Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II
Summary
Oxidative stress inhibits the repair of photodamaged photosystem II (PSII). This inhibition is due initially to the suppression, by reactive oxygen species (ROS), of the synthesis de novo of proteins that are required for the repair of PSII, such as the D1 protein, at the level of translational elongation. To investigate in vitro the mechanisms whereby ROS inhibit translational elongation, we developed a translation system in vitro from the cyanobacterium Synechocystis sp. PCC 6803. The synthesis of the D1 protein in vitro was inhibited by exogenous H2O2. However, the addition of reduced forms of elongation factor G (EF-G), which is known to be particularly sensitive to oxidation, was able to reverse the inhibition of translation. By contrast, the oxidized forms of EF-G failed to restore translational activity. Furthermore, the overexpression of EF-G of Synechocystis in another cyanobacterium Synechococcus sp. PCC 7942 increased the tolerance of cells to H2O2 in terms of protein synthesis. These observations suggest that EF-G might be the primary target, within the translational machinery, of inhibition by ROS.
Introduction
Photosystem II (PSII) is a macromolecular complex that utilizes light energy to withdraw electrons from water and drive the transport of electrons. However, this complex is very susceptible to inactivation by light. Exposure of photosynthetic organisms to strong light often results in strong inhibition of the activity of PSII. This phenomenon is referred to as photoinhibition (Powles, 1984; Prásil et al., 1992; Aro et al., 1993; Nishiyama et al., 2006). The primary damage by light occurs, upon absorption of light, at the oxygen-evolving complex of PSII (Hakala et al., 2005; Ohnishi et al., 2005). In particular, UV and blue light are effective in damaging the manganese cluster, which catalyses the oxidation of water. Subsequent damage occurs at the reaction centre upon absorption of light by chlorophylls and carotenoids, resulting in critical damage to the D1 protein, the protein that, together with the D2 protein, constitutes the reaction centre (Ohnishi et al., 2005). Thus, photodamage to PSII is a purely light-dependent event and can occur under light at any intensity (Gombos et al., 1994; Tyystjärvi and Aro, 1996; Anderson and Chow, 2002).
Photosynthetic organisms have developed a rapid and efficient system for repair of photodamaged PSII. Such repair involves several steps, namely, proteolytic degradation of the D1 protein, synthesis de novo of the precursor to D1 (known as pre-D1), incorporation of pre-D1 into the PSII complex, maturation of the D1 protein via the carboxy-terminal processing of pre-D1, and the assembly of the oxygen-evolving complex (Aro et al., 1993; 2005). The activity of PSII depends on the balance between the rate of photodamage to PSII and the rate of repair of damaged PSII, and photoinhibition of PSII becomes apparent when the rate of photodamage exceeds the rate of repair (Nishiyama et al., 2006).
The light-driven reactions of photosynthesis produce reactive oxygen species (ROS) as inevitable by-products. The photosynthetic transport of electrons produces the superoxide radical (O2–), hydrogen peroxide (H2O2), and the hydroxyl radical, while the transfer of excitation energy produces singlet-state oxygen (singlet oxygen; 1O2) (Asada, 1999). Under normal light conditions, the elevated levels of these various ROS are reduced to tolerable levels by antioxidative systems that include ROS-scavenging enzymes and antioxidants (Asada, 1999). Absorption of strong light by the photosynthetic machinery promotes the production of ROS. Elevated levels of ROS that exceed the capacity of the antioxidative systems give rise to oxidative stress (Asada, 1999).
The action of ROS in the photoinhibition of PSII has been the subject of some controversy. Initial studies suggested that ROS and, in particular, 1O2 might be the primary cause of photodamage to PSII (Vass et al., 1992; Keren et al., 1997). However, examination of photodamage and repair separately has revealed that photodamage to PSII is a purely light-dependent event (Hakala et al., 2005; Ohnishi et al., 2005), as described above, and is not mediated by ROS (Nishiyama et al., 2001; 2004; Allakhverdiev and Murata, 2004). ROS, including H2O2 and 1O2, act primarily by inhibiting the repair of PSII (Nishiyama et al., 2001; 2004; Allakhverdiev and Murata, 2004). Moreover, inhibition of repair has been attributed to the suppression, by ROS, of the synthesis de novo of proteins that are required for the repair of PSII, such as the D1 protein (Nishiyama et al., 2001; 2004). Analysis of polysomes has revealed that ROS inhibit the synthesis of the D1 protein de novo at the elongation step of translation (Nishiyama et al., 2001; 2004). Thus, it seems likely that a protein or proteins that are involved in translational elongation might be the target of inhibition by ROS.
Among the various proteins involved in translational elongation, elongation factors are known to be sensitive to oxidation. In Escherichia coli, elongation factor G (EF-G) has been identified as one of the proteins that are most abundantly oxidized, as assessed by the formation of carbonyl groups, in cells treated with H2O2 (Tamarit et al., 1998) as well as in cells that lack a superoxide dismutase (Dukan and Nyström, 1999). The eukaryotic counterpart of EF-G, elongation factor 2, is selectively oxidized by H2O2 in Saccharomyces cerevisiae (Cabiscol et al., 2000) and by cumene hydroperoxide and H2O2 in rat liver (Ayala et al., 1996; Parrado et al., 2003). However, chemical properties of the oxidation of elongation factors by these oxidants remain unclear. Furthermore, effects of their oxidation on the activity of translation remain to be elucidated. Inability of the disruption of genes for such elongation factors has prevented the studies to clarify their action in translation under oxidative stress in vivo.
