Transcription activation of a UV-inducible Clostridium perfringens bacteriocin gene by a novel σ factor
Summary
Expression of the plasmid-encoded Clostridium perfringens gene for bacteriocin BCN5 was shown to depend in vivo and in vitro on the activity of UviA protein. UviA, also plasmid-encoded, proved to be an RNA polymerase σ factor and was also partly autoregulatory. The uviA gene has two promoters; one provided a UviA-independent, basal level of gene expression while the stronger, UviA-dependent promoter was only utilized after the cell experienced DNA damage. As a result, BCN5 synthesis is induced by treatment with UV light or mitomycin C. UviA is related to a special class of σ factors found to date only in Clostridium species and responsible for activating transcription of toxin genes in Clostridium difficile, Clostridium tetani, and Clostridium botulinum.
Introduction
Clostridium perfringens, a common pathogen of humans and domestic animals, is responsible for several medical conditions ranging from relatively mild food poisoning to the potentially life-endangering gas gangrene (Finegold, 1977). Many C. perfringens isolates produce antibacterial proteins, called bacteriocins (Tagg et al., 1976), and the analysis of bacteriocin-encoding plasmids has played an important role in the development of C. perfringens genetics. Garnier and Cole (1986) showed that C. perfringens strain CPN50, harbouring the plasmid pIP404, secretes bacteriocin BCN5 following UV irradiation. Such irradiation induces a high rate of transcription of a 4 kb segment of pIP404 that includes two contiguous transcription units, uviAB and bcn (Garnier and Cole, 1986; Garnier and Cole, 1988). The bcn gene, encoding BCN5, is transcribed from three promoters (P1, P2, P3) while transcription of uviAB initiates at two promoters (P4, P5). Garnier and Cole (1986; 1988) hypothesized that the uviAB operon might encode proteins needed for BCN5 synthesis or secretion or for immunity to the bacteriocin (Brefort et al., 1977), but no direct evidence for the involvement of uviAB gene products in BCN5 accumulation has appeared. The detection of similarity between UviA and some DNA binding proteins, including members of the σ70 family of RNA polymerase σ factors (Lonetto et al., 1992), suggested that UviA might play a regulatory role in BCN5 gene expression.
The σ subunits of RNA polymerase associate with the core enzyme subunits (α, β, and β′) and allow RNA polymerase to bind to specific sequences within the promoter region and initiate transcription (Travers and Burgess, 1969). Most bacterial species encode a major σ factor used for transcription of genes needed for intermediary metabolism and macromolecular synthesis, and one or more alternative σ factors specific for genes whose products allow the cell to adapt to changes in nutrient availability or environmental stress (Wösten, 1998).
Most σ factors in Eubacteria belong to the σ70 family, named for the principal σ factor of Escherichia coli. However, there is diversity within this family (Lonetto et al., 1992). The so-called extracytoplasmic function (ECF) σ factors, for instance, form a distinct group within the σ70 family (Lonetto et al., 1994), and even among the ECF-related σ factors one can discern coherent subgroups. For instance, TcdR (previously named TxeR or TcdD), the σ factor required for toxin gene expression in Clostridium difficile (Mani and Dupuy, 2001), is quite distinct from other members of the ECF group. Two other regulators of toxin synthesis, Clostridium tetani TetR and Clostridium botulinum BotR, are related to TcdR (Moncrief et al., 1997; Marvaud et al., 1998; Dineen et al., 2004). It has recently been demonstrated that TetR and BotR act as σ factors and are required for transcription of the neurotoxin genes in C. botulinum and C. tetani respectively (Raffestin et al., 2004). The −35 regions of target gene promoters for TcdR, TetR and BotR are nearly identical, with a conserved ‘TTTACA’ hexanucleotide motif (Fig. 1 and Raffestin et al., 2004). Thus, these three proteins, which control production of important toxins in several major pathogenic Clostridium species, seem to constitute a specific group (group 5) within the σ70 family (Helmann, 2002).
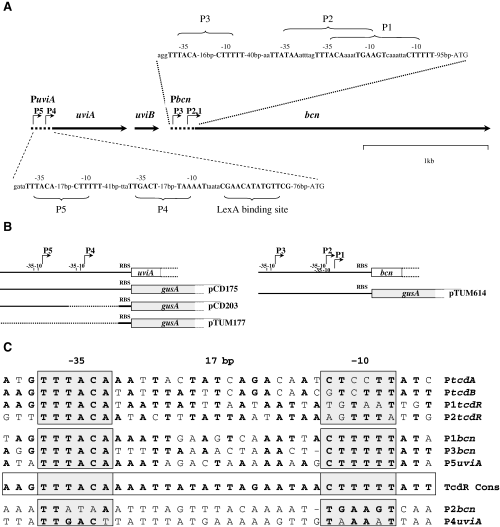
A. Genetic organization and partial restriction map of a 4 kb segment from the UV-inducible region of pIP404 (Garnier and Cole, 1988). Nucleotide sequences of promoter regions of uviA and bcn are indicated and the principal promoter elements (−35, −10 and LexA binding motif) are shown in bold. B. Schematic representation of the promoter regions indicating the portions of the uviA and bcn regulatory regions that were fused to the gusA reporter gene in the fusion vector pTUM177 (see Experimental procedures). C. Sequence comparison of the promoter regions of the C. difficile toxin A (PtcdA) and toxin B (PtcdB) genes, the putative promoters of the C. difficile tcdR gene, and the UV-regulated promoters of C. perfringens. With the exception of the tcdR promoters, the −35 and −10 regions are based on determination of the transcriptional start site in vivo (Dupuy and Sonenshein, 1998; Garnier and Cole, 1988; Mani et al., 2002). The promoter consensus sequences for the TcdR subfamily σ factors are shown.
