Identification of the core transmembrane complex of the Legionella Dot/Icm type IV secretion system
Summary
Type IV secretion systems (T4SS) are utilized by a wide range of Gram negative bacteria to deliver protein and DNA substrates to recipient cells. The best characterized T4SS are the type IVA systems, which exhibit extensive similarity to the Agrobacterium VirB T4SS. In contrast, type IVB secretion systems share almost no sequence homology to the type IVA systems, are composed of approximately twice as many proteins, and remain largely uncharacterized. Type IVB systems include the Dot/Icm systems found in the pathogens Legionella and Coxiella and the conjugative apparatus of IncI plasmids. Here we report the first extensive characterization of a type IVB system, the Legionella Dot/Icm secretion apparatus. Based on biochemical and genetic analysis, we discerned the existence of a critical five-protein subassembly that spans both bacterial membranes and comprises the core of the secretion complex. This transmembrane connection is mediated by protein dimer pairs consisting of two inner membrane proteins, DotF and DotG, which are able to independently associate with DotH/DotC/DotD in the outer membrane. The Legionella core subcomplex appears to be functionally analogous to the Agrobacterium VirB7-10 subcomplex, suggesting a remarkable conservation of the core subassembly in these evolutionarily distant type IV secretion machines.
Introduction
Legionella pneumophila is an opportunistic bacterium that replicates within freshwater protozoan hosts (Rowbotham, 1980). Accidental inhalation of aerosols containing L. pneumophila can lead to pneumonia when the organism replicates within alveolar macrophages (Molofsky and Swanson, 2004). In both protozoa and macrophages, growth of L. pneumophila requires a set of 26 genes named ‘dot’ (defect in organelle trafficking) or ‘icm’ (intracellular multiplication) that encode a type IV secretion system (T4SS) (Segal et al., 1998; Vogel et al., 1998; Segal and Shuman, 1999). T4SS include traditional plasmid conjugation systems and adapted conjugation systems, which are used by many bacteria to deliver effector molecules to host cells.
Adapted conjugation systems are found in a wide spectrum of pathogens (Burns, 2003). The most extensively characterized is the VirB type IVA secretion system in the plant pathogen Agrobacterium tumefaciens. This T4SS is composed of 11 VirB proteins (VirB1 through VirB11) and a putative ATPase, VirD4 (Christie et al., 2005). Extensive biochemical and genetic analyses of the VirB complex have lead to a detailed understanding of how the secretion apparatus assembles and functions in substrate translocation (Christie and Cascales, 2005). In contrast to this well-characterized system, little is known about the L. pneumophila Dot/Icm type IVB secretion system. The Dot/Icm T4SS is composed of approximately twice as many proteins, most of which have no detectable homology to proteins of the A. tumefaciens type IV system, making predictions of their functions difficult.
Although our understanding of the L. pneumophila secretion system is not as advanced, some progress has been made in characterizing the Dot/Icm proteins (summarized in Table 1). To date, five proteins have been localized to the L. pneumophila cytoplasm: DotB, IcmQ, IcmR, IcmS and IcmW (Coers et al., 2000; Dumenil and Isberg, 2001; Sexton et al., 2004a). DotB is a hexameric ATPase with homology to A. tumefaciens VirB11 protein and plays a role in the assembly of the L. pneumophila T4SS as well as in the selection of substrates for secretion (Sexton et al., 2004a; 2005). The IcmS and IcmW proteins interact with each other and function as type IV adaptors, targeting secreted substrates to the T4SS (Coers et al., 2000; Bardill et al., 2005; Ninio et al., 2005). IcmS also interacts with the LvgA protein, and this heterodimer may function as a second type IV adaptor complex (Vincent and Vogel, 2006). Purified IcmQ is able to form pores in membranes and is bound in the cytoplasm by its chaperone IcmR (Dumenil and Isberg, 2001; Dumenil et al., 2004).
| Proteina | Size (aa) | kDa | Predicted localizationb | Localization (wild type)c | Informationd | Referencee |
|---|---|---|---|---|---|---|
| DotA | 1048 | 113 | IMf | IM | Polytopic membrane protein | Roy and Isberg (1997) |
| DotB | 377 | 42 | Cytoplasm | Cytoplasm | ATPase/PilT and VirB11g homology | Sexton et al. (2004a) |
| DotC | 303 | 34 | IM or OMh | OM | Putative lipoprotein | Yerushalmi et al. (2005) |
| DotD | 163 | 18 | IM or OM | OM | Putative lipoprotein | Yerushalmi et al. (2005) |
| DotE/IcmC | 194 | 20 | IM | |||
| DotF/IcmG | 269 | 30 | IM | OM | Interacts with secreted substrates | Luo and Isberg (2004) |
| DotG/IcmE | 1048 | 108 | IM | OM | VirB10g homology | |
| DotH/IcmK | 360 | 39 | Periplasm | Periplasm and OM | Andrews et al. (1998) | |
| DotI/Icml | 212 | 23 | IM | IM | Andrews et al. (1998) | |
| DotJ/IcmM | 94 | 11 | IM | |||
| DotK/IcmN | 189 | 21 | IM or OM | OM | Putative lipoprotein | Yerushalmi et al. (2005) |
| DotL/IcmO | 783 | 87 | IM | IM | VirD4g homology/T4CP/essential gene | Buscher et al. (2005) |
| DotM/IcmP | 376 | 43 | IM | IM | Essential gene | |
| DotN/IcmJ | 208 | 24 | Cytoplasm or IM | Cytoplasm and IM | Essential gene | |
| DotO/IcmB | 1009 | 112 | Cytoplasm | IM | Andrews et al. (1998) | |
| DotP/IcmD | 132 | 14 | IM | IM | ||
| DotU | 261 | 30 | IM | IM | Accessory factor (interacts IcmF) | Sexton et al. (2004b) |
| DotV | 180 | 20 | IM | Homology to DotE | Sexton et al. (submitted) | |
| IcmF | 973 | 111 | IM | IM | Accessory factor (interacts DotU) | Sexton et al. (2004b) |
| IcmQ | 191 | 22 | Cytoplasm | Cytoplasm | Pore forming molecule | Dumenil and Isberg (2001);Dumenil et al. (2004) |
| IcmR | 120 | 13 | Cytoplasm | Cytoplasm | Chaperone for IcmQ | Dumenil and Isberg (2001);Dumenil et al. (2004) |
| IcmS | 114 | 13 | Cytoplasm | Cytoplasm | Adaptor for substrates | Coers et al. (2000);Ninio et al. (2005) |
| IcmT | 86 | 9.9 | IM | Bitar et al. (2005) | ||
| IcmV | 151 | 18 | IM | |||
| IcmW | 151 | 17 | Cytoplasm | Cytoplasm | Adaptor for substrates | Coers et al. (2000);Ninio et al. (2005) |
| IcmX | 466 | 51 | Periplasm | Periplasm | Matthews and Roy (2000) | |
| LvgA | 208 | 27 | Cytoplasm | Cytoplasm | Adaptor for substrates | Vincent and Vogel (2006) |
- a. The protein name is shown as Dot or Icm if only one names exists or as a Dot/Icm if both names exist. For the sake of brevity, we have chosen to only use the Dot names when appropriate.
