Food vacuole-associated lipid bodies and heterogeneous lipid environments in the malaria parasite, Plasmodium falciparum
Summary
The malaria parasite Plasmodium falciparum induces a sixfold increase in the phospholipid content of infected erythrocytes during its intraerythrocytic growth. We have characterized the lipid environments in parasitized erythrocyte using the hydrophobic probe, Nile Red. Spectral imaging with a confocal microscope revealed heterogeneous lipid environments in parasite-infected erythrocytes. An insight into the nature of these environments was gained by comparing these spectra with those of triacylglycerol/phospholipid emulsions and phospholipid membranes. Using this approach, we identified a population of intensely stained particles of a few hundred nanometers in size that are closely associated with the digestive vacuole of the parasite and appear to be composed of neutral lipids. Electron microscopy and isolation of food vacuoles confirmed the size of these particles and their intimate association respectively. Lipid analysis suggests that these neutral lipid bodies are composed of di- and triacylgycerols and may represent storage organelles for lipid intermediates that are generated during digestion of phospholipids in the food vacuole. Mono-, di- and triacylglycerol suspensions promote β-haematin formation, suggesting that these neutral lipid bodies, or their precursors, may also be involved in haem detoxification. We also characterized other compartments of the infected erythrocyte that were stained less intensely with the Nile Red probe. Both the erythrocyte membrane and the parasite membrane network exhibit red shifts compared with the neutral lipid bodies that are consistent with cholesterol-rich and cholesterol-poor membranes respectively. Ratiometric imaging revealed more subtle variations in the lipid environments within the parasite membrane network.
Introduction
The malaria parasite spends part of its life cycle inside the erythrocytes of its human host. These terminally differentiated cells provide a refuge from the immune system but pose logistical difficulties with respect to obtaining the nutrients needed for growth and for the disposal of waste products. Mature human erythrocytes contain 310–350 mg ml−1 (20 mM) haemoglobin (Hellerstein et al., 1970) but no internal organelles and no machinery for the de novo synthesis or trafficking of proteins or membranes. The parasite degrades at least 75% of the erythrocyte haemoglobin during intraerythrocytic growth (Loria et al., 1999) to provide a source of amino acids and to create sufficient space for growth and division. The intraerythrocytic parasite feeds using a cytostome (mouth) to ingest small packets of haemoglobin from the host cytoplasm. The haemoglobin-containing vesicles are transported to an acidic food vacuole (FV), where the surrounding membrane is degraded by phospholipases. The haemoglobin is then digested by the action of a series of proteases (Rosenthal and Meshnick, 1996). As a consequence of this diet of haemoglobin, the parasite produces large amounts of free haem. Haem is a toxic molecule that can disrupt membranes, inhibit enzymic processes and initiate oxidative damage (Loria et al., 1999; Tilley et al., 2001; Campanale et al., 2003). It is detoxified by conversion into an insoluble pigment, known as haemozoin (Slater and Cerami, 1992; Sullivan, 2002).
The parasite also needs to obtain a source of lipids. During intraerythrocytic development, the phospholipid content increases sixfold as the parasite generates the membranes needed for growth and division (Beaumelle and Vial, 1988; Simoes et al., 1992). The host erythrocyte lacks lipid biosynthetic activity although it does have a limited phospholipid remodelling capacity (Vial and Ancelin, 1992; Mitamura and Palacpac, 2003). The parasite undertakes some short-chain fatty acid biosynthesis (using a type II pathway) as well as mevalonate-independent isoprenoid biosynthesis in the apicoplast (Jomaa et al., 1999; Waller et al., 2000; Surolia and Surolia, 2001). However, the bulk of the fatty acid and polar head group building blocks appear to be scavenged from the host (Mitamura and Palacpac, 2003; Vial et al., 2003). Using these intermediates, the parasite is capable of extensive synthesis and remodelling of phospholipids. The membranes generated by the parasite are enriched in phosphatidylcholine and phosphatidylethanolamine and depleted in cholesterol and sphingomyelin compared with the host membrane (Vial et al., 2003). The host cell membrane also undergoes some remodelling and exhibits a decrease in the level of polyunsaturated phospholipids (Vial et al., 2003).
After infection by Plasmodium falciparum, there is also an accumulation of triacylglycerol (TAG) (Vial and Ancelin, 1992). This is surprising as neutral lipids such as TAG are barely detectable in uninfected erythrocytes (Vial and Ancelin, 1992; Nawabi et al., 2003) and P. falciparum lacks the ability to oxidize fatty acids for energy (Holz, 1977). Recently, Palacpac et al. (2004) undertook biochemical analyses that showed pronounced accumulation of TAG in mature-stage parasites. These authors visualized lipid structures in P. falciparum-infected erythrocytes with the hydrophobic probe, Nile Red, and reported that these lipid bodies are associated with the parasitophorous vacuole (PV).
The absorption and fluorescence properties of Nile Red are known to be sensitive to environmental factors such as polarity (Greenspan and Fowler, 1985; Sackett and Wolff, 1987) and Nile Red is known to label membranes in addition to neutral lipid particles (Greenspan et al., 1985). We have combined the spatial resolution of the confocal microscope with spectral imaging of the Nile Red probe in order to characterize the various lipid compartments within P. falciparum-infected erythrocytes and to determine the location of amphipathic and neutral lipid components. We find that the lipid bodies are associated with the parasite FV rather than the PV and provide evidence that the lipid components of these structures, or their precursors, play an important role in the detoxification of haem in the parasite FV.
Results
Nile Red labelling of infected erythrocytes
Recently Palacpac et al. (2004) showed pronounced synthesis and accumulation of TAG in mature-stage parasites. They also reported that lipid bodies were visualized in P. falciparum-infected erythrocytes using the hydrophobic probes, Nile Red and Sudan III.
We have examined these lipid structures using two different labelling protocols. We initially cultured parasitized erythrocytes in the presence of Nile Red for a period of at least 12 h before examination by fluorescence microscopy. We found that the growth of 3D7 parasites was unaffected by concentrations of Nile Red from 1 ng ml−1 to 100 µg ml−1. Indeed, the labelled parasites were able to reinvade and grow at similar rates to control parasites (incubated with an equivalent volume of methanol) for up to 3 weeks. However, concentrations of Nile Red above 10 µg ml−1 resulted in some vesiculation of the parasite and host cell membranes (data not shown). Therefore, concentrations of ≤10 µg ml−1 were chosen to give maximum fluorescence while maintaining the integrity of the parasite membranes. In some experiments, cultures of parasitized erythrocyte were labelled with Nile Red and examined immediately. Similar results were obtained using either labelling protocol.
