tRNAGlu wobble uridine methylation by Trm9 identifies Elongator's key role for zymocin-induced cell death in yeast
Summary
Zymocin-induced cell death in Saccharomyces cerevisiae requires the toxin-target (TOT) effector Elongator, a protein complex with functions in transcription, exocytosis and tRNA modification. In line with the latter, trm9Δ cells lacking a tRNA methylase specific for wobble uridine (U34) residues survive zymocin and in excess, the Trm9 substrate tRNAGlu copies zymocin protection of Elongator mutants. Phenotypes typical of a tot3/elp3Δ Elongator mutant are absent from trm9Δ cells but copied in a tot3Δtrm9Δ double mutant suggesting that Elongator acts upstream of Trm9. Consistent with Elongator-dependent tRNA modification being more important to mRNA decoding than Trm9, SUP4 and SOE1TRNA suppressors are highly sensitive to loss of Elongator and tRNA U34 hypomodification. As Trm9 overexpression counteracts the effect of high-copy tRNAGlu, zymocin suppression by high-copy tRNAGlu may reflect tRNA hypomethylation of trm9Δ cells. Thus, Trm9 methylation may enable recognition of tRNA by zymocin, a notion supported by a dramatic reduction of tRNAGlu levels in zymocin-treated cells and by cytotoxic zymocin residues conserved between bacterial nucleases and a tRNA modifying GTPase. In sum, Trm9 is a bona fideTOT pathway component whose methylation may be hijacked by zymocin to target tRNA function and eventually, mRNA translation.
Introduction
The trimeric (αβγ) zymocin complex from Kluyveromyces lactis imposes a cell cycle block on Saccharomyces cerevisiaeThat prevents G1 exit and budding (Stark and Boyd, 1986; Butler et al., 1991a; Schaffrath and Meinhardt, 2005). Based on conditional expression, toxicity resides within zymocin's smallest γ-subunit (γ-toxin) and screens for growth in the presence of exo-zymocin or endogenously expressed γ-toxin have distinguished non-target (class I) from toxin-target (class II) kti (K. lactistoxin-insensitive) mutants in S. cerevisiae (Butler et al., 1991b; 1994). Analysis of class I resistance has revealed that zymocin docking requires the chitin synthesis gene KTI2/CHS3, a notion congruent with in vitro exo-chitinase and chitin-binding capacities of holo-zymocin (Butler et al., 1991c; Jablonowski et al., 2001a). Upon cell surface recognition, γ-toxin potentially becomes activated by plasma membrane H+-ATPase, the KTI10/PMA1 gene product, and enters the cytosol in a manner dependent on sphingolipid synthesis gene KTI6/IPT1 (Mehlgarten and Schaffrath, 2004; Zink et al., 2005).
As for the intracellular toxin target process (TOT), 10 class II KTI genes imply a complex response pathway or a multifactorial target (Butler et al., 1994). Indeed, analysis of tot (γ-toxin target) mutants suggests that TOT overlaps with Elongator, a hexameric protein complex with multiple roles in RNA polymerase II (pol II) transcription, exocytosis and tRNA modification (Otero et al., 1999; Frohloff et al., 2001; Jablonowski et al., 2001b; Winkler et al., 2001; Li et al., 2001; Huang et al., 2005; Rahl et al., 2005). Consistently, Elongator inactivation and removal of Kti11–14, Sit4, Sap185 and Sap190, collectively Elongator-relevant proteins, nullify zymocin (Frohloff et al., 2001; 2003; Jablonowski et al., 2001b,c; 2004; Fichtner et al., 2002a,b; 2003; Fichtner and Schaffrath, 2002; Mehlgarten and Schaffrath, 2003). Based on pleiotropic, hypersensitive phenotypes to various stresses, kti and tot mutations identify an essential role for Elongator in stress tolerance, while under standard conditions, Elongator is dispensable for life (Otero et al., 1999; Frohloff et al., 2001; Jablonowski et al., 2001b). tot-phenotype induction as a result of Elongator hyperphosphorylation in cells that lack or deregulate Sit4 phosphatase activity implies that Elongator function may be subject to phosphoregulation (Jablonowski et al., 2001c; 2004). In line with a transcriptional role, Elongator assists pol II in vitro by virtue of its histone acetylase (HAT) activity and associates with unspliced mRNA transcripts (Wittschieben et al., 1999; Winkler et al., 2002; Kim et al., 2002; Gilbert et al., 2004).
In addition, biochemical and genetic evidences connect novel Elongator functions with cytoplasmic roles in exocytosis regulation and tRNA modification (Rahl et al., 2005; Huang et al., 2005). As for the latter, uridine modification at the tRNA wobble position (U34) requires holo-Elongator and Kti11–Kti13 (Huang et al., 2005). Hence, Elongator mutants abolish anticodon U34 modification in at least 11 tRNA species, a scenario likely to cause mistranslation and pleiotropic tot-phenotypes observable in Elongator-minus cells (Huang et al., 2005). Indeed, Elongator mutants no longer promote UAA stop codon read-through by SUP4, a mutant tRNATyr with a G34 to U34 replacement, suggesting that Elongator impacts anticodon/codon interaction and mRNA decoding (Huang et al., 2005).
Here, we show that, in excess, tRNAGlu suppresses zymocin. Intriguingly, zymocicity requires Trm9, a tRNA methylase specific for U34 methylation of the Elongator substrates tRNAGlu and tRNAArg (Kalhor and Clarke, 2003). Class II kti1 mutants are rescued by a wild-type TRM9 allele and trm9Δ cells survive zymocin as efficiently as tot-mutants. However, trm9Δ cells fail to express phenotypes typical of Elongator and epistasis analysis places Trm9 downstream of Elongator's role in tRNA modification. Hence, Trm9 and tRNAGlu identify the importance of tRNA function for zymocin-induced cell death, a notion supported by a dramatic decline of tRNAGlu levels upon zymocin treatment. In sum, our data suggest that the TOT effector role of the Elongator complex enables zymocin to recognize the Trm9 methylase substrate tRNAGlu. Eventually, this may cause cessation of mRNA translation and cell death.
Results
tRNA methyltransferase Trm9, a novel zymocin sensitivity determinant
In addition to toxin resistance, Elongator and class II kti11–13 mutants abolish tRNA wobble uridine (U34) modification (Huang et al., 2005). Studying the zymocin responses of additional tRNA modification mutants (mod5Δ, trm2Δ, trm7Δ or trm9Δ), solely trm9Δ cells survived exo-zymocin in killer eclipse and toxin-plate assays (Fig. 1A). Survival of trm9Δ cells was indistinguishable from a tot3Δ/elp3Δ mutant lacking Elongator subunit 3 (Fig. 1A) and like the latter, trm9Δ cells resisted GAL1-driven expression of intracellular γ-toxin, a scenario deadly for TRM9 cells (Fig. 1B). Related with Elongator-dependent tRNA U34 modification, the TRM9 gene product methylates U34 of tRNAGlu and tRNAArg, two Elongator-targets (Kalhor and Clarke, 2003; Huang et al., 2005). In search for the class II gene KTI1 (Butler et al., 1994), TRM9 complemented kti1 cells in single-copy and restored toxin sensitivity (Fig. 1C). Together with kti1 trm9Δ diploids resisting zymocin just as kti1 and trm9Δ haploids and 4/0 (zymocin-resistant/-sensitive) spore segregation (not shown), we conclude that KTI1 and TRM9 are allelic. In sum, shared roles for Elongator and Trm9 in zymocicity underline that the tRNA methylase is zymocin-relevant.
