Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae
Summary
The transcriptional regulation of membrane fatty acid composition in the human pathogen Streptococcus pneumoniae is distinct from the systems utilized in the model organisms Escherichia coli and Bacillus subtilis. The genes encoding the components of type II fatty acid biosynthesis cluster at a single location within the S. pneumoniae genome, and the second gene in this cluster (SPR0376) encodes a transcription factor (FabT) that belongs to the MarR superfamily. Derivatives of S. pneumoniae strain D39 were constructed that lacked functional FabT. This strain had significantly elevated levels of saturated fatty acids and longer chain lengths than the control strain, was unable to grow at pH 5.5 and had increased sensitivity to detergent. Eliminating FabT function increased the expression levels of all of fab genes with the notable exception of fabM. FabT was purified and bound to the DNA palindrome located within the promoter regions of the fabT and fabK genes within the cluster. The analysis of cells with increased expression of individual genes leads to a model where the physical properties of the S. pneumoniae membrane is controlled primarily by the activity of FabK, the enoyl reductase, which diverts intermediates to saturated fatty acid formation, in contrast to E. coli where FabB, an elongation condensing enzyme, pulls the pathway in the direction of unsaturated acid synthesis.
Introduction
Fatty acid biosynthesis is a vital facet of bacterial physiology and is carried out by a series of enzymatic steps, each encoded by a different gene known as the type II fatty acid synthase (Cronan and Rock, 1996; Rock and Cronan, 1996). Although the paradigm established in Escherichia coli provides a template for understanding the system, there exists considerable diversity within the prokaryotes (Marrakchi et al., 2002a; Cronan, 2003). Although Streptococcus pneumoniae and E. coli both produce the same spectrum of saturated (SFA) and unsaturated (UFA) fatty acids, there are clear differences in the enzymology of the two systems. In E. coli, unsaturated fatty acid synthesis requires the action of two gene products, fabA the essential dehydratase/isomerase and fabB an elongation condensing enzyme (Cronan and Rock 1996; Rock and Cronan, 1996). S. pneumoniae lacks both of these genes (Hoskins et al., 2001; Tettelin et al., 2001), and instead employs a unique enzyme with only an isomerase function encoded by the fabM gene (Marrakchi et al., 2002b). Disruption of the fabM gene in Streptococcus mutans leads to the absence of UFA, and the abnormal fatty acid composition of the mutant membrane results in changes in several membrane-related activities, including increased transporter gene expression and ATPase activity (Fozo and Quivey, 2004b). Another difference is at the enoyl-acyl carrier protein (ACP) reductase step. E. coli uses the NADH-dependent FabI, whereas S. pneumoniae expresses a flavoprotein, FabK (Heath and Rock, 2000; Marrakchi et al., 2003). These differences mean that the branch point in UFA synthesis in S. pneumoniae is located one step downstream from the branch point in E. coli (Marrakchi et al., 2002b). Although it is clear that the interplay between the elongation condensing enzymes FabB and FabF in E. coli control the UFA:SFA and 18:16 carbon fatty acid ratios (Cronan and Rock, 1996; Rock and Cronan, 1996), there is no information on how these parameters are regulated in organisms that contain only a single FabF type of condensing enzyme.
Genetic regulation of the type II system was first established in the E. coli model system. The fabA and fabB genes are tightly regulated by two transcriptional regulators: FadR, the activator, and FabR, the repressor (for reviews, see Lu et al., 2004; Schujman and de Mendoza, 2005). Both factors belong to the TetR superfamily of transcriptional regulators. FadR was discovered as a repressor of the β-oxidation regulon (Overath et al., 1969; DiRusso and Nunn, 1985), and subsequently found to also act as an activator of fabA and fabB transcription (Henry and Cronan, 1991; 1992; Campbell and Cronan, 2001). Thus, FadR has a dual function acting as a repressor of β-oxidation genes and an activator of the two genes required for UFA synthesis (Cronan and Subrahmanyam, 1998). The ligand that controls FadR DNA binding is long-chain acyl-CoA (DiRusso et al., 1992; 1998; Raman and DiRusso, 1995; Cronan, 1997; van Aalten et al., 2000; 2001; Xu et al., 2001). The second E. coli regulator, FabR, was predicted by bioinformatic analysis (McCue et al., 2001), and the regulatory role was confirmed by the construction of a fabR deletion mutant (Zhang et al., 2002). FabR binds to the promoter region of fabA and fabB genes and acts as a transcriptional repressor, and deletion of fabR increases the expression of these two genes leading to an increased level of UFA. Thus, Gram negative bacteria exert combinatorial control over the two key genes that determine the spectrum of fatty acid structures produced by the pathway and the biophysical properties of the membrane.
The situation in Gram positive bacteria is different and more diverse. Like E. coli, the Bacillus subtilis fatty acid biosynthetic genes are scattered throughout the chromosome and this organism has a transcription factor belonging to the same protein family as the E. coli regulators, called FapR, that functions as a global regulator of fatty acid and phospholipid biosynthetic genes (Schujman et al., 2003; Schujman and de Mendoza, 2005). FapR is a repressor and deletion of fapR results in significant upregulation of the target genes leading to membrane compositional alterations and a cold sensitive phenotype. The human pathogen, S. pneumoniae lacks a FadR, FapR or FabR homologue and has a distinctive clustering of the fab genes within the genome coupled with an atypical mechanism for UFA biosynthesis (Marrakchi et al., 2002b) (Fig. 1A). The second gene in the fab cluster, fabT (Fatty Acid Biosynthesis Transcriptional regulator), is predicted to encode a helix–turn–helix DNA binding protein belonging to the MarR superfamily of transcriptional regulators (Sulavik et al., 1995). There are two DNA palindromes located in the promoter regions of fabT and fabK (Fig. 1A). The sequences of these palindromes are very similar, and their location suggests they are binding sites for a transcriptional repressor. The goal of this study was to evaluate the physiological role of FabT in the regulation of UFA production by examining the effect of deleting this transcription factor on gene expression of the fab cluster and membrane fatty acid composition.
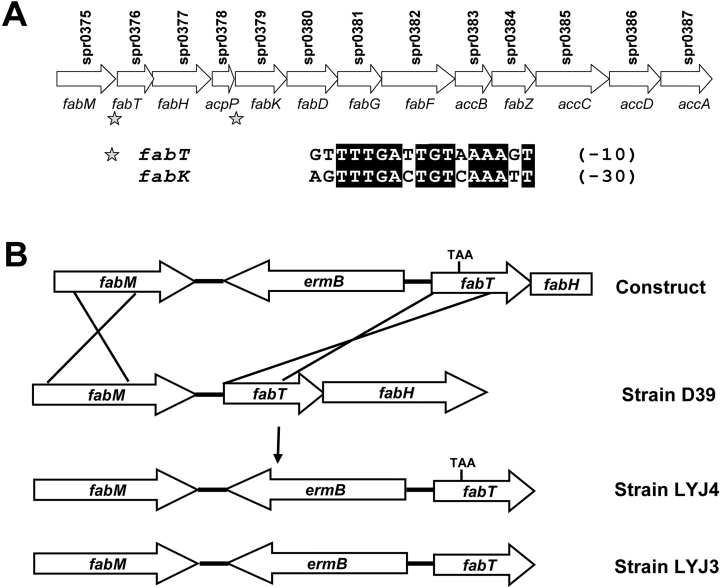
Organization of the S. pneumoniae fab gene cluster and the strategy used to generate the FabT-deficient mutant. A. All the genes required for type II fatty acid biosynthesis are located in a single cluster in the S. pneumoniae genome. The gene names are given below the arrows and their gene designations are above. The asterisks indicate the location of the palindromes identified in the promoter regions of fabT and fabK genes. The alignment of these DNA sequences is shown under the gene organization chart. The numbers in parenthesis are the distances between the palindromes and the ribosomal binding sites in base pairs. B. Strategy used to generate the fabT mutant strain LYJ4. A pUC19 vector containing the ermB gene flanked by 857 bp upstream and 1000 bp downstream genomic sequence containing the missense mutation in fabT (construct) was generated as described under Experimental procedures. Linear DNA was used to transform S. pneumoniae stain D39 and two strains were obtained from screening the recombinants. Strain LYJ4 has the missence mutation in fabT creating a premature stop codon and a strain that lacks the expression of a functional FabT protein, and strain LYJ3 does not (control). Both strains have identical ermB cassettes inserted in the intergenic region between fabM and fabT.