Nile Red labelling patterns were initially examined by epifluorescence microscopy using a standard rhodamine filter cube (Fig. 1A). In very early-stage parasites, a ring of fluorescence around the central nucleus was observed (Fig. 1A, a). This may represent endoplasmic reticulum (ER) labelling as the pattern resembles that seen with ER Tracker (data not shown). There was also some very faint staining of the erythrocyte membrane (see below). In young trophozoites, diffuse fluorescence was observed in the parasite cytoplasm, probably representing the ER, with stronger fluorescence surrounding the FV (Fig. 1A, b). As the parasite matures and begins to undergo schizogony (Fig. 1A, c–f), a segmented pattern of labelling, probably representing ER, surrounding individual merozoites was discerned. In addition, there were intense spots of fluorescence (indicated by white arrow) that may represent lipid stores. In contrast to the report of Palacpac et al. (2004), we found that these lipid-rich structures were often closely associated with the FV. The bright fluorescent structures correspond to ‘dark’ regions in the bright field images (black arrow).
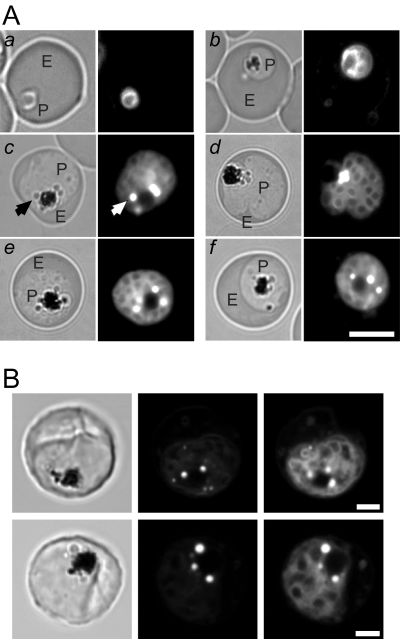
Nile Red labelling of P. falciparum-infected erythrocytes. A. Bright field (left) and fluorescence images (right) of erythrocytes infected with (a) ring (b) young trophozoite and (c–f) mature trophozoite and schizont-stage parasites recorded with an epifluorescence microscope using a rhodamine filter set. The P. falciparum-infected erythrocytes (3D7 strain) were maintained in culture in the presence of Nile Red at a final concentration of 1 µg ml−1. P denotes parasite and E denotes the erythrocyte compartment. An intense fluorescence spot is indicated by a white arrow and the corresponding ‘dark’ region in the bright field image by a black arrow. Scale bar = 5 µm. B. Schizont-infected erythrocytes examined by confocal microscopy. Bright field images are shown on the left. The middle images were recorded with 488 nm excitation and emission wavelengths of 540–600 nm. The images on the right were recorded with 543 nm excitation and with emission wavelengths of 600–680 nm. Scale bars = 2 µm.
More detailed investigations of Nile Red labelling patterns were performed using a confocal microscope. The relative intensity of the intense spots of fluorescence compared with the surrounding labelling depends on the combination of the excitation line and emission wavelengths utilized in the generation of the images (Fig. 1B). In particular, the intense spots of fluorescence were more evident when the excitation light and emission wavelengths were relatively blue shifted (Fig. 1B, middle) whereas other structures within the confines of the parasite become more evident when using more red-shifted wavelengths (Fig. 1B, right). Nile Red exhibits shifts in its absorption and fluorescence spectra depending on environmental factors such as polarity (Dutt et al., 1990; Ghoneim, 2000; Han et al., 2003). In particular, it has previously been reported that Nile Red stains both membranes and lipid bodies and that membranes can be preferentially detected as a red-shifted emission (Greenspan et al., 1985). Our results therefore indicate that Nile Red is staining different lipid environments in parasite-infected erythrocytes with the intensely staining spots representing more hydrophobic environments.
Spectral and ratiometric imaging of Nile Red-labelled structures
To gain a greater insight into the characteristics of the various structures labelled by Nile Red, we have utilized the spectral imaging capability of the Leica TCS-SP2 confocal microscope and measured the spectral properties of Nile Red in membrane dispersions and emulsions. Images were collected using 514 nm excitation with an emission bandwidth of 10 nm over the wavelength range 535–675 nm. Phosphatidylcholine dispersions labelled by Nile Red were visualized as structures of various sizes (Fig. 2A, b). The fluorescence intensity appears relatively uniform in different regions of the membrane cross-section (Fig. 2A, b, green images) and the average emission spectrum of these dispersions exhibits an emission maximum at 610–615 nm (Fig. 2A, a, squares). However, it was noted that different regions of the membrane structures produced different spectra. This spatial variation of the Nile Red emission spectrum was visualized by generating ratiometric (R) images from the spectral series as outlined in Experimental procedures. Regions of the membrane possessing blue-shifted spectra (larger R values) are evident as orange to yellow using the look-up-table shown to the right of this figure. A striking feature of the R images of phosphatidylcholine membranes is that regions of membrane that are horizontal in the plane of the page (yellow arrows) possess blue-shifted spectra compared with those that are orientated vertical to the page (purple arrows).