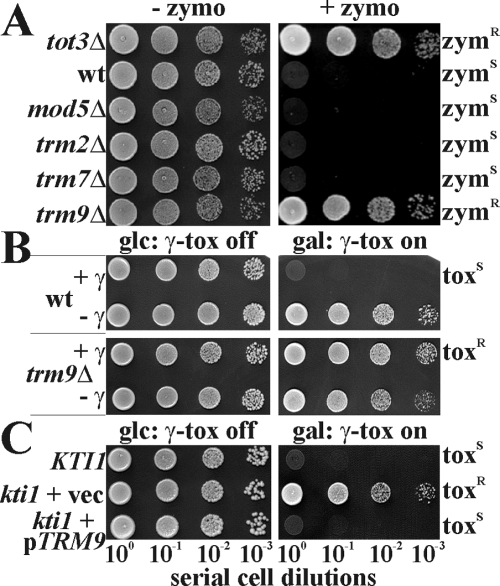
Disruption of TRM9/KTI1 copies class II zymocin resistance of Elongator mutants. A. Yeast killer toxin plate assays. Zymocin sensitive wild-type strain (wt: BY4741) and the resistant Elongator mutant (tot3Δ: Y02742) were tested with the indicated tRNA modification mutants on 60% (v/v) zymocin (+zymo) and control (–zymo) plates. Lack of growth indicates sensitivity (zymS), growth equals resistance (zymR). B. γ-Toxin assay. The indicated yeast strains transformed with GAL1::γ-toxin vector pHMS14 (+γ) or pHMS22 control (–γ) were spotted onto glucose (glc) and galactose (gal) medium. Gal+ phenotypes distinguish γ-toxin resistance (toxR) from sensitivity (toxS). C. TRM9 complements kti1 cells. Mutant kti1 (ARB1) cells carrying YEplac111 (vec) or YEplac111-TRM9 (pTRM9) were tested against γ-toxin expression (see B) and compared with parental KTI1 cells (LL20).
Epistasis places Trm9 downstream of Elongator
Toxin resistance of trm9Δ and tot3Δ cells suggests linkage between Elongator, Trm9, tRNA and zymocin. As wobble U34 modification of tRNAGlu and tRNAArg is Elongator- and Trm9-dependent (Kalhor and Clarke, 2003; Huang et al., 2005), we assayed tot3Δ Elongator and trm9Δ mutants for phenotypes and genetic epistasis. With the exception of tot3Δ-like sensitivity to calcoflour white (CFW) (Fig. 2A), trm9Δ cells hardly expressed tot-phenotypes, grew normally at 30°C or 37°C and proved caffeine-tolerant (Fig. 2A). trm9Δ cells resisted the antibiotic sparsomycin (Fig. 2A) and, albeit considerably more sensitive than wild type, they coped better than tot3Δ cells with the translation indicator drug anisomycin (Dinman et al., 1997) or with caffeine at 37°C (Fig. 2A). Thus, although less severe than Elongator removal, a TRM9 deletion can prove harmful in combination with thermal and chemical stressors, a notion supported by trm9Δ sensitivity to paromomycin at 37°C (Kalhor and Clarke, 2003). In line with milder phenotypes of trm9Δ cells, two out of 11 Elongator-dependent tRNAs are Trm9 substrates (Kalhor and Clarke, 2003), a hierarchy supported by TOT3-TRM9 epistasis. While a single trm9Δ mutant lacked clear-cut tot-phenotypes, viability of tot3Δ single and tot3Δtrm9Δ double mutants ceased at 39°C and in response to caffeine stress (Fig. 2B). In sum, loss of TRM9 is non-essential and less severe than a TOT3 deletion, notions supported by epistasis that places Elongator upstream of the Trm9 tRNA methylase.
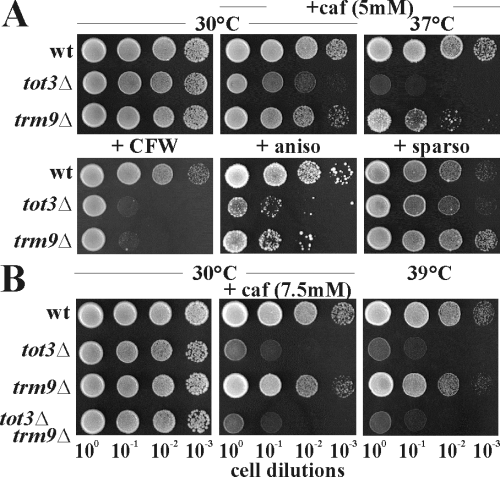
The Trm9 methylase acts downstream of Elongator. A. trm9Δ and tot3Δ phenotypes. Tests for sensitivity to drugs and antibiotics involved serial dilutions of wild-type (wt: LS20), trm9Δ (DJY09) and tot3Δ (FFY3) mutants on YPD medium (control: 30°C) or plates supplemented with caffeine (caf: 7.5 mM), Calcofluor White (CFW: 50 µg ml−1), anisomycin (aniso: 20 µg ml−1) or sparsomycin (sparso: 20 µg ml−1). Thermosensitivity at 37°C was tested in combination with 5 mM caffeine, a condition shown to elicit tot-phenotypes typical of Elongator mutants (Rahl et al., 2005). B. Deletion of the Elongator HAT subunit gene TOT3/ELP3 is epistatic over a trm9Δ null-allele. Phenotypes of trm9Δ (DJY09) or tot3Δ (FFY3) single mutants were compared with a trm9Δtot3Δ double mutant (DJY093). Assays involved sensitivity to 7.5 mM caffeine (middle panel) or at 39°C (right panel).
