The Aspergillus pH-responsive transcription factor PacC regulates virulence
Present addresses: Faculty of Science and Engineering, Saint Louis University-Madrid Campus, Avenida del Valle, 34, Madrid 28003, Spain; ‡Department of Clinical Microbiology, Trinity Centre, St James's Hospital, Dublin 8, Ireland.
Summary
The ability of a pathogen to adapt to the host environment is usually required for the initiation of disease. Here we have investigated the importance of the Aspergillus nidulans PacC-mediated pH response in the pathogenesis of pulmonary aspergillosis. Using mutational analysis, we demonstrate that, in neutropenic mice, elimination of the A. nidulans pH-responsive transcription factor PacC, blocking the ambient pH signal transduction pathway or prevention of PacC proteolytic processing acutely attenuates virulence. Infections caused by these alkali-sensitive mutants are characterized by limited growth in vivo and a reduction of inflammatory cell infiltration. In stark contrast, constitutive activation of PacC causes increased mortality marked by extensive fungal invasive growth. PacC action is therefore required for, and able to enhance virulence, demonstrating that the A. nidulans pH-responsive transcription factor PacC plays a pivotal role in pulmonary pathogenesis.
Introduction
The incidence of severe mycoses is steadily rising, concomitant with growing numbers of immunosuppressed patients. Recent epidemiological and autopsy surveys have shown that invasive pulmonary aspergillosis (IPA) has become the single most frequent cause of death attributed to fungal infection (Groll et al., 1996; McNeil et al., 2001; Clark and Hajjeh, 2002). IPA is a major opportunistic mycosis complicating haematopoietic stem cell treatment for haematological malignancy (Marr et al., 2002a), solid organ transplantation (Patterson et al., 2000) and other immunocompromised states (Marr et al., 2002b). Reported mortality rates exceeding 90% in some groups underline the urgent need for new therapeutic interventions (Denning, 1996; Lin et al., 2001).
Aspergillus fumigatus (Latgé 1999; Brakhage and Langfelder, 2002) is the most frequently isolated pathogen but other pathogenic species include Aspergillus flavus, Aspergillus niger, Aspergillus terreus and Aspergillus nidulans (Baddley et al., 2003; Husain et al., 2003). A. nidulans has particular virulence in patients having chronic granulomatous disorder (CGD), an inherited defect of NADPH oxidase in which phagocytes fail to generate reactive antimicrobial oxidants (Segal et al., 1998; Johnston, 2001; Dotis et al., 2003; Dotis and Roilides, 2004). In contrast to A. fumigatus, genetic manipulations are easily achievable in A. nidulans, which has been the subject of intense genetic scrutiny since the late 1940s (Pontecorvo et al., 1953). Among the wealth of available A. nidulans mutants (http://www.fgsc.net/), many can only be feasibly selected using non-wild-type backgrounds or result from point or multiple mutations, making them almost impossible to select and/or construct in less-easily manipulated members of the genus. This legacy of genetic analysis has enormous potential for aiding our understanding of pathogenicity, provided the selection of isogenic reconstituted strains is possible.
Several fungal attributes have demonstrated roles in the development of IPA including nutrient availability (Tang et al., 1994; D’Enfert et al., 1996; Brown et al., 2000; Liebmann et al., 2004), regulation of amino acid biosynthesis (Krappmann et al., 2004), nitrogen metabolite derepression (Hensel et al., 1998), nitrogen signalling (Panepinto et al., 2003) and conidial pigment formation (Langfelder et al., 1998). The consensus of current opinion attributes Aspergillus virulence to the coupling of successful adaptation to physical conditions encountered within the host and resistance to or tolerance of host defences (Latgé 1999). The adaptive response to environmental pH is a fungal regulatory mechanism (Arst and Peñalva, 2003a,b) with relevance to both of these processes. Its impact on pathogenesis in the context of pulmonary infection has not been the subject of previous investigation.
In addition to general homeostatic responses to alterations in ambient pH many fungi possess a genetic regulatory system that acts through a conserved family of PacC/Rim101 transcription factors to control the expression of a subset of pH regulated genes (Arst and Peñalva, 2003a). Considerable progress has been made in characterizing this system in A. nidulans in which the transcription factor PacC controls acid- and alkaline-structural gene expression according to extracellular ambient pH (Caddick et al., 1986; Tilburn et al., 1995). At alkaline pH, stepwise proteolysis of PacC generates the active form of the protein, PacC27, which acts positively on expression of alkaline genes, and negatively on acidic genes (Diéz et al., 2002; Arst and Peñalva, 2003a). Alkaline pH is signalled by the protein products of six pal genes including two potential plasma membrane pH sensors, PalH and PalI (Arst et al., 1994; Denison et al., 1998; Negrete-Urtasun et al., 1999), and a signalling protease, probably PalB (Denison et al., 1995), which catalyses the pH-dependent first proteolytic step of PacC processing (Diéz et al., 2002). Signalling proteolytic cleavage of PacC is assisted by the binding of PalA to two YPXL/I motifs on either side of the signalling protease box (Vincent et al., 2003) and the action of two other Pal proteins, encoded by palC and palF, whose functions are unclear (Caddick et al., 1986; Maccheroni et al., 1997; Negrete-Urtasun et al., 1999). All identified components of the A. nidulans PacC regulatory system are conserved in the more commonly pathogenic A. fumigatus (http://www.tigr.org/tdb/e2k1/afu1/).
We hypothesized that PacC mediated pH-dependent gene expression would be important in vivo both in terms of enabling acidophilic, soil-dwelling fungi such as Aspergilli to accommodate the alterations in ambient pH encountered during lung colonization and in terms of controlling the appropriate synthesis of potentially virulence-enhancing substances such as proteases and toxins. Evidence to support this hypothesis can be found from early studies of murine intraperitoneal injection with A. nidulans (Purnell and Martin, 1971) where a noted association between alkaline phosphatase production and virulence can now be interpreted as a correlation between virulence and A. nidulans pH signal transduction. In addition, recent chemical–genetic interaction data have shown that mutants lacking components of the homologues regulatory system in Saccharomyces cerevisiae are hypersensitive to the antifungal drug fluconazole (Parsons et al., 2004), suggesting that perturbation of this system may help treat disease.
