Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum
Summary
The Apicomplexan parasite responsible for the most virulent form of malaria, Plasmodium falciparum, invades human erythrocytes through mutiple ligand–receptor interactions. Some strains of P. falciparum are sensitive to neuraminidase treatment of the host erythrocyte and these parasites have been termed sialic acid-dependent as they utilize receptors containing sialic acid. In contrast, other strains can efficiently invade neuraminidase-treated erythrocytes and hence are sialic acid-independent. The molecular interactions that allow P. falciparum to differentially utilize receptors for merozoite invasion are not understood. The P. falciparum reticulocyte-binding protein homologue (PfRh or PfRBL) family have been implicated in the invasion process but their exact role is unknown. PfRh1, a member of this protein family, appears to be expressed in all parasite lines analysed but there are marked differences in the level of expression between different strains. We have used targeted gene disruption of the PfRh1 gene in P. falciparum to show that the encoded protein is required for sialic acid-dependent invasion of human erythrocytes. The ΔPfRh1 parasites are able to invade normally; however, they utilize a pattern of ligand–receptor interactions that are more neuraminidase-resistant. Current data suggest a strategy based on the differential function of specific PfRh proteins has evolved to allow P. falciparum parasites to utilize alternative receptors on the erythrocyte surface for evasion of receptor polymorphisms and the host immune system.
Introduction
Plasmodium falciparum is a protozoan parasite that causes the most severe form of malaria in humans resulting in over two million deaths each year (World Health Organization, 2002). The clinical symptoms of malaria arise from rapid expansion of the parasite in the erythrocyte of the host during the asexual phase of the parasite life cycle. The invasive merozoite forms are released from parasitized erythrocytes and these rapidly invade new cells in a tightly controlled process involving a cascade of events including multiple receptor–ligand interactions and apical reorientation of the parasite with the host cell (Gratzer and Dluzewski, 1993; Barnwell and Galinski, 1998). The merozoite contains a specialized apical complex that includes the rhoptry and microneme organelles that play a central role in invasion including storage and release of parasite ligands required for binding to host receptors. After apical reorientation of the merozoite a tight junction is formed between host receptors and parasite ligands that moves progressively towards the posterior end of the parasite until host cell membrane fusion occurs (Aikawa et al., 1978).
Some strains of P. falciparum cannot invade neuraminidase-treated erythrocytes efficiently and depend on sialic acid-containing receptors for merozoite invasion (sialic acid-dependent) whereas other strains can efficiently invade these enzyme-treated host cells (sialic acid-independent) (Miller et al., 1977; Howard et al., 1982; Pasvol et al., 1982; Pasvol, 1984). This has been confirmed with field isolates from India (Okoyeh et al., 1999) and Africa (Baum et al., 2003a) where invasion of some isolates were sialic acid-dependent and others -independent. The ability to utilize specific receptors on the erythrocyte appears to be mediated by reliance of the merozoite on different ligands during the invasion process (Barnwell and Galinski, 1998). The mechanism involved is not understood although differential expression of specific ligands does play a role (Duraisingh et al., 2003a). The ability of P. falciparum to utilize different patterns of receptors on the erythrocyte surface for merozoite invasion would be an important mechanism for the parasite to respond to polymorphisms and evade the host immune system.
To date two families of ligands involved in merozoite invasion have been described. First, the Duffy binding-like (DBL) family includes at least three members: EBA-175 (Sim et al., 1990) and its paralogues, EBA-140 (BAEBL) (Mayer et al., 2001; Thompson et al., 2001) and EBA-181 (JESEBL) (Gilberger et al., 2003). Second, the reticulocyte binding-like (RBL) family includes at least five members in P. falciparum: PfRh1, PfRh2a, PfRh2b, PfRh3 and PfRh4 (Rayner et al., 2000; 2001; Taylor et al., 2001; Triglia et al., 2001; Kaneko et al., 2002). The RBL family of merozoite ligands are related to the Plasmodium vivax reticulocyte binding proteins-1 and -2 (RBP-1 and RBP-2) and the rodent malaria, P. yoelii, Py235 proteins. Both PvRBP1 and RBP2 (Galinski et al., 1992) and at least one member of the Py235 proteins (Ogun and Holder, 1996) have been shown to bind their relevant host erythrocyte. The P. falciparum ligands EBA-175 (Sim, 1995), EBA-140 (Mayer et al., 2001; Thompson et al., 2001), EBA-181 (Gilberger et al., 2003) and PfRh1 (Rayner et al., 2001) are also known to bind human erythrocytes. Given the red blood cell (RBC) binding function of the P. falciparum Rh1 protein, these and the closely related Plasmodium reichenowi RBLs have also been called normocyte binding proteins (NBPs) (Rayner, 2001; 2004).
The DBL family members, EBA-175 (Sim et al., 1994), EBA-140 (Mayer et al., 2001; Thompson et al., 2001) and EBA-181 (Gilberger et al., 2003), bind to erythrocytes in a sialic acid-dependent manner. EBA-175 binds to the sialo-glycoprotein glycophorin A (Sim et al., 1994) whereas EBA-140 (BAEBL) binds to glycophorin C (Mayer et al., 2002; Lobo et al., 2003; Maier et al., 2003). Although EBA-175 and EBA-140 are important ligands in merozoite invasion, they are not absolutely essential as the genes can be disrupted in parasite lines that invade by either sialic acid-dependent or -independent processes (Duraisingh et al., 2003b; Maier et al., 2003). EBA-181 binds to putative receptor E on the erythrocyte surface and although it has not been directly demonstrated to function in merozoite invasion the gene encoding this protein cannot be disrupted in some parasite lines and may be essential (Gilberger et al., 2003). It would appear that while individual ligands are not absolutely required for merozoite invasion there is a minimal level of ligand binding required for efficient invasion (Duraisingh et al., 2003b).
The PfRh1 (NBP1) protein of the RBL family also binds erythrocytes in a sialic acid-dependent manner and a putative receptor Y has been defined (Rayner et al., 2001). In contrast, PfRh2b has not been demonstrated to bind directly to erythrocytes; however, targeted gene disruption has shown that it is required for a novel invasion pathway that utilizes a sialic acid-independent receptor Z (Duraisingh et al., 2003a). All PfRh proteins are expressed in merozoites except PfRh3 which appears not to be expressed at any life-cycle stages (Taylor et al., 2001) and are located at the apical end consistent with a role in ligand–receptor interactions (Duraisingh et al., 2003a). PfRh2a and PfRh2b show a remarkable degree of variant expression where some parasites express no detectable levels of these proteins (Taylor et al., 2002; Duraisingh et al., 2003a) and it has been suggested that this provides a mechanism of phenotypic variation to allow utilization of alternate receptors for invasion of merozoites into human erythrocytes (Rayner et al., 2000; Triglia et al., 2001).