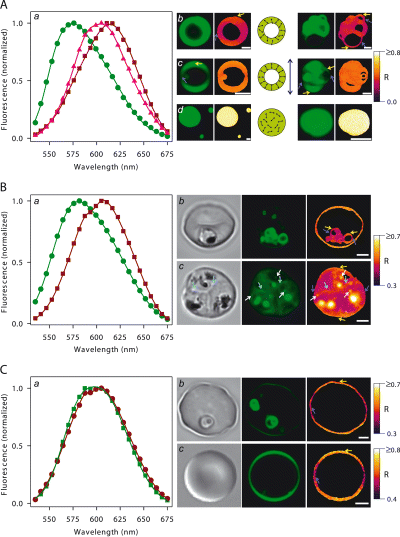
Spectral properties of Nile Red-labelled lipid structures in model systems and in parasite-infected erythrocytes. A series of images of Nile Red-labelled samples for spectral analysis were recorded as described in Experimental procedures. Panels a represent average emission spectra recorded from a number of lipid structures or cells. Representative bright field (grey scale), fluorescence (green) and ratiometric (R) images (with look-up-tables on the extreme right of each panel) are shown in b–d. A. Model membrane systems. Panel a represents spectra for dispersions of phosphatidylcholine (squares), phosphatidylcholine/cholesterol (triangles) and phosphatidylcholine/trioleoylglycerol (circles). Two representative fluorescence images of these three types of dispersions with their corresponding R images are shown in b–d respectively. Scale bars = 1 µm. Yellow arrows in images point to membrane regions orientated in the horizontal plane of the page whereas the purple arrows point to membrane regions orientated in the vertical plane of the page. The double-headed dark blue arrow corresponds to the plane of polarization of the excitation light. The schematic diagrams represent possible orientations of the absorption dipole of Nile Red (double-headed arrows) in cross-sections for each of the three model systems. Those molecules containing their absorption dipole in the same direction as the plane of polarization of the excitation light are more likely to be excited and hence exhibit fluorescence. B. Nile Red staining of the parasite compartment in infected erythrocytes. Panel a represents averaged emission spectra arising from the intensely fluorescent spots within the parasites (circles) and from all compartments of the intracellular parasite except the intense spots (squares). Panel b shows a young trophozoite. The fluorescence image (green) has been enhanced using the gamma function to visualize the weak staining of the erythrocyte membrane. Two spectrally scanned series of images were obtained at different photomultiplier gains and the subsequent R images combined to permit a direct comparison of the erythrocyte and parasite R values. Panel c is an erythrocyte multiply infected with mature trophozoites. The white arrows point to small structures possessing intermediate R values compared with the intensely fluorescent spots and general membrane staining. The light blue arrows point to stretches of membrane possessing larger R values relative to the surrounding staining. The purple and yellow arrows point to vertically and horizontally oriented regions of membrane respectively. Scale bars = 2 µm. C. Nile Red staining of the erythrocyte membrane in infected and uninfected erythrocytes. Panel a represents averaged emission spectra from infected (circles) and uninfected (squares) erythrocytes. Panels b represent an erythrocyte infected with two rings. The movement of the rings precluded their analysis in the R image. The fluorescence image was generated from a single-line scan rather than from a spectral series and has been enhanced using the gamma function to visualize the weaker staining of the erythrocyte membrane in relation to the rings. Panels c represent an uninfected erythrocyte. The purple and yellow arrows point to vertically and horizontally oriented regions of membrane respectively. Scale bars = 2 µm.
The incorporation of 50 mol% cholesterol into the phosphatidylcholine membranes was associated with a blue shift in the Nile Red emission spectrum giving an emission maximum of 595 nm (Fig. 2A, a, triangles). There was also an almost complete abolition of the spatial variation in the R values (Fig. 2A, c). Moreover, the addition of cholesterol produced a striking effect on the fluorescence images of the membrane cross-sections (Fig. 2A, c, green images). In particular, stretches of membrane that are orientated horizontally in the plane of the page (yellow arrows) appear brighter than those orientated vertically in the plane of the page (purple arrows). Similar non-homogenous fluorescence patterns have been observed in polarized images of eosin-labelled band 3 and carbocyanine dyes in erythrocyte membranes (Blackman et al., 1996; Sund et al., 1999). It is therefore likely that this pattern of fluorescence reflects the anisotropic orientation of Nile Red within the membrane bilayer and the use of a polarized laser (polarization vertical in the plane of the page as shown by the double-headed dark blue arrow in Fig. 2A). This presumably leads to preferential excitation of Nile Red molecules that have their absorption dipole orientated in the same direction. Because the absorption dipole of Nile Red lies along the long axis of the molecule (Dutt et al., 1990), this indicates that the Nile Red associated with the membrane is arranged with its long axis perpendicular to the plane of the membrane. The schematic diagram in Fig. 2A illustrates the likely orientation of the probe in the different model systems.
Trioleoylglycerol/phosphatidylcholine emulsions stained by Nile Red were visualized as circular cross-sections of various sizes with a uniform fluorescence intensity and R value across the structure (Fig. 2A, d). The spectrum shows a highly blue-shifted emission maximum of ≈575 nm (Fig. 2A, a, circles). These general trends in the spectral shifts of Nile Red are consistent with previous fluorometric determinations of Nile Red in lipid dispersions and in various solvents (Greenspan and Fowler, 1985). In particular, a blue shift in the spectrum is consistent with a general decrease in the polarity of the microenvironment. In the case of the TAG/phospholipid emulsions, this reflects the incorporation of the Nile Red within the hydrophobic core of the particle, whereas in the case of the phospholipid/cholesterol dispersions this reflects the decreased water penetration into the bilayer centre in the presence of cholesterol (Marsh, 2001).
We next examined the spectral properties of the various Nile Red-labelled structures in parasite-infected erythrocytes. When the intense spots of fluorescence were excluded from the analysis, the average emission spectrum of the parasite-associated Nile Red exhibited an emission maximum of 605 nm (Fig. 2B, a, squares). This is similar to that observed for the phosphatidylcholine dispersions. Again, it was noted that the spectrum obtained was dependant on the region of the parasite that was examined. Figure 2B (b) shows a young trophozoite that does not yet possess the intense spots of fluorescence. The R image generated from the spectral series of this cell exhibits the same spatial variation as observed in the phosphatidylcholine dispersions. These properties indicate that the weakly labelled structures represent the parasite membrane network.
The intense spots of fluorescence in mature parasites exhibit an emission maximum of 580 nm (Fig. 2B, a, circles), similar to that observed in the TAG/phospholipid emulsions. Nile Red is commonly used as a stain of lipid stores in cells such as adipocytes (Greenspan et al., 1985; Toscani et al., 1990) and tends to concentrate in neutral lipid droplets such as stores of TAG and cholesterol esters (Greenspan et al., 1985). Thus, the intensely fluorescent spots observed in the infected erythrocytes are likely to represent storage organelles of this nature and are hereafter referred to as neutral lipid bodies (NLB).