To initiate an investigation of this hypothesis we have exploited available A. nidulans mutants to construct a series of prototrophic A. nidulans pH regulatory mutants and cognate isogenic reconstituted strains. Here we show that pacC and palB loss of function mutants are attenuated for virulence in a respiratory model of murine infection, demonstrating that A. nidulans Pal-mediated pH signalling and PacC-regulated gene expression are essential for the development of IPA. We further show that a pacC gain of function mutant is increased in virulence and that constitutive activation of PacC can confer virulence to a palB loss of function mutant. These data demonstrate a pivotal role for PacC in A. nidulans pathogenicity.
Results
Construction and characterization of prototrophic A. nidulans pacC and palB mutants
Five prototrophic A. nidulans mutants (Table 1), each having a different perturbation of the pH regulatory system, were constructed either by meiotic crossing of classically constructed, auxotrophic mutants (Dorn, 1965; Denison et al., 1995; Tilburn et al., 1995; Diéz et al., 2002; Fernández-Martínez et al., 2003) to wild-type strains or by A. nidulans protoplast transformation.
| Strain designation | Genotype | Nucleotide changea | Mutant proteinb(amino acids) | Source or reference |
|---|---|---|---|---|
| B7 | palB7 | palB (G2856T) | PalB (1–791e) | Denison et al. (1995) and M.A. Peñalva (pers. comm.) |
| B7Rd | palBR | palB (A2858C) | This study | |
| C209 | pacC+/–209 | pacC (T2451C) | PacC (L498S) | Diéz et al. (2002) |
| C209Rd | pacCR | pacC (G2434A) | This study | |
| C14 | pacCc14 | pacC (C2437A) | PacC (5–492e) | Tilburn et al. (1995) |
| C14Rd | pacC+ | pacC (G2434A) | This study | |
| C6309 | pacC–6309; pacCc63 | pacC (ΔC832, A833T; T1734G) | No PacC | Fernández-Martínez et al. (2003) |
| C6309Rd | pacCR | pacC(G2434A) | This study | |
| B7C11 | palB7; pacCc11 | palB (G2856T); pacC (G2579T) | PalB (1–791e); PacC (5–540e) | This study and Tilburn et al. (1995) |
- a . PacC nucleotide numbering is consistent with Tilburn et al. (1995).
- b . PacC translation initiates at Met codon 5 of the ORF (Mingot et al., 1999) but residues are numbered from Met codon 1 to avoid inconsistency between pre- and post-1999 publications.
- d . R denotes silently mutated reconstituted allele.
- e . Stop codon after last residue shown.
To test the effect on A. nidulans virulence of eliminating PacC and therefore abolishing PacC-regulated gene expression (though retaining functional pH signalling) a pacC null strain, C6309, was constructed. C6309 is devoid of PacC because of a frameshift in the initiating methionine codon of the pacC gene (Fernández-Martínez et al., 2003). We also examined the requirement for PacC processing without impairing ambient pH signalling. This was achieved by using a prototrophic strain, C209, with a single proteolysis-preventing missense mutation in the pacC gene (Diéz et al., 2002). The effect of blocking ambient pH signalling in vivo was determined using strain B7, which has a loss of function mutation in the palB gene. Strain B7 is unable to catalyse the pH-dependent initial proteolytic cleavage of PacC, which normally occurs in response to alkaline pH (Diéz et al., 2002).
To test the effect of pH-independent constitutive processing of PacC two approaches were taken. A prototrophic mutant having a C-terminal truncation of PacC was constructed in an otherwise wild-type background, strain C14. C14 does not require Pal ambient pH signalling functionality for PacC processing as mutational truncation of the PacC protein in this strain renders it permanently accessible to the subsequent pH-independent processing protease (Tilburn et al., 1995; Diéz et al., 2002). We constructed a second strain with similarly constitutive PacC processing, B7C11, this time in a pH signalling deficient background. B7C11 was made, in the B7 genetic background, by replacing pacC with a polymerase chain reaction (PCR)-amplified pacC gene from a pacCc11 mutant and directly selecting for growth at pH 8. The pacCc11 mutation results in a stop codon truncating PacC after amino acid residue 540 (Tilburn et al., 1995). It is important to note that there is no practicable selection method in an A. nidulans wild-type strain for selecting directly transformants having any of the mutations used in this study. pal– transformants can only be directly obtained with a realistic probability using an areA repressed (areAr) mutant (as pal − mutants suppress the inability of areAR mutants to utilize γ-aminobutyrate as nitrogen source) and the same is true of pacC null mutations for which a slow growth phenotype would be an additional handicap (Arst et al., 1994; Fernández-Martínez et al., 2003). pacC non-processible mutations can only be obtained at a reasonable frequency by γ-aminobutyrate utilization selection in an areA repressed homozygous diploid in order to select additionally for partial dominance (Diéz et al., 2002) and pacC constitutive transformants can only be obtained with a realistic probability by suppression of a pal– mutation (Tilburn et al., 1995).
To control for variation in genetic backgrounds between strains we systematically reconstituted the lesion in each A. nidulans mutant by homologous integration, in single copy, of a silently mutated version of the appropriate gene and used direct selection for expected phenotypes (see Experimental procedures). Thus isogenic sets of mutant and reconstituted A. nidulans strains were generated with the advantage that strains having reconstituted alleles could be distinguished in the possible presence of contaminating wild-type strains.