In this paper, we show that UviA is also an alternative RNA polymerase σ factor of the TcdR group and that it is required for the activation of the UV-inducible bacteriocin gene of C. perfringens and for its own expression.
Results
UviA activates bcn expression and is autoregulatory in C. perfringens
The UV-inducible bcn promoters P1 and P3 are not recognized in an in vitro transcription system by the major form of C. perfringens RNA polymerase, suggesting that transcription from these promoters requires a positive regulatory factor or a special σ factor (Garnier and Cole, 1988). To test this hypothesis and to assess the possibility that UviA is the factor in question, a DNA fragment containing the bcn promoter region (including promoters P1, P2 and P3) was fused to the gusA reporter gene of E. coli in the fusion vector pTUM177 (Mani and Dupuy, 2001). The resulting plasmid (pTUM604) was introduced into C. perfringens strain SM101, a pIP404-free strain. Two versions of the fusion strain were created. One carried the plasmid pTUM423, a derivative of pTRKL2 that includes the uviA gene under the control of its natural promoters, and the other carried the vector pTRKL2.
As uviA gene expression is highly induced by DNA damage (Garnier and Cole, 1988), the resulting strains were treated with the DNA damaging agent mitomycin C, which is known to induce bacteriocin synthesis in certain strains of C. perfringens (Mahony, 1977). While a promoter-less gusA construct (pTUM177) failed to show any detectable activity whether UviA was expressed or not, the Pbcn–gusA fusion showed high-level expression, but only in the presence of UviA and in a manner that was dependent on the dose of mitomycin C (Fig. 2A). In the absence of the uviA gene, the Pbcn–gusA fusion was not expressed to a detectable extent at any mitomycin C concentration used (Fig. 2A). These results indicate that UviA greatly stimulates transcription from the bcn promoter region.
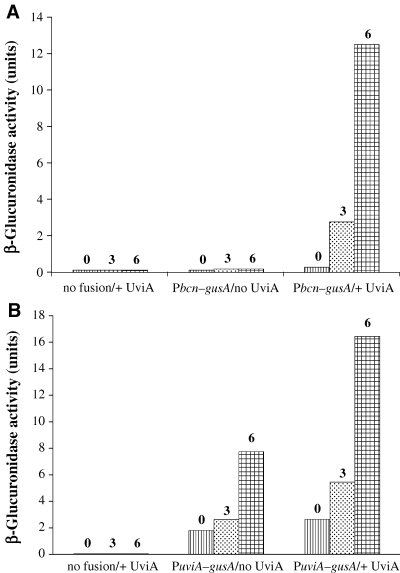
Activation of Pbcn–gusA (A) and PuviA–gusA (B) fusions by UviA. Plasmids carrying the bcn and uviA promoters, translationally fused to the gusA reporter gene, were introduced into C. perfringens with or without the mitomycin C-inducible uviA gene in trans. Strains were grown to mid-exponential growth phase and exposed to subinhibitory concentrations of mitomycin C (0, 3 or 6 µg ml−1 as indicated). Samples were removed after 3 h for measurement of β-glucuronidase activity. In control experiments, cells carrying the fusion vector alone were assayed. The values reported for this representative experiment did not vary by more than 15% in two to three additional trials.
To determine if UviA also activates it own expression, a DNA fragment containing the uviA promoter region (including promoters P4 and P5) was fused to the gusA reporter gene (creating pTUM175), and introduced into derivatives of C. perfringens strain SM101 that either carried the uviA gene or not. In the absence of UviA, a low level of PuviA–gusA expression was observed; such expression was increased in the presence of mitomycin C (Fig. 2B). When the uviA gene was present and was induced by treatment with mitomycin C, a higher level of GusA activity was observed, indicating that UviA activates uviA gene expression.
Overexpression and purification of UviA
To know how UviA functions biochemically, we cloned a version of the uviA gene in which the coding sequence had been extended to include six histidine codons at the C-terminus and then expressed the recombinant gene in E. coli. To avoid formation of inclusion bodies, we grew the cells at low temperature (as we had performed previously for C. difficile TcdR; Mani and Dupuy, 2001; Mani et al., 2002). His-tagged UviA was then purified in a single step using a nickel affinity column (see Experimental procedures). The identification of the purified UviA protein was confirmed by immunoblot analysis using antibodies to the His-tag (data not shown).
