Nuclear fusion occurs during mating in Candida albicans and is dependent on the KAR3 gene
Summary
It is now well established that mating can occur between diploid a and α cells of Candida albicans. There is, however, controversy over when, and with what efficiency, nuclear fusion follows cell fusion to create stable tetraploid a/α cells. In this study, we have analysed the mating process between C. albicans strains using both cytological and genetic approaches. Using strains derived from SC5314, we used a number of techniques, including time-lapse microscopy, to demonstrate that efficient nuclear fusion occurs in the zygote before formation of the first daughter cell. Consistent with these observations, zygotes micromanipulated from mating mixes gave rise to mononuclear tetraploid cells, even when no selection for successful mating was applied to them. Mating between different clinical isolates of C. albicans revealed that while all isolates could undergo nuclear fusion, the efficiency of nuclear fusion varied in different crosses. We also show that nuclear fusion in C. albicans requires the Kar3 microtubule motor protein. Deletion of the CaKAR3 gene from both mating partners had little or no effect on zygote formation but reduced the formation of stable tetraploids more than 600-fold, as determined by quantitative mating assays. These findings demonstrate that nuclear fusion is an active process that can occur in C. albicans at high frequency to produce stable, mononucleate mating products.
Introduction
Since the identification of a mating type-like (MTL) locus (Hull and Johnson, 1999) led to the discovery of mating in Candida albicans (Hull et al., 2000; Magee and Magee, 2000), significant progress has been made in understanding the regulation and cell biology of the mating process in this organism. One of the most interesting facets of mating in C. albicans is the role of a phenomenon known as white-opaque switching. First observed in the 1980s by Soll and colleagues, the term ‘white-opaque switching’ refers to the ability of certain isolates of C. albicans to switch between growing as white, hemispherical colonies and growing as grey, flat colonies (Slutsky et al., 1987). The ability of a strain to carry out white-opaque switching is governed by the MTL locus: a and α cells can switch, but a/α cells cannot (Lockhart et al., 2002; Miller and Johnson, 2002).
The control of white-opaque switching by the MTL locus makes intuitive sense as opaque-phase cells are the mating competent form of C. albicans; opaque cells mate more than 106 times as efficiently as white-phase cells (Miller and Johnson, 2002). Transcriptional profiling of white-phase and opaque-phase cells has shown that these phases differentially regulate around 400 genes (Lan et al., 2002; Tsong et al., 2003). Several genes implicated in mating are upregulated in the opaque phase, suggesting that opaques are primed to mate. However, approximately one-third of the genes regulated by the white-opaque switch are implicated in metabolism, suggesting that a major role of the white-opaque transition may be to allow C. albicans to adapt to different microenvironments in the host (Lan et al., 2002). It is possible that the white to opaque transition directs mating to a particular host niche.
Mixing of opaque a and α cells was shown to result in polarized cell projections, indicating that cell signalling between mating partners occurs by secreted pheromones, similar to mating in Saccharomyces cerevisiae (Miller and Johnson, 2002; Lockhart et al., 2003a). Several groups recently identified the gene (MFα) encoding the α-pheromone produced by α opaque cells (Lan et al., 2002; Bennett et al., 2003; Lockhart et al., 2003b; Newport et al., 2003; Panwar et al., 2003). Addition of a synthetic peptide corresponding to the mature α-pheromone caused a opaque cells to form mating projections; concomitant with these morphological changes, 60–70 genes were upregulated (Bennett et al., 2003).
Cytological studies of the mating process have been carried out in some detail using natural isolates of C. albicans that are a or α strains. In these studies, several of the mating steps parallel those observed for S. cerevisiae, including the formation of polarized projections in response to mating pheromones, fusion of a and α cells, and subsequent daughter cell budding from the zygote (Lockhart et al., 2003a). Unlike the case for S. cerevisiae, however, the nuclei were not observed to fuse; instead they moved towards each other following cell fusion but then appeared to back away from each other. Typically, only one of the parental nuclei would be inherited by the daughter cell, although sometimes both parental nuclei would enter the daughter cell, only to be segregated from one another in future cell divisions. On the surface, these observations appeared to be at odds with results from other laboratories, where the products of mating – whether from laboratory-derived strains or from clinical isolates – were tetraploid cells with a single nucleus (Hull et al., 2000; Magee and Magee, 2000; Legrand et al., 2004). In these latter studies, mating was scored by selecting for prototrophic mating products that had acquired genetic markers from both parents. Although these mating products were tetraploid in DNA content and mononucleate, many cell divisions had taken place since the initial mating event, and it was difficult to deduce exactly when and with what efficiency nuclear fusion had occurred.
To reconcile the cytological and genetic observations of mating in C. albicans, it was suggested that karyogamy might be a relatively rare occurrence, but one that was simply selected for in the genetic experiments. An alternative explanation held that the different strains used in the cytological versus the genetic experiments differed in their abilities to undergo nuclear fusion (Johnson, 2003; Lockhart et al., 2003a). A third possibility for the seemingly contradictory observations concerns the media conditions used in the mating studies, as these differed between the cytological and genetic studies. It is possible, for example, that growth conditions determine whether nuclear fusion occurs with high or low efficiency.
To address the points raised above, we describe cytological and genetic analyses of mating cells from C. albicans carried out using the standard laboratory strain SC5314 as well as several different clinical isolates. We analysed mating both cytologically and genetically and compared mating on different media. Using a variety of techniques, including time-lapse microscopy, we find that mating and karyogamy can occur efficiently on laboratory media, without the requirement for genetic selection. The efficiency of karyogamy depends both on the strains involved and on the media on which mating is carried out. To demonstrate that nuclear fusion is an active process in C. albicans, we show that the C. albicans KAR3 gene, which encodes a microtubule-based motor protein, is necessary for efficient nuclear fusion as observed cytologically and genetically. These results show that nuclear fusion is part of the mating process in C. albicans and that the Kar3 motor protein is required for this event.
Results
High efficiency of mating in C. albicans on laboratory media
In previous studies, we employed a quantitative mating assay to determine the frequency of mating between C. albicans strains (Miller and Johnson, 2002). In this assay, one mating strain was defective in adenine metabolism while the partner strain was defective in uracil metabolism. Following mixing of these auxotrophic strains, only cells that had mated could grow on medium lacking both adenine and uracil. The frequency of mating was then determined by the fraction of cells able to grow on media lacking both adenine and uracil (the mating products) compared with the number of cells able to grow on media lacking either adenine or uracil (the parental cells). Using this method, it was typically observed that approximately 20% of opaque-phase a and α cells mated on YEPD agar plates during a 6 day incubation period (Miller and Johnson, 2002).