We next tested the ability of each strain to grow in vitro across a pH range of 6–8 under a variety of nutritional or stress-inducing conditions. These conditions included carbon catabolite derepression/gluconeogenic metabolism resulting from utilization of 1% (v/v) ethanol as carbon source, nitrogen metabolite derepression resulting from utilization of 1 mg ml−1 uric acid as nitrogen source, phosphate derepression, addition of 7% (v/v) fetal calf serum (FCS), osmotic stress resulting from 1 M NaCl and oxidative stress through addition of 0.003% (v/v) hydrogen peroxide (Table 2). These new data are consistent with pH being the over-riding physical factor influencing the growth properties of these mutants. Radial growth resulting from inoculation of 105 conidia was measured after 48 h of incubation at 37°C (Table 2). The reconstituted strains, C6309R, C209R, B7R and C14R displayed comparable growth capabilities on all media tested, confirming the absence of any additional mutations having pH-dependent growth phenotypes in the background of any strain used in this study. Consistent with previous observations (Peñalva and Arst, 2004) C6309 shows an overall reduction in radial growth at all pHs and on all media tested. Absence of PacC (C6309) allowed slow growth at elevated pH but the absence of PacC processing attributed to defective ambient pH signalling (B7) or the presence of a proteolysis-resistant PacC protein (C209) completely prevented growth beyond pH 7.2 on minimal medium in vitro (Table 2). The B7 growth phenotype was completely rescued by introduction of the C-terminally PacC truncating mutation, pacCc11, in strain B7C11 (Table 2). A. nidulans mutants, C14 and B7C11, able to process PacC constitutively, have comparable growth rates to reconstituted strains with no demonstrable growth advantage in vitro at any pH.
| Growth medium | Mean radial growth rate (µm h−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B7 | B7R | B7C11 | 209 | 209R | 6309 | 6309R | C14 | C14R | |
| Minimal medium | |||||||||
| pH 6 | 0.28 | 0.31 | 0.25 | 0.28 | 0.28 | 0.17 | 0.31 | 0.28 | 0.35 |
| pH 7.2 | 0 | 0.29 | 0.25 | 0 | 0.26 | 0.06 | 0.28 | 0.24 | 0.29 |
| pH 8 | 0 | 0.2 | 0.19 | 0 | 0.21 | 0.06 | 0.19 | 0.21 | 0.23 |
| 1% (v/v) ethanol as carbon source | |||||||||
| pH 6 | 0.24 | 0.3 | 0.21 | 0.27 | 0.27 | 0.14 | 0.27 | 0.25 | 0.32 |
| pH 7.2 | 0 | 0.26 | 0.12 | 0 | 0.27 | 0.04 | 0.24 | 0.24 | 0.27 |
| pH 8 | 0 | 0.1 | 0.11 | 0 | 0.15 | 0 | 0.15 | 0.17 | 0.16 |
| 0.1 mg ml−1 uric acid as nitrogen source | |||||||||
| pH 6 | 0.31 | 0.33 | 0.24 | 0.27 | 0.27 | 0.24 | 0.3 | 0.29 | 0.33 |
| pH 7.2 | 0.08 | 0.27 | 0.25 | 0.07 | 0.18 | 0.08 | 0.29 | 0.28 | 0.29 |
| pH 8 | 0 | 0.23 | 0.21 | 0 | 0.21 | 0.04 | 0.22 | 0.22 | 0.22 |
| 7% (v/v) FCS | |||||||||
| pH 6 | 0.37 | 0.38 | 0.26 | 0.34 | 0.3 | 0.22 | 0.37 | 0.29 | 0.38 |
| pH 7.2 | 0.07 | 0.37 | 0.24 | 0.09 | 0.31 | 0.08 | 0.34 | 0.27 | 0.35 |
| pH 8 | 0 | 0.32 | 0.24 | 0 | 0.3 | 0.06 | 0.31 | 0.26 | 0.31 |
| 1 M NaCl | |||||||||
| pH 6 | 0.23 | 0.24 | 0.22 | 0.23 | 0.24 | 0.07 | 0.24 | 0.22 | 0.24 |
| pH 7.2 | 0.09 | 0.06 | 0.09 | 0.06 | 0.08 | 0.03 | 0.08 | 0.07 | 0.09 |
| pH 8 | 0 | 0.07 | 0.08 | 0 | 0.29 | 0 | 0.08 | 0.06 | 0.08 |
| Low phosphate | |||||||||
| pH 6 | 0.33 | 0.3 | 0.31 | 0.3 | 0.3 | 0.1 | 0.3 | 0.31 | 0.27 |
| pH 7.2 | 0.12 | 0.21 | 0.23 | 0.1 | 0.2 | 0.07 | 0.22 | 0.21 | 0.2 |
| pH 8 | 0.08 | 0.19 | 0.19 | 0.1 | 0.17 | 0.07 | 0.19 | 0.19 | 0.18 |
| 0.003% (v/v) H2O2 | |||||||||
| pH 6 | 0.25 | 0.3 | 0.25 | 0.23 | 0.29 | 0.08 | 0.28 | 0.25 | 0.31 |
| pH 7.2 | 0.13 | 0.24 | 0.21 | 0.11 | 0.22 | 0.04 | 0.24 | 0.19 | 0.24 |
| pH 8 | 0.08 | 0.19 | 0.19 | 0.1 | 0.17 | 0.07 | 0.19 | 0.19 | 0.18 |
- 105 conidiospores from each strain were spotted, in triplicate, in a volume of 1 µl onto Aspergillus minimal media buffered to pH 6, 7.2 or 8.0 with a final concentration of 200 mM phosphate in the presence or absence of 1% (v/v) ethanol as sole carbon source, 0.1 mg ml−1 uric acid as sole nitrogen source, 7% (v/v) foetal calf serum (FCS), 1 M NaCl or 0.003% (v/v) H2O2. Low phosphate medium (Caddick and Arst, 1986) was buffered using 20 mM Tris-HCl pH 8 or pH 7.2, or 2-morpholinoethanesulphonic acid (MES) pH 6.0. Colony diameters were measured following incubation for 72 h at 37°C and mean radial growth rate calculated. No significant deviation from the mean was observed for any colony.