SUP4 and SOE1 tRNA suppressor genes require Elongator, not Trm9 function
Elongator defects abolish read-through of ochre mutations (ade2–1 and can1–100) by SUP4, a tRNATyr suppressor mutant (Huang et al., 2005). There are γ-toxin resistant tot3/elp3 Elongator mutations that lack typical tot-phenotypes and map outside the HAT-relevant domain B (Wittschieben et al., 2000; Frohloff et al., 2001; Jablonowski et al., 2001b). To study the impact of these Elongator separation of function mutants onto SUP4 suppression, we compared them with zymocin resistant tot3Δ and trm9Δ null-alleles in the SUP4 background (Fig. 3A). trm9Δ cells performed like SUP4 (ade+, canS), while tot3Δ cells antisuppressed SUP4 (ade–, canR) (Fig. 3A). All tot3 mutants abolished SUP4 read-through of can1–100 (canR) whereas SUP4 antisuppression at ade2–1 (ade–) selectively occurred with pDJ9, pDJ10, pDJ12, pFA1 and pFA2 (Fig. 3B). Wild-type pTOT3 suppression (ade+) was copied by pDJ7/pDJ8 and to lesser degrees by pDJ11/pFA3 (Fig. 3B). So, SUP4TRNA suppression is particularly Elongator-dependent, while Trm9 removal hardly plays a role. The failure of less compromized tot3 alleles (pDJ7, pDJ8, pDJ11, pFA3) to impact SUP4 resembles a trm9 minus-like scenario. Indeed, under conditions that induce bona fide tot3Δ phenotypes, these Elongator HAT variants copy wild-type properties and zymocin resistance of trm9Δ cells (Jablonowski et al., 2001b). The tRNA suppressor SOE1 specifies a mutant tRNAGluThat decodes lysine and hence suppresses E-K missense mutations of the deoxythymidylate kinase gene (cdc8–1ts) (Su et al., 1990). As with SUP4, loss of Elongator in SOE1tot3Δ cells antisuppressed SOE1 and caused thermosensitivity at 36°C similar to cdc8–1ts cells on their own (Fig. 3C). As judged from zymocin resistance and thermotolerance at 36°C of a tot3 knock-out in parental CDC8 cells (Fig. 3C), this effect is unlikely due to tot3Δ-induced thermosensitivity. In fact, combined thermo/chemostress (2 mM caffeine at 36°C) was able to elicit the tot3 Elongator defect which was fully complemented by pTOT3, the plasmid-encoded wild-type gene of Elongator subunit 3 (Fig. 3C). Similar to SUP4trm9Δ cells (Fig. 3A), SOE1 suppression was intact in trm9Δ cells at 36°C (Fig. 3C) and slightly affected by 2 mM caffeine at 36°C (Fig. 3C) reinforcing our findings that Trm9 removal can be become harmful in conjunction with chemical and thermal stressors (Fig. 2A). Finally, SOE1 suppression was insensitive to zymocin-protective TOT3 mutations encoded by pDJ7, pDJ8, pDJ11, pDJ12 and pFA3 (Fig. 3C). Collectively, compared with antisuppression of SOE1 and SUP4 by Elongator removal, under unstressed conditions, the methylase Trm9 is dispensable for nonsense or missense suppression by mutant tRNATyr or tRNAGlu. So, Elongator tRNA U34 modification, not Trm9 U34 methylation, impacts mRNA decoding by suppressor tRNAs.
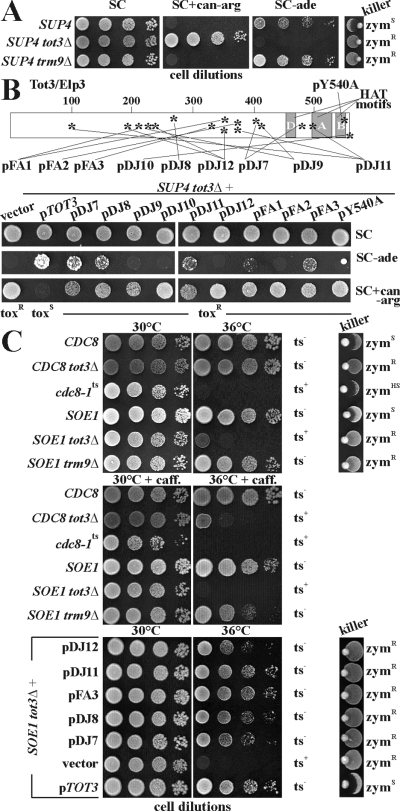
Antisuppression of missense (SOE1) and nonsense (SUP4) tRNA suppressors distinguishes Elongator from trm9Δ defects. A. SUP4 nonsense suppression relies on Elongator, not on Trm9. Dilutions of the indicated strains were tested on SC, SC+can-arg and SC-ade media to check for suppression of the can1–100 and ade2–1 ochre mutations (Huang et al., 2005). Zymocin responses were tested by killer eclipse assays (right panel). Eclipse formation of S. cerevisiae next to K. lactis AWJ137 (killer) indicates sensitivity (zymS). Resistance (zymR) is unique to the SUP4tot3Δ (UMY2916) and SUP4trm9Δ (DJY90) mutants. B. Elongator defects due to mutations in the HAT gene TOT3/ELP3 modulate SUP4 suppression. The SUP4tot3Δ strain (UMY2916) was transformed with vector control (vector), wild-type TOT3 (pTOT3), or the indicated mutagenized TOT3 alleles (pDJ7-12, pFA1-3 and pY540A; for description see Table 2) and tested for SUP4 performance (see A). The tot3 mutations (Jablonowski et al., 2001b) are shown in the Tot3 sketch with HAT domains D, A and B (Wittschieben et al., 2000). C. Trm9 methylase, not the Elongator complex, is dispensable for missense suppression of cdc8–1ts by SOE1. The indicated strains were tested at temperatures permissive (30°C) or restrictive (36°C) for cdc8–1ts viability (Su et al., 1990). Combined thermo/chemostresses [36°C and 2 mM caffeine (caff.)] were to elicit elongator phenotypes (Rahl et al., 2005). Plasmids used for tot3Δ complementation are shown in B. Killer assays (see above) were performed at 36°C. Zymocin hypersensitivity is denoted by zymHSThermotolerance or sensitivity is indicated by ts– and ts+ respectively.
tRNAGlu yields class II toxin resistance in multicopy
We studied the roles of Elongator and Trm9 substrate tRNAs for zymocicity. Among tRNAGly, tRNAArg, tRNALys, tRNAPro, tRNALeu, tRNATyr, tRNAGlu and mutants of the latter two (SUP4 and SOE1) tested, wild-type and mutant tRNAGlu exclusively suppressed zymocin in multicopy (Fig. 4A). In line with previous data (Butler et al., 1994), the specific effect of tRNAGlu was reproduced with distinct tRNAGlu loci (B, E2, E3 and G1) (Hani and Feldmann, 1998) on their own or in combination (Fig. 4A). Consistent with multicopy maintenance, cellular tRNAGlu levels were found to be upregulated in reverse transcription polymerase chain reaction (RT-PCR) studies suggesting that zymocin suppression correlates with tRNAGlu overproduction (Fig. 4B). In contrast, tRNAGly levels and 18S rRNA (RDN18) expression remained unaltered in these multicopy tRNAGlu cells (Fig. 4B). Toxin protection by high-copy tRNAGlu was similar to Elongator defects including resistance to exo-zymocin and intracellular γ-toxin (Fig. 4C). This means that excess tRNAGlu and removal of tRNAGlu modifiers (Trm9 or Elongator) copy class II toxin resistance, collectively evidence for an altered toxin-target process. High-copy tRNAGlu does not mimic Elongator inactivation, as unlike tot3Δ cells, excess tRNAGlu had no impact on tolerance to caffeine or thermostress (Fig. 4C). In sum, based on toxin survival in the absence of tot-phenotypes, high-copy tRNAGlu cells resemble trm9Δ rather than Elongator-minus cells (Fig. 4C). This identifies tRNAGlu as a bona fideToxin response component downstream of the Elongator complex and the tRNA methylase Trm9.