To determine the mating efficiency of opaque-phase cells on a variety of different laboratory media, we performed similar quantitative mating experiments using auxotrophic strains (see Experimental procedures). The results of these experiments are shown in Table 1. Consistent with previous studies, we observed approximately 20% of cells had mated on solid YEPD medium after 4 days of incubation at 22°C, although only 1.6% of these cells had mated after 24 h (Table 1A). Substitution of mannitol for glucose (YEPM plates) increased the mating efficiency more than fourfold in the first 24 h of mating (6.8% of cells now mated) and more than threefold after 4 days (78% of cells now mated). By far, the most efficient mating occurred on spider medium (Liu et al., 1994). As shown in Table 1A, after 24 h of mating on spider plates, 40% of cells had mated and this increased to almost 90% after 4 days. In contrast, synthetic complete media complemented with either glucose (SCD) or mannitol (SCM) produced the lowest levels of mating. The addition of 10% serum to YEPD plates increased the mating efficiency from 1.6% to 7.7% after 24 h, although levels of mating after 4 days were similar in the presence or absence of serum (24% and 21% respectively).
| A | ||
|---|---|---|
| Solid media | 1 day (% mating) | 4 days (% mating) |
| YEPD | 1.6 | 21 |
| YEPM | 6.8 | 78 |
| SCD | 0.14 | 7.3 |
| SCM | 0.2 | 25 |
| Lee’s + D | 6.5 | 79 |
| Lee’s + M | 5.5 | 63 |
| YPD + 10% serum | 7.7 | 24 |
| Spider | 40 | 89 |
| B | |
|---|---|
| Liquid media | 1 day (% mating) |
| YEPD | 0.016 |
| YEPM | 0.47 |
| SCD | 0 (<1 in 10 6) |
| SCM | 0.2 |
| Lee’s + D | 0.055 |
| Lee’s + M | 0.18 |
| YPD + 10% serum | 0.011 |
| Spider | 1.1 |
- A quantitative mating assay (see Experimental procedures) was used to determine the mating efficiency of C. albicans on (A) solid media, and (B) liquid media. The entries in the table show the averages of two mating experiments, one carried out between CHY439 and CHY477, and the other between CHY257 and MMY278. Based on the range of values, we believe that differences greater than threefold represent significant effects of media composition on mating.
We also carried out similar quantitative mating experiments using C. albicans strains grown in liquid culture (see Table 1B). Overall, we observed much lower rates of mating in liquid culture than on solid media (compare Table 1A and B). This may be because the effective concentration of the two mating strains was much lower under our liquid culture conditions than on solid media (see Experimental procedures). However, the general trends in mating efficacy in liquid culture were similar to those observed with mating on solid media, with mating in spider medium the most efficient. Also, media containing mannitol generated more mating products than media containing glucose for all media tested. We also noted that no mating was detected in SCD medium (<1 in 106 cells mated), so that, in liquid, mating is reduced more than five orders of magnitude in this medium compared with spider medium.
These results show that media compositions can affect the efficiency of mating by several orders of magnitude in C. albicans. Previously, Chen et al. (2002) used a semi-quantitative mating assay to look at the mating efficiency of white-phase cells growing for 6 days on several different media. These authors found that mating was completely inhibited on SCD medium but was rescued by substitution of glucose with mannitol. We find that mating is also extremely low between opaque-phase cells on this medium and that substitution of glucose with mannitol also rescues mating efficiency. The most significant finding of our quantitative mating experiments is that a large fraction of C. albicans cells undergo mating on solid spider medium within 24 h (approximately 40% of cells mated). The high efficiency of mating on spider medium has allowed to us to efficiently follow the mating process in C. albicans using cytological methods, as described below.
Identification of mating zygotes
Lockhart et al. (2003a) recently described in some detail the cytological stages of mating using C. albicans strains isolated from patients. As described in Introduction, nuclear fusion was not observed during mating of these strains. To determine whether nuclear fusion occurred in the isogenic laboratory strains used in this study, we examined mating between a and α derivatives of SC5314. We first carried out these experiments in spider medium, as this generated the highest frequency of mating products, as described above. Using the technique of Lockhart et al. (2003a), we labelled a cells with the vital dye rhodamine-conjugated concanavalin A (rhodamine-ConA), which stains cells red, and labelled α cells with the vital dye fluorescein isothiocyanate-conjugated ConA (FITC-ConA), which stains cells green. After 7–24 h of incubation on spider medium, cells were collected and fixed for analysis by fluorescent microscopy. Figure 1 shows cells at different stages of zygote formation. In Fig. 1A and B, mating a and α cells have generated mating projections that have made contact with each other but have yet to undergo cell fusion. Note that the polarized cell growth that occurs after the mixing of the a and α cells is not stained by the vital dyes. In Fig. 1C, the mating projections appear to have fused at the point of contact to generate a zygote. Consistent with previous observations (Lockhart et al., 2003a), cell fusion was not observed between cells of the same mating type. At the later time points, as shown in Fig. 1D–F, fusion between the a and α cells gave rise to a zygote from which a daughter cell budded.
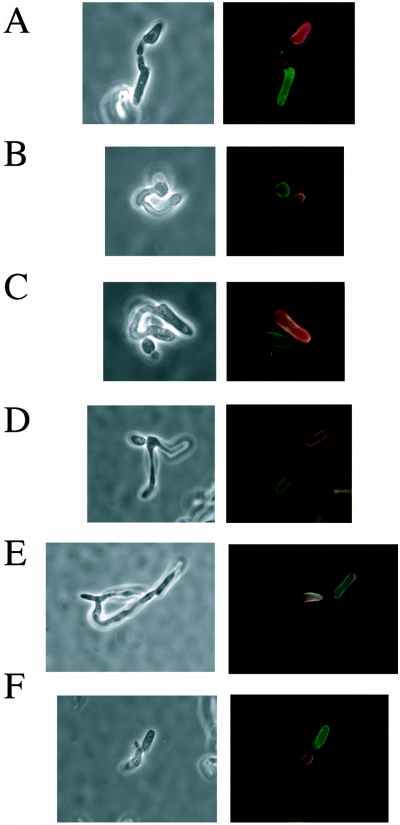
Mating zygotes in C. albicans formed by the fusion of a and α cells. C. albicans SC5314-derived a cells (RBY731) were stained with rhodamine-ConA and α cells (RBY734) were stained with FITC-ConA (see Experimental procedures). Labelled a and α cells were mixed and incubated on spider medium for 7 h (A–C) or 24 h (D–F), before fixing and cytological analysis. Each of the examples includes a phase-contrast image and a fluorescent image (in which the rhodamine and FITC images have been superimposed).