The difference in the emission spectrum of the NLB compared with the weakly stained parasite membrane system is evident in the R image of an erythrocyte multiply infected with mature trophozoites (Fig. 2B, c). The NLB exhibit bright fluorescence and high R values (i.e. they appear white using the look-up-table associated with this figure). A number of spots with lower R values compared with the brightly stained NLB are also evident in the R image (white arrows). These correspond to small, faint spots in the green fluorescence image (white arrows). The lower R values of these bodies may be the result of mixing with the fluorescence signal from the surrounding parasite membrane. Alternatively, they may represent NLB in the process of being formed or of being depleted that possess intermediate lipid environments compared with the intensely fluorescent NLB.
The spatial variation in the emission spectrum of Nile Red is also evident in the membrane network of the mature-stage infected erythrocyte shown in Fig. 2B (c). In particular, the top and bottom regions of the image (yellow arrows in R image) are blue shifted compared with the sides (purple arrows in R image). These regions correspond to the outer limits of the parasite and probably represent fluorescence arising from the PV membrane and/or the parasite plasma membrane. The R values within the parasites appear to be more evenly distributed although there are some regions (indicated by the light blue arrows) that display higher R values. These regions also appear to correspond to the outer limits of the parasites (see light blue arrows in corresponding bright field and fluorescence images). These results indicate the presence of heterogeneous lipid environments within the parasite membrane network.
Nile Red also stains the red cell membrane although with about 10-fold less intensity compared with the parasite membrane network. This is evident in the fluorescence (green) images of infected erythrocytes (Fig. 2B and C, b). In these images, the Nile Red signal from the erythrocyte has been enhanced using the gamma function. Analysis of the spectral characteristics of Nile Red in the host cell membrane required the use of 5–10 µg ml−1 of the fluorophore during labelling, and a washing step to remove the background fluorescence. A striking feature of the labelled erythrocytes is the non-homogenous intensity observed across the membrane cross-section (see green fluorescence image in Fig. 2C, b). This is similar to the pattern observed in phosphatidylcholine/cholesterol dispersions (Fig. 2A) and is consistent with the known cholesterol content of the erythrocyte membrane.
The emission spectra of Nile Red in the host membranes of a number of uninfected and infected erythrocytes was recorded and averaged. There were no obvious differences between uninfected and infected erythrocytes (Fig. 2C, a), or between erythrocytes infected with ring- and trophozoite-stage parasites (data not shown). The large overlap in signal from the parasite and host membranes in mature trophozoites and schizonts prevented analysis of these cells.
The emission maximum of the Nile Red-labelled erythrocyte membranes (≈590 nm) is blue shifted compared with that of the parasite membrane network, again consistent with the presence of cholesterol in the former. The spectral differences between these membranes are evident in the R image of the young trophozoite shown in Fig. 2B (b; a largely magenta parasite versus a largely orange erythrocyte membrane). However, the Nile Red-labelled erythrocyte membranes exhibit a more pronounced spatial variation in spectral properties than the phosphatidylcholine/cholesterol model membranes. In particular, the brightest regions in the cross-sections are blue shifted compared with the fainter regions (yellow and purple arrows in Fig. 2C), indicating a somewhat less ordered microenvironment in the biological membrane.
Neutral lipid bodies are associated with the parasite FV
An examination of the fluorescence images shown in 1, 2 (and many others) indicated a tendency for the lipid bodies to be closely associated with the parasite FV. This association was investigated by isolating the FV of 3D7 strain parasites after very gentle disruption of the parasitized erythrocytes on ice (Saliba et al., 1998). To follow the enrichment process, the FV preparation was characterized using an anti-serum recognizing P. falciparum chloroquine resistance transporter (PfCRT), which has previously been shown to be FV associated (Fidock et al., 2000; Zhang et al., 2002). This anti-serum was generated against a recombinant fragment representing amino acids 1–97 of PfCRT. It recognizes a band with an apparent molecular mass of 43 kDa in parasite preparations (Fig. 3A, lane e). This agrees with previous findings (Fidock et al., 2000; Zhang et al., 2002) using an antibody raised against a 15 residue peptide in the C-terminal region of PfCRT. The anti-serum also recognizes a minor cross-reactive species with an apparent molecular mass of ≈55 kDa (Fig. 3A, lane e). A species with an apparent molecular mass of 85 kDa was also observed in some preparations. This appears to be a dimer of PfCRT as the level of this species was decreased at lower loadings of the sample. Immunofluorescence microscopy shows that the anti-serum mainly labels the FV with less intense labelling of small structures in the parasite cytoplasmic and some weak background fluorescence (Fig. 3B).
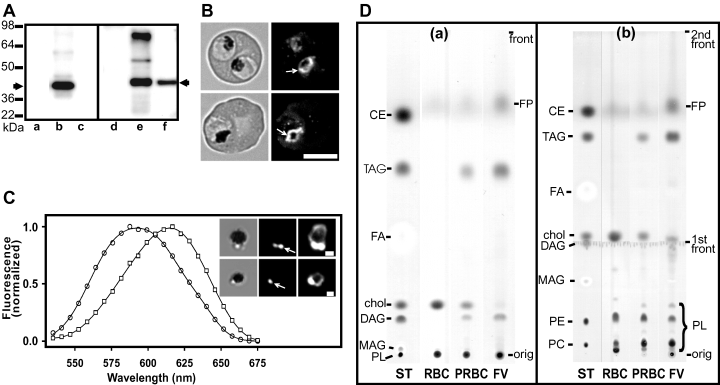
Characterization of a FV preparation with anti-PfCRT, Nile Red labelling and thin-layer chromatography. A. Assessment of the FV enrichment protocol. Erythrocyte membranes (lanes a and d), saponin-lysed infected erythrocytes (lanes b and e), and purified FV (lanes c and f) were subjected to SDS-PAGE (12% acrylamide) and transferred to polyvinylidene difluoride. The membranes were probed with either rabbit anti-PfERC (1:1000; lanes a–c) or rabbit anti-PfCRT (1:500; lanes d–f), followed by horseradish peroxidase-conjugated anti-rabbit IgG and visualized using enhanced chemiluminescence reagent. The arrows represent PfERC and PfCRT monomer. B. Immunofluorescence analysis of PfCRT in infected erythrocytes. Parasitized erythrocytes (3D7 strain) were smeared on glass slides, fixed with acetone : methanol (1:1) and probed with affinity-purified rabbit anti-PfCRT anti-serum followed by fluorescein-labelled anti-rabbit IgG. Left panels are bright field images and right panels are fluorescence images. The arrows point to the FV. Scale bar = 5 µm. C. Spectral properties of Nile Red in isolated FV. FV preparations were incubated in the presence of Nile Red (4 µg ml−1) and a spectral series of images collected using the Leica TCS-SP2 confocal microscope. The inset shows bright field images on the left and fluorescence images obtained with different photomultiplier settings (the right panel represents an overexposed image). The emission spectra of the intense spots (circles) and of the weaker fluorescence surrounding the haemozoin (squares) are shown. Scale bar = 1 µm. The arrows point to the NLB. D. TLC analysis of lipid classes in saponin-lysed uninfected or parasitized erythrocytes and isolated FV. Samples were separated using a single mobile phase (a) or a combination of two mobile phases (b) as described in Experimental procedures. The lipid standards (10 µg each) are phosphatidylcholine (PC), phosphatidylethanolamine (PE), monoacylglycerol (MAG), diacylglycerol (DAG), cholesterol (chol), fatty acid (FA), triacylglycerol (TAG) and cholesterol ester (CE). ST denotes standards, RBC is erythrocyte membranes, PRBC is lysed parasitized erythrocytes, FV is purified food vacuoles, PL is phospholipid, FP is ferroprotoporphyrin IX = reduced haem.