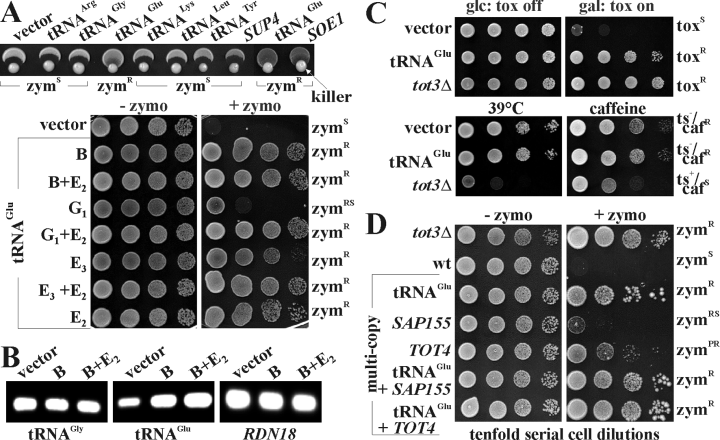
Higher-than-normal levels of tRNAGlu confer class II zymocin resistance. A. The indicated tRNA genes were tested in high-copy for zymocin responses in strain LS20 by eclipse (upper panel) or by zymocin plate assays [lower panel, right: + zymo (50%) (v/v)]. Zymocin-free medium (lower panel, left: – zymo) served as a growth control. Reduced zymocin sensitivity (zymRS) is indicated, for other phenotypes refer to Fig. 1. Distinct tRNAGlu genes are denoted by their respective chromosomal loci (B, E2, E3 and G1). B. Multi-copy tRNAGlu loci B and E3 correlate with upregulated tRNAGlu levels. Total RNA of the indicated cells was prepared and subjected to tRNA and rRNA expression analysis using RT-PCR analysis. Individual tRNAGly or tRNAGlu species and the 18S rRNA gene (RDN18) are shown. C. Excess tRNAGlu protects against intracellular γ-toxin without affecting Elongator. The indicated strains were transformed with GAL1::γ-toxin vector pHMS14 (upper panels) and grown on glucose (glc) or galactose (gal) medium. Gal+ and Gal– equal, respectively, γ-toxin resistance (toxR) and sensitivity (toxS) (see Fig. 1). For tot-phenotypic studies, the strains were incubated at 39°C (lower panel, left) or grown in the presence of 5 mM caffeine (lower panel, right). Tolerance to thermo- (ts–) or chemo-stress (cafR) is distinguished from sensitivity (ts+ or cafR). D. Excess tRNAGlu out-competes zymocin protection associated with Elongator deregulation. Zymocin protection due to overproduction of Tot4 and Sap155, two Elongator-related proteins, is intensified by multicopy tRNAGlu. Zymocin-free (left panel) and -supplemented (right panel) plates were used to assay response modulation in dependency of the indicated multicopy tRNAGlu, SAP155, TOT4 gene dosage or combinations thereof. zymPR stands for a partial resistant phenotype.
Consistent with tRNAGlu modification requiring Elongator and Trm9, zymocin suppression by multicopy tRNAGlu resembled resistance of tot3Δ or trm9Δ cells but was out-competed by higher doses of the toxic compound (not shown). Compared with zymocin suppression by Elongator phospho-deregulation, i.e. multicopy SAP155 (coding for a Sit4 phosphatase subunit) (Jablonowski et al., 2004) or high-copy TOT4 (coding for an Elongator partner) (Fichtner et al., 2002a), the effect of high-copy tRNAGlu was stronger (Fig. 4D). Combined with high-copy tRNAGlu, however, protection by multicopy TOT4 or SAP155 cells almost compared with survival of an Elongator tot3Δ mutant (Fig. 4D). Additive zymocin resistance due to combining tRNAGlu overexpression with Elongator deregulation may mimic tRNAGlu undermodification as observed in Elongator and trm9Δ mutants.
High-copy tRNAGlu can be antagonized by TRM9 overexpression
If the high-copy tRNAGlu effect resulted from tRNA undermethylation, a surplus of tRNAGlu may exceed the methylation capacity of Trm9. To address this, we tested the effects of a conditional GAL1::HA-TRM9 allele towards zymocin suppression by high-copy tRNAGlu. Irrespective of tRNAGlu dosage, glucose-repression of GAL1::HA-TRM9 protected against zymocin and Trm9 depletion copied methylase-minus trm9Δ cells (Fig. 5A and B). In comparison, toxin suppression by tRNAGlu in TRM9 cells was slightly less effective than in glucose-grown GAL1::HA-TRM9 or trm9Δ cells (Fig. 5A). Contrast this with galactose. Here, toxin suppression by high-copy tRNAGlu in TRM9 cells almost entirely vanished upon inducing GAL1::HA-TRM9 by galactose (Fig. 5A and B). Together with the finding that resistance of trm9Δ cells on galactose was non-responsive to tRNAGlu copy number (Fig. 5A), we conclude that the induced GAL1::HA-TRM9 gene antagonizes the effect of high-copy tRNAGlu. This suggests that high-copy tRNAGlu out-competes the methylase and that in excess, Trm9 is capable of restoring tRNAGlu methylation and zymocin sensitivity. So, tRNAGlu suppression by ectopic TRM9 expression reinforces the importance of U34 methylation for zymocicity and strongly suggests that toxin suppression by high-copy tRNAGlu correlates with tRNA undermethylation.
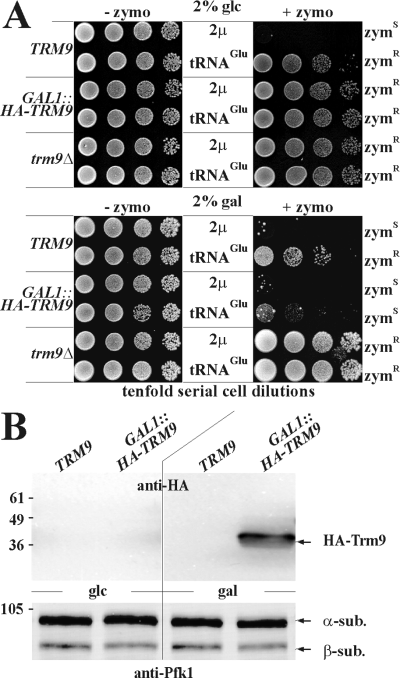
Conditional GAL1::HA-TRM9 expression antagonizes toxin suppression by excess tRNAGlu. A. The indicated strains were transformed with vector only (2µ) or multicopy tRNAGlu plasmid pYF1 and plated onto control medium without zymocin (–zymo) or medium containing 50% (v/v) zymocin (+zymo). Depending on the carbon source supplied, expression of the GAL1::HA-TRM9 allele was repressed on glucose (glc) or induced on galactose (gal). For zymocin phenotypes, see Fig. 1. B. Expression of GAL1::HA-TRM9 is carbon source-responsive. Identical amounts of protein extracts from glucose- (glc) or galactose- (gal) grown TRM9 (LS20) and GAL1::HA-TRM9 (DJYG-H9) strains were immuno-probed in Western blots using anti-HA and anti-Pfk1 antibodies. The latter served as a loading control to follow phosphofructokinase α- and β-subunit expression (arrows).