In this study, we have used targeted gene disruption in a sialic acid-dependent P. falciparum strain to determine the role of PfRh1 in merozoite invasion. This has shown that PfRh1 function is required for sialic acid-dependent invasion in P. falciparum. These results suggest that differential function of PfRh1 provides the parasite population with the ability to utilize different patterns of receptors and provides a mechanism to evade erythrocyte receptor polymorphism and host immune responses.
Results
Expression of PfRh1 and PfRh2a/2b in P. falciparum
It has previously been shown that PfRh2a and PfRh2b are differentially expressed in P. falciparum parasites and some do not have detectable protein (Taylor et al., 2002; Duraisingh et al., 2003a). Expression of the related protein PfRh1 has been analysed in three P. falciparum lines and an apparent processed product of 195 kDa (as against the expected 358 kDa intact protein) was identified in FCB1 and 3D7 parasites, whereas this fragment was not detectable in immunoblots of supernatants from 3D7, FCB1 or T996 parasites (Taylor et al., 2002). In order to determine the pattern of PfRh1 expression in a broad range of isolates from different geographical regions, we used culture supernatants from W2mef, FCR3, T994, FCB1, Pf120, K1, 7G8, D10, HB3, T996 and 3D7 in Western blots with anti-Rh1 antibodies (Fig. 1A and B). A protein band of ≈240 kDa was readily detected in supernatants from W2mef, FCR3 and FCB1. Smaller peptides were also observed in some samples that may represent processing or degradation products of a larger PfRh1 precursor. Identical results were obtained using supernatants from the same parasite lines with anti-Rh1-B antibodies (Fig. 1A) that were raised to a different region of the PfRh1 protein and these results confirmed the specificity of the anti-Rh1 and anti-Rh1-B antibodies (data not shown). The difference in size between the previously published 195 kDa fragment (Taylor et al., 2002) and the 240 kDa fragment observed here probably relates to differences in high-molecular-weight markers used and differences in concentrations of acrylamide used in SDS-PAGE gels. In contrast, the 240 kDa PfRh1 band, while detectable in longer exposures, appeared to be present in lower amounts in the other isolates tested when compared with the control protein SERA5 (Fig. 1B). The SERA5 protein is expressed late in the erythrocytic cycle (Miller et al., 2002), and a processed fragment of ≈50 kDa is shed into the culture supernatant after merozoite invasion (Hodder et al., 2003). To confirm the size of the processed PfRh1 fragment at 240 kDa and ensure that the 3D7 parasite actually expresses PfRh1, a Western blot of schizont material from the Tak994, 3D7 and FCR3 parasites was probed with anti-Rh1 antibodies. A band at 240 kDa representing the PfRh1 processed fragment was present in both Tak994 and 3D7, but was overexpressed in the FCR3 parasite, confirming the result on FCR3 supernatants (Fig. 1B).
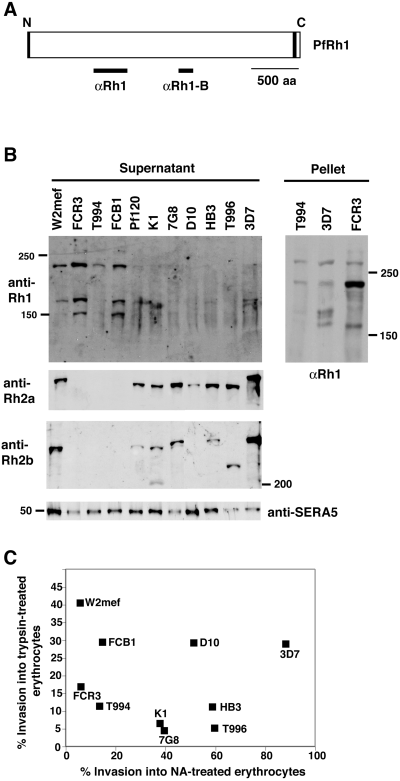
Relationship between PfRh2a/Rh2b and PfRh1 expression with invasion into enzyme-treated erythrocytes in P. falciparum clones. A. Structure of the PfRh1 protein showing the signal sequence (black shading) at the N-terminus and the transmembrane domain (black shading) at the C-terminus. The location of the fusion proteins used to raise the anti-Rh1 and anti-Rh1b antibodies used in this study is shown. B. Enriched culture supernatants from 11 parasites and three schizont pellet samples from three parasites were probed with anti-PfRh1, anti-PfRh2a, anti-PfRh2b and anti-SERA5 antibodies. The location of the 50, 150, 200 and 250 kDa markers is shown. C. Parasites were assayed for their ability to invade trypsin-treated (1 mg ml−1 final) and neuraminidase-treated erythrocytes. For each parasite, the ability to invade trypsin-treated erythrocytes (vertical axis) is plotted against its ability to invade neuraminidase (NA)-treated erythrocytes (horizontal axis).
To compare the expression of PfRh1 with other members of the RBL family of proteins, we used the same parasite supernatants in Western blots and probed with anti-PfRh2a antibodies (Duraisingh et al., 2003a). The expected protein band of ≈300 kDa was detected in W2mef, Pf120, K1, 7G8, D10, HB3, T996 and 3D7; however, this protein showed no apparent expression in FCR3, T994 and FCB1 (Fig. 1B). This pattern of expression was similar to that observed when Western blots of the same supernatants were tested with anti-PfRh2b antibodies except that D10 supernatants showed no PfRh2b protein. D10 has been shown previously to lack the PfRh2b gene (Triglia et al., 2001). Generally, parasites in which the 240 kDa PfRh1 protein was present at higher levels in the supernatant did not express PfRh2a/2b. However, there are clearly exceptions as W2mef expresses detectable levels of all three proteins.