Immunoblots of erythrocyte membranes, lysed parasite-infected erythrocytes and purified FV were probed with anti-PfCRT and an antibody against P. falciparum ER-located calcium-binding protein (PfERC) as a marker of ER contamination. Anti-PfERC recognizes a band of 40 kDa in parasite preparations (Fig. 3A, lane b) as reported previously (La Greca et al., 1997). Neither of the anti-sera recognized proteins in uninfected erythrocyte preparations (Fig. 3A, lanes a and d). Analysis of the FV preparation revealed the presence of PfCRT and the absence of PfERC, indicating enrichment of the parasite FV without ER contamination (Fig. 3A, lanes c and f).
The FV preparation was incubated in the presence of Nile Red at a final concentration of 4 µg ml−1 and examined by fluorescence microscopy. The bright field images (Fig. 3C, inset) show the presence of dark haemozoin crystals surrounded by poorly refracting halos, which appear similar to images of isolated FV reported previously by Saliba et al. (1998). The FV in the Nile Red-stained samples were typically associated with one to three intensely stained structures that exhibited emission spectra (Fig. 3C, circles) that approached those observed for NLB in intact parasitized erythrocytes. Overexposure of the same images revealed the presence of a low-intensity circle of fluorescence surrounding the haemozoin (Fig. 3C, inset, right). The spectrum from this compartment, which is presumably the FV membrane, exhibited an emission maximum of 615 nm (Fig. 3C, squares) as expected for a membrane compartment.
To further characterize the composition of the NLB, we have used thin-layer chromatography (TLC) to analyse the lipid classes in purified FV and in saponin-lysed uninfected or parasitized erythrocytes. Samples were separated using hexane : di-isopropyl ether : acetic acid (64:40:4) alone (Fig. 3D, a) or after initial separation to front 1 using chloroform : methanol : water (50:20:3) (Fig. 3D, b). The former solvent system allows good separation of neutral lipids but the phospholipids remain at the origin. The latter system separates the phospholipids but diacylglycerol (DAG) is difficult to visualize as it runs close to the first front. The chromatographs of the uninfected erythrocyte membrane extract indicate that this sample has relatively more cholesterol than the other samples but no neutral lipid. In contrast, spots corresponding to DAG and TAG were apparent in the extracts of parasitized erythrocytes and enhanced in the isolated FV. The two-stage chromatography method allowed separation of the phospholipid classes. These data indicate an enrichment of phosphatidylcholine and phosphatidylethanolamine, depletion of sphingomyelin (the slowest migrating phospholipid spot) and relative depletion of cholesterol, in the parasitized erythrocytes as have been reported previously (Vial and Ancelin, 1992). Semi-quantitative analysis of the amido black staining to estimate the relative amounts of TAG compared with phospholipid (Fig. 3D, b) indicates that there is a significant (2.2-fold) enrichment of TAG in the FV preparation compared with the parasitized erythrocytes. The fastest migrating spot is not cholesterol ester, which is absent from infected erythrocytes (Nawabi et al., 2003). Analysis of extracts of erythrocyte cytosol suggests that this spot may correspond to a small amount of reduced haem in the samples. In contrast, haematin/haemozoin remains at the origin as seen in the FV preparation (Fig. 3D, b).
The intensely stained NLB evident in the microscopic images (1, 2) are on the order of a few hundred nanometers in size (i.e. they approach the limit of the resolution available with optical microscopy imposed by the diffraction of light). An examination of electron micrographs of mature stage-infected erythrocytes (A4 strain) revealed that some sections (≈10%) contained small lipid droplets that are predominately located adjacent to the FV (Fig. 4). These lipid droplets are characterized by their low electron density, homogeneous appearance and the absence of a limiting unit membrane as has been observed in other cell types (Charron and Sibley, 2002; Robertson et al., 2003). Similar structures were observed in three other parasite strains, FCR3, D4 and T996 (data not shown). The size of these FV-associated lipid droplets is in the range expected from the microscopic images of the live cells.

Transmission electron microscopy of lipid bodies in parasitized erythrocytes. A. Section through an erythrocyte containing a late trophozoite-stage parasite showing a weakly stained body (arrow) associated with the FV. N, nucleus. Scale bar = 1000 nm. B. Detail of the neutral lipid body (arrow). Scale bar = 200 nm.
Neutral lipids promote β-haematin formation
Fitch et al. (1999) have shown that monooleoylglycerol and, to some extent, dioleoylglycerol and other lipid mixtures can enhance β-haematin formation in vitro, however, they found that TAG was not active. Given that the NLB appear to be comprised of both DAG and TAG and that these structures are closely associated with the FV where haemozoin formation takes place, we have re-examined the ability of mono-, di- and triacylglycerols to promote β-haematin formation. Under the conditions examined (150 µM haematin mixed with sonicated lipid in 90 mM sodium acetate, pH 5), addition of monooleoylglycerol yielded up to 55%β-haematin during the 24 h incubation (Fig. 5). This is in general agreement with previous reports (Papalexis et al., 2001). By comparison, in the absence of lipids, about 8% of the haematin was converted to β-haematin. Mono- and dimyristoylglycerol were also efficient enhancers of haematin crystallization and were effective at a lower concentration. Trimyristoylglycerol and trioleoylglycerol also enhanced β-haematin formation although interestingly the level of enhancement decreased at higher levels of the TAG (Fig. 5).