- b. Based on analysis of a Kyte-Doolittle hydrophilicity plot.
- c. Experimental determination by ultracentrifugation and sucrose gradient separation.
- d. Homology and/or motifs.
- e. Only most relevant references listed.
- f. Inner membrane.
- g. Agrobacterium tumefaciens type IV secretion protein.
- h. Outer membrane.
Four of the Dot/Icm proteins have been shown to localize to the inner membrane and one to the periplasm. The inner membrane proteins are DotA, DotL, DotU and IcmF. DotA is a large polytopic inner membrane protein of unknown function (Roy and Isberg, 1997). DotL, a member of the type IV coupling protein family, is distantly related to the A. tumefaciens VirD4 protein and has been proposed to serve as the inner membrane receptor for secreted substrates (Buscher et al., 2005). DotU and IcmF function as accessory factors that stabilize the secretion apparatus (Sexton et al., 2004b). IcmX is the only known periplasmic Dot/Icm protein (Matthews and Roy, 2000); the outer membrane component(s) of the Dot/Icm apparatus have not yet been identified.
To date, only individual Dot/Icm proteins of this T4SS have been examined. We report the first comprehensive biochemical and genetic examination of the L. pneumophila T4SS. We have identified a subcomplex consisting of DotC, DotD, DotF, DotG and DotH, bridging the inner and outer membranes of L. pneumophila. This L. pneumophila Dot/Icm subcomplex is analogous to the A. tumefaciens VirB7-10 subcomplex and suggests a conserved core structure in these evolutionarily diverse secretion systems.
Results
Localization of the L. pneumophila Dot/Icm proteins
As an initial step in characterizing the molecular architecture of the Dot/Icm secretion apparatus, we determined the subcellular localization of the majority of the Dot/Icm proteins in the wild-type L. pneumophila strain Lp02 using biochemical approaches that have proven successful in the study of the A. tumefaciens VirB T4SS (Thorstenson et al., 1993). Because L. pneumophila cells grown in vitro become virulent at the transition from late exponential to early stationary phase (Byrne and Swanson, 1998), we analysed the localization of the Dot/Icm proteins at this stage of growth. Cells grown in liquid media were fractionated based on ultracentrifugation to separate soluble from membrane-associated proteins and Triton X-100 (TX-100) solubility to differentiate inner from outer membrane proteins (Nikaido, 1994). Most Gram-negative bacterial inner membrane proteins are soluble in the non-ionic detergent TX-100, whereas outer-membrane proteins are typically insoluble.
Legionella pneumophila cells were separated into five fractions: total proteins (T), soluble proteins (S), total membrane proteins (M), TX-100 extractable proteins (E) and TX-100 non-extractable proteins (N). Samples were analysed by Western blotting using antibodies that recognize 21 of the 26 Dot/Icm proteins and one T4SS substrate, SdeC (Fig. 1). Using this method, we were able to divide the Dot/Icm proteins into three subcellular classes: soluble proteins (Fig. 1A), inner membrane proteins (Fig. 1B), and outer membrane proteins (Fig. 1C). The purity of the fractions obtained by this method was confirmed using antibodies to proteins known to localize to each compartment (Fig. 1D).
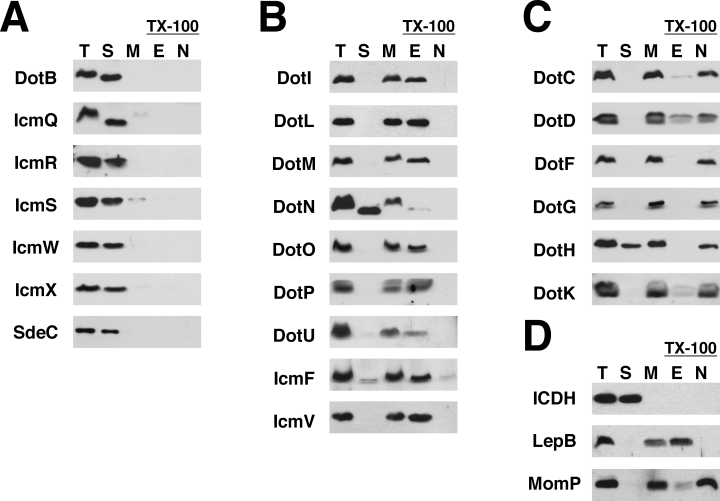
Localization of the Dot/Icm proteins by ultracentrifugation and Triton X-100 solubility. L. pneumophila strain Lp02 was grown to late exponential phase and cells were fractionated using ultracentrifugation and Triton X-100 (TX-100) extraction. Protein fractions were resolved by SDS-PAGE followed by Western blotting using antibodies specific to the proteins listed to the left of each blot. Each blot contains the following fractions: total proteins (T), soluble proteins (S), total membrane proteins (M), TX-100 extractable proteins (E), and TX-100 non-extractable proteins (N). The fractionation results have been grouped together as:A. Soluble proteins (cytoplasm and periplasm), including the secreted substrate SdeC.B. TX-100 extractable proteins (likely inner membrane).C. TX-100 non-extractable proteins (likely outer membrane).D. Fractions were assayed for the presence of the following control proteins to insure the quality of the separation techniques: isocitrate dehydrogenase (ICDH) (soluble), signal peptidase LepB (inner membrane), and MomP (outer membrane). The data shown are representative of three independent experiments.
Our results are consistent with the predicted localizations of many of the Dot/Icm proteins (Table 1) and confirm several reported findings (Coers et al., 2000; Matthews and Roy, 2000; Dumenil and Isberg, 2001; Sexton et al., 2004a; Buscher et al., 2005). However, several proteins did not localize as predicted, which proved to be valuable in understanding their function (see below). For example, DotF and DotG were TX-100 non-extractable, although they are predicted to be inner membrane proteins. Two other proteins, DotH, a predicted outer membrane protein, and DotN, a predicted inner membrane protein, were atypical in that they localized to both the membrane and the soluble fractions. As the soluble fraction in Fig. 1 contains both cytoplasmic and periplasmic proteins, we resolved the soluble fraction by osmotic shock (Witholt et al., 1976). This technique revealed that the soluble DotH is in the periplasm, whereas the soluble DotN is in the cytoplasm (Fig. S1). In summary, six Dot/Icm proteins were mostly soluble, nine were TX-100 extractable, indicating they are found in the inner membrane, and six were TX-100 non-extractable, suggesting they are present in the outer membrane.