Plasmodium falciparum strains expressing PfRh1 in the absence of PfRh2a/2b, or overexpressing PfRh1 in the presence of PfRh2a/2b, invade erythrocytes via sialic acid-dependent pathways
The variation in the ability to invade neuraminidase-treated erythrocytes by both laboratory parasites and field isolates is well documented (Binks and Conway, 1999; Okoyeh et al., 1999). In order to determine whether the level of PfRh1 expression correlated with the pattern of receptor usage on the erythrocyte by merozoites, we tested the ability of the different parasite strains to invade either neuraminidase- or trypsin-treated erythrocytes. Parasite strains that expressed lower levels of PfRh1 were generally able to invade neuraminidase-treated erythrocytes more efficiently than those that overexpressed (W2mef, FCR3, FCB1) the PfRh1 protein (Fig. 1C). The parasite strains W2mef, FCR3, FCB1 and T994 invaded neuraminidase-treated erythrocytes at between 5% and 14% whereas the efficiency for the other parasites that express both PfRh1 and PfRh2a/2b was much higher (38–90%). In contrast, invasion into trypsin-treated erythrocytes showed no correlation with respect to expression of PfRh1, PfRh2a or PfRh2b. This suggested that parasites that overexpress PfRh1 (W2mef, FCR3, FCB1) either in the absence (FCR3, FCB1) or in the presence (W2mef) of PfRh2a/2b, or express normal levels of PfRh1 in the absence of PfRh2a/2b (Tak994), show a sialic acid-dependent invasion of erythrocytes. On the other hand, parasites that express normal levels of PfRh1 in the presence of PfRh2a/2b (Pf120, K1, 7G8, D10, HB3, Tak996 and 3D7) show a more sialic acid-independent invasion of erythrocytes.
Targeted disruption of the PfRh1 gene in P. falciparum
In order to determine the role of PfRh1 in merozoite invasion, we decided to disrupt the PfRh1 gene in a number of parasite strains that either invade erythrocytes predominantly in a sialic acid-dependent manner or are sialic acid-independent in the invasion process. W2mef, FCR3 and Tak994 parasites either overexpress or express normal levels of PfRh1 and invade neuraminidase-treated erythrocytes inefficiently, whereas 3D7 which expresses normal levels of this protein can invade these enzyme-treated cells efficiently (Fig. 1B and C) (Duraisingh et al., 2002; Taylor et al., 2002). To disrupt PfRh1 in these parasites, we constructed the plasmid pHtkΔRh1 that would integrate and disrupt the gene by double recombination cross-over, a construct similar to that used to disrupt this gene in 3D7 parasites, to derive 3D7ΔRh1 (Fig. 2A) (Duraisingh et al., 2003a). This plasmid was transfected into Tak994, W2mef and FCR3 and selected with WR99210 and ganciclovir. Integration of the pHtkΔrh1 plasmid into the Tak994 genome was confirmed by Southern blotting, and cloned lines were obtained. Hybridization of the PfRh1 probe (Pr) to genomic DNA (gDNA) from parental Tak994 revealed a 5.1 kb hybridizing fragment corresponding to the endogenous PfRh1 gene (Fig. 2B). When the same probe was hybridized to DNA from the cloned lines T994ΔRh1-5′c1 and T994ΔRh1-5′c2, hybridizing bands of 7.3 and 1.7 kb were obtained. In contrast, hybridizing bands of 5.7 and 1.1 kb were obtained for the cloned lines T994ΔRh1-3′c1 and T994ΔRh1-3′c2 (Fig. 2B). This confirmed that the PfRh1 gene in Tak994 had been disrupted independently by single cross-over recombination events. The pattern of bands seen in the clones indicated that the recombination events had occurred either in the PfRh1 5′ flank (T994ΔRh1-5′c1/2) or in the PfRh1 3′ flank (T994ΔRh1-3′c1/2) of the transfection plasmid. Despite the plasmids not integrating via the expected double cross-over recombination, the PfRh1 gene was disrupted in the transfected parasite clones. Additionally, integration of the plasmid in either the 5′ or 3′ flank provided two sets of independent transfectants to confirm any phenotypic changes in merozoite invasion.
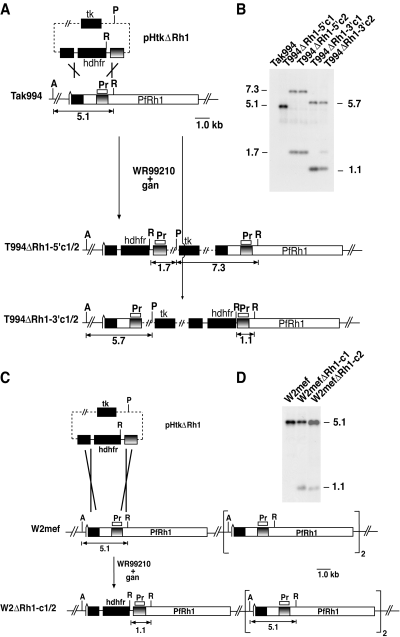
Disruption of the PfRh1 gene. A. The pHTkΔRh1 plasmid has the human dhfr gene (hdhfr) cassette flanked by two target sequences for homologous recombination within the PfRh1 gene. The plasmid also includes the thymidine kinase (tk) gene cassette for negative selection with ganciclovir. Two cloned transfected lines (T994ΔRh1-5′c1 and T994ΔRh1-5′c2) are shown and were derived by single cross-over recombination at the 5′PfRh1 flank while the other lines (T994ΔRh1-3′c1/2) arose by single cross-over recombination at the 3′PfRh1 flank. The PfRh1 genes are shown with the relevant restriction enzyme sites: A, AflII; P, PvuI; R, EcoRI. The probe (Pr) used in (B) is shown. B. Southern blotting of genomic DNA from Tak994 and T994ΔRh1-5′c1, T994ΔRh1-5′c2, T994ΔRh1-3′c1 and T994ΔRh1-3′c2 digested with a mixture of AflII, PvuI and EcoRI. The sizes are in kb. Hybridization of the PfRh1 probe (Pr) to genomic DNA from parental Tak994 revealed a 5.1 kb hybridizing fragment corresponding to the endogenous PfRh1 gene. When the same probe was hybridized to DNA from the cloned lines T994ΔRh1-5′c1 and T994ΔRh1-5′c2 hybridizing bands of 7.3 and 1.7 kb. In contrast, hybridizing bands of 5.7 and 1.1 kb were obtained for the cloned lines T994ΔRh1-3′c1 and T994ΔRh1-3′c2. C. Disruption of the PfRh1 gene in W2mef parasites. The plasmid pHTkΔRh1 was used to target the PfRh1 locus in W2mef parasites. The presence of multiple copies of the PfRh1 gene in this parasite is shown. Two cloned transfected lines (W2ΔRh1-c1 and W2ΔRh1-c2) that have arisen by double cross-over recombination are shown. The restriction enzymes used are as in (A). D. Southern blotting of genomic DNA from W2mef, W2ΔRh1-c1 and W2ΔRh1-c2 digested with the same restriction enzymes as in Fig. 2. The sizes are in kb. Hybridization of the PfRh1 probe to digested DNA from wild-type W2mef revealed a 5.1 kb band (as in the case of Tak994) corresponding to the endogenous PfRh1 gene (B). In contrast, the same probe with DNA from W2ΔRh1-c1 and W2ΔRh1-c2 revealed the original endogenous PfRh1 band at 5.1 kb but also another band at 1.1 kb. The appearance of the 1.1 kb band indicated that the PfRh1 gene had been disrupted by a double cross-over recombination.
