the product cloned into pGEM/T Easy. The resulting construct pSA302 was transformed into the flhDC and fliA promoter fusions. To confirm that YpIII PflhDC::lux pSA302 and YpIII PfliA::lux were AHL-negative cell-free supernatant extracts were analysed by TLC as described above.
To complement the ypsR/I and ytbR/I mutations the primers SA160, SA161, SA164 and SA165 were used to introduce HindIII restriction sites to the upstream promoter region and SalI restriction sites downstream of the coding regions of ytbR or ytbI. Primers SA162, SA163, SA166 and SA167 were used to create similar PCR products for ypsR or ypsI but introduced unique SalI and EcoRI restriction sites. Using the engineered restriction sites ytbR/ypsR and ytbI/ypsI PCR products were cloned into the low-copy-number vector pHG327 (Stewart et al., 1986) to give the corresponding double R or double I mutant complementing plasmids pSA290 and pSA291. An EcoRI/SalI fragment removed ypsR from pSA290 and ypsI from pSA291 to give pSA292 and pSA293 containing ytbR and ytbI, respectively, while restriction with HindIII/SalI removed ytbR from pSA290 and ytbI from pSA291 to give pSA294 and pSA295 containing ypsR and ypsI respectively. pSA220 was used to complement the flhDC mutant. The complementing plasmid was then introduced into the appropriate QS or flhDC mutants.
Construction and analysis of ypsI, ypsR, flhDC and fliA promoter fusions
In order that we had a promoter-less luxCDABE cassette in a multisite polylinker we cut luxCDABE out of pSB417 (Winson et al., 1998b) as a BamHI–StuI fragment, created a blunt end at the BamHI site and cloned the fragment into SmaI restricted pBluescript II to give pBluelux.
PCR primers were designed to amplify the promoter regions of ytbI, ytbR (YtbIF, YtbIR, YtbRF, YtbRR; Table S2) ypsI and ypsR (IF2 IR2 and RF1 RR1; Atkinson et al., 1999) and the resulting PCR products were cloned into either pGEM/T Easy (ytbI/R) or pUC57/T (ypsI/R). PytbI or PytbR were excised as EcoRI–SpeI fragments from pGEM/T Easy and cloned into pBluescript to give pBlue::PytbI and pBlue::PytbR. The luxCDABE cassette was excised from pBluelux and cloned into either pBlue::PytbI or pBlue::PytbR as a SacI fragment after which the PytbI::luxCDABE and PytbR::luxCDABE cassettes were cloned as blunt end fragments into pKNG101 (Kaniga et al., 1991) to give pHP276 and pHP277. PypsI or PypsR were excised as XbaI–PstI fragments from pUC57/T and cloned into pKNG101 (Kaniga et al., 1991) to give pKNG::PypsI and pKNG::PypsR. The luxCDABE cassette from pBluelux was then excised as a PstI fragment and cloned into either pKNG::PypsI or pKNG::PypsR to give pSA278 or pSA279. Single crosses into the parent, ytbI, ytbR, ypsI and ypsR mutants were performed as previously described (Atkinson et al., 1999) to give the corresponding PytbI::luxCDABE, PytbR::luxCDABE, PypsI::luxCDABE or PypsR::luxCDABE promoter fusions (Table S1).
PCR primers were also designed to amplify flhDC (Mot4 and Mot7) and fliA (Mot1 and Mot2) from Y. pseudotuberculosis, and the resulting PCR products were cloned into pGEM/T Easy (Promega) to give pSA220 and pSA221. Once sequenced new primers suitable for amplifying the promoter regions of flhDC (Mot7 and Mot11) and fliA (Mot1 and Mot10) were designed and the PCR products cloned into pGEM/T Easy. PflhDC and PfliA were excised from pGEM/T Easy as SalI–SacII fragments and cloned into pBluescript SK+. The promoter-less luxCDABE cassette was then removed from pBluelux as a SacI fragment and cloned downstream of PflhDC and PfliA to give pBlue PflhDC::luxCDABE and pBlue PfliA::luxCDABE. PflhDC::luxCDABE and PfliA::luxCDABE were then cloned into pDM4 (OToole et al., 1996) as SalI fragments to give pSA200 and pSA208. Single crosses into the parent, ypsR, ypsI, ytbR, ytbI, ypsI/ytbI, ypsR/ytbR mutants were performed as previously described (Atkinson et al., 1999) to give the corresponding PflhDC::luxCDABE or PfliA::luxCDABE promoter fusion. Bioluminescence was determined as a function of cell density with a combined automated luminometer/spectrophotometer (Anthos Lucy I). Overnight seed cultures were diluted 1:200 in fresh LBmops + antibiotics and grown for 3 h. Exactly 200 μl of the culture adjusted to a starting optical density (OD)600 of 0.01 was then added to the wells of a microtitre plate. Luminescence and OD at 405 nm (OD405) of the cultures was automatically determined every 30 min for 24 h and presented as relative light units per unit of OD405 (light per unit cell). For every promoter fusion assay each sample was assayed in triplicate on the microtitre plate, and each experiment was carried out at least twice. For some experiments, synthetic AHLs were added either singly or as mixtures, AHLs were added to the promoter fusion assays at concentrations of 100 nM along with the appropriate AHL-free solvent controls prior to Lucy analysis.
Microscopic analysis of motility in the flhDC and fliA promoter fusions in the relevant mutants were carried out as previously described (Atkinson et al., 1999).
Intracellular accumulation of AHLs
To determine whether exogenously supplied AHLs are internalized by the Y. pseudotuberculosis parent and ypsI/ytbI mutant, two approaches were taken. First, the AHL-negative ypsI/ytbI mutant was transformed with the AHL biosensor plasmid pSB401(Winson et al., 1998b), grown overnight at 22°C, diluted 1 in 50 and grown for 3–4 h before being inoculated in 96-well microtitre plates in the absence or presence of C6-HSL or 3-oxo-C12-HSL (100 nM). The plates were incubated for 12 h at 22°C in a Lucy Anthos II combined spectrophotometer/luminometer. The maximum light output was determined as a function of cell population density (RLU/OD492). The tritiated 3-oxo-C12-HSL radiolabel experiments were carried out essentially as described previously (Atkinson et al., 2006a). Briefly, 10 ml overnight cultures (grown at 22°C) of the Y. pseudotuberculosis parent and ypsI/ytbI mutant were harvested and re-suspended in 250 μl of KG buffer (10 mM potassium phosphate (pH 7.0), 0.03% glycerol). Tritiated 3-oxo-C12-HSL (2.70 TBq/mmol) was added to a final concentration of 100 nM in a final volume of 300 μl. The bacteria were incubated at 22°C for 20 min and then centrifuged through 75 μl of Nyosil (Nye Lubricants, New Bedford, MA) containing 25% w/v trichloroacetic acid and 10% v/v glycerol. The supernatant was removed and the bacterial pellet was re-suspended in 150 μl of KG buffer. Equal volumes of both the supernatant and the pellet fractions were counted in a Topcount NXT microplate scintillation and luminescence counter (Packard Bioscience UK).