Results
Construction of the fabT mutant in S. pneumoniae
FabT was proposed to have a transcriptional regulatory role in the fab gene cluster based on bioinformatics analysis (Lu et al., 2004) (Fig. 1A), and our approach to testing this hypothesis was to construct a strain lacking a functional FabT protein to examine the effect of the lack of FabT on membrane fatty acid composition and mRNA synthesis from the fab cluster. Our first approach to construct a fabT knockout mutant was simply to replace the fabT gene with an antibiotic resistance cassette. However, we consistently failed to obtain recombinants, although control replacements of nonessential S. pneumoniae genes were routinely obtained. This result suggested that fabT was either an essential gene or a component of a transcriptional unit containing an essential gene. Thus, the transcripts arising from the fabT-fabH-acpP gene cluster were investigated by Northern blot analysis using fabT, fabH and acpP probes (Fig. 2). Two bands at 1.8 kb and 1.5 kb were detected when the blots were developed with fabT and fabH probes. These two bands correspond to the predicted transcript sizes for fabT-fabH-acpP and fabT-fabH. The acpP probe revealed a strong 300 bp band that corresponded to the predicted acpP transcript and a faint 1.8 kb band corresponding to the fabT-fabH-acpP transcript. These data established that fabT and fabH were co-transcribed and suggested that any attempt to replace fabT with an antibiotic resistance cassette would disrupt the transcription of fabH, a gene known to be essential for fatty acid synthesis and cell growth (Revill et al., 2001; Lai and Cronan, 2003). Furthermore, the replacement of fabT with a cassette containing a promoter to support the expression of fabH and downstream genes would compromise the analysis of the role of fabT. The results also illustrate that acpP either has its own strong promoter or has a tertiary structure that stabilized the acpP mRNA. The high steady state level of acpP mRNA was consistent with the fact that this cofactor is one of the most abundant proteins in a bacterial cell (Cronan and Rock, 1996).
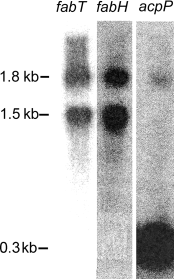
Analysis of the mRNA transcripts derived from the fabT-fabH-acpP gene cluster. RNA samples from exponentially growing strain D39 were isolated and the full coding sequences of fabT, fabH and acpP were used to prepare the 32P-labelled probes. Higher molecular weight species were not detected above the fabT-fabH-acpP transcript indicating that longer transcripts containing these mRNAs either did not exist or were too unstable to detect. Northern blotting was carried out with the individual probes as described under Experimental procedures.
We used the strategy outlined in Fig. 1B to knockout FabT protein function. This approach was designed to maintain the integrity of the fabT-fabH transcript and introduce a missence mutation for a premature stop codon into the fabT gene to prevent the translation of a functional FabT protein. This approach allowed us to determine if fabT is auto-regulated. We inserted the ermB resistance cassette into the fabM-fabT intragenic region for selection, followed by the screening of erythromycin-resistant recombinants by genomic DNA sequencing to identify those recombinants harbouring the stop codon mutation along with the resistance cassette. As anticipated, not all of the recombination events produced strains that contained the insert plus the fabT mutation. This was a distinct benefit to the work, because in addition to have the parental strain D39 was a baseline, this process produced an isogenic control strain that was wild-type for fabT with an identical ermB insertion. The control isolate was named strain LYJ3 and the strain that lacked expression of a functional FabT protein was named strain LYJ4. We were unable to detect a significant difference between strains D39 and LYJ3 in any of the parameters we measured in this work. The introduction of the stop codon in fabT had the potential to interfere with the expression of downstream genes like fabH. However, this was not an issue based on the mRNA measurements by microarray and the specific activity of FabH protein in cell extracts (see below).
Aberrant fatty acid production in the strain lacking a functional FabT
The fatty acid composition of strains D39 and LYJ3 were essentially identical, establishing that the presence of the antibiotic insertion element has little effect on the product distribution of the pathway (Table 1). The 18:1 fatty acid was cis-vaccenate (18:1Δ11), rather than oleate (18:1Δ9), based on gas chromatography analysis with a capillary column described in Experimental procedures. Strain LYJ4 produced significantly more SFA than strain LYJ3. The most pronounced difference was the increase in stearate (18:0) from 4.0% to 26.8% of the total fatty acids with little change in the palmitate (16:0) content leading to a decrease in the 160:18:0 ratio from 5.7 to 0.7. The increase in 18:0 was at the expense of palmitoleate (16:1), and consequently the UFA:SFA ratio changed markedly from 2.6 in strain LYJ3 to 0.9 in strain LYJ4. The chain length ratio (C18:C16) is a third index for evaluating fatty acid composition and this parameter increase from 0.8 to 3.5. We verified the conclusion that the fatty acid compositional changes arose from alterations in de novo biosynthesis by labelling strains LYJ3 and LYJ4 with [14C]acetate. The lipids were extracted and the distribution of label in the fatty acid methyl esters determined by argentation thin-layer chromatography. The UFA:SFA ratio was 2.7 in strain LYJ3 and 0.8 in strain LYJ4, illustrating that the strain lacking a functional FabT protein was deficient in the production of UFA. Also, the 16:1 comprised 25% of the label in strain LYJ3 and only 7.5% of the label in strain LYJ4, demonstrating the deficiency of 16:1 synthesis in strain LYJ4. There was no difference in total acetate labelling between the two strains.
| Fatty acids | S. pneumoniae strains | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D39 (Parent) | LYJ3 (Control) | LYJ4 | LYJ4/pfabM | LYJ4/pfabT | LYJ4/pfabF | LYJ3/pfabT | LYJ3/pfabF | LYJ3/pfabK | LYJ3/pfabKF | LYJ3/pfabM | LYJ3+ Cerulenina | LYJ4+ Cerulenina | |
| 14:0 | 1.3 ± 0.1 | 1.4 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 1.5 ± 0.9 | 0.5 ± 0.0 | 1.6 ± 0.9 | 0.8 ± 0.3 | 4.0 ± 0.1 | 3.4 ± 1.2 | 2.7 ± 0.7 | 17.6 ± 3.7 | 11.1 ± 1.3 |
| 16:0b | 22.3 ± 0.7 | 22.6 ± 0.8 | 19.1 ± 1.9 | 25.1 ± 0.8 | 19.8 ± 5 | 20.6 ± 5.6 | 25.3 ± 2.4 | 20.7 ± 1.1 | 51.8 ± 9.6 | 50.2 ± 1.7 | 28.1 ± 4.4 | 34.2 ± 2.0 | 47.5 ± 1.5 |
| 16:1 | 28.8 ± 5.1 | 31.3 ± 6.1 | 3.0 ± 0.2 | 2.7 ± 0.7 | 24.8 ± 5 | 2.3 ± 0.3 | 24.8 ± 1.3 | 18.2 ± 4.7 | 17.3 ± 6.5 | 16.4 ± 1.7 | 24.8 ± 1.3 | 26.8 ± 1.5 | 22.4 ± 2.7 |
| 18:0 | 4.2 ± 0.3 | 4.0 ± 0.7 | 26.8 ± 3.7 | 30.3 ± 6.3 | 5.6 ± 0.3 | 33.6 ± 5.8 | 4.5 ± 0.3 | 6.6 ± 2.1 | 9.4 ± 3.9 | 10.7 ± 1.5 | 4.5 ± 0.3 | 7.7 ± 3.6 | 6.3 ± 1.8 |
| 18:1 | 43.4 ± 1.6 | 40.7 ± 6.5 | 50.7 ± 5.5 | 40.9 ± 5.1 | 48.8 ± 1.1 | 43.2 ± 8.4 | 43.9 ± 4.9 | 53.7 ± 1.9 | 17.5 ± 7 | 19.4 ± 3.0 | 43.9 ± 4.9 | 13.6 ± 0.4 | 12.5 ± 1.7 |
| UFA:SFAc | 2.6 | 2.6 | 0.9 | 0.8 | 2.8 | 0.8 | 2.2 | 2.6 | 0.5 | 0.6 | 2.2 | 0.7 | 0.5 |
| (16:0):(18:0)d | 5.3 | 5.7 | 0.7 | 0.8 | 4.4 | 0.6 | 5.6 | 3.1 | 5.5 | 4.7 | 6.2 | 4.4 | 7.5 |
| (C18):(C16)e | 0.9 | 0.8 | 3.5 | 2.5 | 1.2 | 3.4 | 1.0 | 1.6 | 0.4 | 0.5 | 0.9 | 0.3 | 0.3 |
- Strains were grown in TY medium buffered at pH 7.0 and the cells harvested when they reached mid-log phase of growth (Table S2). The lipids were extracted, fatty acid methyl esters prepared and the compositions determined by gas-liquid chromatography as described under Experimental procedures. When added, cerulenin was present at 20 µM.