UviA enables holoenzyme and core enzyme of RNA polymerase to bind to the bcn promoter region
Even though UviA stimulates transcription from the bcn promoter in vivo, no direct interaction between UviA and the regulatory region of the bcn gene could be detected by a gel mobility shift assay (Fig. 3A and B). This result suggests that UviA is not a conventional positive regulator. As a similar result was obtained for interaction of TcdR and the C. difficile tox gene promoters (Mani and Dupuy, 2001), we were led to test the possibility that UviA acts, like TcdR, as a sigma factor. If so, UviA should enable RNA polymerase to bind to the bcn promoter and initiate transcription. To test binding, gel mobility shift assays were performed with RNA polymerase purified from C. perfringens (Katayama et al., 1999) and a DNA fragment encompassing the bcn promoter region. C. perfringens RNA polymerase (likely to be a mixture of the major holoenzyme form, other holoenzyme forms and core enzyme) was only able to bind to the bcn promoter fragment in the presence of added UviA, as shown by formation of heparin-resistant RNA polymerase–DNA complexes (Fig. 3A). The same sample of RNA polymerase without added UviA could bind well to the promoter region of the C. difficile glutamate dehydrogenase (gdh) gene, a gene expressed during exponential growth phase (Fig. 3C).
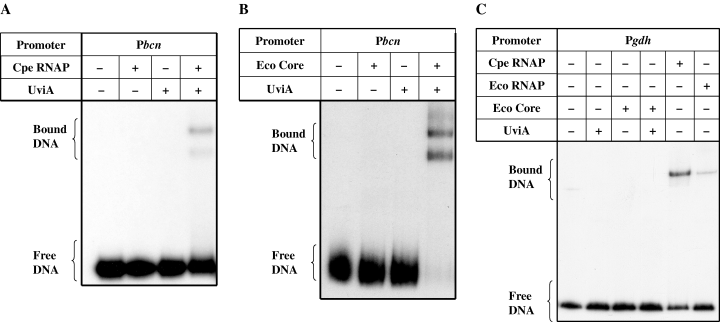
Gel mobility retardation of bcn (A and B) and gdh promoters (C). A DNA fragment containing the bcn promoter region was incubated with C. perfringens RNA polymerase (A) or E. coli RNA polymerase core enzyme (B), alone or after preincubation with UviA (see Experimental procedures). A DNA fragment containing the gdh promoter (C) was incubated with C. perfringens RNA polymerase or E. coliσ70 RNA polymerase alone, or with E. coli core enzyme with or without preincubation with UviA.
UviA was also able to permit interaction of E. coli core enzyme with the bcn promoter (Fig. 3B). Core enzyme, whether alone or mixed with UviA, was unable to shift the mobility of the gdh promoter-containing fragment, whereas E. coliσ70 holoenzyme, like C. perfringens RNA polymerase, was able to bind to the gdh promoter region (Fig. 3C). These results provide direct biochemical evidence that UviA is required for the interaction of RNA polymerase specifically with the bcn promoter region.
UviA activates RNA polymerase to transcribe from the bcn promoters in an in vitro transcription system
When a DNA fragment including the bcn promoter region and extending to a position within the coding sequence was mixed with UviA and RNA polymerase from C. perfringens in the presence of the substrates of the enzyme, transcripts of ∼130 nt , ∼140 nt and ∼213 nt were synthesized (Fig. 4B). Similar results were obtained with a mixture of UviA and E. coliσ70 RNA polymerase (Fig. 4B). The sizes of the transcripts corresponded closely to those predicted from primer extension analysis of in vivo RNA (Garnier and Cole, 1988). The P1 transcript was much more abundant than the others (Fig. 4B), as is also true in vivo (Garnier and Cole, 1988). In the absence of UviA, no bcn transcripts were detectable (Fig. 4B) whereas C. perfringens and E. coliσ70 RNA polymerase holoenzymes were able to transcribe from the gdh promoter without added UviA (Fig. 4A). These results confirm that UviA interacts with RNA polymerases and show that UviA activates transcription from the bona fide bcn promoters. An anomaly in our experiments is the failure of the mixture of UviA and core RNA polymerase to transcribe from the P2 promoter. C. perfringens RNA polymerase and E. coli holoenzyme both recognized P2 in the presence of UviA. One possibility is that P2 transcription requires an additional factor that is absent in the core enzyme preparation.
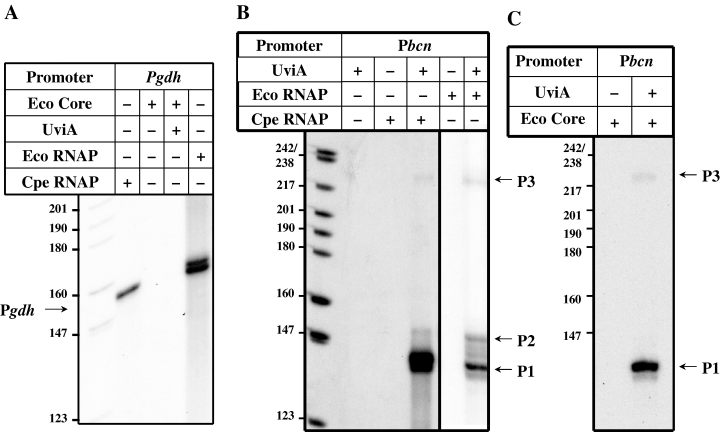
Transcription activation of bcn in vitro. Run-off transcription reactions were performed using E. coliσ70 RNA polymerase holoenzyme (A, B) or C. perfringens RNA polymerase (A, B) or E. coli RNA polymerase core enzyme (C) and DNA fragments containing either the gdh promoter (Pgdh) (A) or the bcn promoter (Pbcn) (B, C) incubated in the absence or presence of UviA. The expected sizes of run-off transcripts (indicated by arrows) are: Pgdh, 150 nt; P1bcn, 130 nt; P2bcn, 140 nt and P3bcn, 213 nt. Note that the mobility of the bands in A was skewed during electrophoresis; the bands in the second and fifth lanes had essentially the same mobility.