γ-Toxin residues conserved in nucleases and tRNA modifiers mediate cytotoxicity
In common with a requirement of tRNAGlu for zymocin, colicin-type toxins are known to attack tRNAs and tRNA cleavage causes cessation of amino-acylation and mRNA translation (Tomita et al., 2000). Although bioinformatics failed to show similarity between full-length γ-toxin and these colicin tRNAses, γ-toxin partially aligned with Mycoplasma mycoides and Campylobacter upsaliensis peptides involved in nucleic acid metabolism or modification: exodeoxyribonuclease V (ExoV) α-subunit, ATP-dependent nuclease (AddB) and a tRNA modifying GTPase (TrmE) (Westberg et al., 2004; Fouts et al., 2005; Scrima et al., 2005) (Fig. 6A). Intriguingly, random γ-toxin mutagenesis identified (among others) five cytotoxically relevant substitutions within the addB/ExoV overlap and eight replacements in the TrmE segment (Fig. 6A). Of these, seven affected identical, three conserved and another three non-conserved residues (Fig. 6B). Strikingly, the Q34N, N42S and I45M as well as the R151K, V162A and G164D substitutions map (in pairwise combination) to both the AddB/ExoV and the TrmE overlaps (Fig. 6A and B). On assaying these with other γ-toxin mutants in the GAL1-expression system (Fig. 1) all but wild-type toxin lacked cytotoxic capacity (Fig. 6B). Hence, conserved residues between γ-toxin, AddB, ExoV and TrmE appear to be functionally relevant, a notion largely supported by RT-PCR and Northern studies showing that zymocin-treated wild-type cells undergo a massive decline in cellular tRNAGlu levels (Fig. 6C; not shown) while global tRNA or rRNA expression remains unaffected (Fig. 6C). As expected from the epistasis analysis between trm9Δ and Elongator defects, tot3Δ mutants were found to cause trm9Δ-like tRNAGlu protection in the presence of zymocin (Fig. 6C). Whether this means that Trm9 methylation of Elongator-dependent tRNA species facilitates recognition and cleavage of tRNAGlu by the γ-toxin is currently investigated.
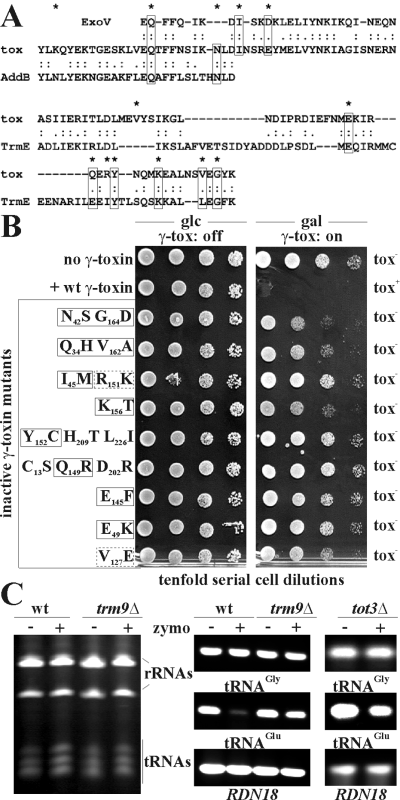
Zymocin impacts tRNAGlu levels and cytotoxicity overlaps with tRNA modifying and nucleolytic proteins. A. Partial alignment (top) between γ-toxin (residues 19–66), exodeoxyribonuclease V (ExoV) α-subunit (residues 124–151) and ATP-dependent nuclease (AddB) (residues 60–86). Partial alignment (bottom) between γ-toxin (residues 114–166) and tRNA modifying GTPase (TrmE) (residues 151–216). Identical residues (‘:’) are distinguished from conserved (‘.’) ones. Cytotoxic γ-toxin residues are indicated by asterisks, conserved ones between ExoV, AddB and TrmE (see B) are boxed. B. Identification of cytotoxic γ-toxin residues by random mutagenesis and cell death assays. Cytotoxicity of the indicated γ-toxin variants (fused to the GAL1 promoter) was assessed in strain LS20 with vector (no γ-toxin) and wild-type GAL1::γ-toxin (+wt γ-toxin) controls. Following glucose-to-galactose shifts, lack of killing activity (tox–) was distinguished from cell death induction (tox+). Cytotoxic γ-toxin residues conserved within the ExoV, AddB or TrmE polypeptides (A) are indicated by solid, non-conserved ones by dashed boxes. Cytotoxic residues that are not marked map to other γ-toxin regions. C. Zymocin reduces tRNAGlu levels. Total RNA of wild type, trm9Δ and tot3Δ cells was prepared in the absence (–) or presence (+) of zymocin (zymo) and subjected to tRNA and rRNA expression analysis using 6% urea/PAGE (left panel) or RT-PCR (right panels). rRNA and tRNA pools are marked in the left panel, while expression of individual tRNAGly or tRNAGlu species and the 18S rRNA gene (RDN18) are shown in the right panels.
Discussion
Elongator-dependent tRNA modification operates prior to tRNA methylation by Trm9
Elongator mutants express zymocin resistance and a defect in U34TRNA modification (Frohloff et al., 2001; Huang et al., 2005). Here, we identify a novel zymocin role for the tRNA U34 methylase Trm9. By several criteria, Trm9 acts downstream of Elongator. First, trm9Δ cells hardly suffer from Elongator-minus phenotypes but behave in many regards (see below) like wild type. Second, a double trm9Δtot3Δ mutant phenocopies a single tot3Δ Elongator mutant implying that tot3Δ is epistastic over trm9Δ. Third, among 11 Elongator-dependent tRNAs, just two (tRNAArg, tRNAGlu) are identified Trm9 substrates (Kalhor and Clarke, 2003; Huang et al., 2005). Finally, with tRNA suppressors (SUP4 or SOE1) being highly sensitive to Elongator-removal, Trm9 plays a minor role in mRNA decoding. In line with Crick's revised wobble hypothesis, U34 modification limits A/G recognition in the third codon position, while U34 hypomodification relaxes anticodon/codon interaction (Lim and Curran, 2001). Hence, mistranslation due to tRNA hypomodification in totΔ cells may cause their previously described pleiotropic phenotypes (Frohloff et al., 2001; Huang et al., 2005). In support, pronounced anisomycin-sensitivity indicates a translational defect and accurate mRNA decoding relies on modified tRNA nucleobases (Yarian et al., 2002). Although non-essential for life, a TRM9 deletion harms viability upon exposure to a combination of thermal and chemical stresses. U34 methylation of tRNAArg plays a role in anticodon/codon recognition (Weissenbach and Dirheimer, 1978) and mild anisomycin-sensitivity of trm9Δ cells suggests a minor effect on translational fidelity. Hence, similar to (but less severe than) tot3Δ cells, a trm9Δ mutant expresses caffeine-sensitivity at 37°C, a phenotype supported by paromomycin-sensitivity at 37°C (Kalhor and Clarke, 2003). Thus, Trm9 tRNA methylation appears particularly relevant under heat shock.