In the present study, we examined the effects of oxidative stress on EF-G using a translation system in vitro that we had prepared from the cyanobacterium Synechocystis sp. PCC 6803 (hereafter Synechocystis) as part of our efforts to identify the molecular mechanisms whereby ROS inhibit translational elongation. We found that inhibition of translational activity by H2O2 was attributable to the reversible oxidation of EF-G. Furthermore, overexpression of EF-G of Synechocystis in another cyanobacterium, Synechococcus sp. PCC 7942, increased the tolerance of the latter to H2O2 in terms of protein synthesis.
Results
Synthesis of the D1 protein in a translation system derived from Synechocystis
To investigate the mechanism whereby ROS inhibit the synthesis of the D1 protein, we developed a translation system in vitro using Synechocystis. We prepared an extract, which included thylakoid membranes, from cells that had been grown to exponential phase in light at 80 μE m−2 s−1 at 32°C. The mRNA encoding the D1 protein was generated by transcription in vitro of the psbA2 gene, which included the coding region plus 31 bp of the 5′-untranslated region and 139 bp of the 3′-untranslated region (Fig. 1A). Incubation of the cell extract with psbA2 mRNA and appropriate substrates for translation markedly stimulated the synthesis of protein de novo (Fig. 1B). The addition of mRNA for green fluorescent protein (GFP), which included the coding region and a Shine–Dalgarno sequence, to the translation system also resulted in the enhanced synthesis of protein de novo (Fig. 1B). The translational activity observed in the absence of exogenous mRNA was due to the synthesis of proteins encoded by intrinsic mRNAs that had been included in the extract. Inclusion of 1 M glycine betaine and 25% (w/v) glycerol in the medium used for preparation of the cell extract was essential for subsequent high translational activity. Further extension of the 5′-untranslated region of psbA2 up to 130 bp did not alter the translational activity (data not shown), suggesting that the original 31 bp sequence in the 5′-untranslated region might have been sufficient for the initiation of translation.
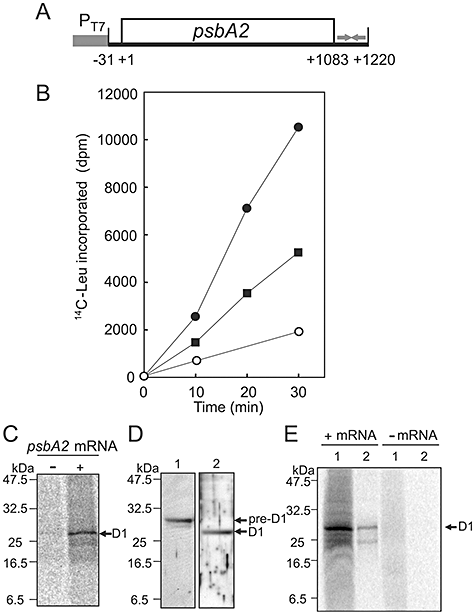
Synthesis of the D1 protein in vitro in the translation system derived from Synechocystis.A. Schematic representation of the DNA template for transcription of the psbA2 gene. The coding region, the T7 promoter (PT7) and the putative terminator are indicated by an open box, a filled box and arrows respectively. Translation is initiated at nucleotide +1.B. Levels of [14C]Leu-labelled products of translation in vitro in the presence of psbA2 mRNA (●) and of mRNA for GFP (▪) and in the absence of exogenous mRNA (○). Radioactivity is indicated as disintegrations per minute (dpm), as determined by liquid scintillation counting.C. Autoradiographs of products of translation in vitro in the presence of the psbA2 mRNA (+) and in its absence (−) after translation for 30 min.D. Immunoblotting analysis of pre-D1 (lane 1) with the antibody against pre-D1 and mature D1 (lane 2) with the antibody against mature D1 in thylakoid membranes that had been prepared directly from cells.E. Detection of newly synthesized D1 in the PSII complex. Translation in vitro in the presence of the psbA2 mRNA (+) and in its absence (−) was performed with [35S]Met/Cys and an extract from mutated cells that expressed histidine-tagged CP47. After the reaction, thylakoid membranes were isolated from the extract and the PSII complex was purified. Lane 1, thylakoid membranes; lane 2, the PSII complex.
Autoradiography after electrophoresis of the products of translation in vitro confirmed the synthesis of the D1 protein (Fig. 1C). The synthesized protein migrated to the same position as the mature D1 protein, which was detected by immunoblotting analysis in thylakoid membranes that had been prepared directly from cells (Fig. 1D), suggesting that pre-D1 had been appropriately processed in vitro. The pre-D1 protein is incorporated during its translation into thylakoid membranes and assembled with other components of the PSII complex (Aro et al., 1993; 2005). After the carboxy-terminal extension of pre-D1 has been removed by a luminal protease, CtpA, the mature D1 protein becomes functional in the PSII complex (Anbudurai et al., 1994). To examine the localization of the synthesized D1 protein, we synthesized this protein in an extract that had been prepared from mutated cells that expressed histidine-tagged CP47, a component of PSII (Sakurai et al., 2006), and then we purified the PSII complex (Fig. S1). The labelled D1 protein was detected within the purified PSII complex (Fig. 1E), indicating that the newly synthesized pre-D1 had been incorporated into the PSII complex and processed appropriately.