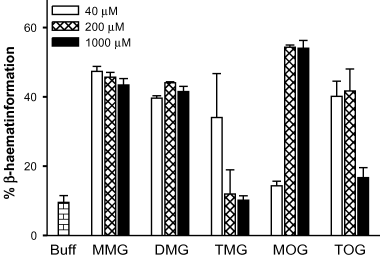
Enhancement of β-haematin formation in vitro by neutral lipids. Samples of haematin (150 µM) were incubated for 24 h at 37°C, in 90 mM sodium acetate, pH 5.0 in the absence (Buff) or in the presence of the indicated concentrations of mono-, di- or trimyristoylglycerol (MMG, MDG or TMG, respectively) or mono- or trioleoylglycerol (MOG or TOG respectively). Error bars correspond to the variations observed in duplicates.
Discussion
Nile Red labelling distinguishes a number of lipid compartments in infected erythrocytes
In this study, the structures in P. falciparum that are labelled by the fluorescent probe, Nile Red, were examined using a confocal microscope with spectral scanning capabilities. The spectra were compared with those observed for Nile Red in synthetic phospholipid dispersions and neutral lipid emulsions. Nile Red was shown to stain a variety of structures within the infected erythrocyte. The most intensely stained were lipid bodies present in mature trophozoites and schizonts. The intense fluorescence of the NLB was superimposed on a weaker staining of the parasite membrane system, although the two types of structures could be clearly distinguished based on their emission maxima as illustrated using ratiometric imaging.
Nile Red associated with the parasite and erythrocyte membranes exhibited a number of interesting properties. Non-uniform fluorescence intensity was evident in the confocal sections of the erythrocyte membrane. A similar topographical variation in intensity was apparent for Nile Red in phosphatidylcholine/cholesterol dispersions but was less pronounced in pure phospholipid liposomes. This effect presumably reflects an anisotropic orientation of the probe within the membrane bilayer and reports the increased order of membranes in the presence of cholesterol (Yeagle, 1985; Hofsass et al., 2003). As a result, Nile Red molecules occupying regions of the membrane that lie perpendicular to the plane of polarization of the exciting light (i.e. with the absorption dipole of Nile Red parallel to the plane of polarization) are more efficiently excited and hence have a greater fluorescence intensity compared with other regions of the membranes. This effect was less obvious in the parasite membrane network. This may reflect a lower cholesterol content, which in turn permits a more heterogeneous population of Nile Red molecules.
Similarly, we could clearly distinguish a blue shift in the emission spectrum of Nile Red in the erythrocyte membrane compared with that in the parasite membranes. Again, this shift is likely to reflect differences in cholesterol content given that the erythrocyte membrane contains ≈50% cholesterol, whereas cholesterol is greatly depleted in parasite membranes (Fig. 3D; Vial et al., 2003). Cholesterol increases the order of the fatty acyl chains in the bilayer and limits access of water to the hydrophobic core of the membrane (Marsh, 2001).
Further spectral differences were evident within the parasite membrane network that could reflect differences between the PV membrane/parasite plasma membrane and the intracellular parasite membranes. It is likely that some host lipid components are incorporated into the PV membrane during invasion (Dluzewski et al., 1995) and there is evidence for trafficking of raft components between the host cell membrane and the PV membrane (Haldar et al., 2002). Our ratiometric imaging data are consistent with these reports of a more ‘host-like’ lipid environment in the PV membrane.
In summary, the spectral analysis indicates that Nile Red is capable of providing information about different lipid compartments of P. falciparum-infected erythrocytes. Previous studies of lipid composition have relied on physical separation of the parasite and host membranes (Vial et al., 2003) or have measured general membrane microviscosity in cuvette-based measurements (Howard and Sawyer, 1980). The ability to collect spatially resolved spectral data using the confocal microscope circumvents the difficulties of obtaining clean membrane preparations and allows ready visualization of erythrocytes infected with live parasites at different stages of development.
Role of NLB in infected erythrocytes
Levels of TAG (but not cholesterol ester) have been shown to increase significantly after plasmodial infection (Nawabi et al., 2003; Vial et al., 2003; Palacpac et al., 2004). Palacpac et al. (2004) used Nile Red to visualize NLB in parasitized erythrocytes and reported that the lipid structures are associated with the secretory pathway and accumulate in the PV. ER-associated NLB have also been reported in Toxoplasma gondii and other cells (Murphy and Vance, 1999; Charron and Sibley, 2002). However, our examination of a number of Nile Red-labelled cells by fluorescence microscopy indicated that the NLB structures are largely associated with the FV. Electron microscopy confirmed this association and provided no evidence for the association of NLB with the PV. The physical attachment of the NLB to the FV was confirmed by co-isolation with the FV in an enrichment protocol that was monitored with an antibody against the FV protein, PfCRT. The enrichment of TAG (and DAG) in these purified FV was confirmed by TLC analysis of lipid classes. Thus it is likely that the PV-associated Nile Red reported by Palacpac et al. (2004) represents staining of the parasite membrane network. The enhanced spectral discrimination offered by our system may have facilitated identification of the different parasite compartments. While this article was under review, another article providing EM evidence for the presence of NLB in P. falciparum-infected erythrocytes has been published (Vielemeyer et al., 2004).
The close association of NLB with the FV suggests that they may function in processes occurring within this organelle. The FV is the site of degradation of haemoglobin, which is endocytosed from the host cell cytosol. The digestion process results in the production of potentially toxic free haem. An important pathway for the detoxification of haem is the formation of crystals of haemozoin, the characteristic malarial pigment present within the FV. Haemozoin and its synthetic equivalent, β-haematin, are comprised of a repeating array of coordinated dimers, with the ferric iron of each haematin chelated onto the carboxyl side chain of its partner, held together in a crystalline matrix by hydrogen bonding interactions (Pagola et al., 2000). It has been suggested that histidine-rich proteins may contribute to the catalysis of haemozoin formation in vivo (Sullivan et al., 1996; Francis et al., 1997); however, the levels of these proteins in the FV appear to be too low to provide sufficient catalytic activity (Papalexis et al., 2001; Akompong et al., 2002). Bendrat et al. (1995) proposed that specific lipid components in parasite preparations may also contribute to the catalysis of haemozoin formation in vivo. We considered the possibility that neutral lipid components of the NLB might assist in the crystallization of haematin in the FV. Mono- and dioleoylglycerol have previously been shown to promote the formation of β-haematin (Fitch et al., 1999; Papalexis et al., 2001; Pandey et al., 2003), but trioleoylglycerol was reported not to promote β-haematin formation (Fitch et al., 1999). We found, however, that freshly sonicated TAG suspensions do promote haematin crystallization, particularly at lower lipid to haematin ratios. The mechanism of catalysis is likely to involve presentation of haematin in a soluble form that is suitable for the formation of the coordinated β-haematin dimers that form the units of the crystal. The lower concentrations of TAG may promote the close association of haematin molecules and enhance the formation of β-haematin dimers. The differences between our findings and those of Fitch et al. (1999) probably derive from the use of lower levels of TAG and the preparation of sonicated suspensions possessing increased surface area.