Although Triton X-100 solubility is a well-established technique for separating membrane proteins in Gram-negative bacteria, some proteins have been identified that exhibit aberrant localization when assayed by detergent solubility (Nikaido, 1994; Daefler and Russel, 1998; Castanie-Cornet et al., 2006). Therefore, to independently determine the membrane localization results obtained in Fig. 1, we analysed the subcellular localization of the Dot/Icm membrane proteins using a different technique, sucrose density gradient ultracentrifugation. L. pneumophila total membrane proteins were isolated and subjected to ultracentrifugation through a sucrose density gradient (25–55% sucrose, w/w). As before, inner membrane proteins could be differentiated from outer membrane proteins based on the localization of the controls LepB and MomP (Fig. 2). Of the nine proteins shown to be in the inner membrane by TX-100 solubility, all nine were confirmed to be in the inner membrane by sucrose density gradient ultracentrifugation (Fig. 2). Of the proteins shown to be in the outer membrane (Fig. 1A), all were present in the outer membrane fractions of the sucrose gradient (Fig. 2 and data not shown for DotK). Notably, both DotF and DotG appeared in the outer membrane protein fractions by both techniques, in spite of their predicted inner membrane localization, suggesting that they either are in fact outer membrane proteins or that they perhaps associate with the outer membrane.

Localization of Dot/Icm proteins by sucrose density gradient ultracentrifugation. Lp02 membrane proteins were isolated by ultracentrifugation, applied to the top of a sucrose gradient and centrifuged at 170 000 g for 20 h. Fractions were collected from the bottom of the gradient and analysed by Western blotting using the antibodies listed to the left of each blot. Antibodies that recognize the control proteins LepB and MomP detect inner membrane fractions (right side of the blots) and outer membrane fractions (left side of the blots). The data shown are representative of at least two independent experiments.
Effects of dot/icm mutations on the stability of other Dot/Icm proteins
Based on the observation that proteins that interact often exhibit decreased stability in the absence of their binding partner (Hapfelmeier et al., 2000; Possot et al., 2000), we examined a collection of in-frame dot/icm deletions with the goal of identifying potential protein interactions (strains listed in Table S1). We compared the levels of Dot/Icm proteins in a wild-type strain to the individual dot/icm deletions by Western blotting. Wild-type levels of each Dot/Icm protein are indicated in the first lane (Lp02) (Fig. 3). A strain lacking all of the dot/icm genes (indicated by a Δ) did not express any of the Dot/Icm proteins and each individual dot/icm deletion did not express its corresponding Dot/Icm protein (Fig. 3).
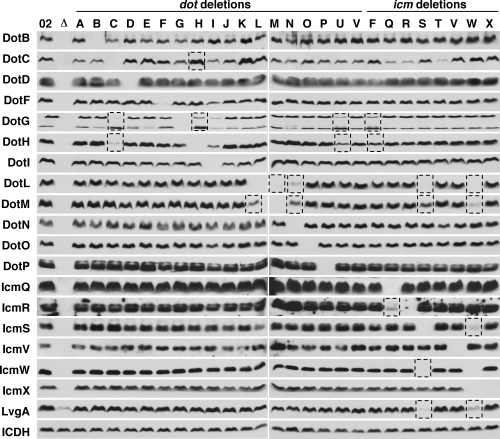
Effects of dot/icm deletions on the stability of other Dot/Icm proteins. Extracts of wild-type L. pneumophila and various dot/icm deletions were analysed by Western blotting using antibodies specific to the proteins listed to the left of each blot. The strains used are designated at the top of the figure and include: wild-type strain Lp02 (02), strain JV4044 that is missing all dot/icm genes (Δ), and 26 individual dot/icm deletions. The dot/icm deletions are designated by the last letter in the name for the dot genes (left side of the figure) or the icm genes (right side of the figure). The dotL, dotM and dotN deletions were constructed in a ΔdotA strain, as these genes are essential in the wild-type strain background (see Appendix S1). Two DotG-specific bands were observed in the DotG blots; the upper band corresponds to full-length DotG and the lower band corresponds to a breakdown product. Equivalent numbers of cells were used as indicated by the loading control ICDH. Strains utilized in this figure are listed in Table S1. Dashed boxes indicate stability effects noted in the text. The data shown are representative of three independent experiments.
In general, we observed that the steady-state levels of most of the Dot/Icm proteins were not affected by other dot/icm deletions. For example, deletions in other dot/icm genes did not affect proteins levels of DotO, DotP and IcmQ (Fig. 3). However, in a few specific cases, we did observe alterations in the protein levels of various Dot/Icm proteins. Loss of icmS had a profound effect on the levels of IcmW and likewise loss of icmW had an effect on the levels of IcmS (Fig. 3), confirming the reciprocal stability effect of these type IV adaptor proteins (Ninio et al., 2005). Similarly, the IcmR protein requires its binding partner, the pore-forming protein IcmQ, for its stability (Fig. 3) (Dumenil and Isberg, 2001; Dumenil et al., 2004).
In addition to these known interactions, our analysis revealed several novel stability effects. The first includes DotG and DotH, two proteins observed in the outer membrane fractions. The ΔdotH mutation had a profound effect on levels of DotG, although the effect of dotG deletion on DotH was more subtle (Fig. 3). In addition, DotG and DotH stability required the presence of the lipoprotein DotC (Fig. 3). In contrast, DotC levels were increased in many dot/icm deletions including the ΔdotH mutant. DotC was the only protein assayed that was affected in this manner. Finally, deletion of the macromolecular chaperones DotU and IcmF also resulted in decreased stability of DotG and DotH, further supporting a connection between DotG and DotH (Fig. 3) (Sexton et al., 2004b).
The second major result observed in this analysis involved the type IV coupling protein DotL, DotM and DotN. DotL was destabilized in the ΔdotM and ΔdotN mutants and DotM was destabilized in the ΔdotL and ΔdotN mutants (Fig. 3). These three proteins have previously been proposed to interact, because they share the unusual trait of being essential for the viability of L. pneumophila (Buscher et al., 2005). Finally, both DotL and DotM levels were also dependent on the presence of the type IV adaptors IcmS, IcmW and LvgA. This dependence may be related to delivery of substrates to the secretion complex through interactions with the coupling protein DotL (C.D. Vincent and J.P. Vogel, unpubl. data).