- a . Cerulenin was added at 20 µM.
- b . Number of carbons: number of double bonds.
- c . (16:1 + 18:1)/(16:0 + 18:0).
- d . (16:0)/(18:0).
- e . (18:0 + 18:1)/(16:0 + 16:1).
Studies have established a relationship between membrane biophysical properties, as measured by fatty acid composition, and stress tolerance. Exposing S. mutans to acidic pH (Fozo and Quivey, 2004a,b) and Salmonella enterica (Sampathkumar et al., 2004) to alkaline pH both induced alteration in membrane fatty acid composition. Furthermore, the inactivation of the fabM gene, the essential enoyl-ACP isomerase (Marrakchi et al., 2002b), in S. mutans leads to disappearance of unsaturated fatty acids and the inability to grow at acidic pH (Fozo and Quivey, 2004b). Therefore, we examined the ability of strain LYJ4 to grow at different pH values (Table S2). Strain LYJ4 actually grew slightly faster at pH 7.5 than strain LYJ3 (Table S2). Strains D39 and LYJ3 grew to stationary phase at pH range from 5.5 to 8.5, whereas strain LYJ4 was unable to grow at pH 5.5. The fatty acid composition of strains LYJ3 and LYJ4 were determined at each pH value (Fig. 3). The UFA:SFA ratio in control strain LYJ3 was highest between pH 7.0 and 7.5 and decreased at both high and low pH values (Fig. 3A). Strain LYJ4 had a lower UFA:SFA ratio at all pH values examined, although like the control strain this parameter was highest at pH 7.5. A second parameter measured was the 18:16 carbon ratio (Fig. 3B). This ratio in the control strain LYJ3 was highest at pH 5.5 and lowest at pH 8.5, but there was little change over this range. In contrast, strain LYJ4 had significantly higher levels of 18-carbon fatty acids at all pH values and diverged considerably from the control strain LYJ3 at pH 7.0 and below. Both strains had similar minimal inhibitory concentrations (MICs) for ampicillin and vancomycin. We also examined the sensitivity of strain LYJ4 to detergent lysis and hydrogen peroxide (Fig. S1). Strain LYJ4 was significantly more sensitive to deoxycholate than strain LYJ3 (Fig. S1A). However, strain LYJ4 was not more sensitive than the control to hydrogen peroxide challenge (Fig. S1B). The introduction of the pfabT plasmid into strain LYJ4 restored the strain's deoxycholate resistance to match the control strain (Fig. S1C).
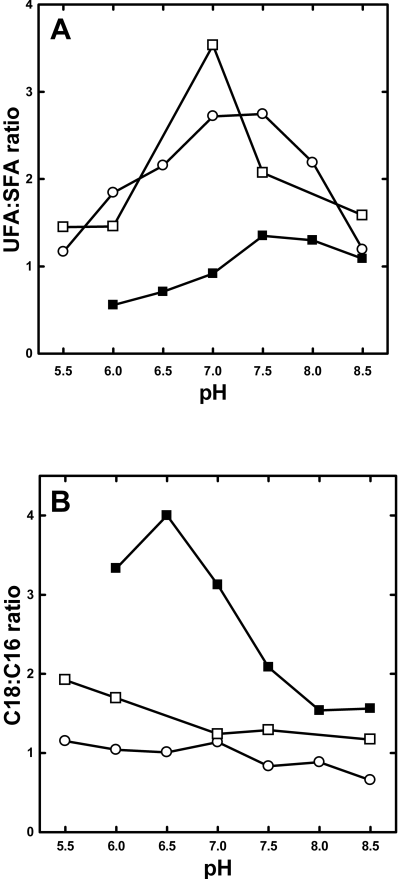
Fatty acid compositions of S. pneumoniae strains grown at different pH values. Strains LYJ3, LYJ4 and LYJ4/pfabT were grown in TY medium buffered at the indicated pH to mid-log phase as tabulated in Table S2. Total lipids were extracted and fatty acid methyl esters were analysed by gas chromatography as described under Experimental procedures and the UFA:SFA (Panel A) and C18:C16 ratios were calculated as described in Table 1. Strain LYJ3, ○; strain LYJ4, ▪; and strain LYJ4/pfabT, □.
We attempted to alter the fatty acid composition of the strains by growth in the presence of exogenous fatty acids. Growth of strain LYJ4 in the presence of 10 µg ml−1 16:1 increased the 16:1 content of the cell from 3% to 6% of the total; however, the UFA:SFA ratio was 1.0. Thus, while exogenous fatty acids are incorporated into S. pneumoniae, their uptake was too inefficient to significantly modify the membrane phospholipid fatty acid composition.
fabT regulation of gene expression
Microarray analysis was carried out to compare the gene expression levels in strains LYJ4 and LYJ3 to obtain a global view of the genes altered by the elimination of FabT. The absence of FabT at pH 7.0 led to the upregulation of 81 genes and downregulation of four genes more than twofold (Table S1). The key result was that all the fab genes, with the exception of fabM, were significantly upregulated (Fig. 4A). These data indicated that FabT functioned as a repressor of the fab cluster genes. Another gene predicted to be involved in membrane lipid biosynthesis, SPR1465, exhibited a 2.7-fold increase in its expression. This gene has similarity to the E. coli 1-acyl-glycerol-3-phosphate acyltransferase (plsC) gene. The other genes that were altered by FabT inactivation encoded hypothetical proteins (27), transport and binding proteins (15), DNA metabolism proteins (8), protein synthesis proteins (7), and other (11) (Table S1). It seemed unlikely that these changes were due to a direct effect of FabT on their promoters. Rather, the expression level changes of non-fab genes may reflect a cellular response to the change in membrane fatty acid composition. Many of the upregulated proteins were transport components whose expression levels may change to compensate for a more rigid, less unsaturated phospholipid composition. This idea is supported by the recent finding that deletion of the fabM gene, which eliminated UFA production in S. mutans, resulted in the upregulation of several ion transport systems (Fozo and Quivey, 2004b).
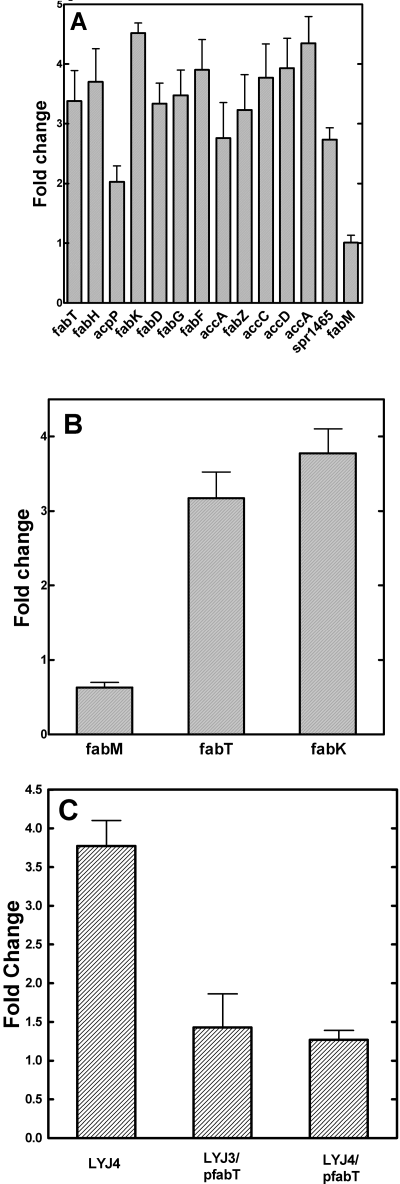
Upregulation of the fab gene cluster in strain LYJ4. A. Gene expression level changes within the fab gene cluster revealed by microarray analysis. Experiments were carried out using RNA samples isolated from three independent growth experiments at pH 7.0, and data from replicate spots on the same array were averaged yielding a total of 12 determinations of the gene expression pattern in each strain. Details of the microarray analysis and statistical treatment are found under Experimental procedures. A complete list of the genes altered in strain LYJ4 is located in Table S1. B. Expression levels of fabT, fabM and fabK mRNA in strains LYJ3 and LYJ4 were determined by RT-PCR. Oligonucleotide primers and probes for RT-PCR were designed with Primer Express 2.0 software, and the experiments were executed as described under Experimental procedures using mRNA isolated from triplicate cultures grown in TY medium at pH 7.0 and harvested when the culture reached mid-log phase (Table S2). The level of gyrA mRNA was used as the internal normalization control. C. FabT overexpression did not suppress fabK expression below that observed in the wild-type strain. Strains LYJ3, LYJ4, LYJ3/pfabT and LYJ4/pfabT were grown to mid-log phase and mRNA extracted. RT-PCR quantification was performed as described in Experimental procedures and the levels normalized to those found in strain LYJ3. The level of gyrA mRNA was used as the internal normalization control.