Integration of the pHtkΔRh1 plasmid into the W2mef genome was also confirmed by Southern blotting and cloned lines obtained. Hybridization of the PfRh1 probe to digested DNA from wild-type W2mef revealed a 5.1 kb band (as in the case of Tak994) corresponding to the endogenous PfRh1 gene (Fig. 2C). In contrast, the same probe with DNA from W2ΔRh1-c1 and W2ΔRh1-c2 revealed the original endogenous PfRh1 band at 5.1 kb but also another band at 1.1 kb. The appearance of the 1.1 kb band indicated that the PfRh1 gene had been disrupted by a double cross-over recombination (Fig. 2D). Analysis of the FCR3 transfectants showed results similar to those observed for W2mef suggesting that the transfection plasmid had indeed integrated into PfRh1 via double recombination (data not shown). However, as only W2mef and FCR3 parasites had maintained the endogenous 5.1 kb band we considered it likely that both W2mef and FCR3 parasites had more than one copy of PfRh1 initially, and that not all of these copies had been disrupted (Fig. 2D). Subsequent mapping of the W2mef and Tak994 transfectants confirmed this interpretation (data not shown).
Disruption of the PfRh1 gene in Tak994 leads to loss of expression of the protein
In order to determine whether disruption of PfRh1 in Tak994 and W2mef resulted in altered expression of the corresponding protein, we used Western blots of supernatants probed with antibodies (Fig. 3A). Culture supernatants from Tak994, W2mef, T994ΔRh1 and W2mefΔRh1 parasites were separated by SDS-PAGE and probed with anti-PfRh1 antibodies. The expected protein of ≈240 kDa was detected in Tak994 but was absent in the four knockout clones, T994ΔRh1-5′c1/2 and T994ΔRh-3′c1/2. The amount of protein loaded for each supernatant was equalized using anti-SERA5 antibodies as control (Fig. 3A) (Hodder et al., 2003). Western blots with schizont pellets from T994 and T994ΔRh1-5′c1 parasites confirmed the lack of expression of the PfRh1 protein in the knockout parasite (Fig. 3A). Western blots on schizont pellets from the other T994ΔRh1 clones gave identical results (data not shown). In contrast, PfRh1 was detectable in W2mef and the W2mefΔRh1 clones despite integration of the plasmid into the PfRh1 locus. The intensity of PfRh1 reactivity with the antibodies was decreased in the W2mefΔRh1c1/2 consistent with decreased expression of the protein resulting from disruption of one copy of multiple PfRh1 genes. These results clearly show that PfRh1 is not expressed in Tak994ΔRh1-5′c1/2 and Tak994ΔRh1-3′c1.
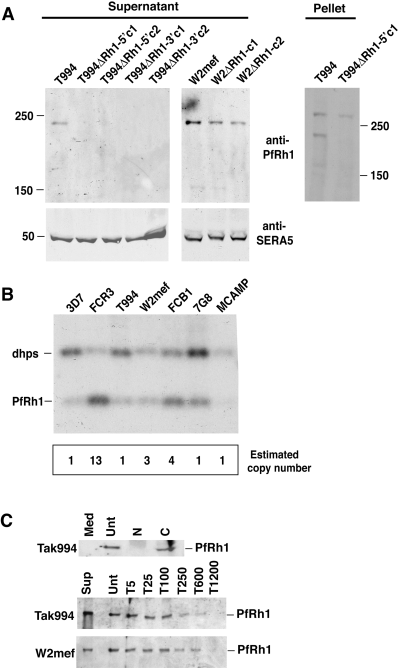
Analysis of PfRh1 expression and gene copy number. A. Loss of PfRh1 expression in T994ΔRh1 but not W2ΔRh1 parasites. Enriched culture supernatants from Tak994, T994ΔRh1-5′c1, T994ΔRh1-5′c2, T994ΔRh1-3′c1 and T994ΔRh1-3′c2, W2mef, W2ΔRh1-c1 and W2ΔRh1-c2 were probed with anti-PfRh1 and anti-SERA5 antibodies. Schizont pellet proteins from Tak994 and T994ΔRh1-5′c1 parasites were also probed with anti-PfRh1 antibodies. Sizes are shown in kDa. B. The PfRh1 gene copy number differs in cloned P. falciparum lines. Southern blotting of genomic DNA from 3D7, FCR3, Tak994, W2mef, FCB1, 7G8 and MCAMP parasites, digested with a mixture of the restriction enzymes XbaI and BglII. The digested DNA was probed with a mixture of two fragments from both the PfRh1 and dhps genes. Below the Southern blot is a panel showing the PfRh1 copy number determination for each parasite. The intensity of the bands in the Southern blot, calculated using a phosphorimager (Molecular Dynamics), were used for the copy number determination. C. Culture medium alone (med) or enriched culture supernatant from Tak994 or W2mef parasites was bound to untreated (Unt), trypsin-treated (at concentrations of 5–1200 mg of protease as shown), neuraminidase-treated (N) or chymotrypsin-treated (C) erythrocytes. After extensive washing, bound proteins were eluted with 250 mM NaCl, separated on SDS-PAGE gels, Western blotted and probed with anti-PfRh1 antibodies. PfRh1 binding to erythrocytes was sensitive to neuraminidase but resistant to chymotrypsin. A series of trypsin concentrations (5, 25, 100, 250, 600 and 1200 µg ml−1) were used to determine the effect on PfRh1 binding.