Because most of the dot/icm genes are encoded in operons, it was possible that some of the effects observed in Fig. 3 were due to polar effects of the deletions on transcription of downstream genes. Attempts to restore the stability effects by complementation were successful in all but the ΔdotI and the ΔicmW mutants. Complementation was observed for the ΔdotC,ΔdotH,ΔdotL, ΔdotM, ΔdotN, ΔdotU, ΔicmF and ΔicmS mutants, indicating these instability results were not due to polarity (Fig. S2). For example, addition of a dotH complementing clone to the ΔdotH mutant restored levels of DotH and DotG. Likewise, addition of dotC to the ΔdotC mutant restored levels of DotC, DotG and DotH. In summary, we noted 20 prominent stability effects involving 10 different dot/icm deletions (Table 2). This comprehensive approach for predicting protein–protein interactions via stability effects has suggested the existence of two major subcomplexes. The first consists of the coupling protein DotL, DotM, DotN and the type IV adaptors IcmS, IcmW and LvgA; the second subcomplex includes DotG, DotH and the lipoprotein DotC.
| Δdot/icm | Proteins affected |
|---|---|
| ΔdotC | DotG, DotH |
| ΔdotH | DotG |
| ΔdotL | DotM |
| ΔdotM | DotL |
| ΔdotN | DotL, DotM |
| ΔdotU | DotGa, DotHa |
| ΔicmF | DotGa, DotHa |
| ΔicmQ | IcmRb |
| ΔicmS | DotL, DotM, IcmWc,d, LvgAd |
| ΔicmW | DotL, DotM, IcmSc,d, LvgAd |
- a. Similar to a previous report (Sexton et al., 2004b).
- b. Similar to a previous report (Dumenil and Isberg, 2001).
- c. Similar to a previous report (Ninio et al., 2005).
- d. Similar to a previous report (Vincent and Vogel, 2006).
DotG interacts with DotF
DotG and DotF are predicted to be inner membrane proteins, yet both localize to the outer membrane (1, 2), suggesting that they are in fact outer membrane proteins or that they may interact with the outer membrane. Based on this unexpected result, we hypothesized that DotG and DotF might interact. Using a bacterial two-hybrid assay (Karimova et al., 1998), we observed an interaction between DotG and DotF (Fig. S3). We also observed that DotG dimerizes, consistent with its limited homology to the VirB10 protein of the A. tumefaciens T4SS, which is known to dimerize (Cascales and Christie, 2004; de Paz et al., 2005). DotF, a protein reported to bind to T4SS substrates at the inner membrane of L. pneumophila (Luo and Isberg, 2004), was also shown to interact with itself (Fig. S3).
DotG and DotF are inner membrane proteins
Because the bacterial two-hybrid system we used relies on reconstitution of adenylate cyclase activity in the cytoplasm, the two-hybrid results demonstrate that the amino-termini of DotG and DotF must be in the cytoplasm. However, DotG and DotF cofractionated with the outer membrane (1, 2). This discrepancy can be explained if DotG and DotF are inner membrane proteins that fractionate abnormally due to interactions with components of the outer membrane. To test this hypothesis, DotG and DotF were expressed individually in a L. pneumophila strain lacking the Dot/Icm secretion apparatus. In contrast to the wild-type strain, DotG and DotF both localized to the inner membrane when expressed in the absence of the other Dot/Icm proteins, implicating the Dot/Icm complex in stable localization of these proteins with the outer membrane (Fig. 4A and B). We further hypothesized that DotH and DotC are required for outer membrane association of DotG and DotF. As predicted, DotG and DotF were no longer observed in the outer membrane fractions of the ΔdotH and the ΔdotC mutants (Fig. 4).
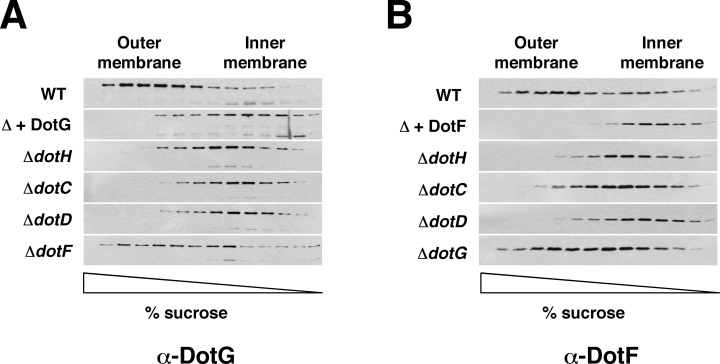
Outer membrane localization of DotG and DotF requires DotC, DotD and DotH. Total membrane proteins isolated from a series of L. pneumophila strains were subjected to sucrose density gradient ultracentrifugation and fractions were analysed by SDS-PAGE followed by Western blotting using antibodies specific to DotG (A) or to DotF (B). Strains used for each gradient are listed to the left of each blot: Lp02 (wild-type), JV5404 (Δ + dotG), JV3563 (ΔdotH), JV3743 (ΔdotC), JV3572 (ΔdotD), JV3579 (ΔdotF), JV5403 (Δ + dotF) and JV3559 (ΔdotG). JV5404 and JV5403 are L. pneumophila strains lacking the Dot/Icm complex (designated in the figure as Δ). Detection of DotG in the dot/icm deletion background required longer exposures because DotG is not completely stable in the absence of other Dot/Icm proteins. Results shown are representative of three independent experiments.
Because the L. pneumophila Dot/Icm complex contains an additional lipoprotein, DotD (Segal et al., 2005; Yerushalmi et al., 2005), we examined the localization of DotG and DotF in a ΔdotD strain. Once again, DotG and DotF both lost their outer membrane association in the absence of DotD (Fig. 4). Although DotG and DotF interact, they did not require each other for outer membrane association (see ΔdotF and ΔdotG in Fig. 4). As a result, it can be concluded that DotG and DotF are inner membrane proteins that associate independently with the outer membrane due to the presence of the DotH, DotC and DotD proteins.
Outer membrane localization domains of DotG and DotF
Our bacterial two-hybrid assays suggested that the amino-termini of DotG and DotF were in the cytoplasm. To confirm the topology of these proteins, PhoA was fused to each protein after its predicted transmembrane domain (Fig. 5A). Alkaline phosphatase is active in the periplasm, but not in the cytoplasm, and thus can be used to determine the topology of inner membrane proteins. Both fusions exhibited PhoA activity, indicating that the carboxy-termini of these proteins are in the periplasm, thus suggesting that the DotF and DotG transmembrane domains are embedded in the inner membrane (Fig. 5B).