To test the ability of UviA to act as a sigma factor, we carried out in vitro run-off transcription assays with the core enzyme form of E. coli RNA polymerase in the presence or absence of UviA. Addition of UviA to E. coli core enzyme stimulated transcription from the P1 and P3 promoters of the bcn gene (Fig. 4C), whereas the core enzyme by itself was unable to activate transcription from either of these promoters (Fig. 4C) or from the gdh gene promoter used as a control (Fig. 4A). It is interesting to note that RNA polymerase holoenzyme reconstituted from E. coli core enzyme and UviA transcribed from the P1 promoter preferentially (Fig. 4C). As UviA is a protein that allows RNA polymerase core enzyme to bind to and initiate transcription from bona fide promoters, it acts by definition as a σ factor.
UviA interacts with the E. coli core RNA polymerase
To verify that UviA interacts with core RNA polymerase, we immobilized E. coli core RNA polymerase on membranes and then incubated the membranes with purified UviA protein or with a crude extract of E. coli strain BL21λDE3 (pET28-b). Retention of UviA was detected using anti-His tag polyclonal antibodies (see Experimental procedures). As shown in Fig. 5, a signal appeared in a dose-dependent manner only when the core enzyme was incubated with UviA (lanes 3–5) and not with a crude extract of E. coli (lane 2). Moreover, no specific binding of anti-His tag antibodies appeared with immobilized core enzyme (lane 1) or with crude extract protein of E. coli immobilized on the filter (lane 7). These results show that UviA is able to interact with RNA polymerase core enzyme in the absence of DNA, a key characteristic of σ factors.
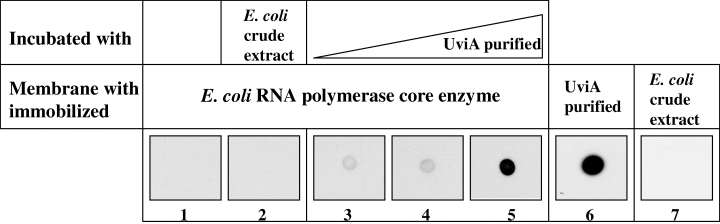
Interaction of purified UviA with E. coli RNA polymerase core enzyme. Core RNA polymerase (0.3 µg; filters 1–5) was spotted onto nitrocellulose membranes and immunoblotted using anti-His tag antibodies after incubation with no added protein (filter 1), crude extract of E. coli (200 µg) carrying the vector pET28-b (filter 2), or 1.5, 3.5 or 15 µg of purified UviA-His6 protein (filters 3–5 respectively). As controls for antibody specificity, purified UviA-His6 protein (10 ng; filter 6) or E. coli crude extract (1 µg; filter 7) were immobilized and immunoblotted.
UviA allows RNA polymerase to bind to the uviA promoter and to initiate transcription from uviA promoters in an in vitro transcription system
σ factors often regulate their own expression (Helmann, 2002). As the in vivo results (Fig. 2B) indicated that UviA is required for its own maximal expression, we tested the role of UviA in recognition of and transcription from the uviA promoters in vitro. Gel mobility shift assays showed that C. perfringens RNA polymerase could bind to the uviA promoter region even in the absence of UviA, but that the binding to the uviA promoter was greatly stimulated when UviA was added (Fig. 6A). Moreover, UviA caused the appearance of complexes that were UviA-specific. These results are consistent with low-level, UviA-independent expression of the PuviA–gusA fusion and the higher expression seen when UviA synthesis was induced (Fig. 2B). When we used E. coli RNA polymerase core enzyme instead of C. perfringens RNA polymerase, no shift in the mobility of the uviA promoter-containing fragment was seen unless UviA was present (Fig. 6B). Thus, UviA seems to act at the uviA promoter region by interacting with the RNA polymerase core enzyme and stimulating binding.
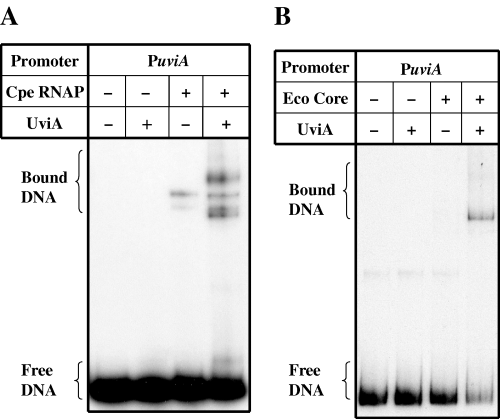
Gel mobility retardation of the uviA promoter. A DNA fragment containing the uviA promoter region was incubated with C. perfringens RNA polymerase (A) or E. coli RNA polymerase core enzyme (B), alone or preincubated with UviA (see Experimental procedures).