The mircoarray results were validated by determining the expression changes of the fabM, fabT and fabK genes using quantitative real-time polymerase chain reaction (RT-PCR) (Fig. 4B). These genes lie downstream of the DNA palindrome sequences proposed to bind FabT (Fig. 1A). The RT-PCR results agreed with the microarray data. The levels of fabT and fabK transcripts increased 3.2- and 3.7-fold respectively, whereas the fabM transcript level was not altered. Thus, fabT, like fapR (Schujman et al., 2003) was auto-regulated. We also measured the enzyme specific activity of FabH in extracts from strains LYJ3 and LYJ4. The activity in strain LYJ4 (0.295 pmol min−1 µg protein−1) was twice higher than in strain LYJ3 (0.14 pmol min−1 µg−1 protein). These data show that the amount of protein produced was on the same order as the increase in fabH mRNA measured in the microarray experiments (Fig. 4A).
The temperature-dependent regulation of membrane fatty acid composition is a universal characteristic in bacterial fatty acid synthases (Russell, 1983; Ulrich et al., 1983). Typically, strains that produce straight-chain UFA and SFA contain lower levels of UFA when grown at higher temperatures compared with cells grown at lower temperatures. Strains LYJ3 and LYJ4 were cultured at 30°C, 37°C and 40°C and their fatty acids profiles were determined (Fig. S2). As anticipated strain LYJ4 consistently had lower levels of UFA and higher levels of 18-carbon fatty acids than strain LYJ3. However, the changes in the UFA:SFA and 18:16 carbon ratios in response to temperature were very similar. These data illustrate that the mechanism(s) that control the temperature-dependent changes in fatty acid composition are independent of FabT.
Perturbation of fatty acid synthesis by increased/decreased expression of individual genes
We verified that the alterations in fatty acid composition in strain LYJ4 were due to the absence of a functional FabT by introducing a plasmid into strains LYJ3 and LYJ4 that expressed a His-tagged version of the wild-type fabT gene. This allowed us to confirm the expression of FabT in this strain by Western blotting (not shown), and the His-tag did not interfere with the DNA binding properties of FabT (see below). Expression of FabT in strain LYJ4 (strain LYJ4/pfabT) rescued growth at pH 5.5 and increased the UFA content at all pH values to approximate the UFA:SFA ratio observed in the control strain LYJ3 (Fig. 3A). We did not detect differences in the level of FabT expression as a function of pH (not shown). The C18:C16 carbon ratio in the fatty acid profile was also restored to control values in strain LYJ4/pfabT (Fig. 3B). We conclude from these data that regulation of fatty acid composition by FabT was essential for the growth of S. pneumoniae at low pH, and that the absence of functional FabT was the sole cause of the biochemical and growth phenotypes exhibited by strain LYJ4.
The fabM gene encodes the essential isomerase for UFA formation (Marrakchi et al., 2002b; Fozo and Quivey, 2004b), therefore we tested whether the expression of plasmid-borne fabM in strain LYJ4 (strain LYJ4/pfabM) would elevate UFA and rescue the pH-sensitive growth phenotype. Strains LYJ3 and LYJ4 were transformed with the pfabM plasmid encoding a His-tagged version of FabM, and the expression of FabM protein was verified by Western blotting (not shown). In cells grown at pH 7.0, the UFA:SFA and the C18 : C16 carbon ratios were not significantly altered in strain LYJ4/pfabM compared with strain LYJ4 (Table 1). Neither were these ratios altered in the control strain LYJ3/pfabM expressing excess FabM (Table 1). Also, the expression of FabM did not permit the growth of strain LYJ4 at pH 5.5. We verified that the expressed FabM protein was active by purifying the tagged protein by affinity chromatography and assaying it for isomerase activity using a reconstituted system described by Marrakchi et al. (2002b) (Fig. S3). Thus, increasing the expression level of the fabM gene had little impact on the UFA production in either the presence or absence of a functional fabT gene. On the surface this result appeared counterintuitive, but it means that normal levels of FabM are saturating with respect to the supply of trans-2-acyl-ACP. The overexpression of the fabA isomerase essential for UFA synthesis in E. coli does not increase UFA synthesis either (Clark et al., 1983).
The elongation class of condensing enzymes is one of the most important determinants of membrane fatty acid composition (Cronan and Rock, 1996), therefore we specifically investigated a role for FabF expression in S. pneumoniae. First, we determined the effect of increased FabF expression by introducing a plasmid-borne fabF gene into strains LYJ3 and LYJ4. The increased expression of FabF in strain LYJ3/pfabF elevated the proportion of 18-carbon fatty acids, but did not significantly alter the UFA content (Table 1). FabF expression in strain LYJ4/pfabF did not alter either the UFA:SFA or 18:16 carbon ratios (Table 1). These illustrate that upregulation of FabF alone cannot explain the composition changes engendered by the elimination of the FabT repressor. Second, the levels of FabF were reduced by growing the strains in medium containing cerulenin concentrations that partially blocked FabF activity. Cerulenin is a specific inhibitor of the elongation class of condensing enzymes (Omura, 1976), and the antibiotic covalently attaches to the active site cysteine and irreversibly blocks FabF activity (Moche et al., 1999). We determined the MIC for cerulenin in S. pneumoniae strains LYJ3, LYJ4 and LYJ3/pfabF using a microbroth dilution method. The MIC for strain LYJ3 was 62.5 µM, whereas the MICs for strains LYJ4 and LYJ3/pfabF were 125 µM. These results were consistent with the higher levels of FabF expressed in these two strains either due to the inactivation of FabT or the introduction of pfabF. We used sub-inhibitory cerulenin concentrations (20 µM) to diminish FabF activity and investigated how the reduction in FabF activity influenced fatty acid composition (Table 1). In the presence of cerulenin, strain LYJ3 produced significantly less UFA than in the absence of cerulenin. Also, cerulenin treatment resulted in more 14-carbon fatty acids and less 18-carbon fatty acyl chains, consistent with a reduction in FabF activity. Cerulenin treatment of strain LYJ4 also decreased the chain length of the fatty acid produced, but the UFA:SFA ratio remained low (Table 1). It is thought that the chain length of fatty acids produced in E. coli was regulated in part by the competition between elongation condensing enzymes and the glycerol-phosphate acyl transferase (Cronan et al., 1975), and the fatty acid composition of the cerulenin-treated cells indicates that this mechanism may also operate in S. pneumoniae. These data show that FabF, like the condensing enzymes of E. coli (Garwin et al., 1980; Cronan and Rock, 1996; Rock and Cronan, 1996; Zhang et al., 2002), plays an important role in controlling the degree of unsaturation and chain length of the fatty acids.
The key enzyme examined with this approach was FabK. Increased expression of the fabK gene alone significantly increased the proportion of SFA in the membrane (Table 1). The coexpression of FabK and FabF in the same cell yielded a fatty acid composition similar to FabK expression alone (Table 1), illustrating that factors other than the activity of FabF contribute to the increased chain length in FabT knockout mutants. This factor was most likely the production of malonyl-CoA for chain elongation via the increased expression level of the four acetyl-CoA carboxylase subunits in strain LYJ4 (Fig. 4). These key findings clearly implicated FabK as the major determinant of the UFA:SFA ratio that arise from the deletion of FabT.