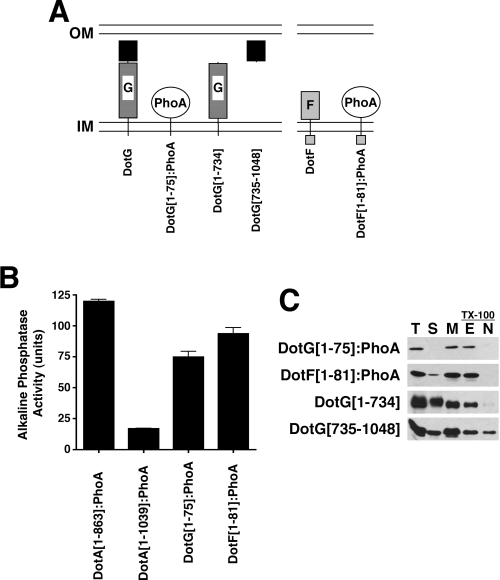
Analysis of DotG and DotF topology and protein–protein interactions.A. Schematic representation of the DotG and DotF proteins. Both proteins contain a transmembrane domain near their amino termini. The DotG transmembrane domain is followed by approximately 50 copies of a 10 amino acid repeated sequence (striped box). The carboxy-terminal ∼300 amino acids of DotG contain homology to the VirB10 protein (solid black box).B. Topology of the DotG and DotF proteins. Alkaline phosphatase activity (expressed in arbitrary units) of the first 75 amino acids of DotG fused to PhoA (DotG[1–75]:PhoA) and the first 81 amino acids of DotF fused to PhoA (DotF[1–81]:PhoA) expressed in wild-type L. pneumophila strain Lp02. PhoA fusions to DotA at amino acid 863 or amino acid 1039 were included as positive and negative controls respectively (Roy and Isberg, 1997).C. Localization of DotG[1–75]:PhoA, DotF[1–81]:PhoA, DotG[1–734] and DotG[735–1048] by TX-100 solubilization. Strains carrying the fusion proteins or truncated DotG clones were fractionated by ultracentrifugation and TX-100 solubility. Localization of the PhoA fusions was assayed by Western blotting with an antibody that recognizes PhoA. DotG fragments were detected using antibodies raised against the amino-terminus (amino acids 1–734) or the carboxy-terminus (amino acids 735–1048) of DotG.
To identify the domains responsible for the outer membrane association, we fractionated the strains expressing the DotG and DotF PhoA fusions. In contrast to full-length DotG and DotF, the PhoA fusions were extractable with TX-100, indicating inner membrane localization (Fig. 5C). To determine the domain of DotG responsible for association with the outer membrane, we assayed the localization of two additional constructs. The first consisted of the amino-terminal 734 amino acids of DotG, which contains the transmembrane domain and the repeat region. The second construct had a cleavable signal sequence from the periplasmic protein IcmX (Matthews and Roy, 2000) fused to the carboxy-terminal 314 amino acid domain of DotG, which possesses the VirB10-like domain (Segal et al., 2005). The amino-terminal DotG fragment localized to the inner membrane, similar to the localization of the DotG[1–75]:PhoA fusion, indicating that the first 734 amino acids of DotG are not sufficient to interact with the outer membrane. In contrast, a significant portion of the carboxy-terminus of DotG was observed in the TX-100 insoluble membrane fraction. Thus, the carboxy-terminal VirB10-like domain of DotG is likely responsible for the observed outer membrane localization of DotG. In summary, we have provided evidence that DotF and DotG are inner membrane proteins that colocalize with the outer membrane of L. pneumophila via interactions between their carboxy-terminal domains and other components of the Dot/Icm apparatus, possibly DotH, DotC and/or DotD.
Outer membrane localization of DotH
To further characterize the putative outer membrane subcomplex, we determined the factors required for the proper localization of DotC, DotD and DotH. The lipoproteins DotC and DotD localize to the outer membrane of wild-type cells as determined by sucrose density gradients (Fig. 2). DotC and DotD also localize to the outer membrane in the absence of the Dot/Icm complex, indicating their localization is not mediated by interactions with other Dot/Icm proteins (data not shown). In contrast, DotH did not cofractionate with the outer membrane when expressed in the absence of the Dot/Icm complex (Fig. 6A). Instead DotH accumulated in the soluble fraction, probably the periplasm, in this strain. This result implies that DotH requires one or more Dot/Icm protein for its localization to the outer membrane. We predicted that the lipoproteins, DotC and DotD, might be responsible. In fact, DotH did not accumulate in the outer membrane in the ΔdotC or the ΔdotD strains (Fig. 6A). However, DotH localization did not require DotF or DotG (see ΔdotF and ΔdotG in Fig. 6A). Thus, wild-type L. pneumophila appears to possess two forms of DotH; a soluble form in the periplasm and an outer membrane associated form that is dependent on the presence of the two lipoproteins DotC and DotD.
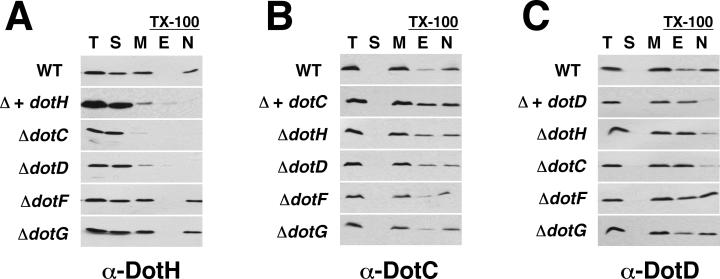
DotH localization to the outer membrane requires the lipoproteins DotC and DotD. Fractionation of Dot/Icm proteins by ultracentrifugation and TX-100 solubility. Protein fractions were analysed by SDS-PAGE followed by Western blotting using antibodies to DotH (A), DotC (B), or DotD (C). Strains that were fractionated are listed to the left of each blot. Lanes contain total proteins (T), soluble proteins (S), total membrane proteins (M), TX-100 extractable proteins (E), or TX-100 non-extractable proteins (N). Strains used were Lp02 (wild-type), JV5405 (Δ + dotH), JV5469 (Δ + dotC), JV5470 (Δ + dotD), JV3743 (ΔdotC), JV3572 (ΔdotD), JV3579 (ΔdotF), JV3559 (ΔdotG) and JV3563 (ΔdotH). JV5405, JV5469 and JV5470 are L. pneumophila strains lacking the Dot/Icm complex (designated in the figure as Δ).