Effects of H2O2 on the synthesis of the D1 protein in vitro
We examined the effects of oxidative stress on the synthesis of the D1 protein in vitro. As the synthesis of the D1 protein is responsible for almost all incorporation of radioactivity (Fig. 1), protein synthesis will hereafter be assumed to reflect the synthesis of the D1 protein. The synthesis of the D1 protein in vitro was strongly suppressed in the presence of 10 mM H2O2 (Fig. 2A). As H2O2-scavenging enzymes, such as catalase and peroxidase, had been concentrated at high levels together with other proteins in the extract during its preparation, the addition of 40 mM NaN3 to the extract was necessary to inhibit the activities of these enzymes. We also observed the inhibitory effect of H2O2 on the synthesis of GFP in vitro (data not shown). Thus, H2O2 inhibited the translational machinery itself. To avoid any artefacts due to NaN3, we prepared an extract from the ΔkatG/Δtpx mutant of Synechocystis, which lacks both catalase peroxidase and thioredoxin peroxidase (Nishiyama et al., 2001). In the extract of ΔkatG/Δtpx cells, the synthesis of the D1 protein was strongly suppressed by addition of 10 mM H2O2 in the absence of NaN3 (Fig. 2B).
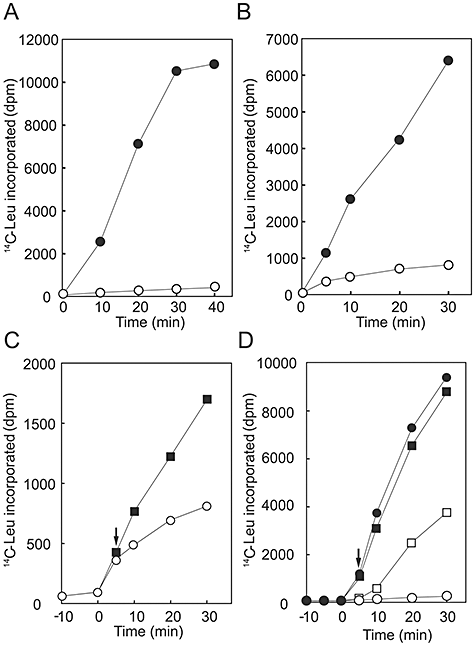
Effects of H2O2 on the synthesis of the D1 protein in extracts from wild-type cells and ΔkatG/Δtpx cells and recovery of the translation reaction from H2O2-induced inhibition.A. Levels of translation products generated in the extract from wild-type cells in the presence of 10 mM H2O2 (○) and in its absence (●). 40 mM NaN3 was added to the extract from wild-type cells to inhibit endogenous catalase and peroxidases.B. Levels of translation products generated in the extract from ΔkatG/Δtpx cells in the presence of 10 mM H2O2 (○) and in its absence (●).C. Effects of catalase on the H2O2-induced inhibition of translation in vitro in the extract from ΔkatG/Δtpx cells. After incubation for 5 min, 8 μg of catalase was added for removal of H2O2 as indicated by an arrow. Levels of translation products generated in the presence of catalase (▪) and in its absence (○) are shown.D. Effects of DTT on the H2O2-induced inhibition of translation in vitro in the extract from wild-type cells. After incubation for 5 min, 4 mM DTT was added to the reaction mixture as indicated by an arrow. Levels of translation products generated in the presence of DTT (□) and in its absence (○) are shown. Effects of DTT on translation in the absence of H2O2 are also shown. ▪, Addition of DTT; ●, no added DTT. The results shown are representative of the results from three independent experiments.
Recovery of translation from H2O2-induced inhibition
To examine the reversibility of the H2O2-induced inhibition of translational activity, we added catalase to the extract of ΔkatG/Δtpx cells after the extract had been treated with 10 mM H2O2. Removal of H2O2 by catalase restored the synthesis of the D1 protein (Fig. 2C), suggesting that the inhibition of translation by H2O2 might be reversible. In addition, synthesis of the D1 protein also resumed when 4 mM dithiothreitol (DTT) was added to an extract of wild-type cells in which translation had been inhibited by the addition of 10 mM H2O2, in the presence of 40 mM NaN3 (Fig. 2D). We postulated that DTT had released the translational machinery from oxidation-induced inhibition. In the absence of oxidative stress, the addition of DTT to the extract had no effect on translational activity (Fig. 2D).
Three EF-G proteins in Synechocystis and their functions
The genome of Synechocystis includes three genes that encode putative EF-G proteins, namely, sll1098, slr1463 and sll0830, but the functions of the products of these genes have not yet been characterized, to our knowledge. We constructed a phylogenetic tree of EF-G proteins with multiple alignments of the deduced amino acid sequences (Fig. S2). Sll1098 is part of the same group common as bacterial forms of EF-G. Slr1463 appears to be homologous to chloroplast EF-G. Sll0830 is located at a distance from the main branch of EF-G proteins.
None of the three genes for EF-G was able to be disrupted in Synechocystis. To investigate their function as EF-G, we examined whether EF-G proteins from Synechocystis might be able to complement the function of EF-G of E. coli in vitro. We prepared the three recombinant EF-G proteins, with a histidine tag, in their reduced forms, and we performed complementation assays using a translation system in vitro, derived from E. coli, in which all components required for translation except EF-G had been mixed. This translation system was inactive without exogenously added active EF-G. The addition of the EF-G protein from E. coli to this translation system allowed the synthesis of dihydrofolate reductase (DHFR) to resume (Fig. 3). The addition of each of Sll1098, Slr1463 and Sll0830 also restored the synthesis of DHFR in vitro (Fig. 3). Thus, Sll1098, Slr1463 and Sll0830 appeared to function as EF-G.
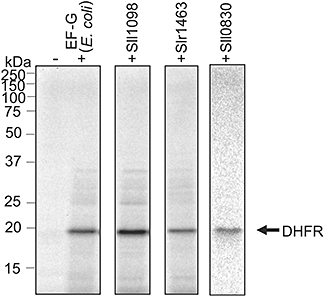
Complementation of EF-G in vitro. Synthesis in vitro of [35S]Met/Cys-labelled DHFR in the translation system, derived from E. coli, that lacked EF-G (see Experimental procedures), in the presence of EF-G from E. coli and from Synechocystis and in their absence, as indicated.