Aspergillus nidulans ambient pH signalling and the pH-responsive transcription factor PacC are essential for virulence
Murine aspergillosis resulting from A. nidulans infection has been described in neutropenic mice (Tang et al., 1994). Although higher infectious doses of A. nidulans are required in order to achieve murine infection as compared with A. fumigatus, the kinetics of infection, based upon survival analyses, appear similar for the two species (Tang et al., 1993; 1994). We chose an infectious dose of 2 × 106 conidiospores, which routinely resulted in 50–60% mortality by day 5 post infection, in dose assessment studies with the wild-type A. nidulans laboratory strain, EBPN17 (data not shown).
We used strains C6309, C209 and B7 to investigate the requirement for the A. nidulans transcription factor PacC, PacC proteolytic processing and ambient pH signalling, respectively, in murine infection. To control for variation in genetic backgrounds between strains, the virulence of each mutant was directly compared with that of its otherwise isogenic reconstituted counterpart, using groups of 15 neutropenic mice (Fig. 1). Growth of A. nidulans mutants in the murine lung and associated inflammation were monitored by sacrificing animals at predetermined time points following infection and examining paraffin-embedded sections stained with GMS for hyphal growth, or H&E for cellular visualization respectively. Conidial deposition in bronchioles and alveoli was observable after 20 h for all mutant and reconstituted strains (data not shown).
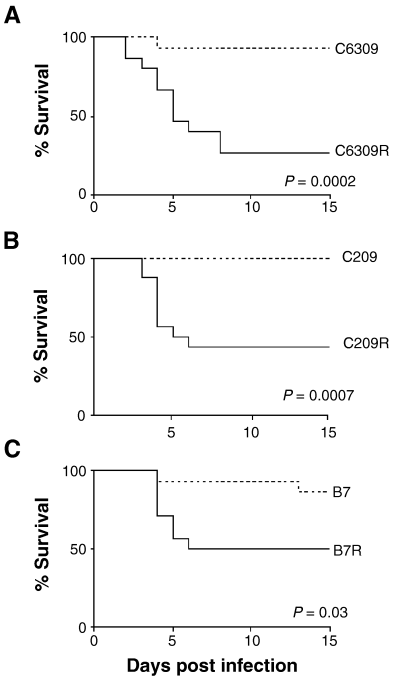
Loss of function A. nidulans pacC and palB mutants are attenuated for virulence. Kaplan–Meier log rank analysis of survival. Groups of 15 neutropenic mice were intranasally infected with 2 × 106 condiospores of A. nidulans C6309 (A), C209 (B) and B7 (C) loss of function pacC and palB mutants (dotted lines), and isogenic reconstituted strains, C6309R (A), C209R (B) and B7R (C) (solid lines).
Only one of 15 mice inoculated with strain C6309 was sacrificed during the course of our experiments. Mortality within this group was therefore 7% by day 15 post infection compared with 73% in mice infected with the reconstituted strain, C6309R (Fig. 1A). The attenuation of virulence in C6309 is significant (P = 0.0002), and directly attributable to the pacC mutation, indicating that PacC is essential for A. nidulans virulence in murine IPA. By 40 h post infection we observed conidial germination in C6309 infected mice (Fig. 2A). Germlings constituted around a third of fungi stained and were confined to conglomerations of conidia and host cells within the bronchioles. Evidence of C6309 conidia and germ tubes were detectable up to 15 days post infection but no A. nidulans was culturable from lung homogenates, and minimal inflammation was associated with such debris (data not shown). In contrast, infection with C6309R resulted in germination of almost all conidia and considerable hyphal growth, accompanied by heavy recruitment of inflammatory cells to sites of infection, after 40 h (Fig. 2C,D). By 60 h post infection hyphae were branched and septate and penetration of surrounding tissue was becoming evident (data not shown).

Fungal burden and inflammation is reduced in mice infected with loss of function pacC and palB mutants. Murine lung sections following infection with A. nidulans pacC or palB loss of function mutants C6309 (A and B), and C209 (E and F) and isogenic reconstituted strains C6309R (C and D) and C209R (G and H). Mice were sacrificed 40 h after infection and lungs were removed immediately and fixed in 4% (v/v) formaldehyde. Lungs were embedded in paraffin prior to sectioning and stained with haemotoxylin and eosin (H&E) (B, D, F and H) or Grocott's methenamine silver (GMS) (A, C, E and G).
Having established that the A. nidulans transcription factor PacC is essential for murine aspergillosis, we next determined whether proteolytic processing of PacC is required during infection by using the processing resistant mutant C209 and its isogenic reconstituted strain C209R (Fig. 1B). C209 was completely attenuated for virulence (P = 0.0007), demonstrating that PacC processing is essential for virulence. Lung sections fixed after 20 h of infection contained many germinating C209 conidia in alveolar spaces and evidence of surrounding inflammation (data not shown). By 40 h post infection isolated germinating C209 conidia were still observable in tissue sections. However, these were few in number and largely restricted to conidial aggregates within bronchioles. At this time ungerminated spores now predominated in C209 infected lungs mostly in or beside alveoli, usually in association with host cells (Fig. 2E), mild recruitment of inflammatory cells was also evident (Fig. 2F). After 60 h, conidia in discrete airway sites had germinated and extended germ tubes, indicating that C209 growth in vivo was not completely prevented but restricted to larger airways and slowed to a sufficient degree to preclude development of fatal infection (data not shown). In contrast, reconstitution of the C209 lesion resulted in considerable hyphal growth and inflammatory infiltration to sites of infection after 40 h (Fig. 2G,H).