The PfRh1 gene copy number differs in P. falciparum lines
To estimate the PfRh1 gene copy number in different P. falciparum strains, we compared the level of hybridization of a probe from this gene and that of dihydropteroate synthase (dhps), a known single-copy gene. Restriction enzyme-digested gDNA from 3D7, FCR3, T994, W2mef, FCB1, 7G8 and MCAMP was used for Southern blotting and hybridized simultaneously with probes to PfRh1 and dhps (Triglia and Cowman, 1994). The result for 3D7, which was presumed to have a single copy of the PfRh1 gene, was taken as indicating a ratio of 1:1 for dhps versus PfRh1 (Fig. 3B). The lower intensity of the PfRh1 band reflected the lower labelling efficiency of the PfRh1 probe versus the dhps probe. The high copy number of PfRh1 in FCR3 was evident and densitometry measurements suggested that it was present in 13 copies compared with 3D7 (Fig. 3B). In contrast, the PfRh1 gene was present as three copies in W2mef and four copies in FCB1 while MCAMP, 7G8 and T994 had a single copy of the gene. These data were consistent with the results obtained from the gene disruption experiments for Tak994, W2mef and FCR3 (Fig. 2B and C). While it is clear that there are multiple copies of the PfRh1 gene in W2mef, FCR3 and FCB1, it was not possible to determine the similarity of each gene although some apparent restriction length fragment polymorphisms were detected in W2mef suggesting differences. Whether these differences are of functional significance remains to be determined. Comparison of PfRh1 gene copy number for W2mef, FCR3, Tak994 and FCB1 with protein expression, as measured by Western blot using anti-PfRh1 antibodies, showed a correlation between gene copy and protein expression.
PfRh1 is an important ligand in sialic acid-dependent invasion
PfRh1 has been shown previously to bind erythrocytes and this occurred via a receptor that is trypsin-resistant and neuraminidase-sensitive (Rayner et al., 2001), but no information was available with respect to chymotrypsin sensitivity. In order to assist in interpreting the functional analysis of Tak994ΔRh1-5′c1/2 and Tak994ΔRh1-3′c1, we first tested the ability of the 240 kDa fragment of PfRh1 from Tak994 to bind erythrocytes that had been treated with trypsin, neuraminidase or chymotrypsin (Fig. 3C). This confirmed that PfRh1 binding to erythrocytes was neuraminidase-sensitive and although relatively resistant to trypsin, binding was affected at high concentration of this protease. PfRh1 bound efficiently to chymotrypsin-treated erythrocytes indicating that the receptor was resistant to this protease (Fig. 3C).
Having constructed Tak994 and 3D7 parasite lines that lacked expression of the PfRh1 protein, we tested the effect of loss of this protein on the ability of merozoites to invade normal human erythrocytes. The growth rate of T994ΔRh1-5′c1/2 and Tak994ΔRh1-3′c1/2 parasites were unchanged relative to the Tak994 parent (data not shown). This suggested that either the PfRh1 protein was not required for merozoite invasion, or the loss of PfRh1 is compensated by the function of other parasite proteins in the knockout lines. This has been described previously in parasites that have lost functional EBA-175 in W2mef (Reed et al., 2000; Duraisingh et al., 2003b) or PfRh2b in 3D7 parasites (Duraisingh et al., 2003a). Loss of function of EBA-175 in W2mef results in the parasites switching to a sialic acid-independent pathway while loss of PfRh2b in 3D7 uncovers a novel sialic acid-independent pathway.
To examine the role of PfRh1 in invasion further, we determined the effect of trypsin treatment of human erythrocytes on the invasion of the Tak994 and 3D7 parents compared with the T994ΔRh1 and 3D7ΔRh1 parasite clones. Trypsin cleaves glycophorin A, C and a third unknown receptor X (Dolan et al., 1994; Thompson et al., 2001; Maier et al., 2003) but leaves many other erythrocyte receptors such as receptors E, Y and Z and glycophorin B unaffected (Dolan et al., 1994; Rayner et al., 2001; Duraisingh et al., 2003a; Gilberger et al., 2003). Loss of PfRh1 protein significantly increases the ability of T994ΔRh1 clones to invade trypsin-treated erythrocytes relative to the Tak994 parent (Fig. 4A). Therefore, there has been compensation of PfRh1 function by other invasion pathways such that the overall efficiency of invasion into untreated erythrocytes remains the same as the parental parasite line.
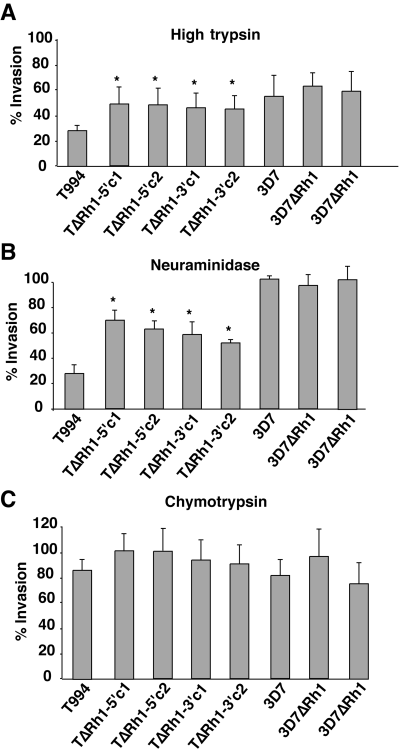
Invasion of Tak994, T994ΔRh1, 3D7 and 3D7ΔRh1 parasites into enzyme-treated erythrocytes. Erythrocytes were treated with high trypsin (A), neuraminidase (B), or chymotrypsin (C), as described in Experimental pricedures, before testing in invasion assays. The percentage invasion for each parasite is based on the invasion of that same parasite into untreated erythrocytes. The data were from three independent experiments in triplicate and errors shown represent 95% confidence limits. In all experiments, the starting parasitaemia was either 0.5% or 0.75% and the final parasitaemia into untreated erythrocytes was more than 3%. The asterisks indicate invasion which is significantly different from that obtained with Tak994 parasites.
Next, we determined the effect of loss of PfRh1 expression on invasion into neuraminidase-treated erythrocytes. Neuraminidase removes sialic acid from erythrocyte receptors such as glycophorin A, B and C, as well as the unknown receptors E and Y (Mayer et al., 2001; Rayner et al., 2001; Gilberger et al., 2003; Maier et al., 2003). Lack of PfRh1 function in T994ΔRh1 parasites resulted in a significantly increased invasion into neuraminidase-treated erythrocytes when compared with the Tak994 parent (Fig. 4B). The Tak994 parent invades both trypsin and neuraminidase-treated erythrocytes at ≈30% efficiency compared with untreated cells while the T994ΔRh1 parasites invade trypsin-treated erythrocytes at 47% and neuraminidase-treated erythrocytes at 58%. A similar analysis of the W2ΔRh1 clones showed virtually no change in invasion when compared with the W2mef parent, a result that is consistent with the continued expression of PfRh1, although at a decreased level (data not shown). The increased ability of T994ΔRh1 parasites to invade both trypsin and neuraminidase-treated erythrocytes reflects a shift in these parasites towards the utilization of receptors that are more neuraminidase- and trypsin-resistant compared with the Tak994 parent. This was in contrast to 3D7ΔRh1 parasites that showed no differences in the ability to invade neuraminidase-, trypsin- or chymotrypsin-treated erythrocytes compared with the 3D7 parental line (Fig. 4A–C). Although normal levels of PfRh1 protein are present in 3D7, it appears to play no functional role in the sialic acid-independent invasion by this parasite. These results, however, are consistent with PfRh1 playing an important role in parasites showing sialic acid-dependent invasion.