Nuclear positioning in mating cells
To determine the position of the nuclei during mating between the isogenic laboratory strains, we fixed mixtures of mating cells after incubation for 7 h or 24 h on spider medium, and stained the cells using the nuclear dye, 4′,6-diamidino-2-phenylindole (DAPI). Cells at various stages of zygote formation were identified and photographed, as shown in Fig. 2. In the initial stages of zygote formation, the tips of the mating projections meet and fuse, by which time the nuclei have migrated close to the ends of the projections (Fig. 2A). After fusion of the mating cells and before daughter cell formation, the nuclei appeared either to fuse or to superimpose one on the other (Fig. 2B). Although at the resolution of these experiments, it was impossible to distinguish between fusion and superposition, we did observe a number of cells with a single DAPI-staining body (e.g. Fig. 2B), and the simple interpretation is that these single spots represent nuclei that have fused. Subsequent daughter cell budding from the zygote is shown in Fig. 2C, with one nucleus typically in the daughter cell bud and one in the parental fusant. Additional budding generated zygotes with several daughter buds still attached (Fig. 2D)
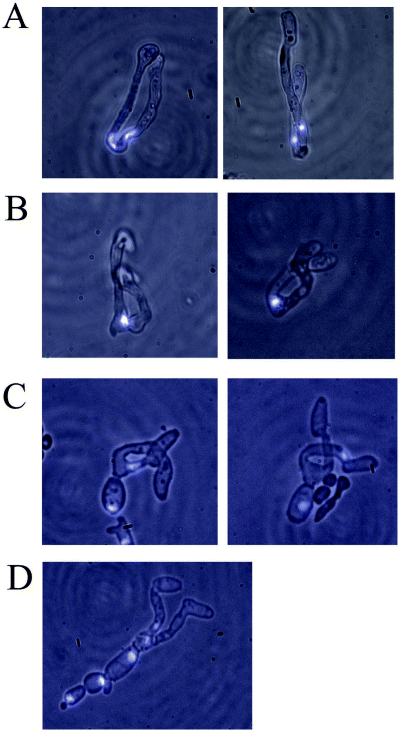
Nuclear positioning during the mating cycle. C. albicans zygotes were generated by the mixing of SC5314-derived a and α cells on spider medium for 7–24 h. Cells were then fixed and stained with DAPI, as described in Experimental procedures. Zygotes at different stages of formation are shown, from initial fusion of the a and α cell, to formation of multiple daughter budded cells. Fluorescent images of nuclei (blue) are superimposed on phase-contrast images.
To investigate the movement of nuclei within living cells during the mating process, we constructed a and α strains in which either the yellow fluorescent protein (YFP) or cyan fluorescent protein (CFP) was expressed as a fusion protein with histone H2B. As can be seen in the experiments that follow, these fusion proteins selectively localized to the nuclei, as expected. In these experiments, the a strain expressed the YFP marker, and the α strain expressed the CFP marker, so that the nuclei of the two mating cells could be clearly distinguished during zygote formation. Figure 3 shows images from a typical mating experiment between the YFP- and CFP-labelled strains. In this experiment, mating was performed between a and α cells mixed and incubated on Lee's medium supplemented with glucose for 18 h (we used Lee's medium in these experiments because spider medium produced high autofluorescence in the CFP channel). Mating cells were then transferred to a coverglass chamber and mating was followed by videomicroscopy (see Experimental procedures). At the start of the videomicroscopy, the a and α cells have fused generating a zygote containing two distinct, fluorescently tagged nuclei (see Fig. 3). In the example shown (which was typical), the two nuclei can be seen migrating towards one another and finally fuse approximately 90 min into the experiment. The YFP and CFP signals colocalized in the zygote for the next 2–3 h, during which time the zygote began to form a daughter cell bud. In this example, the nucleus finally divided at 270 min, approximately at the site of the future daughter cell bud septum. This gave rise to two nuclei (both containing YFP and CFP signals), with one nucleus located in the daughter cell bud and one nucleus in the parent conjugate (Fig 3, 290 min and 310 min time points).
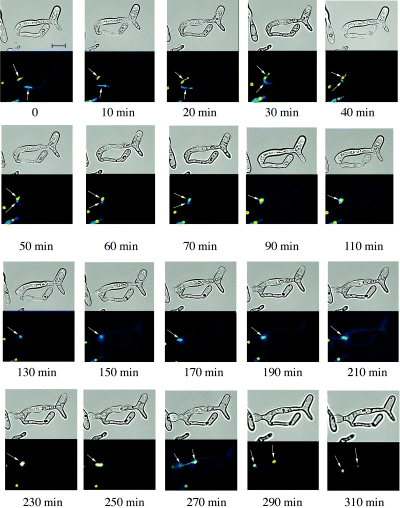
Live imaging of nuclear fusion in mating cells. C. albicans zygotes were prepared by mixing strains RBY1006 (nucleus marked by CFP expression) and RBY1009 (nucleus marked by YFP expression) as described in Experimental procedures. Mating cells were analysed by videomicroscopy to capture differential interference contrast (DIC), YFP and CFP signals. Images show a time-lapse of a mating zygote formed by the fusion of an a and an α cell. The nuclei (indicated by arrows) approach one another, fuse and subsequently divide, leaving one nucleus in the daughter cell and one in the parental conjugate cell. Some differences in fluorescence can be seen during the time-lapse due to re-focusing during the course of the experiment. Scale bar, 10 µm.
These experiments, which used genetically matched strains of C. albicans, demonstrate that following zygote formation, the two nuclei move towards one another and fuse. Eventually, a daughter cell is formed that inherits a single nucleus containing fluorescence from both the a and α parental nuclei. These conclusions differ from experiments carried out with a pair of clinical isolates (Lockhart et al., 2003a) in which nuclear fusion was never observed, and daughter cells typically inherited genetic information from only a single parent.
Genetic analysis of mating zygotes
The cytological studies described above show that nuclear fusion occurs at a high efficiency during mating between strains derived from SC5314. To confirm this result we directly analysed the DNA present in cells derived from mating zygotes. As the parental cells differed in their DNA configuration at the MTL locus, it was possible to determine, using polymerase chain reaction (PCR), whether cells contained DNA derived from the a parent, the α parent, or from both parents (see Experimental procedures).