DotC and DotD become Triton X-100 soluble in the absence of DotH
Based on the lipoprotein requirement of DotH, we examined DotC and DotD in more detail. In contrast to integral outer membrane proteins, outer membrane lipoproteins are often extractable with TX-100, making this technique unreliable for determining the localization of lipoproteins (Daefler and Russel, 1998; Castanie-Cornet et al., 2006). However, TX-100 solubility can reveal if a lipoprotein is bound by another outer membrane protein, because this can enhance the lipoprotein's resistance to TX-100 extraction. In fact, DotC and DotD were slightly sensitive to TX-100 extraction in the wild-type strain and this sensitivity was enhanced when these proteins were expressed in a strain lacking the Dot/Icm complex (Fig. 6B and C). These results suggest that they may interact with another outer membrane Dot/Icm protein.
To determine the protein responsible for the increased resistance to TX-100 extraction, we examined this property in several dot/icm mutant backgrounds. DotC and DotD both displayed increased solubility in a ΔdotH mutant, consistent with their binding DotH in the outer membrane (Fig. 6B and C). Interestingly, both proteins were more soluble in a strain lacking the other lipoprotein, whereas ΔdotF and ΔdotG mutations did not have significant effects on DotC and DotD detergent solubility. Based on these results, we can conclude that DotH requires DotC and DotD to target to the outer membrane, where the proteins appear to exist in a complex, and that DotF and DotG are not directly involved in this process.
Kinetic analysis of DotH localization
To determine the kinetics of DotH outer membrane association, we constructed a plasmid with dotH under the control of a regulated promoter for the purpose of assaying the effects of DotH depletion or induction. As DotH was depleted, we observed that the soluble fraction of DotH decreased at a rate faster than the fate of decrease of membrane-associated DotH (Fig. 7A). After 7 h of growth without inducer, no soluble DotH was detected, whereas the membrane-associated DotH was only moderately decreased. In the converse experiment, assaying the effects of DotH induction, newly synthesized DotH was only observed in the soluble fraction (Fig. 7B). Soluble DotH appeared to reach a maximum steady-state level after 2 h of induction. In contrast, DotH was not observed in the membrane protein fraction until the 2 h time point and did not reach maximum levels in this fraction until 5 h after induction. These results suggest that soluble DotH exists as a precursor in the periplasm prior to its association with DotC and DotD in the outer membrane. We also examined the effects of DotH depletion and induction on DotC and DotD detergent solubility. Concomitant with DotH depletion, DotC and DotD became increasingly susceptible to TX-100 extraction (Fig. 7A). In contrast, DotC and DotD became more resistant to TX-100 upon DotH induction (Fig. 7B), further supporting the existence of a DotC/DotD/DotH complex.
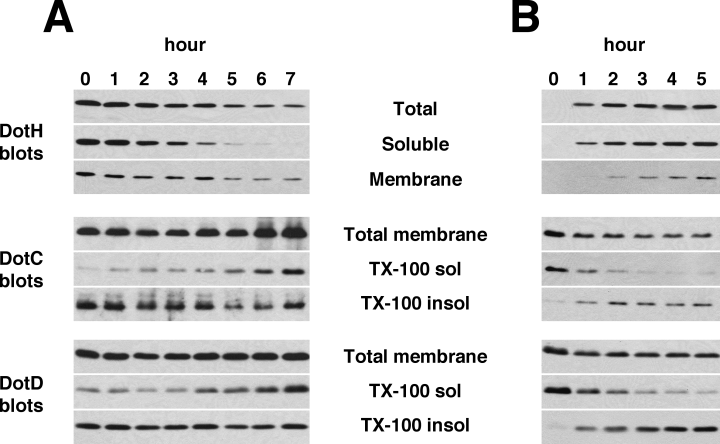
Effects of induction and depletion of DotH.A. Depletion of DotH in Lp02 ΔdotH. Strain JV5507 (ΔdotH + pDotH) was grown to early exponential phase in the presence of the inducer IPTG (100 μM). Cells were collected by centrifugation, resuspended in medium lacking IPTG, and allowed to continue growing at 37°C. Samples of the culture were taken immediately after resuspension in medium without IPTG (time 0) and each hour for 7 h. The cells taken at each time point were then fractionated by ultracentrifugation and TX-100 solubility to localize the DotH, DotC and DotD proteins. Fractionation samples were analysed by Western blotting using antibodies specific to the proteins listed to the left of each blot.B. Induction of DotH in Lp02 ΔdotH. Strain JV5507 (ΔdotH + the dotH complementing clone pJB4424) was grown to early exponential phase in the absence of the inducer IPTG and then induced with 100 μM IPTG. Samples of the culture were taken immediately before induction and every hour after induction for 5 h. The cells taken at each time point were then fractionated by TX-100 solubility to localize the DotH, DotC and DotD proteins. Fractionation samples were analysed by Western blotting using antibodies specific to the proteins listed to the left of each blot. Results shown in A and B are representative of three independent experiments.
Discussion
We report here the first comprehensive examination of the L. pneumophila Dot/Icm T4SS. Using two different fractionation techniques, we report the localization of the majority of the Dot/Icm proteins. We show that five Dot proteins form a subcomplex that bridges the two bacterial membranes. This subcomplex consists of the inner membrane proteins DotF and DotG, which interact and form a subcomplex with the outer membrane proteins DotC, DotD and DotH. These five proteins constitute the ‘core’trans-envelope subcomplex of the L. pneumophila T4SS.
Prior to this study, eight Dot/Icm proteins had been localized experimentally. Here we describe the localization of an additional 14 Dot/Icm proteins, including DotC, DotD, DotF, DotG, DotH, DotI, DotK, DotM, DotN, DotO, DotP, DotU, IcmF and IcmV (Table 1). Four additional proteins, DotE, DotJ, DotV and IcmT, could not be localized due to a lack of antibodies, but are predicted to be inner membrane proteins. Collectively these data support a model of the L. pneumophila Dot/Icm system consisting of five proteins in the cytoplasm, 16 proteins associated with the inner membrane, one periplasmic protein and four outer membrane proteins (Fig. 8). These results were surprising due to the high proportion of inner membrane proteins in this system as compared with type IVA systems. This suggests that the additional complexity of type IVB secretion systems is due to elaboration of the inner membrane portion of the secretion system.