Reversal of the inhibition of translation by H2O2 upon addition of EF-G
We postulated that, if the inhibition of translation by H2O2 is due to the specific oxidation of EF-G, a supply of sufficient and non-oxidized EF-G should restore translational activity. As anticipated, we found that addition of the reduced form of Sll1098 to an extract, derived from Synechocystis, that had been treated with 5 mM H2O2 was able to reverse the inhibition of translation (Fig. 4A). By contrast, the oxidized form of Sll1098, which had been prepared by incubation of the protein with 2 mM H2O2, was unable, initially, to restore translational activity (Fig. 4A, closed triangles). Thus, the oxidized form of EF-G appeared to be non-functional. However, oxidized Sll1098 began to restore translational activity after 15 min, suggesting that a portion of the oxidized Sll1098 might have been reduced in the extract. Each of the reduced EF-G proteins from Synechocystis was able to reverse the inhibition of translation to some extent (Fig. 4). Addition of intact EF-G proteins had no effects on translational activity under non-oxidative conditions (data not shown), suggesting that the recovery of translation was not due to enhancement of translational activity by additional EF-G proteins. Addition of the EF-G protein of E. coli also recovered translational activity but its recovery effect was about 40% of that of Sll1098 (data not shown). No recovery of translation was observed when other types of protein, such as bovine serum albumin and creatine phosphokinase, were added to the H2O2-suppressed translation system (Fig. S3), suggesting that the recovery effect might be specific to EF-G. These results demonstrated that EF-G might be the primary target of inhibition by H2O2 in the translational machinery.
Addition of EF-G proteins to the H2O2-inhibited translation system. Prior to the translation reaction, the extract from wild-type Synechocystis cells was incubated for 10 min in the presence of 5 mM H2O2 and 40 mM NaN3. Components required for the synthesis of the D1 protein were added at zero time. After incubation for 5 min, 5 μg of EF-G protein from Synechocystis was added to the extract as indicated by arrows.A. Effects of the reduced (●) and oxidized (▴) forms of Sll1098.B. Effects of the reduced (●) and oxidized (▴) forms of Slr1463.C. Effects of the reduced form of Sll0830 (●). ○, Addition of buffer used for preparation of EF-G. Values are means ± SD (bars) of results from three independent experiments.
Oxidation of cysteine residues in Slr1463
The reversible inhibition of EF-G by H2O2 suggested a specific oxidation of cysteine residues within the protein. We monitored the redox state of cysteine residues of EF-G by modifying free thiol groups with a maleimidyl reagent, α-maleimidopropyl-ω-methoxy,polyoxyethylene, which has a molecular mass of 5207. Slr1463 has five cysteine residues. Modification of the reduced form of Slr1463 with this reagent retarded its electrophoretic mobility on a gel (Fig. 5A). Incubation of Slr1463 with 500 μM H2O2 prior to the modification resulted in faster mobility (Fig. 5B), suggesting that some of the five cysteine residues were oxidized. However, the addition of 5000 μM DTT to the oxidized Slr1463 recovered its mobility to the same position as the fully reduced protein had (Fig. 5B). It appears that inhibition of EF-G by H2O2 involves a reversible oxidation of specific cysteine residues and, most likely, the formation of a disulphide bond between two cysteine residues.

Effect of H2O2 on the redox state of cysteine residues of Slr1463.A. Patterns on a non-reducing gel of the mobility of Slr1463 that had been treated with a maleimidyl thiol-modifying reagent (+) or untreated (−).B. Effects of H2O2 and DTT on the mobility of Slr1463. A total of 2 μM of Slr1463 was incubated in the presence of 500 μM H2O2 and then incubated in the presence of the indicated concentrations of DTT. After the proteins were treated with the thiol-modifying reagent, they were separated by non-reducing SDS-PAGE.
Overexpression of EF-G in Synechococcus sp. PCC 7942
If the oxidation of EF-G were the rate-determining step in the inhibition of translation by oxidative stress, we would expect that elevated levels of EF-G within the cell might minimize oxidative damage to protein synthesis. To avoid homologous recombination of an exogenous EF-G gene by the same intrinsic gene in Synechocystis, we introduced each EF-G gene of Synechocystis into another cyanobacterium Synechococcus sp. PCC 7942. We obtained mutants that overexpressed Slr1463 and Sll0830 respectively, but failed to construct a plasmid that harboured an expressible sll1098 gene, probably because of the lethal effects of the overexpression of Sll1098 in E. coli.
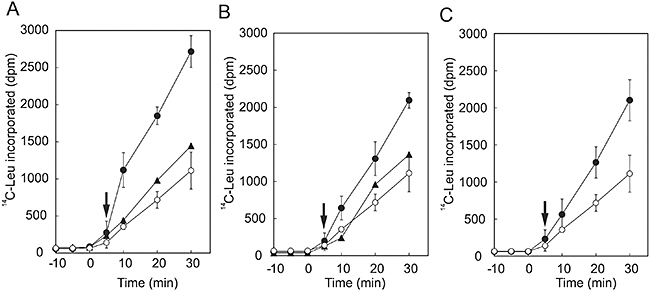
We examined the effects of oxidative stress on the synthesis of proteins de novo in Synechococcus cells that overexpressed EF-G. In the presence of 1 mM H2O2, mutated cells that overexpressed Slr1463 exhibited higher rates of synthesis of proteins de novo than wild-type cells (Fig. 6A). Not only the synthesis of the D1 protein but also the synthesis of almost all proteins in thylakoid membranes was enhanced. Thus, it appeared that elevated levels of EF-G enhanced the tolerance of the translational machinery to oxidative stress in vivo. Overexpression of Sll0830 also enhanced the synthesis of the D1 protein de novo under oxidative conditions (Fig. 6B). In this type of transgenic cell, the synthesis of almost all proteins in thylakoid membranes was enhanced (Fig. 6A). These results demonstrated that overexpression of EF-G increased the tolerance of the overall synthesis of proteins to oxidative stress.