In vitro binding of FabT to the fabT promoter palindrome
His-tagged FabT was expressed, purified by nickel-affinity chromatography followed by gel filtration on a Superdex· 200 HR 16/60 column. Its molecular weight was estimated to be 40 kDa using a panel of globular proteins as standards indicating that FabT behaves as a dimer in solution (Fig. 5A). The His-tag was removed by thrombin cleavage (Fig. 5A) and the purified FabT was used in a DNA binding assay with 32P-labelled fabT palindrome (Fig. 5B). The His-tagged version of FabT, however, did bind equally well to the probe (not shown). FabT bound to the probe in a concentration-dependent manner, and the binding was sequence-specific based on competition with the same and unrelated oligonucleotides (Fig. 5C). Unlabelled probe eliminated the gel shift while the same amount of unspecific double-strand DNA molecule of the same length unable to block the shift. These data demonstrate that FabT bound to two promoter regions within the fab cluster, one in front of fabT and the other in front of fabK (Fig. 1A).
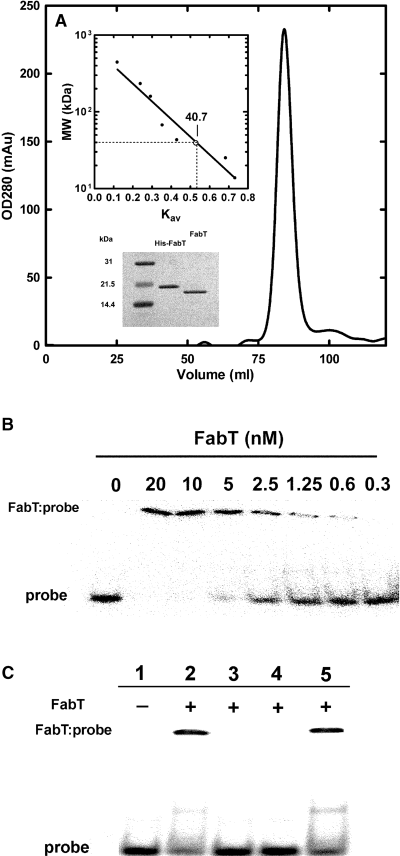
Purification of FabT and its binding to the palindromes in the promoters of fabT and fabK. A. Purification and solution structure of FabT. His-tagged FabT was purified by affinity chromatography and was applied to a Superdex-200 HR 16/60 column equilibrated and eluted by 20 mM Tris-HCl, 100 mM NaCl, 100 mM EDTA, 1 mM DTT. The molecular mass of His-tagged FabT was estimated to be 40.7 kDa based on a standard curve of elution volumes of proteins with known molecular mass (upper inset) indicating that it exists as a dimer in solution. The His-tag was removed by thrombin cleavage to generate the FabT for the DNA binding assays, although we detected no difference between FabT and His-tagged FabT with regard to protein–DNA interactions. Polyacrylamide SDS gel electrophoresis analysis of the purified proteins is shown in lower inset. B. DNA-protein gel mobility shift assays of FabT binding to the fabT DNA palindrome. The fabT probe (0.4 nm) was titrated with increasing concentrations of FabT. The band corresponding to the FabT-probe complex and free probe were indicated. The protein concentrations (based on the dimer molecular weight) are shown above the corresponding lanes. C. DNA competition assay. The complex formed with 0.4 nM 32P-labelled probe and 5 nM FabT (lane 2) was treated with 40 nM unlabelled probe (lane 3), 40 nM of the FabK promoter probe (lane 4), or a scrambled 35 bp double strand of DNA (lane 5). The location of the protein–DNA complex and free probe are indicated. Probe sequences are found in Experimental procedures.
Discussion
The control of membrane fatty acid composition in S. pneumoniae is accomplished by a new version of transcriptional regulation of fatty acid synthesis. FabT belongs to the MarR superfamily and functions as a transcriptional repressor of the entire fab gene cluster, with the notable exception of fabM. The upregulation of fab gene cluster expression by the inactivation of FabT leads to a deficiency in UFA and a marked increase in proportion of 18-carbon fatty acids, particularly stearate, in the membrane. These compositional changes result in an acid-sensitive growth phenotype and decreased resistance to detergent lysis. FabT binds the DNA palindrome located within the promoter regions of fabT and fabK and auto-regulates its own expression. Usually, the location of a transcriptional regulator binding site reflects its function as an activator or a repressor, and the locations of the predicted FabT binding sites in the fabT and fabK promoter regions (−10 and −30 from the ribosomal binding sites respectively) are consistent with its function as a repressor. Analysis of the genomic DNA upstream from the fabM ribosomal binding site reveals the presence of a putative FabT-related sequence (ACTTTGAGTGAAATA) located at −140 bp from the ribosomal binding site, but the function of this region, if any, is unclear. Our microarray and RT-PCR data show that fabM expression is not altered in the strain lacking a functional FabT protein, and thus FabT functions neither as a repressor nor activator of fabM. Similar biosynthetic gene clusters are found in other groups of Gram positive bacteria, such as Enterococcus, Clostridium and Lactococcus. All of these clusters contain a fabT homologue located in the same position within the cluster plus there are highly related DNA palindromes located in the promoter regions of their respective fabT and fabK genes. Thus, FabT regulation of the fab cluster described in this report is likely to be a common feature of these Gram positive bacteria.
Our model for the regulation of UFA synthesis in S. pneumoniae(Fig. 6) assigns an important role for FabK. The regulation of UFA synthesis is envisioned as a combinatorial process involving the activities of the enoyl-ACP reductase (FabK) and the elongation condensing enzyme (FabF). Both enzymatic mechanisms strongly favour the forward reactions reflecting determinant roles of the elongation condensing enzymes (FabB or FabF) and enoyl-ACP reductase (FabI) in initiating new rounds of elongation and completing each round of synthesis in the model type II fatty acid synthase respectively (Heath and Rock, 1995; Cronan and Rock, 1996; Rock and Cronan, 1996). In the elongation condensing enzymes, the release of bicarbonate drives the formation of β-ketoacyl-ACP, and although FabK has not been extensively studied, the large differences in the affinities for NADH and NAD+ in the FabI enoyl-ACP reductase step strongly favour the forward reaction (Heath and Rock, 1995; Sivaraman et al., 2003). In contrast, FabM catalyses the cofactor-independent interconversion of trans-2- and cis-3-C10:1-ACP (Marrakchi et al., 2002b) (Fig. 6). In this respect, FabM is similar to E. coli FabA, which catalyses a similar equilibrium reaction. Therefore, the further utilization for either of these acyl-ACP intermediates depends on the activities of FabK and FabF to pull the reactants towards either UFA or SFA synthesis (Fig. 6). Whereas, FabM carries out the essential isomerization reaction in the formation of UFA (Marrakchi et al., 2002b) and the deletion of the fabM gene eliminates UFA production (Fozo and Quivey, 2004b), increased FabM expression does not alter the UFA:SFA ratio. This somewhat counterintuitive result is consistent with the model (Fig. 6) and is consistent with the conclusion that FabM is saturating with respect to the supply of trans-2-acyl-ACP substrate. It is also reminiscent of a similar finding in the E. coli system where the level of FabA expression, the essential enzyme for olefin formation, is not the determining step in UFA synthesis (Clark et al., 1983). Increased levels of FabK lead to increased SFA production consistent with higher activities of this enzyme pulling the trans-2 intermediate towards the SFA branch of the pathway. On the other side, a decrease in FabF activity brought about by partial inhibition of FabF activity with cerulenin also increases proportion of SFA formed by the pathway due to the diminished capacity of FabF to pull the cis-3 intermediate toward UFA formation. Finally, the observation that increased FabF expression does not increase UFA formation indicates that the basal expression of FabF is saturating with respect to the availability of the cis-3 intermediate. This conclusion is reminiscent of the regulatory network in E. coli where the elongation condensing enzyme, FabB, functions to pull the pathway in the direction of UFA synthesis. Accordingly, the FabR transcriptional repressor in E. coli controls the UFA content primarily by alterations in the expression level of FabB (Zhang et al., 2002). Our hypothesis is that FabT controls UFA synthesis in S. pneumoniae primarily by controlling the level of FabK expression. In E. coli, the competition between elongation by the condensing enzymes and acyl transfer by the acyltransferases is a major determinant of the proportion of 18-carbon fatty acids. Either reduction in acyltransferase activity (Cronan et al., 1975) or an increase in condensing enzyme activity (de Mendoza et al., 1983; Zhang et al., 2002) leads to increased 18-carbon fatty acyl chains in the membrane phospholipids. In our S. pneumoniae analysis, the acyltransferase component(s) has not been addressed. Although there is a gene in S. pneumoniae (SPR1465) that is a homologue of the E. coli 1-acylglycerol acyltransferase (PlsC) and is upregulated 2.7-fold in strain LYJ4, the key first enzyme in phospholipid synthesis, glycerol-phosphate acyltransferase, has not been identified. Filling these gaps in our knowledge of interacting enzymatic partners in membrane phospholipid synthesis in S. pneumoniae will be required to formulate a more complete picture of how membrane fatty acid composition is controlled.