In common with Elongator defects, removal of Trm9 methylase evokes TOT deficiency
Congruent with roles for Elongator and Kti11–13 in wobble U34TRNA modification and zymocin, a TRM9 deletion protects against zymocin and abolishes U34 methylation of tRNAArg and tRNAGlu, two of 11 Elongator-dependent substrate tRNAs (Kalhor and Clarke, 2003; Huang et al., 2005). Resistance to exo-zymocin and intracellular γ-toxin is copied between Elongator-mutants and trm9Δ cells suggesting that they share an altered TOT process. Furthermore, its role in (Elongator-dependent) U34TRNA methylation suggests that Trm9 acts downstream of Elongator in the zymocin pathway. Together with TRM9/KTI1 allelism and a total of nine more class II KTI genes coding for Elongator or Elongator-relevant proteins (Butler et al., 1994; R. Schaffrath, unpubl. data), a robust model has emerged in which Elongator is crucial, but not sufficient per se, for zymocin lethality. Hence, Elongator's role in tRNA modification provides a logical link to tRNA methylation by Trm9 and its requirement for zymocicity (Butler et al., 1994; Frohloff et al., 2001; Kalhor and Clarke, 2003; Huang et al., 2005; Schaffrath and Meinhardt, 2005). In support, multicopy tRNAGlu, a Trm9 substrate, nullifies toxin. Suppression strictly depends on GAA decoding tRNAGlu isoacceptors (tRNAGluUUC). Consistently, tRNAGluCUC (the minor GAG decoding isoacceptor) and 11 tRNA species including tRNAArg, the second Trm9 substrate, showed no effect against zymocin. High-copy tRNAGluUUC is likely to induce tRNAGlu overproduction, a notion supported by increased levels in RT-PCR studies and a previous estimate on fivefold increased tRNAGln levels in high-copy tRNAGln cells (Pure et al., 1985). Provided excess supply of tRNAGlu overloaded the tRNA modification capacities of Elongator or Trm9, a pool of hypomodified tRNAGlu should accumulate. Consistent with tRNA hypomodification in tot3Δ or trm9Δ cells (Kalhor and Clarke, 2003; Huang et al., 2005), this pool of hypomethylated tRNAGlu may protect against zymocin. In support, elevated zymocin doses counteract toxin suppression by high-copy tRNAGlu but remain ineffective towards tot3Δ or trm9 cells. Thus, in contrast to a trm9Δ mutant, a high-copy tRNAGluTRM9 cell still maintains a pool of modified tRNAGlu able to confer zymocin lethality. Our assumption that it is hypomethylated tRNAGlu which abrogates zymocin is supported by the antagonistic effect of a conditional GAL1::HA-TRM9 allele. Its induction on galactose restores zymocicity in high-copy tRNAGlu cells, while Trm9 depletion due to glucose-repression copies trm9Δ-like resistance to zymocin.
tRNAGlu, the zymocin target?
Provided toxin suppression by tRNAGlu reflected its translational role, excess tRNAGlu may correlate with defective synthesis of a protein required for zymocin action. However, the lack of any phenotypes (with the exception of toxin suppression) between multicopy tRNAGlu and wild-type cells, argues against general effects in mRNA decoding. Therefore, we favour an alternative scenario, in which wobble U34 modification of tRNAGlu itself is crucial for zymocin lethality. As a potential target, tRNAGlu may be sequestered or even degraded by the γ-toxin subunit. With a ∼5% genomic frequency of GAA glutamate codons being translated by tRNAGluUUC (Wada et al., 1992), tRNAGluTargeting will certainly affect protein synthesis and compromise cell viability. Similar to mutations of translationally relevant CDC genes, zymocin thus may hijack tRNAGluTo cease cell proliferation, a notion consistent with a G1 block imposed by zymocin (Butler et al., 1991a) and with tRNA targeting as a common strategy employed by microbial toxins or antibiotics. For instance, the aminoglycoside antibiotic neomycin B triggers tRNAPhe cleavage and bacterial colicins act as tRNAses that cleave within tRNA anticodon loops (Tomita et al., 2000; Wrzesinski et al., 2005). As a consequence, tRNA amino-acylation and translation cease (Tomita et al., 2000). Despite any similarity between colicin-type tRNAses and γ-toxin, partial alignments with bacterial polypeptides involved in nucleic acid degradation (AddB, ExoV) or tRNA modification (TrmE) support a potential communication between γ-toxin and tRNA. Notably, our evidence that 10 distinct γ-toxin residues, identical or conserved within the AddB, ExoV and TrmE overlaps, lose cytotoxic capacity upon mutagenesis reinforces this option. Together with our findings that zymocin is capable of inducing a massive decline of tRNAGlu levels in sensitive TRM9 cells while it fails to do so in zymocin resistant trm9Δ cells, tRNAGlu methylation by Trm9 is highly likely to be exploited for recognition by and cytotoxic action of the yeast toxin. It will be vital to assess whether zymocin's lethal strategy (in analogy to E. coli colicin-type tRNAses) is to inhibit mRNA translation via targeting tRNA function (Tomita et al., 2000) or whether, independent of tRNA modification, zymocin may hijack Elongator roles in transcription or exocytosis that involve interactions with RNA polymerase II or GTP exchange factor Sec2 respectively (Otero et al., 1999; Rahl et al., 2005). Although evidence has been provided that RNA polymerase II transcripts and total mRNA levels decrease after zymocin treatment (Frohloff et al., 2001; Jablonowski et al., 2001b), these effects may also be ascribed to toxin-dependent tRNA targeting and dysfunctional translation or to down-stream effects of cell death induction. In fact, as is evident from studies on bacterial transcription factor VirF, reduction of VirF protein at the post-transcriptional level due to translational defects negatively interfered with transcription of VirF-dependent virulence genes (Durand et al., 1997). In line with the notion that Elongator's role in tRNA modification may be particularly zymocin-relevant, tRNAGluUUC is a substrate for both Elongator- and Trm9-dependent U34 modification. Also, in spite of an apparently intact Elongator complex though to be essential for zymocin toxicity (Frohloff et al., 2001; Jablonowski et al., 2001b), trm9Δ cells do survive zymocin. It will be important to study whether translationally relevant mutants other than those compromised in tRNA modification (i.e. Elongator defects and trm9 cells) can support life in the presence of zymocin. This should also dissolve the discrepancy as to whether zymocin targets elongator's role in transcriptional processes or in tRNA functioning.