Discussion
The PfRh protein family of P. falciparum has been implicated in merozoite invasion and may play an important role in determining the pattern of host receptor utilization (Rayner et al., 2001; Duraisingh et al., 2003a). Many strains of P. falciparum utilize sialic acid-containing receptors such as glycophorin A and C for invasion to which parasite ligands EBA-175 and its paralogues can bind (Table 1). However, it has not been clear what determines the sialic acid dependence of different strains and whether the PfRh proteins are involved. Here we have used specific gene knockouts of the PfRh1 gene to show that the protein is required for sialic acid-dependent invasion and that loss of function is associated with a switch to utilization of alternative invasion pathways. The availability of different parasite ligands to compensate the loss of previously dominant ligand–receptor interactions provides a mechanism for the parasite to escape host immune responses and polymorphic host receptors during the invasion process.
| Ligand | Receptor | Phenotypea | Reference |
|---|---|---|---|
| EBA175 | GlyA | TSNSCSb | Sim et al. (1994) |
| ? | GlyB | TRNSCS | Dolan et al. (1994) |
| EBA181 | E | TRNSCS | Gilberger et al. (2003) |
| EBA140 | GlyC | TSNSCR | Maier et al. (2003); Mayer et al. (2003) |
| ? | X | TSNRCR | Dolan et al. (1994) |
| PfRh1 | Y | TRNSCR | Rayner et al. (2001); this work |
| PfRh2b | Z | TRNRCS | Duraisingh et al. (2003a) |
| ? | Non-GlyB | TRNS | Gaur et al. (2003) |
- a . Receptor phenotype represents the sensitivity (S) or resistance (R) of each receptor to trypsin (T), neuraminidase (N) and chymotrypsin (C).
- b . Although glycophorin A is chymotrypsin sensitive, its function can be assayed using chymotrypsin-treated erythrocytes where a substantial proportion of receptor is retained on the host cell surface (Duraisingh et al., 2003a).
PfRh1 is an important ligand for sialic acid-dependent invasion
The PfRh1 ligand binds to a neuraminidase-sensitive, chymotrypsin- and trypsin-resistant receptor which has been referred to previously as receptor Y (Rayner et al., 2001). Merozoite invasion of Tak994 parasites is chymotrypsin-resistant, neuraminidase-sensitive; however, it is also trypsin-sensitive suggesting that other ligands contribute to overall merozoite invasion. This ligand(s) would bind to a receptor that is neuraminidase and trypsin-sensitive. The most likely parasite ligands are EBA-175 and EBA-140 as EBA-181 (Gilberger et al., 2003) and PfRh2b (Duraisingh et al., 2003a) utilize trypsin-resistant receptors and other invasion pathways mediated by unknown parasite ligands do not fit with the required properties (Table 1) (Dolan et al., 1994; Gaur et al., 2003). EBA-175 and EBA-140 bind to glycophorin A and C, respectively, and these interactions are neuraminidase- and trypsin-sensitive suggesting that these ligands are important in sialic acid-dependent invasion (Sim, 1995; Thompson et al., 2001; Duraisingh et al., 2003b). However, EBA-175 can be disrupted in W2mef and this is associated with a switch to sialic acid-independent invasion (Duraisingh et al., 2003b) while disruption of EBA-140 in the same parasite line has little phenotypic effect (Maier et al., 2003). Also analysis of polymorphisms in a large number of field isolates has shown that EBA-175 is under strong diversifying selection unlike EBA-140 (Baum et al., 2003b). This suggests that EBA-175 is a more important ligand than EBA-140 in sialic acid-dependent invasion and acts with PfRh1 to determine the overall receptor usage (Table 1). This is consistent with PfRh1 and EBA-175 together being the major determinants of sialic acid-dependent invasion.
It has been proposed that the PfRh proteins play a role in the apical interaction of the merozoite to sense host receptors on the erythrocyte to activate the invasion process (Barnwell and Galinski, 1998; Duraisingh et al., 2003a). While this is possible, the similarity of functional properties for PfRh1, PfRh2b and EBA-175 with respect to invasion of enzyme-treated erythrocytes suggests these proteins may play an equivalent role at some stage in the invasion process. It has been postulated that EBA-175 is involved in the tight junction (Hodder et al., 2003), based on its relationship to the Plasmodium knowlesi and P. vivax Duffy binding proteins, and allows connection either directly or indirectly with the actin-myosin motor to provide the link between the host and merozoite and the force required for invasion (Adams et al., 2001). Currently, there is no direct evidence showing either EBA-175 or the PfRh proteins within the tight junction; however, their importance in the invasion process would be consistent with a role in this process.
The function of PfRh1 in sialic acid-dependent invasion is linked to the presence of increased amounts of the 240 kDa processed peptide in the supernatant suggesting that proteolysis of this protein is important for its function. All parasites examined so far express the 240 kDa processed PfRh1 fragment in both schizont material and parasite supernatants by Western blot. Increased amounts of this processed product in sialic acid-dependent parasites such as W2mef, FCR3 and FCB1 arises from amplification of the PfRh1 gene. On the other hand, many parasites lack expression of the PfRh2a/2b proteins. The mechanism for this lack of protein expression results from absence of the appropriate transcript, even though in these parasite lines, the genes are still present (Taylor et al., 2002). The general conclusion from the examination of many parasite strains with respect to their PfRh1/2a/2b expression and their invasion pattern is that parasites expressing PfRh1 in the absence of PfRh2a/2b (FCR3, FCB1, Tak994) or overexpressing PfRh1 in the presence of PfRh2a/2b (W2mef) show more sialic acid-dependent invasion. The corollary to this is that parasites expressing PfRh2a/2b invade in a sialic acid-independent manner, when there is no amplification of the PfRh1 gene (Pf120, K1, 7G8, D10, HB3, T996 and 3D7).