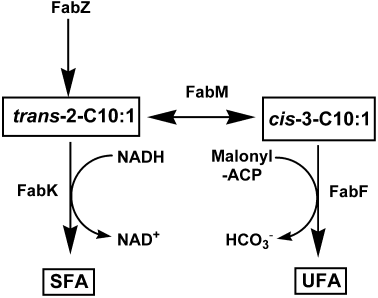
Model for the regulation of the UFA:SFA ratio in S. pneumoniae. FabZ dehydrates the β-hydoxyacyl-ACP intermediates to the trans-2-acyl-ACP. FabM carries out the essential isomerase reaction in the pathway and interconverts the trans-2- and cis-3-C10:1-acyl-ACP. The UFA:SFA ratio is determined by the competition between FabK and FabF for the substrates for the FabM reaction. The FabK enoyl-ACP reductase pulls the pathway towards SFA synthesis by utilizing the trans-2-C10:1 intermediate, whereas FabF pulls the pathway towards UFA synthesis by utilizing the cis-3-C10:1 intermediate.
FabT is not the only regulatory system that impacts on fatty acid composition in S. pneumoniae. YycF (VicR) is an essential response regulator in S. pneumoniae that controls the expression of a wide number of genes including the expression of the fab gene cluster (Mohedano et al., 2005). Enforced overexpression of yycF results in a 1.75–2-fold increase in the fabK-accA segment of the gene cluster and a 1.5–2-fold downregulation of fabT-fabH-acpP operon. The fabM gene was the least affected of the fab genes. However, alterations in fatty acid biosynthetic genes were not detected in cells depleted of YycF where genes whose expression changed twofold or less were excluded from the analysis (Ng et al., 2003). This finding may not be in conflict with the work of Mohedano et al. (2005) who find only a 1.75-fold change for most of the fab genes that were regulated by YycF overexpression. The Mohedano et al. (2005) study identified a potential regulatory site for YycF in the promoter region of the fabT-fabH-acpP operon that may also function as a repressor. The transcriptional changes from YycF overexpression resulted in a modestly altered fatty acid composition with an increase in the proportion of 18-carbon fatty acids including 18:0 and 18:1; however, there was no change in the UFA:SFA ratio. Although YycF is a likely regulator of fab gene expression, the changes in the levels of transcription of the fab genes by this two-component regulator are much less pronounced than we observe with FabT inactivation (Table 1; Fig. 4), thus it is unlikely that alteration in fatty acid composition is one of the essential functions of YycF. Also, regulation by FabT does not explain the temperature-dependent fatty acid compositional changes (Fig. S2), suggesting either the presence of another regulator or that temperature control is an intrinsic property of the expressed enzymes. The latter appears the most tenable hypothesis because temperature regulation in the E. coli model system is an intrinsic property of the elongation condensing enzymes (Garwin and Cronan, 1980; Garwin et al., 1980; de Mendoza et al., 1982; de Mendoza and Cronan, 1983).
A major deficiency in our knowledge is the identity of the ligand(s) that control the DNA binding of the transcription factors that regulate fatty acid synthesis. In the case the FadR activator of E. coli, it is clear that long-chain acyl-CoA is the ligand and the mechanism for regulated DNA binding is known at atomic resolution (DiRusso et al., 1992; 1998; Raman and DiRusso, 1995; Cronan, 1997; van Aalten et al., 2000; 2001; Xu et al., 2001). However, it is not known what controls the DNA binding of either E. coli FabR (Zhang et al., 2002) or B. subtilis FapR (Schujman et al., 2003) repressors. Likewise, we have failed to uncover a ligand for the regulation of FabT in S. pneumoniae, a member of the MarR family of transcription factors. MarR is dimeric winged-helix protein that acts as a repressor of a multidrug resistance operon (Martin and Rosner, 1995), and occupation of the MarR ligand binding site by salicylic acid releases the factor from DNA (Oancea and Meyer, 1998). Thus, it seems likely that FabT DNA binding is also controlled by a small molecule. However, we have analysed the usual suspects in our FabT gel shift assay including various acyl-ACPs, acyl-CoAs, phospholipids, free fatty acids, nucleotides, etc. The most promising candidate would appear to be malonyl-CoA, because the transcription level of the fap regulon correlates with the intracellular level of this intermediate (Schujman et al., 2003). However, the binding of malonyl-CoA binding to the FabT, FabR or FapR repressors has not been directly demonstrated. It is possible that the regulator is not in the lipid biosynthetic pathway, but rather a molecule that functions to co-ordinate the rate of lipid synthesis with other major branches of metabolism, or in the case of S. pneumoniae, perhaps adaptation to growth at low pH.
Experimental procedures
Materials and bacterial strains
Restriction enzymes and other molecular biology reagents were from Promega Life Science, Life Technologies and New England Biolabs. Antibiotics and other chemicals were purchased from Sigma. The S. pneumoniae D39 and E. coli strains that were used in this study and their relevant genotypes are listed in Table 2. S. pneumoniae R6 microarrays were provided by the PFGRC at TIGR (http://pfgrc.tigr.org). DNA sequencing, microarray analysis and oligonucleotide synthesis were performed by the Hartwell Center at St. Jude.
| Stains | Genotype/description | Reference/source |
|---|---|---|
| S. pneumoniae | ||
| D39 | Type 2, virulent, capsulated | Avery et al. (1944) |
| LYJ3 | D39 with an ermB cassette was inserted between fabM-fabT | This study |
| LYJ3/pDL278 | LYJ3 + the empty expression plasmid | This study |
| LYJ3/pfabT | LYJ3 + the fabT expression plasmid | This study |
| LYJ3/pfabM | LYJ3 + the fabM expression plasmid | This study |
| LYJ3/pfabF | LYJ3 + the fabF expression plasmid | This study |
| LYJ3/pfabK | LYJ3 + the fabK expression plasmid | This study |
| LYJ3/pfabKF | LYJ3 + the fabK-fabF expression plasmid | This study |
| LYJ4 | LYJ3 with fabT[A71T, T74A] mutations | This study |
| LYJ4/pDL278 | LYJ4 + the empty expression plasmid | This study |
| LYJ4/pfabT | LYJ4 + the fabT expression plasmid | This study |
| LYJ4/pfabM | LYJ4 + the fabM expression plasmid | This study |
| LYJ4/pfabF | LYJ4 + the fabF expression plasmid | This study |
| LYJ4/pfabK | LYJ4 + the fabK expression plasmid | This study |
| E. coli | ||
| InvαF′ | Strain used to construct all vectors | Invitrogen |
| pET15b-fabT | His-tagged fabT expression vector | This study |
| Plasmids | ||
| pDL278 | E. coli–S. pneumoniae shuttle vector | LeBlanc et al. (1992) |
| pfabT | His-tagged fabT coding sequence in pDL278 | This study |
| Expressed from the DNA polymerase I promoter | ||
| pfabM | His-tagged fabM coding sequence in pDL278 | This study |
| Expressed from the DNA polymerase I promoter | ||
| pfabF | His-tagged fabF coding sequence in pDL278 | This study |
| Expressed from the DNA polymerase I promoter | ||
| pfabK | His-tagged fabK coding sequence in pDL278 | This study |
| Expressed from the DNA polymerase I promoter | ||
| pfabKF | fabK and His-tagged fabF coding sequence in pDL278 | This study |
| Expressed from the DNA polymerase I promoter | ||
Escherichia coli strains were grown in Luria–Bertani (LB) medium. S. pneumoniae strains were grown in Todd–Hewitt broth supplement with 0.5% yeast extract at pH 7.0 (TY medium) for all experiments unless indicated otherwise. Overnight cultures were grown from −70°C glycerol stocks. The following day fresh cultures were started by diluting the overnight cultures 1:100.