It is possible that the NLB represent the site of β-haematin formation in vivo. However, given that these structures are most prominent in more mature-stage parasites, it is more likely that aggregates of DAG and other TAG precursors that accumulate in the FV before incorporation into NLB play a role in haem detoxification during the early trophozoite stage of infection. Indeed, DAG levels have been reported to be maximal in the trophozoite stages but are apparently converted to TAG by the schizont stage (Nawabi et al., 2003). These data are of considerable interest as Plasmodium depends on β-haematin formation to avoid haem toxicity. It may be possible to target anti-malarials to these lipid-rich aggregates to interfere with β-haematin formation. In this context, it is interesting to note that hydrophobic quinolines such as mefloquine are more active against cultures than predicted from their expected accumulation ratio based on charge effects (Tilley et al., 2001).
Although TAG are most often associated with energy storage, they have been shown in Saccharomyces cerevisiae to have a role in storage, mobilization and transport of the building blocks for structural lipids (for review, see Murphy and Vance, 1999). TAG stores may play a similar role in Plasmodium sp. because the parasites do not possess the enzymes required for fatty acid oxidation (Holz, 1977; Gardner et al., 2002). It is known that P. falciparum needs to generate large amounts of phospholipids in schizogony to produce the membranes needed for multiplication within the erythrocyte host. In addition, there must be a large turnover of phospholipids during the growth of the parasite as it ingests the host haemoglobin through the cytostome. The endocytic compartments that are formed are surrounded by a double membrane originating from the parasite plasma membrane and the PV membrane. The outer membrane of the vesicles fuses with the FV membrane, whereas the inner membrane is thought to be degraded by phospholipases (Yayon et al., 1984; Slomianny, 1990). We propose that the phospholipid breakdown products are assembled into TAG and that precursors in the formation of the TAG serve as an important catalyst of β-haematin formation. A diagrammatic representation of this putative process is shown in Fig. 6.
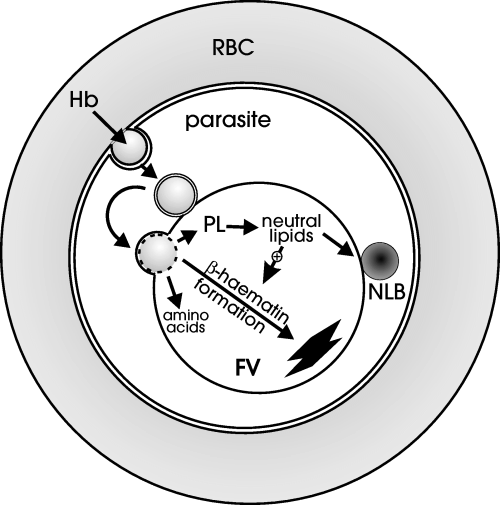
Diagrammatic representation of the digestion of haemoglobin (Hb)-containing vesicles in the parasite FV with the release of amino acids, lipid components and free haem. We propose that the phospholipids (PL) are degraded and the components are reconstituted to form neutral lipids. These neutral lipids promote the formation of β-haematin. We also propose that the NLB function as storage organelles for lipid building blocks until they are needed for membrane synthesis during schizogony.
It is also likely that the NLB serve as a depot of lipid components that can be rapidly mobilized to supply the growing parasite with fatty acids and acylglycerols for generation of phospholipid membranes as suggested by Palacpac et al. (2004). P. falciparum is capable of synthesis of phospholipids via the Kennedy pathway using serum-derived fatty acids, as well as conversion of TAG to essential phospholipids (Vial et al., 2003). Most of the NLB visualized were intensely fluorescent and on the order of a few hundred nanometers in size. However some late-stage schizonts possessed smaller, fainter structures at or below the resolution limit of light microscopy. These may represent NLB that are being consumed as part of the formation of the extensive membrane systems of the daughter merozoites.
In recent years, the development of transfection techniques to permit studies of green fluorescent protein chimeras has led to major advances in our understanding of protein trafficking in malaria parasite-infected erythrocytes whereas our understanding of the organization and mobilization of lipids has lagged somewhat behind. Probes such as Nile Red represent a tool that allows us to study the organization of lipid components in real time in live cells. This work gives insights into some of the lipid structures in this important human pathogen and points to possible new targets for malaria chemotherapy.
Experimental procedures
Parasites
Plasmodium falciparum strains (3D7 and A4) were cultured as previously described (Raynes et al., 1996). The FVs of P. falciparum parasites (3D7 strain) were isolated using minor modifications of a published method (Saliba et al., 1998). Briefly FVs were released from saponin-lysed parasitized erythrocytes by suspending in ice-cold water, pH 4.5 and triturating through a 27-G 1.2 cm needle. After centrifugation, the pellet was further disrupted by trituration in 2 mM MgSO4, 100 mM KCl, 10 mM sodium phosphate, pH 7.4, containing 10 µl of 5 mg ml−1 Dnase 1 (Roche) and collected by centrifugation through 42% Percoll containing 0.25 M sucrose, 1.5 mM MgSO4, pH 7.