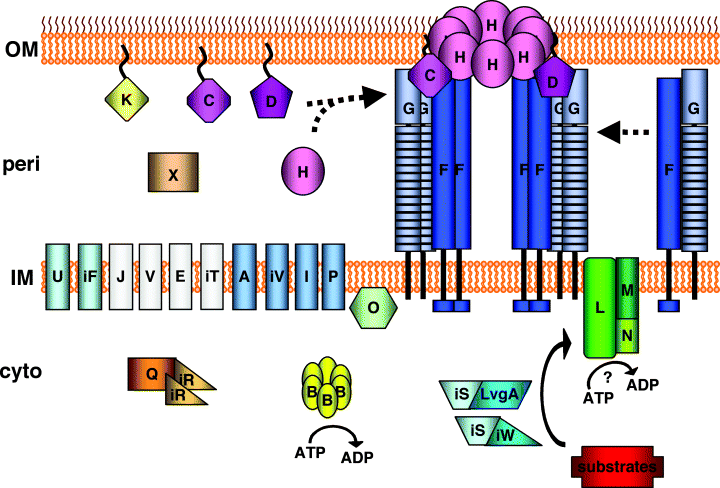
Model showing the DotC/DotD/DotF/DotG/DotH core transmembrane subcomplex. Dot/Icm proteins are labelled using the last letter of their name. Dot proteins are indicated with a single letter whereas Icm proteins with the same last letter as a Dot protein are prefaced with the letter ‘i’. Proteins that have been experimentally localized are shown in colour; the four proteins not localized experimentally but predicted to be inner membrane proteins are shown in white (DotJ, DotV, DotE and IcmT). A potential assembly pathway is shown consisting of three steps: (i) lipoproteins DotC and DotD mediate outer membrane localization of DotH, (ii) DotF and DotG interact in the inner membrane, and finally (iii) the DotC/DotD/DotH/DotG/DotF subcomplex is formed, spanning the inner and outer membrane and constituting the ‘core’ of the Dot/Icm secretion apparatus.
Two proteins of the proposed core Dot/Icm subcomplex, DotF and DotG, displayed unexpected protein localization in the wild-type L. pneumophila strain Lp02. Both proteins are predicted to be inner membrane proteins, yet they localized with the outer membrane when analysed by TX-100 extraction or sucrose gradient centrifugation. We propose that these proteins are in fact inner membrane proteins that cofractionate with the outer membrane due to association with a DotC/DotD/DotH subcomplex. This proposal is based on the following pieces of evidence. First, sequence analysis reveals that DotF and DotG each contain a typical non-cleavable, hydrophobic transmembrane domain that is strongly predicted to target the protein to the inner membrane. Second, adenylate cyclase fusions to DotF or DotG amino termini are active in a bacterial two-hybrid assay, thus indicating these domains are present in the cytoplasm. Third, amino-terminal fragments of DotF and DotG colocalize with the inner membrane in Lp02, suggesting the carboxy-terminus of each protein is responsible for the outer membrane association. Consistent with this, targeting just the carboxyl-terminal domain of DotG into the periplasm allows it to associate with the outer membrane. Fourth, both DotF and DotG expressed alone in a strain lacking the other dot/icm proteins fractionate in the inner membrane by sucrose gradient centrifugation. This strongly suggests the proteins are not directly interacting with the outer membrane itself, nor are they colocalizing with the outer membrane due to interaction with a periplasmic structure such as peptidoglycan, but rather that they are interacting with one or more Dot/Icm proteins in the outer membrane. Finally, because deletion of just dotC, dotD, or dotH also results in DotF and DotG fractionating with the inner membrane, DotC, DotD, or DotH are likely responsible for the observed localization of DotF and DotG. In totality, these data demonstrate that DotF and DotG are inner membranes proteins that cofractionate with the outer membrane due to interactions of their carboxy-termini with DotC, DotD, or DotH in the outer membrane.
The function of DotG is not known. One hint comes from the limited homology between DotG and the A. tumefaciens VirB10 protein. Like DotG, VirB10 is known to interact with inner membrane components (VirB8 and the ATPases VirB4 and VirD4) and outer membrane proteins (VirB7 and VirB9) (Christie and Cascales, 2005). Both DotG and VirB10 have similar topological arrangement to the Escherichia coli protein TonB, which transduces energy from the inner membrane to outer membrane components of a number of transport systems (Cascales and Christie, 2004; Wiener, 2005). DotG may function in a similar manner to transfer energy from the inner to the outer membrane, perhaps energizing DotH as the secretion pore in the outer membrane. Supporting this hypothesis, VirB10 undergoes a structural transition in response to ATP hydrolysis by the inner membrane proteins VirD4 and VirB11, suggesting a potential mechanism for energy transfer to the outer membrane (Cascales and Christie, 2004).
DotF interacts with DotG and exhibited a similarly unexpected intracellular localization (1, 2, 4). DotF is a 269 amino acid protein that contains an ∼50 amino acid highly charged cytoplasmic domain, a single hydrophobic transmembrane domain and an ∼200 amino acid periplasmic domain. DotF has been shown to interact with T4SS substrates in the cytoplasm (Luo and Isberg, 2004). Like DotG, DotF was only observed to associate with the outer membrane fractions in the presence of DotC, DotD and DotH. Interestingly, not only did we find that DotF and DotG behave similarly, we also found that they interact with each other. These data may indicate that DotF and DotG work together to mediate the early steps of Dot/Icm substrate export. For example, substrate binding by DotF may activate DotG. Alternatively, DotF may transduce a signal back to the cytoplasmic face of the inner membrane after a substrate has been exported, thus downregulating DotG activity.
The interaction of DotF and DotG with the outer membrane is mediated by DotC, DotD and DotH. DotC and DotD were recently shown to be lipoproteins, although no specific role was assigned to these proteins (Yerushalmi et al., 2005). Likewise, little was known about the function of DotH (Watarai et al., 2001). Here we report that the lipoproteins DotC and DotD play a critical role in targeting DotH to the outer membrane. At present, we have been unable to prove a direct biochemical interaction between DotH and the lipoproteins due to an inability to solubilize DotH in any detergent other than harsh ionic detergents such as sodium dodecyl sulphate. However, the increased resistance of DotC and DotD to extraction by TX-100 in the presence of only DotH is consistent with the lipoproteins directly interacting with DotH in the outer membrane.