Individual zygotes were picked from 24 h mating mixes (on spider or Lee's medium) using a glass needle attached to a micromanipulator. These cells were deposited on YEPD plates (non-selective media), grown into colonies and then re-streaked for single colonies. PCR was used to analyse the MTL configuration of these colony-purified progeny. As shown in Table 2, progeny cells derived from mating between the isogenic strains RBY731 (a/a) and RBY734 (α/α) typically contained genetic information from both parental strains (93% of zygotes picked from spider media gave rise to progeny containing alleles from both parent strains). Similarly, a high percentage of zygotes (80%) formed from mating of the isogenic strains CHY257 (Δa/α) and MMY278 (Δα/a) on spider medium gave rise to progeny cells that contained alleles from both parent strains.
| Opaque a strain | Opaque α strain | Mating on spider medium, % diparental | Mating on Lee's medium, % diparental |
|---|---|---|---|
| RBY731 (a/a) | RBY734 (α/α) | 93 (26/28) | 88 (22/25) |
| MMY278 (a/Δα) | CHY257 (α/Δa) | 80 (16/20) | 28 (10/36) |
| P37005 (a/a) | WO-1 (α/α) | 10 (2/20) | <4 (0/23) |
| P37005 (a/a) | RBY734 (α/α) | 53 (8/15) | ND |
| RBY731 (a/a) | WO-1 (α/α) | 58 (11/19) | ND |
| RBY731 (a/a) | 19F (α/α) | 95 (21/22) | ND |
| RBY731 (a/a) | P78048 (α/α) | 92 (22/24) | ND |
| 12C (a/a) | RBY734 (α/α) | 44 (7/16) | ND |
| P75063 (a/a) | RBY734 (α/α) | 32 (7/22) | ND |
| 12C (a/a) | 19F (α/α) | <6 (0/16) | ND |
- Individual mating zygotes were micromanipulated and progeny cells were analysed by PCR to determine their DNA configuration at the MTL locus (see Experimental procedures). Cells that contained alleles from both parents were designated as being diparental. The number of zygotes analysed is shown in parentheses.
- ND, no data.
We confirmed that these progeny cells were mononuclear and tetraploid by staining the cells with DAPI and performing FACS analysis on the cells. In total, FACS was performed on 10 recombinant progeny; nine of them were found to have the DNA content of a tetraploid cell (see examples in Fig. 4), while one was intermediate between diploid and tetraploid (data not shown). The latter mating product probably underwent chromosome loss following mating, as instability of tetraploid strains has previously been reported (Bennett and Johnson, 2003). DAPI staining of the 10 tetraploid progeny indicated that they all contained a single nucleus (see examples in Fig. 4).
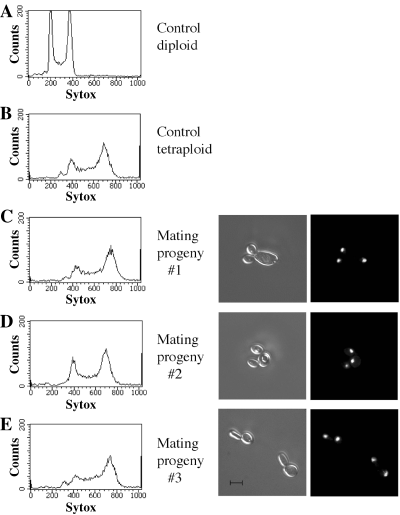
Progeny cells derived from crosses between wild-type strains are tetraploid and mononuclear. Mating zygotes were generated by the mixing of SC5314-derived a and α strains (MMY278 and CHY257) on spider medium. Zygotes were micromanipulated and the resultant progeny cells analysed by DAPI staining and FACS analysis to determine the number of nuclei present and the ploidy of the cells. As shown in the three examples, the mating progeny were mononuclear (i.e. contained a single DAPI-staining body), and tetraploid, indicating that nuclear fusion had occurred during mating. The control diploid strain is RBY731 and the control tetraploid strain is RBY18. For the microscopy, DIC images are shown on the left and fluorescent images are shown on the right. Scale bar, 5 µm.
In addition to analysing zygote cells (conjugate cells either with or without a daughter cell bud), we were also successful in micromanipulating daughter cells away from the parental conjugate cell. Progeny cells from these daughter cells were also analysed by PCR and shown to be recombinant, i.e. they contained genetic information from both parental strains, in 10/11 cases.
These genetic experiments confirm that nuclear fusion occurs rapidly and efficiently in the zygote to generate daughter cells that are mononuclear and tetraploid; moreover, the experiments show that nuclear fusion, as monitored genetically, occurs readily in the absence of genetic selection.
Nuclear fusion in clinical isolates
Thus far, the experiments in this article were performed with isogenic strains derived from SC5314. We next turned to experiments utilizing other strains of C. albicans. We first examined zygotes formed by mating between the clinical isolates WO-1 and P37005, as these strains did not appear to undergo nuclear fusion in the cytological studies (Lockhart et al., 2003a). In this case, we observed that mating of the clinical isolates, when carried out under the same conditions as for the laboratory strains, gave rise only rarely (10% of zygotes) to progeny containing genetic information from both parents. Instead, the majority of the progeny had the MTL configuration of one parent or the other. These genetic results indicate that nuclear fusion occurs in the majority of zygotes formed between isogenic laboratory strains, but occurs rarely in mating between the two clinical isolates, WO-1 and P37005. To further investigate this difference, we mated the clinical isolates against the laboratory-derived strains. Mating of RBY734 (α/α) with P37005 (a/a) generated zygotes of which 53% gave rise to cells with diparental genotypes, while mating of RBY731 (a/a) with WO-1 (α/α) produced zygotes of which 58% of progeny had diparental genotypes (see Table 2). These values are intermediate between those of the isogenic strains mating (93%) and the clinical strains mating (10%), suggesting that the clinical strains WO-1 and P37005 are defective in some aspect of nuclear fusion, and that this defect is partially rescued by crossing with a more robust mating partner.
To extend this analysis to other clinical isolates of C. albicans, we performed several additional crosses, as shown in Table 2. Once again, PCR was performed to determine whether recombinant progeny resulted from each cross (see Experimental procedures). In crossing the laboratory a/a and α/α strains to the different clinical isolates, we observed a large difference in the ability of the zygotes to undergo nuclear fusion. For example, crosses between RBY731 (SC5314-derived a-type mater) and either isolate19F or isolate P78048 produced zygotes that underwent nuclear fusion with a high efficiency, as evidenced by the high percentage of recombinant progeny from those zygotes (95% and 92% respectively). In contrast, crosses between RBY734 (SC5314-derived α-type mater) and either isolate 12C or isolate P75063 generated zygotes (as observed under the microscope) in which nuclear fusion occurred less than half of the time (44% or 32% of progeny cells from these zygotes were recombinant respectively). Finally, a cross between clinical isolates 12C and 19F generated zygotes in which no nuclear fusion events were detected (0/16 zygotes gave recombinant progeny cells).
These results suggest that while mating between certain isolates of C. albicans produces zygotes in which nuclear fusion occurs efficiently, other combinations of strains are clearly inefficient at undergoing nuclear fusion. The ability to undergo nuclear fusion does not appear to correlate with the relatedness of the strains in a given cross. Previous studies have suggested that C. albicans isolates can be assigned to at least five groups, or clades (I, II, III, E and SA), based on genetic similarities (Soll and Pujol, 2003). For example, the laboratory strain SC5314 is a member of clade I (A. Davidson and F. C. Odds, personal communication), and mating strains derived from it undergo mating fusion efficiently. Similarly, isolates 12C and 19F both belong to clade I, yet there was no evidence for fusion between the nuclei of these two strains during mating. In contrast, crosses between RBY734 (clade I) and P75063 (clade SA), or between RBY731 (clade I) and WO-1 (clade II) underwent nuclear fusion at a significant rate (32% and 58% respectively).