The identification of some strains of P. falciparum that have multiple copies of PfRh1 has important implications for the function of these genes. PfRh2a and PfRh2b are identical along their length except for the 3′ region that is unrelated and they appear to have arisen by gene duplication (Rayner et al., 2001; Triglia et al., 2001). Although the proteins encoded by these two genes are closely related, they are functionally distinct. The generation of multiple copies of PfRh1 in some parasites would provide new PfRh genes to generate ligand diversity for the development of novel receptor specificities providing an important advantage for the parasite.
Compensation of PfRh1 function by other invasion pathways
Previously, gene disruption of parasite ligands such as EBA-175 and PfRh2b has resulted in a shift in utilization of different host receptors for merozoite invasion to compensate the loss of function for dominant ligands (Duraisingh et al., 2003a,b). Loss of EBA-175 expression in W2mef resulted in a switch from sialic acid-dependent to -independent invasion whereas loss of PfRh2b in 3D7 parasites uncovered a novel sialic acid-independent invasion pathway. The functional replacement of PfRh2b and EBA-175 in the invasion process would provide sufficient affinity of ligand binding to their cognate receptors to activate subsequent steps in the invasion process and allow the parasite to grow and expand at a normal rate. A similar shift in receptor utilization occurred with loss of PfRh1 function in Tak994 resulting in parasites that utilize host receptors that are more neuraminidase- and trypsin-resistant. While these properties may be a summation of more than one ligand–receptor interaction, there are none currently described that would fit this pattern of enzyme sensitivities, suggesting that the Tak994ΔRh1 parasites have switched to a novel invasion pathway.
In order to investigate whether any of the remaining PfRh or ebl transcripts were upregulated in the mutant parasites, transcripts from the Tak994ΔRh1 and Tak994 parent parasites were analysed by microarray using an Affymetrix P. falciparum chip (Fig.S1) (Le Roch et al., 2003). Apart from the expected downregulation of the PfRh1 transcript in the mutant parasite, no other PfRh or ebl transcripts were altered in transcription level. These data were confirmed with Western blots of proteins from both Tak994ΔRh1 and Tak994 parent parasites with the result that no PfRh or EBA proteins were altered in their expression levels between the two parasites (data not shown).
What determines the sialic acid dependence of P. falciparum strains?
PfRh1 and EBA-175 are important ligands for sialic acid-dependent invasion whereas other proteins such as PfRh2b are involved in sialic acid-independent invasion pathways (Duraisingh et al., 2003b). Here we show that parasites that express PfRh2a/2b, in general, invade erythrocytes in a sialic acid-independent manner. On the other hand, parasites that overexpress PfRh1 as a result of gene amplification, or express PfRh1 in the absence of PfRh2a/2b, invade erythrocytes in a sialic acid-dependent fashion. We propose a ‘limited space’ model to explain sialic acid-dependent or -independent invasion of different parasite strains (Fig. 5). Tak994 parasites express only PfRh1 and so are sialic acid-dependent. 3D7 parasites express PfRh1, PfRh2a and PfRh2b, and invasion is sialic acid-independent, as PfRh2a/2b proteins are present at the apical tip. On the other hand, the W2mef parasite that expresses both PfRh2a and PfRh2b also has an amplified Pfrh1 gene and so overexpresses PfRh1 at the apical tip of the merozoite. If the assumption is made that there is only a limited space at the apical tip to insert PfRh proteins, then the W2mef parasite will usually express the PfRh1 protein as the PfRh1 gene is amplified, which leads to the W2mef parasite being sialic acid-dependent (Fig. 5). The Tak994ΔRh1 parasite that lacks PfRh1, PfRh2a and PfRh2b becomes less sialic acid-dependent than the Tak994 parent as it has lost the PfRh1 protein. However, invasion of erythrocytes still proceeds, either because ebl proteins are present that partially complement function or because there are other as yet unidentified proteins that compensate loss of the PfRh family (Fig. 5).
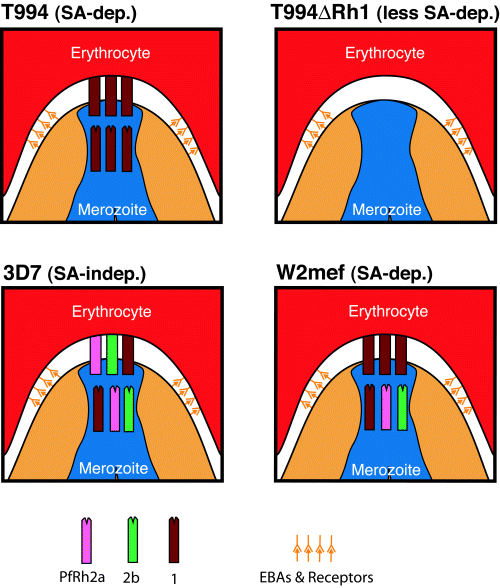
Limited space hypothesis: a model for parasite invasion via sialic acid (SA)-dependent or -independent pathways. The PfRh1/2a/2b proteins are represented by coloured blocks as shown. Erythrocyte binding proteins (EBAs) and their receptors are shown in orange. The limited space model proposes that the apical tip of the merozoite is a confined space such that few PfRh proteins can be inserted. As a result, the PfRh proteins actually making contact with the erythrocyte reflect the proportion of each PfRh protein produced by that parasite. For example, the W2mef parasite is SA-dependent as its PfRh1 gene is amplified threefold, leading to a ratio of PfRh1:2a:2b of 3:1:1.
The most likely reason for differential function of PfRh proteins within the P. falciparum population would be to allow different patterns of receptor utilization for merozoite invasion and this would be important for evasion of host immune responses against specific ligands as well as to circumvent host receptor polymorphisms on the erythrocyte (Duraisingh et al., 2003a). Receptors on the erythrocyte are highly polymorphic within the human population and heterogeneity has been selected to protect against severe malaria (Miller et al., 1994). Additionally, during development and ageing of the erythrocyte, there is variable expression of specific receptors such as glycophorin A. The availability of merozoites within the population with different functional PfRh proteins such as PfRh1 and PfRh2b would provide an array of ligands that would allow successful invasion of at least some in the face of any host receptor polymorphism encountered as well as specific host immune responses that may block function of one or more ligand–receptor interactions. This would increase the ability of the parasite to infect the host and therefore the potential for transmission to subsequent hosts and this has important implications for the design of vaccines using proteins that are involved in the invasion process.