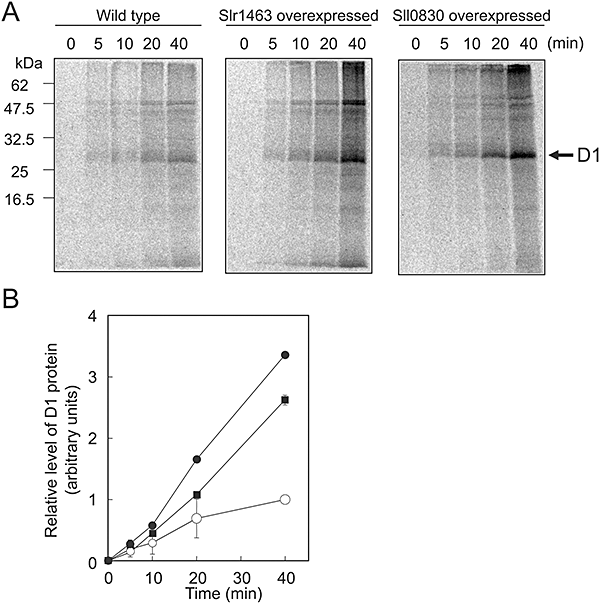
Effects of the overexpression of EF-G on the synthesis of proteins de novo under oxidative stress in Synechococcus sp. PCC 7942. Cells were labelled with [35S]Met at 32°C for the indicated times in light at 1.5 mmol photons m−2 s−1 in the presence of 1 mM H2O2. Thylakoid membranes were isolated and the labelled proteins were analysed.A. Labelled proteins from wild-type, Slr1463-overexpressing and SII0830-overexpressing cells.B. Levels of D1 in wild-type and EF-G-overexpressing cells. ○, wild-type cells; ▪, Slr1463-overexpressing cells; and ●, Sll0830-overexpressing cells. Values are means ± SD (bars) of results from three independent experiments.
Discussion
Development of a translation system in vitro from Synechocystis
In the present study, we developed a translation system in vitro from Synechocystis that allowed us to investigate the way in which translation is inhibited by oxidative stress. The pre-D1 protein was synthesized in our translation system and was inserted into thylakoid membranes that were present in the translation system (Fig. 1). Detection of the mature D1 protein in the PSII complex confirmed that the newly synthesized pre-D1 had been assembled with other proteins of PSII and that its carboxy-terminal extension had been appropriately removed (Fig. 1). Thus, it appeared that our translation system had preserved the ability to repair of PSII via the series of reactions that includes degradation of damaged D1 protein, insertion of newly synthesized pre-D1 into the PSII complex, and processing of pre-D1. This translation system should be a powerful tool with which to identify factors that are involved in the repair of PSII in vitro, as well as to investigate the mechanisms of the cyanobacterial translation, which are far from being fully understood.
Translation systems have been developed from various species and their organelles, for example, E. coli (Kigawa et al., 1999; Shimizu et al., 2005), Bacillus subtilis (Inaoka et al. 2001), Drosophila melanogaster (Tuschl et al., 1999), wheat germ (Madin et al., 2000), and the chloroplasts of tobacco (Hirose and Sugiura, 1996) and pea (Kasai et al., 2004). Each of these translation systems requires a reducing reagent, such as DTT, to drive the translation reaction. However, the translation system that we derived from Synechocystis did not require DTT (Fig. 2D). Thus, it is very likely that some reducing compounds were present initially in our system. Such reducing compounds might be products of the light-driven reactions of photosynthesis and might include reduced forms of ferredoxin and thioredoxin. Recently, a translation system in vitro was developed from another cyanobacterium, Synechococcus sp. PCC 6301, and this translation system required DTT (Mutsuda and Sugiura, 2006). As the extract for translation prepared from Synechococcus sp. PCC 6301 was derived from cells that had been grown in darkness for 12 h, levels of such reducing compounds in their extract might have been lower than in ours. The requirement for reducing power of translational reactions suggests that the translational machinery must be protected from oxidation if it is to remain operative.
Inhibition of translation by H2O2
Among the sequence of steps that lead to the synthesis of the D1 protein de novo, translation of psbA mRNA is known to be sensitive to inhibition by ROS, which include H2O2, O2– and 1O2, in Synechocystis (Nishiyama et al., 2001; 2004). The present study demonstrated that the synthesis of the D1 protein in our translation system in vitro was inhibited by H2O2 (Fig. 2). While the presence of 2 mM H2O2 in a dilute suspension of cells completely inhibited the synthesis of the D1 protein de novo (Nishiyama et al., 2001), 10 mM H2O2 was required for inhibition of the synthesis of the D1 protein in vitro (Fig. 2). This apparent robustness of translation system in vitro in the presence of H2O2 was due, most likely, to the presence of high concentrations of catalase and peroxidases, which accumulated together with other proteins in the extract during its preparation and were not totally inactivated by NaN3. Even in the extract prepared from mutant ΔkatG/Δtpx cells, which lack both catalase peroxidase and thioredoxin peroxidase, other types of peroxidase might have been present at high levels and, as a consequence, 10 mM H2O2 was required for inhibition of translation.