Finally, we also observed that media composition influenced the efficiency of nuclear fusion in some of the mating crosses. For example, while strains RBY731 and RBY734 underwent nuclear fusion readily during mating on both spider medium (93%) and Lee's medium (88%), the strains CHY257 and MMY278 demonstrated efficient nuclear fusion only on spider medium (80%) and not on Lee's medium (28%). Several of the zygotes picked from the mating of CHY257 (Ade+) and MMY278 (Ade–) strains on Lee's medium, grew up as colonies that were approximately half red (Ade–) and half white (Ade+), also indicating that although cell fusion occurred, nuclear fusion did not (data not shown). Nuclear fusion of the clinically isolated strains WO-1 and P37005 also occurred less frequently on Lee's medium (no nuclear fusion events detected) than on spider medium (10%).
We conclude from these studies that a wide variety of C. albicans strains undergo nuclear fusion as a part of the mating process, but that the efficiency of nuclear fusion varies among mating pairs and different environmental conditions.
Deletion of the KAR3 gene in C. albicans inhibits the formation of stable tetraploids during mating
In S. cerevisiae, a number of gene products have been identified that are required for nuclear fusion to occur during mating (for review, see Rose, 1996). These include the KAR3 gene, which encodes a microtubule-based motor protein that is required for migration of the nuclei towards one another before nuclear fusion. In the absence of KAR3, the nuclei fail to associate with one another and fusion is blocked (Meluh and Rose, 1990). We examined a homologue of KAR3 in the sequenced genome of C. albicans (GenBank Accession No. AY182242), which shares 35% identity with its S. cerevisiae counterpart. To determine whether the C. albicans KAR3 gene plays a role in mating, we deleted both alleles of the gene and analysed the resultant strains for mating efficiency. As shown in Table 3, deletion of KAR3 from both mating partners reduced the formation of stable tetraploids more than 600-fold (41% mating efficiency between wild-type strains and 0.063% mating efficiency between Δkar3 strains). Similar to the situation in S. cerevisiae, the efficiency of mating between a wild-type KAR3 strain and a Δkar3 strain was similar to the efficiency of mating between two wild-type strains (approximately 40%). These results show that, for C. albicans, bilateral matings between Δkar3 mutants lead to a greatly reduced formation of stable tetraploids, but that the presence of KAR3 in one mating partner is sufficient for wild-type levels of mating (Table 3).
| Cross | Genotypes in cross | Mating efficiency (%) | ||
|---|---|---|---|---|
| Ade– | Ura– | |||
| 1 | a WT | × | α WT | 41 ± 7 |
| 2 | a kar3/kar3 | × | α WT | 38 ± 6 |
| 3 | a WT | × | αkar3/kar3 | 41 ± 10 |
| 4 | a kar3/kar3 | × | αkar3/kar3 | 0.063 ± 0.01 |
- A quantitative mating assay (see Experimental procedures) was used to compare the mating efficiency of wild-type and Δkar3/Δkar3 mutant strains. Strains were mated for 24 h on spider medium. Strains used are: a WT Ade– (MMY278); akar3/kar3 Ade– (MMY928); α WT Ura– (CHY257); αkar3/kar3 Ura– (MMY917). Average mating efficiencies and standard deviation of the mean are reported for four experiments using individual opaque colonies from each strain.
- WT, wild type.
The C. albicans KAR3 gene is required for efficient nuclear fusion
It seems reasonable to suppose that the mating defect of the Kar3 mutants results from a block in nuclear fusion, and we tested three predictions of this idea: (i) the efficiency of zygote formation should not be affected by deletion of KAR3, (ii) the presence of a single nucleus in zygotes formed between Δkar3 strains should be rare and (iii) micromanipulation of zygotes from Δkar3 crosses should give rise to progeny cells that are a or α diploid cells, but rarely a/α tetraploid cells.
The efficiency of zygote formation was determined for crosses between wild-type strains (CHY257 + MMY278) and between Δkar3 strains (MMY917 + MMY928). Approximately equal numbers of zygotes were observed in both wild-type and mutant crosses after 24 h on spider medium (1.8% of the cells were zygotes in wild-type matings while 2.0% of the cells were zygotes in matings between the Δkar3 strains). These results indicated that the KAR3 gene was not required for any of the steps in mating leading to cell fusion. (We note that the number of zygotes at this time point does not represent all of the cells that have undergone mating by this stage, as some have already mated and undergone subsequent cell divisions).
We also microscopically examined zygotes formed by the mating of wild-type or Δkar3 strains. As described earlier (Fig. 2), mating between wild-type laboratory strains often results in zygotes that contain a single nucleus (as evidenced by a single DAPI-staining body). We therefore compared the frequency with which single nuclei were observed in both wild-type and Δkar3 zygotes. Mating zygotes were chosen that had undergone cell fusion but were yet to produce a daughter cell. DAPI staining of the nuclear material in these zygotes revealed that most of the wild-type zygotes contained a single DAPI-staining body (13/22), while some zygotes contained two or three nuclei (6/22 and 3/22 respectively). In contrast, only 1/20 of the Δkar3 zygotes appeared to have a single nucleus, with the remaining zygotes containing either two nuclei (13/20), three nuclei (5/20) or four nuclei (1/20). Although we are unsure as to its significance, we also observed that C. albicansΔkar3 strains appeared to proceed through the mating process faster than their wild-type counterparts, as daughter cell buds appeared earlier on Δkar3 zygote cells.
Finally, we also micromanipulated individual zygotes from wild-type and Δkar3 crosses performed on spider media and analysed the configuration of MTL alleles present in progeny cells. Once again, crosses between wild-type strains gave rise to a high proportion of progeny cells (95%) that contained DNA from both parental strains, indicative of nuclear fusion having occurred at an early step of mating. In contrast, of 60 zygotes picked from crosses between Δkar3 strains, none gave rise to recombinants; instead, all of them contained DNA markers from only one of the two parent strains, indicating that nuclear fusion had not occurred. To confirm that the defect in nuclear fusion resulted from the absence of the KAR3 gene product, one copy of the KAR3 gene was reintroduced into the kar3/kar3 knockout strain (see Experimental procedures). Mating of an addback strain with a kar3/kar3 deletion strain gave 13% (12/92) recombinant progeny, as determined by PCR. Although complementation was not complete, 13% recombinant progeny is significantly higher than that observed for the kar3/kar3 × kar3/kar3 crosses (<1.6%). In this regard, we note that only a single copy of KAR3 was reintroduced and full complementation would not necessarily be expected. Taken together, these experiments support the conclusion that the C. albicans KAR3 gene is required for efficient nuclear fusion during mating.