The ability of UviA to stimulate transcription from the uviA promoter was tested by in vitro transcription assays. In prior work, Garnier and Cole (1988) showed that the P4 promoter of uviA can be recognized by C. perfringens RNA polymerase in vitro, whereas the UV-inducible P5 promoter cannot. As shown in Fig. 7A, a transcript (∼174 nt ) corresponding to the P4 promoter of the uviA gene (Garnier and Cole, 1988) was synthesized by C. perfringens RNA polymerase in the absence of UviA protein. When we added UviA to the C. perfringens RNA polymerase, a second, less abundant transcript (∼240 nt), corresponding to the P5 promoter, appeared and the P4 transcript was diminished (Fig. 7A). Again, the sizes of the transcripts corresponded closely to those predicted from primer extension analysis of RNA extracted from C. perfringens cells (Garnier and Cole, 1988). When we reconstituted RNA polymerase holoenzyme from E. coli core enzyme and UviA, only transcription from the P5 promoter was detected; core enzyme alone was unable to produce any detectable transcripts (Fig. 7B). These results strongly suggest that uviA transcription is under two modes of regulation: a UviA-independent promoter (P4) is utilized by the major form of C. perfringens RNA polymerase and requires no additional factor; the second promoter (P5) requires core RNA polymerase and UviA, but no other factor.
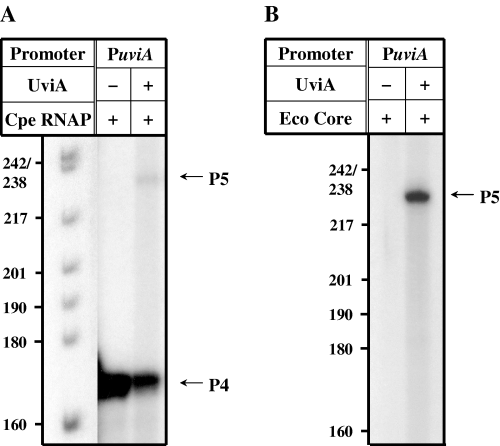
Transcription activation of uviA in vitro. Run-off transcription reactions were performed using C. perfringens RNA polymerase (A) or E. coli RNA polymerase core enzyme (B) and DNA fragments containing the uviA promoter (PuviA) incubated in the absence or presence of UviA. The expected sizes of run-off transcripts (indicated by arrows) are: P4uviA, 174 nt; and P5uviA, 240 nt.
To test directly the distinct roles of the P4 and P5 promoters of uviA in C. perfringens cells, a fragment of DNA containing the uviA promoter region, but lacking promoter P4, was fused to the gusA gene (creating pCD203) and introduced into C. perfringens strains containing either pTUM423 (in which the uviA gene is expressed from its own promoter) or the corresponding vector pTRKL2. As shown in Fig. 8, in the absence of UviA expression in trans, the PuviA–gusA fusion lacking P4 showed no detectable β-glucuronidase activity, whereas a basal level of activity (presumably reflecting UviA-independent expression from P4) was observed with the full uviA promoter fused to the gusA gene. However, when UviA synthesis was induced by treatment with mitomycin C, a high level of GusA activity was observed with both fusions (Fig. 8), suggesting that UviA recognizes the P5 promoter and stimulates uviA gene expression from that promoter.
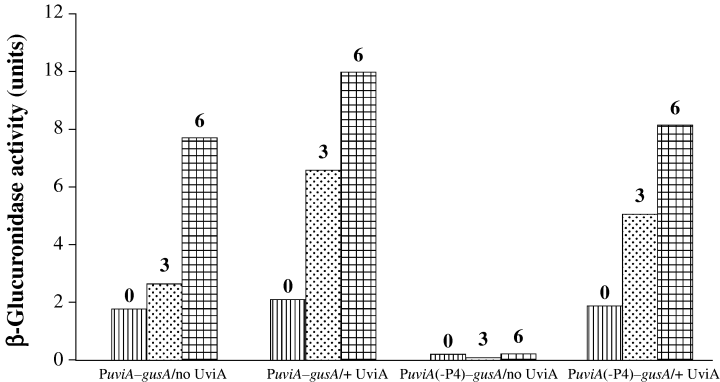
Role of P4 in expression of a PuviA–gusA fusion. DNA fragments containing the wild-type uviA promoter region or a version of the promoter lacking P4 (Garnier and Cole, 1988) were cloned in the reporter fusion vector pTUM177 (Mani and Dupuy, 2001) and introduced into C. perfringens with or without the mitomycin C-inducible uviA gene in trans. Strains carrying the plasmids were grown to mid-exponential growth phase and exposed to subinhibitory concentrations of mitomycin C (0, 3 or 6 µg ml−1 as indicated). Samples were removed after 3 h for measurements of β- glucuronidase activity. The values reported for this representative experiment did not vary by more than 15% in two to three additional trials.
The differential binding of RNA polymerase holoenzyme and core to the P4 and P5 promoter regions was confirmed by gel mobility shift assays with a DNA fragment containing only the P5 promoter. As shown in Fig. 9, both C. perfringens RNA polymerase and E. coli core enzyme were only able to bind to the P5 promoter region in the presence of UviA.
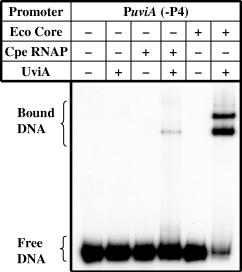
Gel mobility retardation of the uviA promoter region lacking promoter P4. A DNA fragment containing only the P5 promoter of the uviA promoter region was incubated with C. perfringens RNA polymerase or E. coli core enzyme, alone or in the presence of UviA.