Gene targeting strategy for the inactivation of fabT
The gene targeting strategy shown in Fig. 1B was used to generate a fabT stop codon mutation strain of S. pneumoniae D39. The 857 bp fragment upstream of the intergenic region of fabM and fabT was amplified by polymerase chain reaction (PCR) with primers (5′-CCCAAGCTTAGCTGACGCG GTTTGAAGAGATT, a HindIII site is underlined) and (5′-AACTGCAGTTATTTTCCTATAAATTTAGGTC, a PstI site is underlined). The HindIII-PstI digested fragment was cloned into HindIII-PstI site of pUC19. The 1 kb fragment after stop codon of fabM gene was amplified similarly by PCR with primers (5′-CGCGGATCCAAAATCCTTGCATCATTCTTTGA, a BamHI site is underlined) and (5′-CGGGGTACCTAACAA GACACCACCAGCACCATC, a KpnI site is underlined). The amplified PCR product was cloned into PCR 2.1 vector and sequenced. Mutation of Leu25 to stop codon in fabT coding region was performed using QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. Then the BamHI-KpnI digested 1000 bp fragment containing mutated fabT was inserted into the BamHI-KpnI site of the plasmid harbouring the 857 bp upstream fragment. An ermB gene was amplified from plasmid pTCV-lac (Poyart and Trieu-Cuot, 1997) by primers (5′-CGGGATC CGGAAATAAGACTTAGAAGCAAAC, a BamHI site is underlined) and (5′-AACTGCAGCCAAATTTACAAAAGCGACTC, a PstI site is underlined). The BamHI and PstI digested fragment was cloned into the BamHI and PstI site of the plasmid harbouring both the upstream (857 bp) and downstream (1000 bp) PCR fragments, yielding plasmid pUCfabT. This plasmid was digested with AatII and HindIII. The 3.3 kb fragment was purified from 1% agarose gel and used to transform S. pneumoniae D39 in C + Y medium using a standard procedure (Bricker and Camilli, 1999). Erythromycin-resistant clones were selected on tryptic soy agar plates containing 5% sheep blood and 1 µg ml−1 erythromycin (Sigma). The genomic DNA from mutant clones was amplified using primers that annealed outside the DNA region used for mutant construction, and two strains were derived from analysis of the DNA sequence. Strain LYJ3 was fabT wild type with the ermB cassette and strain LYJ4 had an inactivated fabT gene with a missense mutation introducing a premature stop codon and with the ermB cassette in the same location.
RNA analyses of S. pneumoniae strains
RNA samples from exponentially growing strains LYJ3 and LYJ4 were isolated using RNAqueous RNA purification kit (Ambion) essentially as described previously (Haas et al., 2004). Bacteria pellets were dissolved in lysis buffer and shaken for 1 min in the presence of 400 mg of 0.1 mm zirconia-silica beads (BioSpec products) by using a Mini-Beadbeater 3110BX (Biospec). The lysate was centrifuged through a QIAshredder (Qiagen) and processed according to the manufacture's instructions. The full DNA sequences of fabT, fabH and acpP were labelled by Rediprime II kit and 32P-dCTP(Amersham Bioscience). Northern blotting was carried out following standard protocol (Brown and Mackey, 1994). RNA samples from strains LYJ3 and LYJ4 were used for microarray and TaqMan RT-PCR analyses.
Microarray analysis
Microarray experiments were performed by using whole-genome S. pneumoniae cDNA microarrays obtained from the PFGRC at TIGR (http://pfgrc.tigr.org). The S. pneumoniae genome microarray consisted of PCR products representing segments of 2131 open reading frames from S. pneumoniae strain TIGR4 (Tettelin et al., 2001) and 118 unique open reading frames from strains R6 (Hoskins et al., 2001) and G54 (Polissi et al., 1998). Microarray experiments, including RNA quality control, Cy3 and Cy5 dye labelling, hybridization, washing, and scanning, were performed at the Functional Genomics lab, Hartwell Center for Bioinformatics and Biotechnology, St. Jude Children's Research Hospital, by using protocols established by the PFGRC (http://pfgrc.tigr.org/protocols.shtml). RNA samples from both conditions were labelled with monofunctional Cy3 and Cy5 dyes by using an indirect amino-allyl labelling method, combined and hybridized overnight to the printed slides. Slides were washed and scanned by using an Axon 4000B dual channel scanner (Axon) to generate a multi-TIFF image of each slide. Images were analysed by using Axon GenePix 4.1 image analysis software, and the resulting text-data files were imported into Spotfire DecisionSite for Functional Genomics (version 8.0; Spotfire). A series of filtration algorithms were applied to remove spots that consistently generated bad data (based on the frequency with which a particular spot failed to reach a minimum required signal-to-noise ratio and the frequency with which a particular spot was flagged bad by the image analysis software GenePix Pro 4.1). Global normalization was then performed to remove dye-specific bias, and background correction was performed by subtracting the normalized median pixel intensity of the background value from the normalized median pixel intensity of the spot itself. Changes in levels of gene expression were determined by binning Cy5/Cy3 ratios based on logarithm to two (fold changes). As each gene was spotted four times per microarray, only genes whose corresponding spots were not flagged at least 75% of the time were considered.
Statistical analysis of microarray data
Microarray analysis was performed on total RNA isolated from three independent biological replicate experiments and data from the four replicate spots on the same array were averaged. Accuracy and statistical significance of the gene expression differential over the course of the replicate experiments was calculated by using a Student's t-test analysis of variance algorithm available in Spotfire DecisionSite (Kerr and Churchill, 2001). Genes with high levels of significance (P > 0.001) and at least a twofold change were considered up- or downregulated.
Real-time polymerase chain reaction analysis
Oligonucleotide primers and probes for RT-PCR were designed with Primer Express 2.0 software (ABI Prism; PE Biosystems) and made by Hartwell Center at St. Jude. The reverse transcription was performed on total RNA prepared as above after a second step of DNaseI treatment (DNA-free, AMBION). The RT mixture (20 µl) contained 250 ng of total RNA, 10 ng µl−1 random hexamers, 33 units of RNAguard Ribonuclease inhibitor and 20 U µl−1 of Superscript II reverse transcriptase (Invitrogen Life Technologies). Aliquots 1 µl of the reverse transcription reaction were added to the RT-PCR reaction (30 µl) containing 600 nM of each forward and reverse primer and 166 nM of probe. Amplification and detection of specific products was performed with the ABI Prism 7700 Sequence Detection System (PE Applied Biosystems) with the following profile: 1 cycle at 50°C, 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s, and 60°C for 1 min. The critical threshold cycle (CT) is defined as the cycle at which the fluorescence becomes detectable above background and is inversely proportional to the logarithm of the initial template molecules. The CT values were used to calculate the relative number of fabM, fabT and fabK mRNA levels in strains LYJ3 and LYJ4. The quantity of cDNA for each experimental gene was normalized to the quantity of gyrA cDNA in each sample. Each RT-PCR experiment was performed in triplicate aliquots from each of three mRNA samples derived from independent batches of strains grown to mid-log phase (Table S2) at pH 7.0 in TY medium.
Fatty acid composition analysis
Cultures (10 ml) of S. pneumoniae strains were grown to mid-log phase in TY medium adjusted to the appropriate pH with 500 µg ml−1 spectinomycin or 20 µM cerulenin where applicable and harvested by centrifugation. The cell pellet was suspended in 1 ml of water and the lipids were extracted as described by Bligh and Dyer (1959). The lipids were converted to fatty acid methyl esters by the addition of 2 ml of 3% HCl/methanol to the dry extract. The fatty acid methyl esters were identified and quantified using a Hewlett-Packard Model 5890 gas chromatography equipped with a flame ionization detector and a glass column (2 m by 4 mm, internal diameter) containing 3% SP2100 coated on Supelcoport (100/120 mesh) operated at 190°C. We also used the capillary column to analyse the fatty acid composition and distinguish between the Δ9 and Δ11 double positions in the 18-carbon fatty acids. The fatty acid methyl esters were fractionated using a DB-225 capillary column (Agilent Technology, 30 m by 0.536 mm, internal diameter) operated isothermally at 190°C. Fatty acid methyl esters standards were obtained from Matreya.