Discussion
Work in the past several years has revealed that C. albicans readily undergoes mating both in the laboratory and in mouse models of infection. C. albicans has retained a large number of genes required for mating, and the mating process is elaborate. However, the role of mating in C. albicans continues to be a topic of debate, particularly as the population structure of C. albicans is largely clonal, indicating a predominantly asexual mode of reproduction (Graser et al., 1996; Tibayrenc, 1997). Because the existence of an elaborate mating apparatus is difficult to reconcile with the population structure, we felt it necessary to resolve whether nuclear fusion normally occurs when two C. albicans cells mate. If nuclear fusion does not occur, DNA exchange would not take place (or would take place very inefficiently) and the population structure could be easily explained. If, on the other hand, nuclear fusion readily occurred during mating, then reconciling the population structure with the observed mating would require a different explanation. A second reason for resolving the issue of nuclear fusion in C. albicans is the existence of conflicting conclusions regarding this process in the literature, as described in Introduction.
In this article, we show that when mating is carried out with C. albicans strains derived from the standard laboratory strain SC5314, nuclear fusion occurs shortly after cell fusion; the daughter cells that bud off from the zygote are tetraploid and mononucleate. We also show that nuclear fusion in C. albicans requires the microtubule motor protein Kar3, just as it does in S. cerevisiae. The effect of Kar3 can be easily observed in quantitative mating experiments, which score for the stable recombination of genetic markers and therefore require both cell and nuclear fusion. In these experiments, mating of C. albicansΔkar3 strains was reduced 600-fold relative to wild-type strains. However, as in S. cerevisiae, mating between a strain lacking Kar3 and a wild-type strain showed no defect, consistent with Kar3 being diffusible in the zygote. The analysis of the Δkar3 mutant also shows that zygote formation (cell fusion) and nuclear fusion are genetically separable processes and that nuclear fusion is an active process that requires a motor protein.
We also show that the medium on which mating occurs can significantly affect the efficiency of nuclear fusion; for example, nuclear fusion is significantly less efficient on Lee's medium than on spider medium. This observation raises the possibility that nuclear fusion in C. albicans may be regulated by nutritional signals.
Finally, we show that different strains of C. albicans undergo nuclear fusion with greatly varying efficiencies. Whereas the SC5314-derived strains underwent nuclear fusion at high rates (80–95%) when mated on spider medium, crosses between different clinical isolates showed widely ranging rates of nuclear fusion. The efficiency of nuclear fusion between various C. albicans strains ranged from undetectable to more than 90%. The efficiency of fusion did not appear to correlate with the genetic class of the mating strains. For example, mating between two isolates that both belong to the clade I group of strains (see Soll and Pujol, 2003 for description of five clades of C. albicans strains) showed very low rates of nuclear fusion (less than 6%) while mating between an isolate from clade I and an isolate from clade II showed significant rates of nuclear fusion (58%). The process of nuclear fusion does not therefore appear to be responsible for preventing the different clades of C. albicans from recombining with one another.
We also observed that the efficiency of nuclear fusion varied greatly even between different strains within the same clade (again, from more than 90% to less than 6%). These results indicate that within a clade, new mutations have arisen that can inhibit nuclear fusion. Furthermore, nuclear fusion of these isolates cannot be completely complemented by mating with a strain that is proficient in nuclear fusion, indicating a unilateral defect in the fusion process. Complementation of these isolates with budding yeast genes could help to identify the factors responsible for the defects in nuclear fusion.
Based on all these observations, it is also relatively simple to reconcile the conflicting published views of nuclear fusion during mating of C. albicans. The reports concluding that nuclear fusion did not occur were based on crosses between clinical isolates analysed in Lee's medium (Lockhart et al., 2003a; Soll et al., 2003; Soll, 2004). As presented here, the clinical isolates used in these studies (i.e. WO-1 and P37005) show relatively low levels of nuclear fusion compared with other strains; moreover, Lee's medium is not an optimal medium for nuclear fusion. If either the strain or the medium is changed, nuclear fusion occurs at significant levels (see Table 2).
In conclusion, the results presented here show convincingly that nuclear fusion occurs during mating in C. albicans. However, these results raise several interesting possibilities concerning the possible regulation of this process. It is plausible that extracellular signals regulate nuclear fusion independently of cell fusion during mating. Lee's medium differs from spider medium in several aspects (e.g. pH), and it is possible that one of these components regulates nuclear fusion, causing it to be much more efficient in the latter medium. Another intriguing possibility concerns the effects of genetic background on the efficiency of nuclear fusion. It is still too early to ascertain whether C. albicans has a set of genes controlling heterokaryon incompatibility, as has been well documented for certain filamentous ascomycete fungi (for reviews, see Saupe, 2000; Glass and Kaneko, 2003), but it appears that the efficiency of nuclear fusion is not linked to how similar the genetic backgrounds of the two mating strains are. A more likely possibility is that some natural isolates of C. albicans are defective in the expression of components required for nuclear fusion. Consistent with this idea, these strains can be partially ‘rescued’ by mating with a SC5314-derived strain. In any case, the work described in this article clearly documents nuclear fusion as a process that can occur with high efficiency during mating in C. albicans. Thus, we conclude that C. albicans has maintained an elaborate mating apparatus which extends to events that take place after the initial formation of a zygote.
Experimental procedures
Media
Standard laboratory media were prepared as described previously (Guthrie and Fink, 1991). Lee's medium has been described previously (Bedell and Soll, 1979). Lee’s + D and Lee’s + M refer to Lee's medium supplemented with either 2% glucose or 2% mannitol. Spider medium contained 1.35% agar, 1% nutrient broth, 1% mannitol and 0.4% potassium phosphate (pH 7.2) (Liu et al., 1994) and was supplemented with 100 µg ml−1 uridine. SCD and SCM media refer to synthetic complete media supplemented with either 2% glucose or 2% mannitol, and 100 µg ml−1 uridine.