Discussion
The results presented here reinforce the idea that the evolution of Clostridium species has been accompanied by the conserved use of a particular type of σ factor to activate transcription of toxin and bacteriocin genes. TcdR, TetR, BotR and UviA are all likely to be derived from the same ancestral protein (Moncrief et al., 1997; Marvaud et al., 1998; Mani and Dupuy, 2001; Dineen et al., 2004). The original source of this type of σ factor is unknown and need not be an organism closely related to any contemporary Clostridium species. All of these σ factors are encoded within genetic elements that could have been acquired by horizontal gene transfer. UviA and TetR are encoded by plasmids (Finn et al., 1984; Garnier and Cole, 1986), TcdR by a 19 kb pathogenicity locus that may have previously been a mobile genetic element (Braun et al., 1996), and BotR by phages in at least some toxinotypes of C. botulinum (Eklund et al., 1971). The organization of the σ gene and its target gene promoters has also been largely conserved. In most but not all cases, the σ gene lies upstream of the target gene and is transcribed in the same orientation. In all cases examined, the σ gene is autoregulated.
Within the group of TcdR-related (group 5) σ factors, BotR and TetR are very close relatives whereas TcdR and UviA are less closely related to each other or to BotR and TetR. This subdivision notwithstanding, we have found that all four σ factors are sufficiently similar so that they are able to substitute for each other both in vivo and in vitro (B. Dupuy, N. Mani and A.L. Sonenshein, manuscript in preparation). Such conservation of function correlates with near-identity in the −35 recognition sequences of promoters recognized by all four factors (Fig. 1 and Raffestin et al., 2004). Moreover, region 4.2 of the four σ factors, i.e. the region implicated in interaction with the −35 sequence of the promoter, is also well-conserved (Fig. 10). Region 2.4, which interacts with the −10 sequence, is less well-conserved, in keeping with the lower conservation of the −10 sequence in target promoters (Fig. 1).

Alignment of the 4.2 regions of TcdR-like σ factors. The asterisks indicate highly conserved residues.
Although dependence on the UviA σ factor underlies expression of the bcn gene, a second layer of regulation connects bcn expression to DNA damage. UV irradiation and treatment with mitomycin C are known to damage DNA and thereby induce expression of DNA repair genes (the SOS response) (Walker, 1996). The usual mechanism for such induction requires self-destruction of the repressor LexA (Walker, 1996). The accumulation of single-stranded DNA produced by most DNA-damaging agents is sensed by RecA protein, resulting in conversion to its activated form, RecA*. RecA* triggers autoproteolytic cleavage of LexA (Little, 1984), resulting in derepression of SOS genes (Walker, 1996). Interestingly, the repressors of certain temperate phages, such as E. coli phage λ, also self-destruct upon interaction with RecA* (Little, 1984). From the phage's point of view, it may be advantageous to escape lysogeny and pursue the lytic cycle in order to escape from a DNA-damaged cell that may be on the road to death.
Bacteriocin production is also commonly induced by DNA damage. As one function of the SOS system is to block cell division until repair is complete (Walker, 1996), the advantage of bacteriocin secretion to the producer organism may be to inhibit the growth of neighbouring cells while the repair process is given a chance to restore DNA integrity.
The C. perfringens genome encodes a protein with high similarity (56% identity) to Bacillus subtilis LexA and the uviAB operon contains an apparent LexA binding site, CGAACATATGTTCG, perfectly matching the B. subtilis consensus (Dubnau and Lovett, 2002), centred at position −85 with respect to the uviA start codon and overlapping the start site of P4 transcription. Within the putative LexA sequence, a conserved Ala-Gly sequence could be the site of autoproteolysis. The C. perfringens genome also encodes a close homologue (64% identity) of B. subtilis RecA.
The bcn gene appears to have three promoters, all of which are dependent on UviA for activation (Garnier and Cole, 1988 and Fig. 5). P1, the promoter closest to the coding sequence, appears to be the strongest of the three and no condition is known that alters promoter preference. The uviA gene, by contrast, has a UviA-independent promoter (P4) that provides a basal level of uviA expression in the absence of DNA damage (Fig. 2B). Transcription from P4 apparently responds to mitomycin C treatment simply by removal of the repressor, LexA. The level of UviA produced by expression from P4 in the absence of DNA damage is apparently insufficient to drive bcn transcription. The second promoter, P5, is UviA-dependent (2, 7) and, together with P4, drives high-level uviA expression in response to DNA damage (Fig. 2B).
The role of UviB, the second product of the uviAB operon, has never been determined. As BCN5-producing cells are immune to the bacteriocin, UviB might be the immunity protein, but comparison of its sequence to those of other proteins reveals that UviB is similar to BhlA, a product of B. subtilis phage SPβ and annotated as a holin-like protein (Regamey and Karamata, 1998). UviB might therefore be involved in release of BCN5 from the cell. Interestingly, holin-like proteins of a different type are thought to be encoded by the C. difficile pathogenicity locus (Tan et al., 2001) and by the C. tetani plasmid, pE88, which encodes TetR and tetanus toxin (Brüggemann and Gottschalk, 2004).