Finally, to determine whether virulence is dependent upon Pal-mediated PacC proteolysis in vivo we tested the virulence of the pH signalling mutant B7. Infection with this mutant resulted in 13% mortality by day 15 (Fig. 1C), which was significantly lower (P = 0.03) than that caused by the reconstituted strain B7R (47% mortality by day 6) indicating that the presence of PacC is insufficient for murine virulence in the absence of the ambient pH signal. Histological findings were similar to those described for C209 infection with apparent clearance of germinating conidia between 20 and 40 h post infection. Ungerminated conidia, associated with mild inflammatory lesions, predominated in lung sections after 40 h of B7 infection and B7 conidia in airway aggregations had formed young hyphae by 60 h post infection (data not shown). No A. nidulans was culturable from the lungs following sacrifice at 15 days post infection, demonstrating an inability of this mutant to infect invasively the host despite being capable of slow growth in vivo. Histological examination of tissues following infection with the reconstituted strain, B7R, revealed a significant degree of hyphal growth and a marked increase in inflammation compared with infection with B7R but did not differ significantly from that observed with other reconstituted strains.
We cannot comment on relative germination times for our mutants in vivo. This would require a focused time course study and multiple infections with each strain followed by quantitation of germinated versus ungerminated spores for each mutant and an assessment of conidial viability. In the absence of such data it is impossible to estimate the proportions of viable and non-viable conidiospores in histological sections. We have compared the germination rates of these mutants in vitro over the relevant pH range and found no significant differences between mutants at any pH tested (E. Bignell, unpubl.). We were unable to find evidence of invasive growth in the lungs of the three mice that became ill following infection with pacC or palB loss of function mutants. Germinating conidia had become aggregated in airways so sickness in these animals might have been attributable to airway obstruction, assuming that there were no underlying physical or genetic factors.
Constitutive PacC processing proteolysis enhances pathogenicity and promotes invasive growth
In contrast to the attenuation of virulence caused by the PacC or pH signalling defects described above, constitutive processing of PacC, resulting from modest PacC truncation, results in significantly increased mortality (P = 0.02) (Fig. 3A). Infection with strain C14 resulted in 67% mortality by day 4 compared with only 27% mortality with the reconstituted strain C14R at the same time point. Histological examination of lung sections revealed an increase in tissue invasion by fungal hyphae in C14 infected mice compared with the reconstituted strain C14R (Fig. 4). Tissue penetration had occurred extensively by 40 h post infection in sharp contrast to infection with the reconstituted strain where, by the same time point, we only saw germination and limited hyphal growth, the majority of which was restricted to airways and alveolar spaces (Fig. 4). We observed similar results with a second constitutive strain, B7C11 (Fig. 3B), which was significantly more virulent than the recipient strain B7 (P < 0.0001), demonstrating that constitutive PacC processing can confer virulence in the absence of Pal-mediated signalling. Infection with B7C11 resulted in higher mortality (87% by day 14) than either B7 (13% by day 14) or B7R (47% by day 14). Histological examination of lung tissue at 40 h post infection revealed prominent tissue penetration by B7C11 hyphae (Fig. 4), further substantiating the finding that constitutive processing of PacC enhances virulence.

Constitutive PacC activation enhances virulence in both the presence (C14) and absence (B7C11) of Pal-mediated ambient pH signalling. Groups of 30 neutropenic mice were intranasally infected with 2 × 106 condiospores of A. nidulans C14 and B7C11 pacC constitutive mutants (dotted lines) and isogenic reconstituted, C14R, or suppressed, B7C11, strains (solid lines).
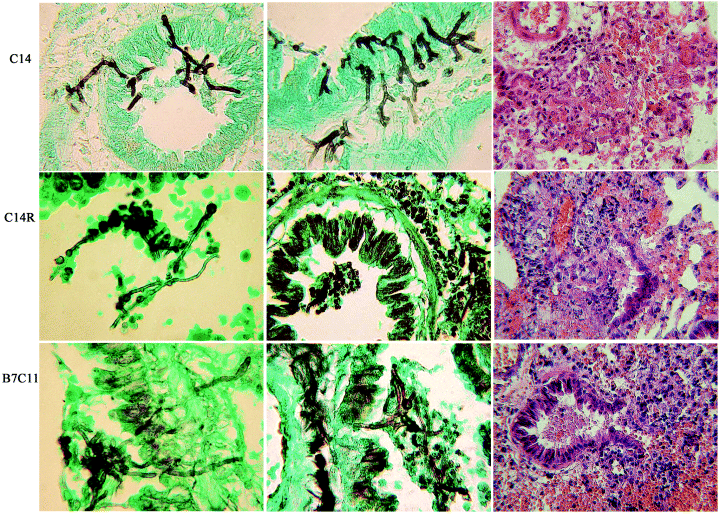
Earlier onset of invasive growth by pacC constitutive mutants. Murine lung following infection with the A. nidulans constitutive pacC mutant, C14, and isogenic reconstituted strain C14R; and the palB7-suppressed strain B7C11 (B7 and B7R strain background). Mice were sacrificed 40 h after infection and lungs were removed immediately and fixed in 4% (v/v) formaldehyde. Lungs were embedded in paraffin prior to sectioning and stained with haemotoxylin and eosin (H&E) or light green and Grocott's methenamine silver (GMS).
Discussion
This is the first study to demonstrate that pH-regulated gene expression, mediated by the conserved fungal transcription factor, PacC, is essential for pulmonary Aspergillus infection. Moreover, we establish that both PacC processing and Pal-mediated pH signalling are required to promote processes integral to pathogenicity and that constitutive activation of PacC enhances virulence.