Strains
The construction of C. albicans strains CAF2-1 (URA3/Δura3::imm343) (Fonzi and Irwin, 1993), CHY257 (a1,a2::hisG-URA3-hisG), CHY477 (a1,a2::hisG-URA3-hisG), CHY439 (α1::hisG-URA3-hisG α2::hisG), MMY278 (α1::hisG-URA3-hisG α2::hisG) (Miller and Johnson, 2002), RBY731 (MTLa/a) and RBY734 (MTLα/α) (Bennett et al., 2003) have been described previously (Table 4). As noted previously, RBY731 and RBY734 were generated by growth of CAF2-1 on sorbose-containing medium (Bennett et al., 2003), which selects for loss of one copy of chromosome 5, the chromosome containing the MTL locus (Janbon et al., 1998). Duplication of the remaining copy of chromosome 5 results in homozygosis of the MTL locus. These strains will therefore be referred to as a/a and α/α strains in this study. In all cases, opaque-phase cells were obtained from white-phase cells by passaging on SCD medium, and opaque cells identified by their darker appearance on the SCD plates.
| Strain | Origin | MTL genotype | Clade | Gene deletions | Reference |
|---|---|---|---|---|---|
| CAF2-1 | Laboratory | a/α | I | None | Fonzi and Irwin (1993) |
| RBY731 | Laboratory | a/a | I | None | Bennett et al. (2003) |
| RBY734 | Laboratory | α/α | I | None | Bennett et al. (2003) |
| RBY1006 | Laboratory | α/α | I | None | This work |
| RBY1009 | Laboratory | a/a | I | None | This work |
| CHY439 | Laboratory | a/Δα | I | URA3 | Miller and Johnson (2002) |
| CHY257 | Laboratory | α/Δa | I | URA3 | Miller and Johnson (2002) |
| CHY477 | Laboratory | α/Δa | I | ADE2 | Miller and Johnson (2002) |
| MMY278 | Laboratory | a/Δα | I | ADE2 | Miller and Johnson (2002) |
| RBY18 | Laboratory | a/α/Δa/Δα | I | None | Bennett et al. (2003) |
| MMY928 | Laboratory | a/Δα | I | KAR3, ADE2 | This work |
| MMY917 | Laboratory | α/Δa | I | KAR3, URA3 | This work |
| MMY958/MMY961 | Laboratory | a/a | I | KAR3 addback | This work |
| WO-1 | Bloodstream | α/α | II | None | Slutsky et al. (1987) |
| P37005 | Mouth | a/a | I | None | Lockhart et al. (2002) |
| 19F | Vagina | α/α | I | None | Lockhart et al. (1996) |
| P78048 | Mouth | α/α | I | None | Pujol et al. (2002) |
| 12C | Mouth | a/a | I | None | Lockhart et al. (1996) |
| P75063 | Bloodstream | a/a | SA | None | Pujol et al. (2002) |
- All laboratory strains are derived from SC5314. C. albicans clade designations are taken from Lockhart et al. (2003a).
The starting strain MMY278 (Δα1α2, Ade–) (Miller and Johnson, 2002) was used to construct the a-type kar3/kar3 strain. MMY278 was plated on 5-fluoroorotic acid (5-FOA) plates to counterselect against the URA3 marker present at the ADE2 locus. The entire open reading frame (ORF) of the first KAR3 allele was disrupted by the technique of Wilson et al. (1999) using the URA3 cassette from pDDB57 (Wilson et al., 2000) and the primers 5′-ACAATTAAGTTTCA AAAAGTTGCCAGACAGGTTTTTTACAATTTTGAAACTACAATCCAATAGTCAATCGTGCACAAGTAGTTTTCCCAGTCACGACGTT-3′ and 5′-TCCAAAACATATATCTGAGCCAATATTT AAATAGATTCTTGTATATAAGTCATGTATGTAAACTATTAACGTAGTAATTATGTGGAATTGTGAGCGGATA-3′. Integration was verified by PCR across the 5′ and 3′ disruption junctions. This strain was then passaged on 5-FOA to recycle the URA3 marker. The second allele of KAR3 was disrupted using the URA3 cassette in the opposite orientation from the first allele and the primers 5′- ACAATTAAGTTTCAAAAAGTTGCCAGA CAGGTTTTTTACAATTTTGAAACTACAATCCAATAGTCAATCGTGCACAAGTATGTGGAATTGTGAGCGGATA-3′ and 5′-TCCAAAACATATATCTGAGCCAATATTTAAATAGATTCTTGTATATAAGTCATGTATGTAAACTATTAACGTAGTAATTAGTTTTCCCAGTCACGACGTT-3′. Correct integration was again verified by PCR across the disruption. Finally, PCR with primers internal to the KAR3 ORF verified that the locus was completely deleted in strain CKFY. This strain was switched to the opaque phase by passaging on SCD medium to yield MMY928 (MTLa/mtlα1mtlα2 kar3::dpl200/kar3::URA3 ade2::HisG/ade2::HisG, opaque).
The starting a/α strain BWP17 (Wilson et al., 1999) was used to construct an α-type kar3/kar3 strain. Strain BWP17 and MMY278 are both derived from CAI4. In this strain, a partial deletion of the KAR3 ORF deletes the C-terminal motor domain of Kar3p. This resulting strain is phenotypically identical to a deletion of the complete kar3 ORF (P. Chua, unpublished results). The first allele of kar3 was disrupted by PCR using the pGEM-HIS cassette (Wilson et al., 1999) and the primers 5′-ATGAAAACCGAGTTGGTTGACCAGGAAA CCAAACGAAGAAAGTTACATGCCCAACTACAAGTTTTCCCAGTCACGACGTT-3′ and 5′-CTTGGTTGCAAACCTCAA AGAGTTTATGGTTTCATTTAAATCCTTGGTAAGTGGTGATATTGTGGAATTGTGAGCGGATA-3′. The second allele was disrupted by PCR using the pRS-ARGΔSpeI cassette (Wilson et al., 1999). Both disruptions were verified by PCR across the disruption junctions and PCR with primers internal to the KAR3 ORF in the resulting strain CKFY71. Both MTLa1 and MTLa2 were then disrupted with the plasmid pCH152 (Hull and Johnson, 1999). This plasmid completely deletes the MTLa1 ORF, and additionally removes the 3′UTR of a2, resulting in an α-type mating strain. The phenotype of this partial deletion is identical to that of an mtla1 mtla2 double mutant with full-ORF disruptions (Tsong et al., 2003). This strain was counterselected on 5-FOA to yield MMY905. This strain was switched to the opaque phase by passaging on SCD medium to yield MMY917 (mtla1, mtla2::HisG/MTLα kar3::HIS1/kar3::ARG4 ADE2/ADE2 ura3::Limm/ura3::imm, opaque).