The requirement of a lipoprotein for proper localization of DotH is reminiscent of secretin proteins found in type II and type III secretion systems (T3SS). Secretins are multimeric outer membrane proteins that form channels in the outer membrane, presumably to allow substrates to be transported out of the cell (Bayan et al., 2006). Similar to DotH, many secretins require a lipoprotein called a ‘pilotin’ that assists in the outer membrane insertion and oligomerization of the secretin. Although DotH does not have homology to the secretin family, this feature of membrane insertion may be a trait common to these diverse secretion systems. Consistent with this idea, the A. tumefaciens VirB9 protein is present in the outer membrane, where it is covalently linked to the lipoprotein VirB7 (Anderson et al., 1996; Spudich et al., 1996; Baron et al., 1997; Christie et al., 2005). An analogy of VirB9 and VirB7 to secretins and pilotins has also been made (Christie et al., 2005). However, it is not known if VirB9 requires VirB7 for outer membrane insertion because VirB9 is not stable in the absence of VirB7. The requirement of two lipoproteins for insertion of an outer membrane protein is unusual, although MxiD, the Shigella T3SS secretin, also requires two lipoproteins for its proper outer membrane association (Schuch and Maurelli, 2001).
In conclusion, we have provided evidence for the existence of a Dot/Icm subcomplex that consists of the outer membrane protein DotH, the outer membrane lipoproteins DotC and DotD, and the inner membrane proteins DotG and DotF. This subcomplex is likely to be assembled in three steps (Fig. 8). First, DotC and DotD mediate the outer membrane insertion of DotH. Second, DotF and DotG insert in the inner membrane and interact. Finally, DotF and DotG interact with the outer membrane subcomplex of DotC/DotD/DotH. Similar to the L. pneumophila T4SS, the A. tumefaciens T4SS has been proposed to contain a core subcomplex that consists of the inner membrane protein VirB8, the membrane spanning protein VirB10, and the outer membrane proteins VirB9 and VirB7. By comparison, DotF may have a role similar to VirB8, and DotG may transduce energy from the ATPases at the cytoplasmic membrane to the outer membrane, similar to the role of VirB10. The DotC/DotD/DotH outer membrane complex may form the outer membrane pore, similar to VirB7/VirB9, which are thought to form a similar role in Agrobacterium. Future characterization of the L. pneumophila subcomplex should reveal important insights into how this T4SS assembles and functions in substrate translocation.
Experimental procedures
Strains and media
All strains utilized in this study were derived from strain Lp02, a streptomycin-resistant, restriction deficient, thymidine auxotroph derived from the L. pneumophila clinical isolate Philadelphia 1 (Berger and Isberg, 1993). All strains were grown on ACES-buffered charcoal yeast extract agar (CYE) or in ACES buffered yeast extract broth, supplemented with thymidine (100 μg ml−1), kanamycin (30 μg ml−1), chloramphenicol (5 μg ml−1) or sucrose (5%) as necessary. Deletion strains were made using the suicide plasmid pSR47S (Merriam et al., 1997). Construction of suicide plasmids is described in Appendix S1, Table S2 and S3.
Immunoblot analysis
Samples were boiled for 5 min in Laemmli sample buffer and separated by SDS-PAGE, followed by transfer to PVDF membranes. Membranes were blocked in BLOTTO (PBS containing 5% non-fat dry milk), washed with wash buffer (PBS containing 0.05% Tween 20) and incubated for 1 h with antibody diluted in BLOTTO. Blots were then washed with wash buffer followed by incubation with secondary goat anti-rabbit or goat anti-mouse antibody conjugated to horseradish peroxidase (Sigma) diluted 1:10 000 in BLOTTO. Blots were subsequently washed with wash buffer prior to development using an ECL detection kit (Amersham Biosciences).
Protein fractionations using Triton X-100 solubility
Late exponential phase cultures of L. pneumophila were fractionated as described (Buscher et al., 2005) with minor modifications. Cells were resuspended in lysis buffer (50 mM Tris pH 8) with protease inhibitor cocktail (Sigma) and lysozyme (0.2 mg ml−1) prior to lysis by French press (14 000 PSI). Lysates were then centrifuged 10 min at 10 000 g to remove unlysed cells. Membrane proteins were separated from cytoplasmic proteins by ultracentrifugation at 100 000 g for 60 min. Inner membrane proteins were solubilized by incubation in buffer containing 50 mM Tris (pH 8), protease inhibitor cocktail, 20 mM MgSO4 and 1% Triton X-100, followed by ultracentrifugation at 100 000 g for 60 min to pellet outer membrane proteins. Triton X-100 insoluble proteins were resuspended in 1 × Laemmli sample buffer. All other fractions were diluted with 2 × sample buffer prior to analysis by SDS-PAGE and Western blotting. All samples were loaded proportionally. The quality of the fractionations was assessed by Western blotting using antibodies recognizing the cytoplasmic protein isocitrate dehydrogenase (ICDH), the inner membrane type I signal peptidase LepB, and the outer membrane protein MomP.
Sucrose density gradient ultracentrifugation
Separation of inner and outer membrane proteins by sucrose density gradient ultracentrifugation was performed as described (Osborn and Munson, 1974). Total membrane proteins were suspended in 25% sucrose (w/w) containing 5 mM EDTA, pH 8.0. Membrane samples were then layered on a 30–55% sucrose gradient and centrifuged at 170 000 g for 20 h at 4°C. Fractions were collected from the bottom of the gradients and analysed by SDS-PAGE followed by Western blotting. Separation of inner and outer membrane proteins was assessed by monitoring separation of the outer membrane protein MomP and the inner membrane protein LepB.
DotH depletion/induction
For DotH depletion experiments, strain JV5507 (ΔdotH + inducible dotH plasmid) was grown to early exponential phase in the presence of the inducer IPTG (100 μM). Cells were then collected by centrifugation and resuspended in prewarmed medium without IPTG and allowed to continue growing. Samples were collected immediately after resuspension (time 0) and each hour after. Prewarmed medium was added at 1 h intervals to maintain the culture in early exponential phase. Cells collected at each time point were fractionated by Triton X-100 solubility to localize the DotH, DotC and DotD proteins. For DotH induction experiments, strain JV5507 was grown to early exponential phase in the absence of IPTG. A sample of the culture was collected immediately prior to the addition of IPTG (100 μM) and each hour after induction. Prewarmed medium containing IPTG (100 μM) was added every hour to maintain the culture in early exponential phase. Cells collected at each time point were fractionated as above.
Acknowledgements
We thank Dr Ralph Isberg for DotF, DotH, DotI and DotO antibodies and Dr Abraham Sonenshein for the ICDH antibody. We also thank Drs Stephen Beverley, Wyndham Lathem, Jessica Sexton, and David Sibley for useful suggestions and critical appraisal of this manuscript. This work was funded by NIH Grant AI48052 to J.P.V.