Translational activity was restored after H2O2-induced inhibition upon addition of catalase or DTT to the translation system, indicating that inhibition of translation by H2O2 is reversible (Fig. 2). This observation is consistent with the reversibility of the H2O2-induced inhibition of the repair of PSII: inhibition by H2O2 of the repair of PSII in a suspension of cells can be reversed by addition of catalase (Nishiyama et al., 2001). Thus, it is likely that inhibition by H2O2 is not due to critical or permanent damage to the translational machinery but is due to site-specific oxidation of some components of this machinery that can easily be reversed by reducing power, for example, the formation of a disulphide bond between two cysteine residues.
EF-G as the primary target of inhibition by H2O2
Analysis of polysomes identified the primary target of inhibition by ROS as the elongation step of translation in Synechocystis (Nishiyama et al., 2001; 2004). In prokaryotes, elongation of translation involves reactions that are catalysed by the ribosome, which consists of more than 50 polypeptides, three RNA molecules, and the three elongation factors EF-Tu, EF-Ts and EF-G. Among these proteins, EF-G is known to be particularly sensitive to oxidation (Tamarit et al., 1998; Dukan and Nyström, 1999; Cabiscol et al., 2000), although the effect of the oxidation of EF-G on translation remains unknown. EF-G catalyses the translocation of peptidyl-tRNA with the simultaneous hydrolysis of GTP (Green, 2000). The genome of Synechocystis has three genes for putative EF-G proteins, none of which has yet been characterized. Complementation assays in vitro revealed that each of the EF-G proteins can function as EF-G (Fig. 3).
Addition of each individual EF-G protein of Synechocystis to the in vitro system in which translation had been inhibited by H2O2 resulted in the recovery of translation (Fig. 4). The reduced forms of these EF-G proteins were effective in restoring translation, while the oxidized forms were initially ineffective (Fig. 4). These observations indicate that replacement of oxidized EF-G by reduced EF-G turned on the translation system that had been turned off by H2O2. Thus, it seems likely that the oxidation of EF-G is the rate-determining step in the inhibition of translation by H2O2. In other words, EF-G might be the primary target of inhibition by H2O2 in the translational machinery. It appears that the recovery of translation from H2O2-induced inhibition is attributable to the reduction of oxidized EF-G by DTT or other reducing compounds present in the translation system.
Possible mechanisms for the regulation of translation via the redox state of EF-G
The finding that EF-G is active in the reduced form but inactive in the oxidized form indicates that the activity of EF-G is regulated by its redox state. The gradual recovery of translation, with time, from H2O2-induced inhibition after the addition of oxidized EF-G suggests that oxidized EF-G can be reduced by reducing compounds that are present in our translation system (Fig. 4). As anticipated, oxidation of EF-G involved the reversible modification of cysteine residues and, most likely, the formation of a disulphide bond between two cysteine residues (Fig. 5).
The EF-G proteins of Synechocystis, namely, Sll1098, Slr1463 and Sll0830, have five, five and three cysteine residues respectively. Slr1463, an EF-G that might phylogenetically be related to the EF-G of the chloroplast, has also been identified as one of proteins that interact with thioredoxin in Synechocystis (Lindahl and Florencio, 2003). Elongation factor 2, the eukaryotic counterpart of EF-G, is also a target of thioredoxin in Arabidopsis thaliana (Yamazaki et al. 2004). It seems likely that the redox state of EF-G and, at least, of Slr1463 might be regulated by thioredoxin via interactions between cysteine residues in Synechocystis. The redox regulation of EF-G would then, in turn, regulate the activity of the translational machinery.
The light-dependent synthesis of the D1 protein is regulated at the translational level in plants (Klein and Mullet, 1987; Zhang and Aro, 2002) and algae (Trebitsh and Danon, 2001) and at both the transcriptional and the translational level in cyanobacteria (Tyystjärvi et al., 2001; 2004). Although details of the mechanisms responsible for the regulation of translation remain to be elucidated, the light-dependent synthesis of the D1 protein in plants is regulated at the elongation step of translation and activation of such synthesis requires reducing equivalents that result from electron transport via photosystem I (PSI) (Kuroda et al., 1996; Zhang et al., 2000). In cyanobacteria, the activation of the synthesis of pre-D1 also requires the transport of electrons via PSI, as well as the synthesis of ATP (Allakhverdiev et al., 2005). Thus, reducing power from PSI appears to be essential to drive the synthesis of the D1 protein in light. Gathering all these observations together, we propose a model for the regulation of the synthesis of the D1 protein as follows. Reducing power, generated from PSI as a result of the photosynthetic transport of electrons, is transmitted to EF-G in a redox pathway via thioredoxin. The resulting reduction of EF-G activates the elongation of translation and switches on the synthesis of the D1 protein. Under oxidative stress, excess ROS might interrupt the normal redox signal by maintaining the oxidation of specific cysteine residues of EF-G, which are the target of thioredoxin, so that the translational elongation of the D1 protein can no longer proceed.
Changes in the tolerance of protein synthesis to oxidative stress upon overexpression of EF-G
Overexpression of EF-G of Synechocystis in Synechococcus sp. PCC 7942 resulted in the enhanced tolerance of protein synthesis to oxidative stress due to H2O2 (Fig. 6). The enhancement of protein synthesis was obvious not only with respect to the D1 protein but also with respect to almost all proteins in thylakoid membranes, suggesting that the elevated levels of EF-G influenced the entire translational machinery. Thus, it seems likely that excess EF-G proteins that are free from oxidation by H2O2 can drive translational elongation, thereby minimizing the inhibitory effects of H2O2 on the translational machinery.