Although the impact of pH adaptation on pulmonary fungal infection had not been addressed prior to this study, such capability might be considered important for successful pathogenesis. Physiological pH might exert a significant degree of stress on soil-dwelling organisms such as Aspergilli, which are likely to be exposed to a wide pH range within microenvironmental host niches as well rapid changes in pH following phagocytosis by macrophages or exposure to neutrophil vacuole contents (Levitz et al., 1999; Newman, 1999; Reeves et al., 2002; Ibrahim-Granet et al., 2003). pH is a signal of demonstrated physiological relevance in murine systemic and vaginal candidiasis where virulence of Candida albicans mutants lacking either of the pH regulated cell surface glycoproteins Phr1 or Phr2 are attenuated according to the pH of the host niche (De Bernardis et al., 1998). Homologues of most of the A. nidulans pH regulatory components (except palC) are present in C. albicans; indeed PHR1 and PHR2 are subject to positive and negative control, respectively, by the C. albicans PacC homologue, Rim101 (Ramon et al., 1999; Davis et al., 2000a). Moreover, rim101 mutants are significantly reduced in virulence and development of kidney pathology in a murine model of systemic candidiasis (Davis et al., 2000b). More recently, a Fusarium oxysporum pacC+/– partial loss of function mutant was shown to have significantly reduced virulence in systemic murine infection compared with the parental wild-type strain (Ortoneda et al., 2004). In this study we used a murine model of IPA and a series of A. nidulans mutants, having pH-adaptive and non-responsive phenotypes, to examine the importance of fungal pH adaptation during pulmonary infection and identify the regulatory components required to mediate this process in vivo.
Our initial observations established that absence of the PacC protein completely attenuates murine virulence and led us to conclude that activities under PacC control are essential for A. nidulans pathogenicity. Given the manner in which activation of PacC occurs in vitro, the next logical step in determining the basis of PacC mediated virulence was to assess the requirement for PacC processing proteolysis during infection. Prevention of ambient pH signalling, by mutation of palB attenuates virulence, demonstrating that the Pal signal transduction pathway is essential for the role of PacC in virulence in vivo, most likely through pH-dependent PacC processing. Further support for this hypothesis is provided by the proteolysis resistant pacC mutant, C209, which is also attenuated for virulence. In addition to alkaline sensitivity null pacC alleles, including 6309 (Tables 1 and 2), exhibit phenotypic features such as cryosensitivity and slow growth with poor conidiation at permissive temperature (Peñalva and Arst, 2004). These features are likely attributed to the total absence of the PacC processed form rather than the full-length form because they are not found in truncation mutants having PacC forms approximating the fully processed form, PacC27 (Peñalva and Arst, 2004). C6309 is less virulent than an isogenic wild-type strain as are a proteolysis resistant pacC mutant, C209, and a pH signal transduction pal mutant, B7. However, neither of the latter two strains exhibit cryosensitivity, slow growth or poor conidiation. Therefore, despite the pleiotropic nature of the pacC null phenotype it is unlikely that the reduced pathogenicity associated with pacC null alleles is solely attributable to the general growth defect demonstrated by C6309. Moreover, we conclude that full Aspergillus virulence can be directly correlated to the presence (and likely the quantity) of processed PacC. Somewhat more of the processed form is detectable in A. nidulans pacCc14 protein extracts under neutral and alkaline conditions as compared with wild-type using electrophoretic mobility shift assays (EMSA) (Orejas et al., 1995; Denison et al., 1998). Thus there appears to be a correlation between increased quantities of processed PacC and hypervirulence. It should nevertheless be noted that, if pHs approaching or exceeding neutrality prevail in vivo most of the wild-type PacC protein will be in the processed form (Orejas et al., 1995).
These data provide significant insight into the relevance of PacC-mediated gene expression in invasive aspergillosis. All mutants having attenuated virulence phenotypes are also alkali-sensitive in vitro. The most obvious interpretation of these survival data is therefore one of prevailing alkalinity in the neutropenic murine lung such that growth and invasion by alkali-sensitive PacC null or processing deficient mutants is prevented. This would agree with a current estimate of airway surface liquid pH, which has been made from healthy murine lungs, at pH 7.28 (Song et al., 2003). However, several lines of evidence argue against such a simplistic interpretation, which would be based upon the unjustified assumption that the tested in vitro growth conditions are not substantially different to those prevailing in vivo. Furthermore any argument that there is a direct correlation between radial growth rate at elevated pH in vitro and pathogenicity is weakened by the hypervirulence of pacC constitutive mutants, which have no demonstrable growth advantage over reconstituted strains at alkaline pH in vitro. In contrast to a complete lack of growth by processing deficient pacC and palB loss of function mutants after 48 h growth on minimal medium in vitro, germination and slow hyphal growth were observable in vivo after 40 h of infection (data not shown). This could be explained by the increased availability or improved uptake of certain substance(s) in vivo as compared with the minimal medium employed in our analysis. Indeed phosphate derepression, nitrogen metabolite derepression, 7% (v/v) serum, 1 M NaCl and exposure to hydrogen peroxide permitted gradual growth in vitro (Table 2). The fact that growth by these strains in vivo was primarily in the bronchioles may be indicative of an inability to adhere to or invade host tissues or attributed to absence of enzymes required for tissue penetration.