Experimental procedures
Yeast strains, media, K. lactis zymocin methods and plasmid transformations
Yeast strains used are listed in Table 1. Yeast was grown in routine yeast extract, peptone and dextrose (YPD) or galactose (YPG) media or in synthetic complete (SC) medium (Sherman, 1991). Testing the effects of caffeine (5–7.5 mM), CFW 20–50 µg ml−1, anisomycin (20–40 µg ml−1) or sparsomycin (100 µg ml−1) involved addition to YPD media. Similarly, thermosensitivity was assayed on YPD plates for 2–3 days at individually indicated temperatures. ade2–1 and can1–100 ochre stop codon suppression by SUP4 gene was assayed after Huang et al. (2005). SOE1 missense suppression of the cdc8–1ts allele (Su et al. 1990) involved permissive (25–30°C) and restrictive conditions (36°C). Zymocin sensitivity tests of S. cerevisiae used eclipse assays (Kishida et al., 1996) together with the K. lactis killer AWJ137 (Table 1). Quantitative killer assays used YPD plates supplemented without or with partially purified zymocin from AWJ137 cultures. S. cerevisiaeTester strains were assayed against zymocin [40–75% (v/v)] for 2–3 days at 30°C. Testing γ-toxin sensitivity involved pHMS14, a conditional GAL1-γ-toxin fusion vector (Table 2), and pHMS22, a GAL1-promoter control (Frohloff et al., 2001). Growth was monitored on galactose [2% (v/v)] or glucose [2% (v/v)] SC media for 3–4 days at 30°C. DNA plasmids constructed in this study to analyse (i) TRM9 function, (ii) kti1 complementation, (iii) toxin suppression by high-copy of tRNAs, TOT4/KTI12 and SAP155, (iv) SUP4- and SOE1-dependent tRNA suppressors, and (v) mutant alleles of the γ-toxin gene are listed in Table 2. Standard yeast transformations used the lithium acetate protocol (Gietz et al., 1992).
| Strain | Relevant genotype | Reference/source |
|---|---|---|
| K. lactis | ||
| AWJ137 | αleu2 trp1[k1+ k2+] zymocin producer, killer | K.D. Breunig |
| S. cerevisiae | ||
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| Y02742 | BY4741 but tot3/elp3Δ::kanMX4 | Euroscarf |
| Y07332 | BY4741 but mod5Δ::kanMX4 | Euroscarf |
| Y04054 | BY4741 but trm1Δ::kanMX4 | Euroscarf |
| Y05127 | BY4741 but trm2Δ::kanMX4 | Euroscarf |
| Y03198 | BY4741 but trm7Δ::kanMX4 | Euroscarf |
| Y00559 | BY4741 but trm9Δ::kanMX4 | Euroscarf |
| W303–1A | MATa ade2–1his3–11, 15 leu2–3, −112 trp1–1 ura3–1 can1–100 | T. Davies |
| CY4029 | W303–1A, but SSD1-v1 | K. Arndt |
| LL20 | MATαleu2–3, 112his3–11, 15 can1 | Butler et al. (1994) |
| LS20 | LL20 but ura3 | Frohloff et al. (2001) |
| ARB1 | LL20 but kti1–1 | Butler et al. (1994) |
| FFY3 | LS20 but tot3/elp3Δ::KlLEU2 | Frohloff et al. (2001) |
| DJY09 | LS20 but trm9Δ::Sphis5 | This study |
| DJY093 | LS20 but trm9Δ::Sphis5+tot3/elp3Δ::KlLEU2 | This study |
| W303–1B | W303–1 A but MATα | Huang et al. (2005) |
| UMY2893 | W303–1B but SUP4 | Huang et al. (2005) |
| UMY2916 | W303 but SUP4 tot3/elp3Δ::kanMX4 | Huang et al. (2005) |
| DJY90 | W303 but SUP4 trm9Δ::Sphis5+ | This study |
| 126 | MATαtrp1–289 ura3–52 leu2–3/112 can1 ade1,2 CDC8 | Su et al. (1990) |
| 199 | MATαtrp1–289 ura3–52 leu2–3/112 can1 ADE1,2 cdc8–1ts | Su et al. (1990) |
| 206 | 199 but SOE1 | Su et al. (1990) |
| DJY206-3 | 199 but SOE1 tot3/elp3Δ::KlLEU2 | This study |
| DJY206-9 | 199 but SOE1 trm9Δ::Sphis5+ | This study |
| DJY126-3 | 126 but tot3/elp3Δ::KlLEU2 | This study |
| DJYG-H9 | LS20 but Sphis5+::GAL1::(HA)-TRM9 | This study |
| Plasmid | Description | Source/reference |
|---|---|---|
| pHMS14 | YCp (CEN/HIS3) carrying GAL1::k1ORF4 (γ-toxin gene) | Frohloff et al. (2001) |
| pHMS22 | YCp (CEN/HIS3) carrying GAL1 promoter only | Frohloff et al. (2001) |
| YCplac33/111 | YCp (CEN/URA3/LEU2) cloning vectors | R. Daniel Gietz |
| pLF16 | YCplac111 (CEN/LEU2) carrying GAL1::k1ORF4 (γ-toxin gene) | This work |
| pLFΔγ | YCplac111 (CEN/LEU2) carrying GAL1 promoter only | This work |
| pToxM1 | pLF16 (CEN/LEU2) with γ-toxin allele (N42S, G164D) | This work |
| pToxM2 | pLF16 (CEN/LEU2) with γ-toxin allele (Q34H,V162A) | This work |
| pToxM12 | pLF16 (CEN/LEU2) with γ-toxin allele (E145F) | This work |
| pToxM6 | pLF16 (CEN/LEU2) with γ-toxin allele (Y152C, H209T, L226I) | This work |
| pToxM31 | pLF16 (CEN/LEU2) with γ-toxin allele (K156T) | This work |
| pToxM11 | pLF16 (CEN/LEU2) with γ-toxin allele (C13S, Q149R, D202R) | This work |
| pToxM29 | pLF16 (CEN/LEU2) with γ-toxin allele (I45M, R151K) | This work |
| pToxM33 | pLF16 (CEN/LEU2) with γ-toxin allele (V127E) | This work |
| PToxM25 | pLF16 (CEN/LEU2) with γ-toxin allele (E49K) | This work |
| pTOT3 | YCplac33 (CEN/URA3) with wild-type ELP3/TOT3 allele | Jablonowski et al. (2001b) |
| pFA1 | YCplac33 (CEN/URA3) with tot3 allele (L215P, N352P) | Jablonowski et al. (2001b) |
| pFA2 | YCplac33 (CEN/URA3) with tot3 allele (Y372C) | Jablonowski et al. (2001b) |
| pFA3 | YCplac33 (CEN/URA3) with tot3 allele (L330S) | Jablonowski et al. (2001b) |
| pDJ7 | YCplac33 (CEN/URA3) with tot3 allele (T406S, Y471N) | Jablonowski et al. (2001b) |
| pDJ8 | YCplac33 (CEN/URA3) with tot3 allele (D266N) | Jablonowski et al. (2001b) |
| pDJ9 | YCplac33 (CEN/URA3) with tot3 allele (T228S, E414D) | Jablonowski et al. (2001b) |
| pDJ10 | YCplac33 (CEN/URA3) with tot3 allele (C-terminal extension) | Jablonowski et al. (2001b) |
| pDJ11 | YCplac33 (CEN/URA3) with tot3 allele (A99P, Y372H, D499Y) | Jablonowski et al. (2001b) |
| pDJ12 | YCplac33 (CEN/URA3) with tot3 allele (Y190N, Q238H, S350R) | Jablonowski et al. (2001b) |