A KAR3 addback strain was constructed using a plasmid that contains the KAR3 ORF together with the CaURA3 gene (pCKFB181). pCKFB181 was constructed by first inserting the 1.2 kb SacII/SpeI fragment of CaURA3 from pGEM-URA3 (Wilson et al., 1999) in between the SacII and SpeI sites of pBS+ (Stratagene), creating plasmid pCKFB127. Then, a PCR product containing KAR3 (together with 1 kb of the upstream region) was generated from genomic DNA using primers 5′-GGGGCTCGAGTGGTTTGTCCCAACTTC TCCTTATT-3′ and 5′-CCCCGATATCGGACTTAAATCTACCC AAGCAGGAT-3′. The PCR introduced XhoI and EcoRV restriction sites (underlined), which were used to insert the PCR product into pCKFB127, resulting in pCKFB181. This plasmid was integrated into Candida strain CKFY by linearization with BamHI (in the KAR3 promoter). Two independent transformants were sorbose-selected to generate a/a derivatives and subsequently passaged on SCD to generate the opaque strains MMY958 and MMY961 (MTLa/akar3::dpl200/kar3::dpl200/KAR3::URA3 ade2::HisG/ade2::HisG, opaque). Addback of the KAR3 ORF was confirmed by PCR.
Strains containing fluorescently labelled nuclei were constructed by expression of histone H2B chimeras with either YFP or CFP. Plasmids containing the YFP and CFP cassettes were gifts of J. Berman (University of Minnesota) (Gerami-Nejad et al., 2001). The YFP or CFP sequence was amplified using oligonucleotides designed to target the fluorescent gene to the 3′ end of the histone H2B gene (HTB in C. albicans) (5′-AGGTGAATTGGCTAAACATGCTGTTTCTGA AGGTACTAGAGCTGTTACCAAATACTCTTCTGCTTCTAAGGTGGTGGTTCTAAAGGTGAAGAATTATT-3′ and 5′-TATT AATATACAATACAAAATAAAACAACAATAATTTGGAGAAATAAACCATTCATGACAAACCTCTCTCTCTAGAAGGACCACCTTTGATTG-3′). The PCR product was used to transform sorbose-selected, a/a and α/α derivatives of CAI4. Correctly integrated constructs were identified by PCR and the strains RBY1006 (CFP-tagged, α/α) and RBY1009 (YFP-tagged, a/a) switched to the opaque phase for analysis in mating experiments.
Quantitative mating assays of C. albicans
Quantitative mating analysis of C. albicans strains was performed as described previously (Miller and Johnson, 2002). Briefly, Ade– and Ura– mating strains in the opaque phase were grown at 25°C overnight in liquid SCD medium and approximately 3 × 107 cells of each strain mixed and deposited onto 0.8 µm nitrocellulose filters using a Millipore 1225 Vacuum Sampling Manifold. The filters were then grown on the surface of plates containing different test media for 1–4 days at 23°C. Cells were collected from the filters and plated onto Ade–Ura– media to select for conjugants and onto Ade– and Ura– plates to monitor each parent population + conjugants.
Quantitative liquid matings were performed similarly, except 3 × 107 cells of each strain were mixed, spun down to remove medium and resuspended in 5 ml of the test liquid media. Cultures were grown for 1 day at 23°C and then plated onto test media as above.
Fluorescent microscopy of mating cells
The fluorescent labelling of opaque a and α cells has been previously described (Lockhart et al., 2003a). In our experiments, liquid cultures of opaque a and α cells were grown in SCD medium at 22°C until the optical density (OD) reached 0.5–1.0 (approximately 1.5–3 × 107 cells per millilitre). Cultures of a cells were then incubated for 30–60 min in 10 µg ml−1 rhodamine-ConA and cultures of α cells were similarly incubated in 10 µg ml−1 FITC-ConA (Vector Laboratories, Burlingame, CA). The cells were washed in SCD to remove unbound probe and approximately 3 × 107 cells of each mating type were mixed and deposited onto nitrocellulose filters, as described above. The filters were placed on the surface of different laboratory media agar plates at 22°C, and cells were taken off the filters at different time points and placed on poly-l-lysine-coated microscope slides to analyse mating. Cells were visualized using an Olympus BX60 microscope with a mercury lamp. Images were recorded using a Photometrics Sensys CCD camera and IP Laboratory Spectrum software. Image processing was performed using Adobe Photoshop and Adobe Illustrator software.
DAPI-stained cells were also obtained from mating cells growing on nitrocellulose filters on agar plates. Cells were taken from the filters and resuspended in phosphate-buffered saline (PBS), pH 7.4. Cells were fixed with formaldehyde (14%) for 30 min, washed with PBS buffer, and then stained for 10 min with DAPI (1 µg ml−1). Cells were washed free of DAPI and mounted onto poly-l-lysine-coated slides. Cells were visualized using a Hamamatsu CCD camera and a Zeiss Axiovert 200M microscope. Image processing was performed using Adobe Photoshop software.
Live imaging of YFP- and CFP-tagged nuclei was performed using cells prepared as described above. YFP- and CFP-tagged strains were mixed and deposited on filters on Lee's medium supplemented with glucose for 18 h at 22°C. Cells were then removed and incubated in Laboratory-tec coverglass chambers (Nalge Nunc International) containing Lee's medium supplemented with glucose and followed by microscopy using a Zeiss Axiovert 200 M microscope. Images were recorded using a Hamamatsu CCD camera and Axiovision software. Image processing was performed using Adobe Photoshop software.
Micromanipulation and analysis of mating zygotes
Opaque a and α cells were mixed and deposited on nitrocellulose filters as described above. Filters were grown on the surface of spider or Lee's medium agar plates for 24 h at 22°C. Cells were picked off the filters and streaked onto yeast extract-peptone-dextrose plates supplemented with 55 µg ml−1 adenine and 100 µg ml−1 uracil (YEPD plates). Mating a and α zygotes were identified using a Zeiss microscope and moved away from the rest of the cells using a glass needle attached to a micromanipulator. Zygotes were allowed to grow up on the YEPD plates for 2–3 days and then streaked for single colonies. PCR was then performed on the single colonies to determine whether they contained DNA markers from both parental cells. In matings between a/a and α/α strains, PCR was used to detect the OBPa and OBPα ORF sequences. These have been shown to be accurate markers of the MTL genotype in the clinical isolates used in this study (Lockhart et al., 2002). The primers used for OBPa were 5′-gtggtcaatggagctgatac-3′ and 5′-acatgtggtcgcccaactcc-3′. The primers used for OBPα were 5′-ccttcaattgcatcgtaagtacc-3′ and 5′-gaagatgactcaggtcatgc-3′. In matings between a/Δα and α/Δa strains, PCR was used to follow all four MTL alleles, as previously described (Miller and Johnson, 2002).
FACS analysis
FACS analysis was carried out as described by Hull et al. (2000).
Acknowledgements
The fluorescent protein cassettes were kindly provided by J. Berman (University of Minneosota) and the clinical isolates of C. albicans were kindly provided by D. Soll (University of Iowa). We thank Robert Moody for assistance with the time-lapse microscopy and A. Davidson and F. C. Odds for communication of results prior to publication. This work was supported in part by grants from the Burroughs Wellcome Fund (993218) and NIH (RO1 AI49187) to A.D.J. M.G.M. is a Howard Hughes Medical Institute Fellow.