The finding that related proteins in four Clostridium species share a common function as sigma factors that activate transcription of toxin or bacteriocin genes raises the possibility that other members of this family remain to be discovered. As genes upstream of the Clostridium novyi alpha toxin gene and the Clostridium sordellii haemorrhagic toxin gene encode proteins with sequence similarity to the TcdR family (C. von Eichel-Streiber, pers. comm.), one can anticipate that these proteins will also prove to be σ factors. Moreover, the genome sequence of C. tetani revealed that this organism encodes three TcdR family members in addition to TetR (Brüggemann et al., 2003). It will be interesting to find the target genes for these potential σ factors as well.
Experimental procedures
Bacterial strains and growth media
Clostridium perfringens strain SM101 (Zhao and Melville, 1998) was grown anaerobically in TY medium or TY supplemented with 1% glucose (TYG), as described previously (Dupuy and Sonenshein, 1998). C. perfringens strains carrying plasmids were grown in medium containing chloramphenicol (20 µg ml−1) or erythromycin (20 µg ml−1) or both as needed for selection and maintenance. The E. coli K-12 strain DH5α (Hanahan, 1983) was used as the recipient for recombinant plasmids and was grown in L broth (Miller, 1972) containing ampicillin (100 µg ml−1) or chloramphenicol (25 µg ml−1) or erythromycin (150 µg ml−1), when appropriate.
Construction of Pbcn–gusA and PuviA–gusA fusions
In order to construct Pbcn–gusA, PuviA–gusA and PuviA (-P4)–gusA transcriptional fusions, the bcn and uviA promoter regions were amplified from C. perfringens CPN50 DNA (Brefort et al., 1977; Canard and Cole, 1989) by polymerase chain reaction (PCR), using, respectively, the oligonucleotide pairs ONM42 and ONM43 (gaattcGAATAG TAAAAGAGAAGATAAG and tctagaCTAAATCCCCTGAAC TAAC), OBD148 and OBD149 (ggtaccTTAAAAAGTGTTAG GATTTGATTT and tctagaACTTTATTTTTTGCATGGTAGA GT) and OBD188 and OBD189 (gaattcTAACCCACAAGCAC CTTT and tctagaTAAACCCAAACAATAAAAAAATAG) (engineered restriction sites underlined and in lower case), and subcloned in the reporter plasmid pTUM177 (Mani and Dupuy, 2001), using the engineered EcoRI and XbaI sites (for Pbcn–gusA), KpnI and XbaI sites (for PuviA–gusA) or EcoRI and XbaI sites [for PuviA (-P4)–gusA] in the PCR products and the corresponding sites in the reporter plasmid vector, pTUM177. The resulting plasmids pTUM614, pCD175, pCD203 and pTUM177 were introduced via electroporation (Dupuy and Sonenshein, 1998) into C. perfringens SM101 carrying either pTRKL2 (O’Sullivan and Klaenhammer, 1993) or pTUM423 (the UviA-expressing plasmid derived from pTRKL2, see below).
Construction of a UviA-expressing plasmid for C. perfringens
The uviA gene was amplified by PCR from C. perfringens strain CPN50 using as primers ONM39 (ttcggatccCTTT TCTATCTTCTATAG) and ONM41 (cgcgaattcggatccCAAT TCAAGCCTAATTTTTGTC) (engineered restriction sites underlined and in lower case), digested with BamHI and cloned in the BamHI site of the broad-host range shuttle plasmid pTRKL2 to yield the plasmid pTUM423. C. perfringens strain SM101 was transformed with pTUM423 by electroporation and the resulting strain (TUM459) was used as a recipient for transformation by gusA fusion plasmids. The resulting C. perfringens strains with various plasmid combinations were grown anaerobically in TY medium to mid-exponential phase and mitomycin C was added as indicated. The minimal inhibitory concentration (MIC) of mitomycin C was determined for C. perfringens strain SM101 to be 12.5 µg ml−1. The cells were incubated further for 3 h at 37°C. The OD600 of each culture was determined and cells were collected by centrifugation. β-Glucuronidase assays were performed as described previously (Dupuy and Sonenshein, 1998).
Expression and purification of UviA
To overproduce UviA for purification, the uviA gene was amplified by PCR from the recombinant plasmid pTG15, carrying a 6.1 kb PvuII-AccI restriction fragment from pIP404 (Garnier and Cole, 1986), and cloned in the expression vector pET28b (Novagen). This construction created a translational fusion adding six C-terminal histidine codons to the uviA coding sequence and placed it under the control of the T7 promoter. The resulting plasmid, pCD96, was introduced into E. coli strain BL21-codon plus (DE3)-RIL (Stratagene), and expression of UviA-His6 was carried out as previously described for TcdR (Mani and Dupuy, 2001). After expression, cells were harvested by centrifugation, resuspended in lysis buffer (20 mM Tris-HCl, 0.3 M NaCl, 10% glycerol, 20 mM imidazole, pH 7.9) and broken by sonication. The soluble fraction was loaded on a 1 ml Ni+-NTA agarose column (Qiagen) and UviA was eluted as previously described for TcdR (Mani et al., 2002). Fractions containing highly purified UviA protein (i.e. the 60 mM imidazole elution fractions) were pooled and dialysed overnight against a buffer containing 50 mM sodium phosphate (pH 8), 0.3 M NaCl and 50% glycerol. UviA purity was assessed by Coomassie blue staining of an SDS-PAGE gel and by Western blot analysis using rabbit polyclonal antibody against the polyhistidine peptide (Santa Cruz Biotechnology).