| pY540A | YCp (CEN/URA3) carrying elp3/tot3 allele (Y540A) | Wittschieben et al. (2000) |
| YEplac181/195 | YEp (2µ/LEU2/URA3) cloning vectors | R. Daniel Gietz |
| pMA3a | pMA3a (2µ leu2d−1) cloning vector | Mick Tuite |
| pDJ41 | YEplac195 (2µ/URA3) carrying TOT4/KTI12 | Jablonowski et al. (2004) |
| CB2643 | YEp24 (2µ/URA3) carrying SAP155 | Luke et al. (1996) |
| pTRM9 | YCplac111 (CEN/LEU2) carrying TRM9 genomic fragment | This work |
| p511–6 | YCp (CEN/TRP1) carrying mutant tRNAGlu locus G1 SOE1 | Su et al. (1990) |
| pYF1 | YEplac181 (2µ/LEU2) carrying tRNAGlu locus E2 | Butler et al. (1994) |
| pJHW17 | YEplac181 (2µ/LEU2) carrying tRNAGlu locus B | Butler et al. (1994) |
| pARB113 | pMA3a (2µ leu2d−1) carrying tRNAGlu locus G1 | Butler et al. (1994) |
| pARB115 | pMA3a (2µ leu2d−1) carrying tRNAGlu locus E3 | Butler et al. (1994) |
| pSZ10 | YEplac195 (2µ/URA3) carrying tRNAArg locus G3 | This work |
| pSZ11 | YEplac195 (2µ/URA3) carrying tRNAGly locus B | This work |
| pSZ14 | YEplac195 (2µ/URA3) carrying mutant tRNAGlu locus G1 SOE1 | This work |
| pSZ15 | YEplac181 (2µ/LEU2) carrying mutant tRNAGlu locus G1 SOE1 | This work |
| pSZ16 | YEplac195 (2µ/URA3) carrying tRNAGlu locus E2 | This work |
| pDJ82 | YEplac195 (2µ/URA3) carrying tRNALys locus D | This work |
| pDJ84 | YEplac195 (2µ/URA3) carrying tRNALeu locus B2 | This work |
| pDJ103 | YEplac195 (2µ/URA3) carrying tRNATyr locus J2 | This work |
| pDJ105 | YEplac195 (2µ/URA3) carrying mutant tRNATyr locus J2 SUP4 | This work |
Targeted gene disruption and epitope-tagging for protein detection
Generating trm9Δ and tot3Δ null-alleles as well as HA epitope-tagged TRM9 alleles under GAL1-promoter control involved PCR manipulations with template plasmids YDp-KlL, YDp-SpH and pFA6a-TRP1-pGAL1–3HA as described (Longtine et al., 1998; Frohloff et al., 2001; Jablonowski et al., 2001a). In addition, TRM9 disruption or epitope-tagging involved knock-out primers FW-ko-TRM9 (5′-GAG CCA AGA AAT AAA AGG TTA AGA ACC AAC ATG GAG ATA AAC CAA GCG GCC GAC GGC CAG TGA ATT CCC GG-3′) and RV-ko-TRM9 (5′-CAA AAT ACA CTG TCT ACC TAT ATA TCA CCT TCA TCT CTT CTG GGC CAC CAA GCT TGG CTG CAG GTC GAC GG-3′) and N-terminal tagging primers F4-TRM9 (5′-GAA GAA ATG GGA ATA AGA GAA AAA ATT CGG TAA TGA ACT GAA CAG AGA TGG AAT TCG AGC TCG TTT AAA C-3′) and R3-TRM9 (5′-ACT TTG TGA ACA TAC TCC TGT TCT TTT TCA GCC GCT TGG TTT ATC TCC ATG CAC TGA GCA GCG TAA TCT g-3′). Manipulations were verified by PCR using ORF primers specific for TOT3 (Frohloff et al., 2001) or TRM9 (FW-TRM9: 5′-GGA TGG TGT CCA AAG GAC CTT GAG G-3′ and RV-TRM9: 5′-TGG GGG GAA ATT CGT GAT GAC CTG G-3′) and by killer assays to test for biological functionality. Detection of tagged proteins by anti-HA (3F10) (Roche) antibodies was as described (Frohloff et al., 2001). Protein loadings were standardized with an antibody specific for yeast Pfk1 subunits α and β (1:10 000 dilution) (kindly provided by Dr J. Heinisch, University of Osnabrück, Germany).
Random γ-toxin gene mutagenesis
Polymerase chain reaction-mediated mutagenesis of the γ-toxin gene (k1ORF4: Stark and Boyd, 1986) involved the protocol of Jablonowski et al. (2001b). PCR reactions utilized pLF16 (Table 2), a pHMS14 derived GAL1-k1ORF4 gene fusion and primers RMgap1 (5′-TGC TTC CGG CTC GTA TG-3′) and RMgap2 (5′-CTT TCA ACA TTT TCG GTT TGT ATT AC-3′) that anneal, respectively, upstream and downstream of the k1ORF4 ATG and TAA codons. Resultant 0.9 kb PCR fragments were cotransformed with BamHI/HindIII-gapped pLF16 into LS20 cells. In vivo gap repair yielded Leu+Transformants that were assayed on galactose for cell death induction. Inactive γ-toxin alleles identified this way, were recovered from E. coli and retransformed into yeast to prove that loss of toxicity was plasmid-associated. Mutant γ-toxin genes were then sequenced on both DNA strands using primers RMgap1 and RMgap2.
RNA, tRNA and RT-PCR methods
Total RNA was isolated from equal amounts of YPD grown cells using the QIAGEN (Hilden, Germany) RNAeasy midi kit. RT-PCR experiments involved equal amounts of total RNA (1–4 µg) with the RevertAidTM kit (MBI Fermentas) for 1 h at 42°C in 20 µl reaction volumes. After first strand cDNA synthesis, 1/20 of the reaction was subjected to PCR (20 cycles) using Taq polymerase (MBI Fermentas) and oligonucleotide primers (10 µM) (available on request). This amplified DNA fragments that derived from transcription by RNA polymerase I (18 S rRNA gene RDN18) or by RNA polymerase III (tRNAGluUUC and tRNAGlyGCC genes). RT-PCR products were separated on 2% agarose gels and stained with ethidium bromide. Global analysis of rRNA and tRNA involved 6% 7 M urea/PAGE as described (Tomita et al., 2000).
Acknowledgements
We thank Drs A. Byström and B. Huang (Umea, Sweden) as well as M. Stark (Dundee, UK), S. Clarke (Los Angeles, USA), J. Svejstrup (Herts, UK), R. Sclafani (Aurora, USA), J. Heinisch (Osnabrück, Germany) and F. Meinhardt (Münster, Germany) for yeast strains, plasmids and antibodies. We are grateful for technical assistance by R. Zabel. The work has been supported by DFG grants (Scha 750/2 and SFB648). A donation by the Fonds der Chemischen Industrie to R.S. is greatly acknowledged.
Note added in Proof
After our paper had been accepted, Lu et al. reported that zymocin indeed cleaves tRNA that undergoes Elongator- and Trm9-dependent modification (Lu, J., Huang, B., Esberg, A., Johansson, M.J., and Byström, A.S. 2005. The Kluyveromyces lactisγ-toxin targets tRNA anticodons. RNA11: 1648–1654).