DNA microarray analysis of Synechocystis revealed that the expression of the three genes for EF-G is constitutive and not affected by strong light, oxidative stress due to H2O2, salt stress, osmotic stress, or high- and low-temperature stress (Y. Kanesaki and N. Murata, pers. comm.). Future studies should be directed towards the full characterization of the specific role of each EF-G protein in the translational machinery. EF-G appears to be the Achilles heel of the translational machinery under oxidative stress. Thus, genetic modification of EF-G that interferes with its specific oxidation should provide a useful strategy with which to attempt to alter the sensitivity of cyanobacteria and chloroplasts to oxidative stress.
Experimental procedures
Organisms and culture conditions
Cells of the wild-type strain of Synechocystis sp. PCC 6803, the ΔkatG/Δtpx mutant (Nishiyama et al., 2001), and the mutant that expressed histidine-tagged CP47 (Sakurai et al., 2006) were grown photoautotrophically at 32°C in BG-11 medium under light at 80 μmol photons m−2 s−1 with aeration by sterile air that contained 1% CO2 (Gombos et al., 1994). Wild-type and mutated cells of Synechococcus sp. PCC 7942 were grown under the same conditions as described above. For maintenance of mutants, appropriate antibiotics, namely, 5 μg ml−1 streptomycin for the histidine-tagged mutant, 20 μg ml−1 kanamycin for ΔkatG/Δtpx, and 10 μg ml−1 ampicillin for EF-G-overexpressing mutants, were included in the culture medium.
Preparation of mRNA templates
The nucleotide sequence of the psbA2 gene of Synechocystis was obtained from the CyanoBase website (http://bacteria.kazusa.or.jp/cyano/cyano.html). The psbA2 gene, together with 31 bp of the 5′-untranslated region and 139 bp of the 3′-untranslated region, was amplified from the genomic DNA of Synechocystis by PCR with the forward and reverse primers 5′-CATCGACAAATACATAAGGAATTA-3′ and 5′-ATGGTAACTGCCCCGGAC-3′ respectively. The resultant DNA fragment was cloned into the pGEM-T vector (Promega, Madison, WI, USA) with the psbA2 gene located downstream of the T7 promoter. Using the resultant plasmid as template, we amplified a DNA fragment by PCR with the T7 primer (Promega) and the reverse primer used for cloning psbA2. The coding region of the gene for GFP (Takara, Shiga, Japan) was cloned into the pBluescript vector (Stratagene, La Jolla, CA, USA) and a typical Shine–Dalgarno sequence (AGGA) was located upstream of the coding region. Using the resulting plasmid as the template, we amplified a DNA fragment by PCR with T7 and T3 primers (Promega). The products of PCR were used as templates for transcription in vitro by T7 RNA polymerase (Toyobo, Osaka, Japan).
Preparation of extracts for translation in vitro
Cells at late exponential phase in 8 l of BG11 medium were harvested by centrifugation at 6000 g for 10 min at 25°C. Pelleted cells were washed with medium A, which contained 50 mM HEPES-KOH (pH 7.5) and 30 mM CaCl2. All subsequent steps were performed at 4°C. Cells were suspended in 15 ml of medium B, which contained 50 mM HEPES-KOH (pH 7.5), 25% (w/v) glycerol, 1 M glycine betaine, 22.5 mM CaCl2, 210 mM potassium acetate, 27.5 mM magnesium acetate, 10.7 mM ammonium acetate, protease inhibitors (protease inhibitor cocktail for use with bacterial cell extracts, Sigma, St Louis, MO, USA), and 0.5 U ml−1 RNase inhibitor (Promega). Cells were broken by homogenization with an equal volume of glass beads in a Bead-Beater (BioSpec Products Inc., Bartlesville, OK, USA) operated at maximum speed for 3 min with a 1 min rest at 30 s intervals. The homogenate was centrifuged twice at 8000 g for 5 min to remove unbroken cells and the supernatant was centrifuged at 50 000 g for 30 min to remove cell debris. The blue-green supernatant that contained thylakoid membranes was designated ‘the extract’. The concentration of proteins in the extract was 25 ± 3 mg ml−1, as determined by Bradford's method (Bradford, 1976) with bovine serum albumin as the standard protein. The concentration of chlorophyll in the extract was 1.0 ± 0.2 mg ml−1, as determined by the method by Arnon et al. (1974). The extract was divided into 400 μl aliquots and stored at −80°C prior to use for translation in vitro.
Translation reaction in vitro
Translation in vitro was performed after 50 μl aliquots of extract had been supplemented with 1.0 mM ATP, 0.75 mM GTP, 0.38 mM cAMP, 68 μM L(-)-5-formyl-5,6,7,8-tetrahydrofolic acid (Sigma), 80 mM creatine phosphate (Nakalai Tesque, Kyoto, Japan), 0.25 μg μl−1 creatine phosphate kinase (Roche, Basel, Switzerland), 0.8 μU μl−1 RNase inhibitor (Promega), 150 nCi [14C]Leu (Amersham, Piscataway, NJ, USA) or 43 μCi [35S]Met/Cys (Amersham), 50 μM of each of the other 19 amino acids, and 100 μg of mRNA. The reaction mixture was incubated at 30°C for designated times in light at 200 μmol photons m−2 s−1. Each reaction was terminated by application of a 10 μl aliquot to filter paper that had been soaked in 10% trichloroacetic acid (TCA) and dried as described previously (Kasai et al., 2004). The filter paper was boiled in 10% TCA for 10 min and washed once with 10% TCA and twice with ethanol. After drying of the paper in the air, the incorporation of [14C]Leu into proteins was quantified by liquid scintillation counting. In additional analysis, the labelled proteins were separated by SDS-PAGE on a 12.5% polyacrylamide gel that contained 6 M urea. The autoradiograph of the gel was obtained with the BAS-2500 system (Fuji Film, Tokyo, Japan).