Current evidence suggests varied regulatory roles for PacC (Peñalva and Arst, 2002). A. nidulans secondary metabolite production can be sensitive to both external pH and pacC mutation (Peñalva and Arst, 2002), for example, alkaline growth conditions and/or alkalinity-mimicking pacCc mutations substantially increase penicillin production (Shah et al., 1991; Espeso et al., 1993) or decrease Aspergillus parasiticus aflatoxin and A. nidulans sterigmatocystin production (Keller et al., 1997). It is therefore feasible that the production of toxic substances under PacC control may contribute to the infection process. Several A. nidulans secreted enzymes have demonstrated PacC dependency including acid and alkaline phosphatases and an alkaline protease, PrtA (Caddick et al., 1986; Tilburn et al., 1995). A. fumigatus Alp, a PrtA homologue, is the major protease secreted by A. fumigatus when incubated with protein at neutral pH and has most recently been implicated in disruption of the actin fibre cytoskeleton and loss of focal adhesion sites in infected lung pneumocytes (Kogan et al., 2004). We have previously shown that a secreted alkaline protease gene, prtA, is subject to positive control by PacC and is upregulated at neutral pH when PacC is constitutively processed (Tilburn et al., 1995). Indirect evidence links proteolytic enzyme production to A. fumigatus pathogenicity (Blanco et al., 2002), but although a correlation can be found between elastase activity and virulence in mice (Kothary et al., 1984), generation of several protease deficient A. fumigatus mutants has failed to identify any single enzyme as contributing significantly to virulence (Latgé 1999 ). Although current data on secreted protease production cannot fully explain Aspergillus virulence, it is feasible that appropriately co-ordinated production of secreted enzymes is important. Fungal proteases can induce local airway inflammation by recruiting inflammatory cells via the activation of epithelial cells. A. fumigatus culture filtrates induce the production of the proinflammatory cytokines IL8, IL6 and MCP-1 and cause cell detachment in pulmonary epithelial cell lines, phenomena that are prevented by inhibition of fungal serine protease activity (Tomee et al., 1997). A. fumigatus culture filtrates from a clinically isolated wild-type strain were significantly more able to prevent neutrophil-mediated hyphal damage in human polymorphonuclear leukocytes than those from an isogenic alkaline protease (Alp) mutant (Ikegami et al., 1998). These findings are pertinent as in addition to restricting growth in vivo, an absence of processed A. nidulans PacC lessened fungal provocation of host inflammatory responses in our hands as evidenced by reduced infiltration of immune cells to sites of lung infection (Fig. 2).
The availability of free iron is a major limiting factor for microbial growth in the human body (Neilands et al., 2004). Recent studies have determined that the ability of A. fumigatus to acquire iron and survive in serum involves siderophore-mediated removal of iron from transferrin implying a role for siderophore biosynthesis in vivo (Hissen et al., 2004). Moreover, A. fumigatusl-ornithine-N5-monooxygenase (SidA), which catalyses the first committed step of hydroxamate-type siderophore biosynthesis, is absolutely essential for virulence (Schrettl et al., 2004). A. nidulans siderophore biosynthesis and uptake are regulated by both iron availability and the PacC-mediated ambient pH regulatory system (Eisendle et al., 2003; Eisendle et al., 2004). Acidity-mimicking mutations, such as that of C6309, downregulate siderophore production at pH 7.0 (Eisendle et al., 2004). However, a deficiency in iron uptake ability attributed to reduced siderophore biosynthesis is unlikely to explain attenuation as Fe2+ supplementation at high pH in vitro was unable to rescue the growth phenotypes of our mutants (data not shown). Loss of function pacC mutants still show weak pH regulation of siderophore biosynthesis (Eisendle et al., 2004) indicating the existence of pH-independent effects on iron uptake. Clearly ambient pH can influence gene expression in ways that are completely independent of the PacC pH regulatory system, e.g. by affecting the uptake of molecules that induce or repress gene expression (Peñalva and Arst, 2002).
Recently genes regulated by the S. cerevisiae PacC homologue Rim101 have been identified (Lamb et al., 2001; Lamb and Mitchell, 2003). The C. albicans homologues of a number of these genes are absolutely required for virulence, e.g. NRG1 (Saville et al., 2003), while others encode functions necessary at specific stages in the infection process, e.g. ARN1 and UTR2 (Heymann et al., 2002; Alberti-Segui et al., 2004). These observations, in tandem with our data, suggest that activities under PacC control have the potential to enhance virulence.
We have presented an examination of the requirement for and nature of PacC action in vivo and demonstrated that activities under PacC control contribute to growth in vivo, tissue invasion and virulence, identifying PacC as a virulence determinant in murine invasive aspergillosis. Characterization of the PacC regulatory realm is in progress in our laboratories and likely to increase greatly our understanding of virulence mechanisms under its control.
Experimental procedures
A. nidulans strains, genetic analysis and phenotypic testing
Genotypes, mutant loci and sequence-derived polypeptides for A. nidulans strains are listed in Table 1. Strains were cultured on Aspergillus complete medium (Cove, 1966) containing 200 mM NaH2PO4. Prototrophic mutants were directly selected from the progeny of meiotic crosses (Pontecorvo et al., 2004) using Aspergillus minimal medium (Cove, 1966) containing 5 mM ammonium (+)-tartrate. Reconstitution of mutants was achieved using silently mutated alleles to enable distinction from wild-type contaminants. For radial growth assays 105 conidiospores from each strain were spotted, in triplicate, in a volume of 1 µl onto Aspergillus minimal media buffered to pH 6, 7.2 or 8.0 with a final concentration of 200 mM phosphate in the presence or absence of 1% (v/v) ethanol, 0.1 mg ml−1 uric acid, 7% (v/v) FCS, 1 M NaCl or 0.003% (v/v) H2O2. Low phosphate medium (Caddick and Arst, 1986) was buffered using 20 mM Tris-HCl pH 8 or pH 7.2, or 2-morpholinoethanesulphonic acid (MES) pH 6.0. Colony diameters were measured following incubation for 72 h at 37°C.
Plasmids
Plasmids were constructed to facilitate reconstitution of mutants. To replace the palB7 lesion in strain B7 with a silently mutated version of palB we constructed plasmid pAnB, containing a 985 bp region of the wild-type A. nidulans palB gene [+2108 to +3093 of the published nucleotide sequence (Denison et al., 1995)]. This region was PCR-amplified from A. nidulans genomic DNA using the oligonucleotide primers (TACTTATACCGCCGTCGTGTC and CCAA CAAGTTTCTGCAGCAT). The amplified fragment was cloned into pGEM-T EASY vector (Promega Corporation). We performed site-directed mutagenesis on pAnB using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and the mutagenic oligonucleotide primer pair (GCGACTCGA TATCTGGCGTCCGA and CCACTCGGACGCCAGATATC GTG) to produce plasmid pAnBS.