Acetate labelling
Strains LYJ3 and LYJ4 were inoculated into TY medium at pH 7.0 containing 1 µCi of [2-14C]-acetate and grown until mid-log phase. The cell pellets were washed with fresh TY medium twice and the total cellular lipids were extracted, and converted to methyl esters. Samples were spotted on 20% silver nitrate-impregnated Silica Gel thin-layer plates and developed three time with toluene at −20°C as described (Cronan, 1967). The distribution of radioactivity was visualized with a PhosphoImager screen, and the UFA (16:1 and 18:1 separately) and SFA were quantified using a Typhoon 9200 and Image Quant5.2 (Amersham Biosciences).
FabH and FabM assays
Strains LYJ3 and LYJ4 were grown to mid-log phase (Table S2) and harvested by centrifugation. Cells were lysed with Triton X-100 as described (Jackowski and Rock, 1981), except that 2500 U ml−1 of mutanolysin was used instead of lysozome. The protein concentration of each supernatant was measured and the specific activity of FabH was determined as described previously (Heath and Rock, 1996).
Strains LYJ3/pdl278, LYJ3/fabM, LYJ4/pDL278 and LYJ4/pfabM were grown to mid-log phase and collected by centrifugation. Cells were resuspended in lysis buffer (20 mM Tris, pH 7.9, 0.5 M NaCl) and passed twice through a French Press. After centrifugation at 100 000 g for 1 h, soluble fractions were incubated with Ni-NTA beads for 1 h at 4°C with shaking. The beads were washed twice with 10 ml lysis buffer and 10 ml lysis buffer with 40 mM imidazole. Then the bound proteins were eluted with lysis buffer plus 200 mM imidazole. FabM reaction was carried out as described previously (Marrakchi et al., 2002b). The reaction mixture contained 100 µM ACP, 1 mM β-mercaptoethanol, 0.1 M sodium phosphate buffer, pH 7.0, 100 µM NADPH, 50 µM octanoyl-CoA, 100 µM [2–14C]-malonyl-CoA (specific activity, 52 mCi mmol−1), MtFabH (1.0 µg), EcFabD (1.0 µg), EcFabG (1.0 µg), SpFabZ (1.0 µg) and SpFabF (3 µg) with 2 µl of elute from each sample in a final volume of 40 µl. The activity of FabM was confirmed by the formation of β-OH C12:1-ACP.
Construction of expression vectors
The expression vectors of fabT, fabM, fabF, fabK and fabKF were constructed using gene splicing by overlap extension method (Horton, 1995). The promoter region (−286 to −1) of DNA polymerase I and the coding regions of fabT, fabM, fabK and fabF genes were amplified with primers containing a PstI site at the beginning and a HindIII site at the end, and then ligated into PCR 2.1 vector. The vectors containing insertions were sequenced, the insert was removed by digestion with PstI and HindIII and the resulting fragment was ligated with an engineered pBAD/Myc-His A empty vector (a new EcoRI site was introduced into the downstream of the poly His coding region by site-directed mutagenesis) that was digested with the same enzymes. The resulting pBAD vectors were digested by PstI and EcoRI and the resulting pieces, which contained the DNA polymerase I promoter, the coding region of desired protein with a 6x-His-tag, were ligated into the pDL278 S. pneumoniae expression vector (LeBlanc et al., 1992) to generate pfabT, pfabM and pfabF. The parent plasmid has a copy number of about 20 (Burne et al., 1999). The DNA polymerase promoter was fused with the sequences of the fabK and fabF in an operon. This vector, pfabKF, was generated by the same method using fabK and fabF as template, which resulted in the expression of FabK and His-tagged FabF from the same plasmid. Transformants of these vectors into strains LYJ3 and LYJ4 were obtained in C + Y medium using standard protocol (Bartilson et al., 2001) and selected on tryptic soy agar plates containing 5% sheep blood and 500 µg ml−1 spectinomycin (Sigma). The expression of these proteins in S. pneumoniae was verified by Western blot using anti-His antibody (Santa Cruz) at 1:600 dilution.
Determination of the MIC
The MICs for cerulenin against S. pneumoniae strains were determined by a broth microdilution method. Bacteria were grown to mid-log phase (Table S2) in TY medium at pH 7.0, with 500 µM spectinomycin for plasmid-bearing strains, and then diluted 3000-fold in the same medium. A 10 µl aliquot of the diluted cell suspension (∼330 cfu) was used to inoculate each well of the 96 well plate (U-bottom with low evaporation lid) containing 100 µl of TY medium with the indicated concentration of cerulenin. The plate was incubated at 37°C for 20 h and the optical density was read with a Fusion Universal Microplate Analyzer (Packard, Canada) at 600 nm. The absorbance was normalized with the solvent treated control which was considered as 100% growth.
Detergent and oxidative stress response
Strains LYJ3, LYJ4 and LYJ4/pfabT were grown in TY medium to a density of mid-log phase (Table S2). Then the cells were treated with either 100 µg ml−1 deoxycholine followed by monitoring the absorbance at 600 nm or 20 mM H2O2 followed by plating 10 µl diluted samples on tryptic soy broth–blood agar plates and counting the colonies at 24 h.
Expression and purification of FabT
The S. pneumoniae fabT gene was amplified by PCR to isolate the gene and introduce appropriate restriction sites, and cloned into the NdeI and BamHI sites of the pET-15b expression vector. This vector was sequenced to verify that there were no errors in the fabT gene and then transformed to E. coli Rosetta competent cells for protein expression. The selected transformant was cultured in LB medium with antibiotic (50 µg ml−1 carbenicillin and 34 µg ml−1 chloramphenicol) at 37°C until the OD600 reached 0.6. Isopropyl-1-thio-β-d-galactopyranoside was added to a final concentration of 1 mM, and incubation continued for a further 3 h at 37°C. Cells were collected by centrifugation (6000 rpm, 4°C, 15 min) and cell pellets were lysed with a French Press. Soluble proteins were applied to a Ni2+-agarose column and washed with 40 mM imidazole, 20 mM Tris-HCl, pH 7.4, 0.5 M NaCl. His-tagged proteins were eluted with 200 mM imidazole in the same buffer. Purified FabT proteins were applied to a Superdex 200 HR 16/60 column (Amersham Biosciences) equilibrated and eluted with 20 mM Tris-HCl, pH 7.4, 100 mM EDTA, 100 mM NaCl, 1 mM DTT. The apparent molecular weight of FabT was estimated using globular protein standards. Protein were quantified by the Bradford method (Bradford, 1976), and the purified protein was stored at −20°C. His-tag was removed from His-FabT using a thrombin kit (Novagen) according to the manufacture's protocol. Briefly, His-tagged FabT was treated with 1 U biotinylated-thrombin at 4°C overnight and next day thrombin was removed by incubating with streptavidin beads for 30 min. The tag was removed from FabT by gel filtration.
Electrophoretic mobility shift assays
The custom-synthesized double-stranded synthetic oligonucleotide corresponding to the palindrome in the fabT promoter 5′-TCTGTCAAATGTTTTGATTGTAAAAGTTTTTTGAA was 32P-labelled using a 5′ end-labelling kit (Invitrogen) and [γ-32P]-ATP (Amersham Bioscience). The sequence of the probe was obtained from the promoter region of fabT that contains the predicted FabT binding palindromes underlined in the middle. His-tagged FabT was treated by thrombin kit (Novagen) according to manufacture's protocol and followed by a gel filtration to remove His-tag. During the DNA binding reactions, 32P-labelled probe and FabT were incubated at room temperature for 20 min in binding buffer containing 10 mM Tris (pH 8.0), 50 mM NaCl, 1 mM EDTA, 10% glycerol, and 1 mM dithiothreitol in a volume of 20 µl. In competition experiments using unlabelled probes, 100-fold excess unlabelled fabT or fabK probe and another unspecific 35 bp double-stranded DNA oligonucleotide were added before adding the 32P-labelled probe. The custom-synthesized double-stranded synthetic oligonucleotide in the fabK promoter that contains the predicted FabT binding palindromes underlined in the middle was: 5′-TTAGGTAATAGTTTGACTGT CAAATTATGGTGAAA. This probe was used to compete the binding of 32P-labelled fabT probe to purified FabT. Samples were separated on DNA retardation gels (Invitrogen). The gels were dried and subjected to autoradiography using a PhosphoImager screen.
Acknowledgements
This work was supported by National Institutes of Health Grant GM34496, Cancer Center (CORE) Support Grant CA 21765, and the American Lebanese Syrian Associated Charities. We thank Jack Sublett and Yong-Mei Zhang for help with the Northern blot analysis and Deepak Kaushal for his expert assistance with the microarray analysis.