Immunoblotting analysis
Proteins in isolated thylakoid membranes, equivalent to 0.5 μg of chlorophyll, were separated by SDS-PAGE on a 12.5% polyacrylamide gel that contained 6 M urea. Immunoblotting was performed with rabbit antibodies raised against a synthetic oligopeptide that corresponded to the AB loop of the D1 protein or the carboxy-terminal extension of pre-D1 from Synechocystis, as described previously (Nishiyama et al., 2001). Each band of immunologically reactive protein was detected with peroxidase-linked second antibodies and enhanced chemiluminescence immunoblotting detection reagents (Amersham).
Preparation of the PSII complex
For detection of the [35S]Met/Cys-labelled D1 protein in the PSII complex, the D1 protein was synthesized in the extract from mutated cells that expressed histidine-tagged CP47. After dilution of 50 μl of the reaction mixture with 450 μl of medium A, thylakoid membranes were pelleted by centrifugation at 15 000 g for 15 min. The pelleted membranes were suspended in 50 μl of medium B that included 1% n-dodecyl-β-d-maltoside (Dojindo, Kumamoto, Japan) and incubated on ice for 1 h. The treated membranes were centrifuged at 15 000 g for 15 min and the supernatant was mixed with 50 μl of Ni-chelated Sepharose beads (Amersham) that had been pre-equilibrated in medium B that contained 0.04% n-dodecyl-β-d-maltoside and 40 mM imidazole. The beads were washed twice with 1 ml of the same medium used for equilibration. The PSII complex was eluted with medium B that contained 0.04% n-dodecyl-β-d-maltoside and 500 mM imidazole.
Preparation of recombinant EF-G proteins
For construction of histidine-tagged EF-G recombinant proteins from Synechocystis, the coding regions of sll1098, slr1463 and sll0830 were amplified from the genomic DNA of Synechocystis by PCR with primers that were designed to create an NdeI site at the start of each gene and an XhoI site at the end (details available upon request). These DNA fragments were cloned into NdeI and XhoI sites of the pET21b vector (Amersham). The resultant plasmids were used to transform E. coli BL21 (DE3) for expression of recombinant proteins. Recombinant proteins were extracted in the presence of β-mercaptoethanol and purified as described previously (Okuda et al., 2004). Purified proteins in the reduced forms were stored in medium C, which contained 20 mM HEPES-KOH (pH 7.5), 50 mM NaCl and 20% (w/v) glycerol. EF-G of E. coli was purchased from Post Genome Institute (Tokyo, Japan).
Complementation assay in vitro
The complementation assay was performed in a reconstituted translation system in vitro, derived from E. coli (Shimizu et al., 2005), in which all components required for translation except EF-G had been mixed (Puresystem; Post Genome Institute). The gene for DHFR was transcribed in vitro and the resultant DHFR mRNA was translated in the presence of 8.6 μCi [35S]Met/Cys and exogenous EF-G (1 μg), according to the instructions from Puresystem.
Modification of thiol group of cysteine residues
The redox state of cysteine residues of Slr1463 was determined as described (Motohashi et al., 2001). To oxidize Slr1463, 2 μM of protein in 50 mM HEPES-KOH (pH 7.5) was incubated for 15 min at 25°C with 500 μM H2O2. The oxidized protein was incubated for 15 min at 25°C in the presence of various concentrations of DTT. Proteins were then precipitated with 10% TCA and collected by centrifugation. The precipitates were washed with acetone and dissolved in a freshly prepared solution containing 1% SDS, 50 mM Tris-HCl (pH 7.5) and 5 mM α-maleimidopropyl-ω-methoxy,polyoxyethylene (Nihon Yushi, Tokyo, Japan). Labelled proteins were separated by non-reducing SDS-PAGE on a 7.5% polyacrylamide gel.
Overexpression of EF-G
The coding regions of genes for EF-G were amplified from the genomic DNA of Synechocystis by PCR with primers that were designed to create an NdeI site at the start of each gene and a PstI site at the end (details available upon request). The resultant DNA fragments were cloned into the NdeI and PstI sites of pAQ-EX1, a shuttle vector that proliferates in both E. coli and Synechococcus sp. PCC 7942 (Akiyama et al., 1998). These plasmids were used to transform Synechococcus sp. PCC 7942.
Labelling of proteins in vivo
Cell cultures were supplemented with 10 nM [35S]Met (> 1000 Ci mmol−1; Amersham) and then incubated at 32°C for designated times in light at 1.5 mmol photons m−2 s−1 as described previously (Nishiyama et al., 2001), with exception of the inclusion of 1 mM H2O2. Labelling was terminated by the addition of non-radioactive methionine to a final concentration of 1 mM and immediate cooling of samples on ice, and then thylakoid membranes were isolated. After separation of proteins equivalent to 0.67 μg of chlorophyll on a 12.5% polyacrylamide gel that contained 6 M urea, labelled proteins on the gel were visualized with the BAS-2500 system and levels of labelled D1 protein were determined densitometrically.
Acknowledgements
This work was supported, in part, by a Grant-in-Aid for Scientific Research (No. 17570040 to Y.N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We are grateful to Dr Hajime Wada (University of Tokyo) for providing the mutant of Synechocystis that expressed histidine-tagged CP47. We thank Professor Masahiro Sugiura and Dr Michinori Mutsuda (Nagoya University) for helpful discussions on translation in vitro. We thank Drs Toru Hisabori and Ken Motohashi (Tokyo Institute of Technology) for helpful discussions on thiol modification. We also thank Mr Masato Hanamura and Ms Ayaka Yamanaka for their skilled technical assistance.