Restoration of pacC loci having wild-type functionality to strains C209, C14 and C6309 was achieved using plasmid p4RC. p4RC was constructed by site-directed mutagenesis of plasmid p4 (Tilburn et al., 1995) using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and the mutagenic oligonucleotide pair (CAATGACGAGCGTCGTA GATACACTGGAGGAACATTGCAGC and GCTGCAATGT TCCTCCAGTGTATCTACGACGCTCGTCATTG).
Restoration of functional A. nidulans genomic loci
The palB7 lesion in strain B7 was reconstituted with a silently mutated version of the corresponding genomic region released from plasmid pAnBS by NotI digestion, prior to A. nidulans protoplast transformation (Tilburn et al., 1995). Putative reconstituted transformants were selected on pH 8 regeneration medium (Negrete-Urtasun et al., 1999), and sequenced appropriately to confirm the presence of the newly introduced silent mutation and absence of the palB7 lesion. Transformants having a single homologous integration event were identified by Southern analysis and a representative strain containing a functionally wild-type palB allele, B7R (Table 1), selected for further analyses.
pacC+/–209, pacCc14 and pacC–6309 lesions were reconstituted in a similar manner with a functionally wild-type pacC locus released from plasmid p4RC, using EcoRI and BamHI, and recipient strains C209, C14 and C6309 respectively. Putative reconstituted transformants were selected on pH 8 regeneration medium (Negrete-Urtasun et al., 1999) for C209 and C6309, or by restored conidiation on pH 6.5 regeneration medium (Tilburn et al., 1995) in the case of C14. Genomic DNA from, C209, C14 and C6309 rescued transformants was sequenced to confirm absence of the original pacC lesions and presence of the newly introduced silent mutations. Transformants having a single homologous integration event were identified by Southern analyses and representative strains, C209R, C14R and C6309R, respectively, chosen for further analysis.
The constitutively active pacCc11 allele (Tilburn et al., 1995) was amplified by PCR using primers (TTCGTACTCG AAACCGTTGT and GCGAACCATTCATGCTCAAA). This DNA fragment was introduced into strain B7 by protoplast transformation followed by selection on pH 8 regeneration medium (Negrete-Urtasun et al., 1999). Genomic DNA from transformants was sequenced to confirm the presence of both the palB7 and pacCc11 mutations. Transformants having a single homologous integration event were identified by Southern analysis and a representative strain, B7C11, was chosen for further analysis.
Virulence studies
Murine infections were performed under UK Home Office Project Licence PPL/70/5361 in dedicated facilities at Imperial College London. Groups of up to 30 outbred male mice (strain CD1, 18–22 g, Harlan Ortech) were housed in individually vented cages and allowed free access to food and water. Mice were immunosuppressed as previously described (Tang et al., 1993). Briefly, intraperitoneal cyclophosphamide (150 mg kg−1, ENDOXANA, Asta Medica) was administered on days −3, −1, +2, and every subsequent third day throughout each experiment. A single dose of hydrocortisone acetate (112.5 mg kg−1, HYDROCORTISTAB, Sovereign Medical) was administered subcutaneously on day −1. All mice received tetracycline hydrochloride 1 mg l−1 and ciprofloxacin 64 mg l−1 in drinking water as prophylaxis against bacterial infection. None of the A. nidulans mutants used for in vivo analysis demonstrated sensitivity to any of the agents administered during infection. These were tested at 10-fold concentrations of estimated tissue concentrations assuming 100% uptake and retention of administered substances, either alone or in combination (data not shown). Aspergillus spores for inoculations were grown on Aspergillus complete medium, containing 5 mM ammonium (+)-tartrate and 200 mM NaH2PO4, for 5 days prior to infection. Conidia were freshly harvested using sterile saline (Baxter Healthcare) and filtered through MIRACLOTH (Calbiochem). Conidial suspensions were spun for 5 min at 3000 g, washed twice with sterile saline, counted using a haemocytometer and resuspended at a concentration of 1–9 × 109 colony forming units (cfu) ml−1. Viable counts from administered inocula were determined following serial dilution by plating on Aspergillus complete medium and growth at 37°C. Mice were anesthetized by halothane inhalation and infected by intranasal instillation of 1–4 × 106 conidia in 40 µl of saline. Mice were weighed every 24 h from Day −3 and visual inspections made twice daily. In the majority of cases the end-point for survival experimentation was a 20% reduction in body weight measured from the day of infection, at which point mice were sacrificed. This usually occurred prior to emergence of other indicators of severe infection such as hunched posture, laboured breathing or a moribund state, which served as additional stand-alone end-points in cases where weight loss did not reach 20%. Viable Aspergilli were recovered from infected lungs following homogenization of lung tissue in 0.5 ml sterile saline using micropestles (Eppendorf). Homogenates were plated on Aspergillus complete medium following 10-fold dilution in sterile saline and incubated at 37°C for 48 h. Lungs for histological sectioning were removed immediately after sacrifice and fixed in 4% (v/v) formaldehyde (Sigma). Lungs were embedded in paraffin prior to sectioning and stained with H&E or light green and Grocott's methenamine silver (GMS).
Statistical analysis
Kaplan–Meier log rank analysis of survival was performed using Prism Software for Macintosh (GraphPad Software).
Acknowledgements
This work was supported by the Chronic Granulomatous Disorder Research Trust (Grant J4G/99/05 to T.R., K.H. and H.N.A.), the Wellcome Trust (Grant 067878 to H.N.A.) and the Biotechnological and Biological Sciences Research Council (Grant 60/P17835 to H.N.A., K.H. and T.R.). We thank Mariana Canedo, Lily Stanton and Phil Muckett for technical assistance and Joan Tilburn and Phillip Hopkins for critical reading of the manuscript, and Miguel Peñalva for drawing our attention to the study by Parsons et al. (2004) and providing the palB7 sequence change.