Experimental procedures
Plasmids and parasites
Genomic DNA from the 3D7 parasite line was used as template to PCR two ≈900 bp fragments from the PfRh1 gene, which were cloned into a vector derived from pHTk, which had a modified multiple cloning site (Duraisingh et al., 2002). Flank 1 was amplified using the primers 5′-GATCAC TAGTTTATGAACCTACCCCTTCAT and 5′-GATCAGATCTAT CAAT ATATATTTCTGGTA and Flank 2 was amplified using the primers 5′-GATCGAATTCATCAAATATGAAGAGCACAT and 5′-GATCCCATGGTTTGGATATATTTTCCCTTA. Flank 1 begins 70 bp into exon 2 of the PfRh1 gene and thus also lacks exon 1 (encoding the signal sequence) and the intron. P. falciparum asexual stages were maintained in human O+ erythrocytes. 3D7 is a cloned line derived from NF54 and was obtained from David Walliker at Edinburgh University. W2mef is a cloned line derived from the Indochina III/CDC strain. The parasites Tak996 and Tak994 from Southeast Asia and FCB1 of African origin were provided by David Baker from the London School of Hygiene and Tropical Medicine. The FCR3 cloned line is of African origin. HB3 and 7G8 are South American cloned lines while D10 was cloned from a Papua New Guinean isolate. MCAMP, K1 and Pf120 are all Southeast Asian lines. Transfection with 80 µg of purified plasmid DNA (Qiagen) and selection for stable transfectants with double recombination cross-over was carried out as described previously (Duraisingh et al., 2002).
Probes and nucleic acids
Genomic DNA was extracted from late stages as described (Triglia and Cowman, 1994). Southern blotting was carried out using standard procedures. Three probes were used in this study. The first PfRh1 probe was used in 2, 3 and was amplified from 3D7 gDNA using the primers 5′-GAAGATAAACATGAATCCAATCC and 5′-TTTTGGATATATT TTCCCTTA. The second PfRh1 probe was used in Fig. 5 and was amplified as above using the primers 5′-TTCTTCTT TTTTCTGTATGATGTC and 5′-GAAAGACAAAACGATGTA CAC. The probe for the dihydropteroate synthase (dhps) gene was amplified using the primers 5′-TAGAACAAAGATT AAATTTTC and 5′-CACATATATTTTTTTGATATA.
Antibodies
A PfRh1 fragment for subcloning into the pGEX vector was amplified by PCR from 3D7 gDNA using the primers 5′-GATCGGATCCGAAGATAAACATGAATCCAATCC and 5′-GA TCCTCGAGTTGTAGAAGATCTATTTCGTGTG. The fusion protein was affinity-purified on glutathione agarose, then used to immunize rabbits. The anti-Rh1 antibodies were affinity-purified on the immunizing fusion protein coupled to Sepharose4B. The PfRh2a- and Rh2b-specific antibodies have been previously described (Rayner et al., 2000). The anti-SERA5 antibody has been previously described (Hodder et al., 2003).
Erythrocyte binding, SDS-PAGE and immunoblot analysis
Culture supernatants enriched in proteins required for merozoite invasion were obtained by synchronization of parasite cultures followed by treatment with trypsin (1.0 mg ml−1) and neuraminidase (25 mU ml−1) in order to prevent reinvasion of erythrocytes after schizont rupture. After 48 h smears were made to confirm the absence of reinvasion and the supernatants were harvested. Total proteins from schizont-stage parasites were obtained by synchronization and further culture until schizonts were present. Parasite proteins were then obtained by saponin lysis of erythrocytes. These proteins are labelled ‘pellet’ in 1, 3. Proteins were separated on 6% (for PfRh proteins) or 10% SDS-PAGE gels (for SERA5 proteins). Western blotting onto nitrocellulose (0.45 µm, Schleicher and Schuell, Germany) was performed according to standard protocols and blots were processed with a chemiluminescence system (ECL, Amersham). Erythrocyte binding assays using enriched culture supernatant from the Tak994 and W2mef parasites were performed as described previously (Triglia et al., 2001). Bound proteins were separated on 6% SDS-PAGE gels, Western blotted, then probed with anti-PfRh1 antibodies.
Erythrocyte invasion assay
Uninfected erythrocytes were treated with enzymes as described previously (Duraisingh et al., 2003a). For the assays, both a low trypsin (66.7 µg ml−1) and a high trypsin (1 mg ml−1) concentration were used for erythrocyte treatments. The invasion assay was carried out essentially as previously described (Reed et al., 2000). Erythrocytes infected with ring stages were synchronized twice followed by pretreatment with high trypsin and neuraminidase to prevent reinvasion into these erythrocytes. Experiments were performed with the addition of new erythrocytes treated with high trypsin (1 mg ml−1), neuraminidase (66.7 mU ml−1), low trypsin (66.7 µg ml−1)/neuraminidase (66.7 mU ml−1) and chymotrypsin (1 mg ml−1). The experiments in Fig. 1C were performed in triplicate in flat-bottomed microtitre plates in hypoxanthine-free medium. Enzyme-treated or untreated erythrocytes at 4% haematocrit were inoculated with infected erythrocytes to give a final parasitaemia of 0.5% and haematocrit of 4% in a total volume of 100 µl well−1. To allow for reinvasion, parasites were incubated for 48 h, after which 3H-hypoxanthine (Amersham) was added to a final concentration of 1 µCi well−1. After an additional 16 h the cells were subjected to a freeze/thaw cycle to facilitate harvesting onto glass fibre filters with the aid of a cell harvester (Packard). Incorporated 3H-hypoxanthine counts were determined in a scintillation counter. Percentage of invasion was calculated in comparison to invasion of the same parasite line into untreated erythrocytes, which was set to 100%. Additionally, invasion assays were set up as described above except that normal medium was used and the reinvasion rate was either determined microscopically by counting ring-stage parasites after 48 h, or determined on a FACScan instrument by counting trophozoite stages after 72 h. Trophozoites were stained with thiazole orange (Retic-COUNT, BD Biosciences).
Acknowledgements
We thank the Red Cross Blood Service (Melbourne, Australia) for supply of red cells and serum. We also thank Elizabeth Winzeler for access to her custom-made P. falciparum Affymetrix arrays. This work was supported by the National Health and Medical Research Council of Australia and the Wellcome Trust. M.T.D. is funded by a Wellcome Trust Advanced Training Fellowship (Tropical Medicine). A.F.C. is a Howard Hughes International Scholar.
Supplementary material
The following material is available from http://www.blackwellpublishing.com/prducts/journals/suppmat/mmi/mmi4388/mmi4388sm.htm
Fig.S1. Microarray analysis of Tak994 versus Tak994ΔRh1.