Gel mobility shift assays
Fragments of 330, 310, 332 and 240 bp, corresponding, respectively, to positions −324 to +6, −267 to +43, −269 to +63 and −366 to −126, with respect to the translational start codon, of the gdh, bcn, uviA and uviA (-P4) promoters, were amplified by PCR from pTUM199 (for the gdh fragment, Mani and Dupuy, 2001) or the recombinant plasmid pTG15 (for the bcn and uviA fragments, Garnier and Cole, 1986), and then end-labelled with T4 polynucleotide kinase (US Biochemicals, Cleveland, OH) and [γ-32P]-ATP (3000 Ci mmol−1; Amersham). The labelled fragments (0.2 nM) were incubated for 60 min at room temperature in 10 µl of glutamate buffer (Mani and Dupuy, 2001) containing 100 nM C. perfringens RNA polymerase (Katayama et al., 1999) or E. coliσ70 RNA polymerase holoenzyme (Epicentre) or E. coli RNA polymerase core enzyme (Epicentre). In some cases, the RNA polymerase was preincubated with a fourfold molar excess of UviA as previously described for TcdR (Mani and Dupuy, 2001). Four microlitres of a heparin dye solution (150 µg of heparin per millilitre, 0.1% bromophenol blue, 50% sucrose) in glutamate buffer was added and the mixture was loaded during electrophoresis on a 4.5% polyacrylamide gel prepared in Tris-borate-EDTA buffer (Mani and Dupuy, 2001). After electrophoresis (2 h at 13 V cm−1), the gel was dried, transferred to filter paper, and analysed by autoradiography.
UviA–core enzyme interaction experiments
Samples (0.3 µg) of E. coli RNA polymerase core enzyme were immobilized on nitrocellulose filters (Amersham) and the membranes were blocked with 5% powdered milk in PBS at room temperature. Filters were then incubated for 2 h at 37°C with 200 µg of whole cell extract of E. coli strain BL21λDE3 (pET28-b) (used as a negative control) or with varying amounts of purified UviA protein. The filters were washed and immunoblotted using anti-His tag polyclonal antibodies (Santa Cruz Biotechnology) and developed using the ECL kit (Amersham). The specificity of the antibodies was checked by immunoblotting membrane filters spotted with 10 ng of purified UviA protein or 1 µg of crude extract of BL21λDE3 (pET28-b).
In vitro run-off transcription assays
In vitro transcription reactions were carried out in a 10 µl reaction volume containing 40 mM Tris-HCl, pH 8.0; 10 mM MgCl2; 0.1 mM EDTA; 1 mM DTT (added freshly); 0.05 M KCl; 0.1 mg BSA per millilitre; 5% (v/v) glycerol; 1 mM MnCl2; 200 µM each of ATP, GTP and CTP; 50 µM unlabelled UTP; 2.5 µCi of [α-32P]-UTP (600 Ci mmol−1, New England Nuclear); 2 U of RNasinTM (Promega); 0.1 U RNA polymerase [C. perfringensKatayama et al., 1999, E. coli holoenzyme (Epicentre), or E. coli core enzyme (Epicentre)] and DNA. To create templates for in vitro run-off transcription reactions, fragments corresponding to the bcn and uviA promoter regions from positions −267 to +41 and −269 to +63, respectively, relative to the translational start points, were amplified by PCR from the recombinant plasmid pTG15 (Garnier and Cole, 1986) and cloned in the plasmid pGEM-T easy (Promega), giving rise to the plasmids pCD91 and pCD92 respectively. pTUM199 (Mani and Dupuy, 2001) was used as the gdh template. These plasmids were digested with SpeI (pCD91 and pCD92) or XbaI (pTUM199) and 2 µg of the purified, linearized DNA was added to in vitro transcription reactions.
To test the effect of UviA, 0.1 U of RNA polymerase (as above) was preincubated for 30 min at 37°C with a fourfold molar excess of purified UviA before addition to the transcription reaction, and the reactions were then incubated for an additional 15 min at 37°C. The reactions were stopped by adding 5 µl of formamide buffer (Sambrook et al., 1989) and then heated at 80°C for 10 min. Five microlitres of samples was loaded directly onto a 5% polyacrylamide/8 M urea-containing gel. Following electrophoresis, the gels were transferred to Whatman filter paper, dried and visualized by autoradiography.
Acknowledgements
We thank B. Belitsky, C. Squires, and S. S. Dineen for helpful discussions and comments on the manuscript. This work was supported by a research grant (GM042219 to A.L.S.) and a project grant (DK34928 to the Tufts University Gastroenterology Research and Secretory Processes Center) from the US Public Health Service, by a postdoctoral fellowship (to N.M.) from the Charles A. King Trust, by a Grant-in-Aid for Scientific Research (to S.K.) from the Ministry of Education, Culture, Sports, Science and Technology (Japan), and by funds from the Institut Pasteur (Paris) (to B.D.).