Thin-layer chromatographic analysis of lipids
Samples of uninfected or parasitized erythrocytes were prepared by lysis with 1% saponin in phosphate-buffered saline containing 5 µg ml−1 streptomycin sulphate for 10 min on ice. Lipids were extracted using a two-phase system comprising chloroform : methanol : water (8:4:3) from ≈109 uninfected erythrocytes, ≈5 × 108 parasitized erythrocytes or from FV prepared from ≈1010 parasitized erythrocytes. Samples corresponding to 30% of these extracts were separated on Silica Gel 60 TLC plates (Merck) using hexane : di-isopropyl ether : acetic acid (64:40:4) either alone or after initial separation using chloroform : methanol : water (50:20:3). Lipid standards (dipalmitoylphosphatidylethanolamine, dimyristoylphosphatidylcholine, myristic acid, cholesterol, cholesterol myristate, mono- and dimyristoylglycerol and trioleoylglycerol; all from Sigma) were prepared in chloroform. The plates were stained with amido black 10B (Sigma) in 1% acetic acid/water (Plekhanov, 1999). The TLC plates were scanned and the images analysed with background correction using NIH ImageJ (http://rsb.info.nih.gov/ij).
Cloning and expression of PfCRT
cDNA from 3D7 strain P. falciparum was prepared as described (Kyes et al., 2000) and kindly provided by Dr Sue Kyes (Institute of Molecular Medicine, Oxford, UK). The full-length Pfcrt sequence was amplified and confirmed by sequencing and a region corresponding to amino acid residues 1–97 of PfCRT was cloned into pGEX5X3 (Amersham Pharmacia Biotech) and expressed in E. coli (BL21 strain). The resultant glutathione S-transferase (GST) fusion protein was purified on a glutathione-Sepharose column (Amersham Pharmacia Biotech), confirmed by N-terminal sequencing and used to generate anti-sera in rabbits as described previously (Adisa et al., 2001).
Western analysis
The anti-GST-PfCRT anti-serum was affinity purified as described previously (Adisa et al., 2001). Preparation of antibodies against PfERC has been described elsewhere (La Greca et al., 1997; Adisa et al., 2001). For immunoblot analyses, erythrocyte membranes, harvested asynchronous P. falciparum-infected erythrocytes and isolated FV were subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE; 4–12% polyacrylamide), transferred to polyvinylidene difluoride, probed with rabbit anti-serum followed by horseradish peroxidase-conjugated anti-rabbit antibody (Sigma) and detected with enhanced chemiluminescence reagent.
Labelling of P. falciparum with the lipid probe, Nile Red
Infected erythrocytes (5–10% parasitaemia, 3% haematocrit) were labelled with Nile Red (Molecular Probes) in culture using a modification of the method of Palacpac et al. (2004). Nile Red was added to a final concentration of 1 µg ml−1, from a concentrated stock in methanol. The final concentration of methanol was 1 vol% and did not affect the growth of parasites. The infected erythrocytes were examined immediately or after culturing for at least 12 h. For measurements of the weak fluorescence arising from the erythrocyte membrane, the cells were centrifuged and resuspended in serum-free culture medium before imaging. Aliquots of labelled cultures were incubated on ice for 30 min before analysis by confocal microscopy. For purified FV, Nile Red was added and samples were incubated on ice for 30 min.
Preparation of membranes and emulsions
Cholesterol, trioleoylglycerol and egg yolk phosphatidylcholine (Sigma) were dissolved in chloroform : methanol (1:1 v/v) and the required amount of each lipid was dispensed into a conical flask. The solvent was allowed to evaporate under nitrogen, and then buffer (0.1 M NaCl, 0.01 M Tris, pH 7.4, 0.02% w/v sodium azide) was added to give a final lipid concentration of 500 µM. The lipids were resuspended by vortexing in the presence of glass beads. Nile Red was added to the lipid suspensions from a methanol stock to a final concentration of 1–4 µg ml−1.
Effect of neutral lipids on β-haematin formation
Haematin (Sigma) was prepared as a stock in 50 mM NaOH on the day of use. The lipids (mono- and trioleoylglycerol, mono-, di- and trimyristoylglycerol; Sigma) were prepared as stocks in chloroform. The required amount of lipid was dispensed into microfuge tubes, the solvent evaporated under nitrogen, and the lipids resuspended in 500 µl of assay buffer (90 mM sodium acetate, pH 5.0) with a 30 s sonication using a Microson ultrasonic cell disrupter (Misonix). Haematin was immediately added to the lipid suspensions and to the controls (assay buffer and an equivalent aliquot of 50 mM NaOH) to a final concentration of 150 µM and the samples were incubated overnight at 37°C with gentle rocking. The β-haematin formed was washed and quantified spectrophotometrically as described by Kalkanidis et al. (2002). The proportion of β-haematin formed in the presence of the lipids was calculated relative to the total haematin present.
Fluorescence microscopy and analysis
Samples were labelled with Nile Red or prepared for immunofluorescence as previously described (Albano et al., 1999; Adisa et al., 2001). The slides were viewed with an Olympus BX50 epifluorescence microscope with a rhodamine filter cube (excitation 530–550 nm bandpass, emission 590 nm long pass) or an inverted Leica TCS-SP2 confocal microscope using 100× oil immersion objectives (1.4 NA). In the latter case, an argon (488 or 514 nm line) or helium-neon laser (543 nm line) equipped with the appropriate dichroics was used for excitation. The TCS-SP2 utilizes a prism rather than barrier filters to select the wavelength range for detecting the fluorescence. Spectral image scans were performed by obtaining a number of images (typically 25) with a 10 nm bandpass across the emission spectrum of Nile Red using an open pinhole (3.3 Airy units) to maximize sensitivity. Emission spectra for defined regions of interest were determined using the Leica software. R images were calculated from the spectral series of images using plugins used in conjunction with NIH ImageJ. Each image in the spectral series was processed using a median filter followed by smoothing (both using a one-pixel radius) to improve the signal/noise ratio. The images corresponding to emission wavelengths 540–600 nm and 540–660 nm were summed as 32 bit images, corrected for background using the average pixel intensity surrounding the cell and converted to 16 bit images. The R image was calculated:
R = 65 535 [(I540−600/I540−660) − min](max − min)
where I540−600 and I540−660 are the 16 bit images obtained by summing the wavelength range indicated in the subscript, and max and min represent scaling factors for presentation of the R image. Only pixels above threshold values (determined for each image) were analysed. Structures that exhibited movement during the scans were cleared in the images. Similarly sections from some images were cleared to assist in the delineation of certain structures within the parasite. Unless otherwise indicated, the fluorescence images shown with the R images were obtained by averaging the spectral series.
Electron microscopy
Samples of A4 strain P. falciparum-infected erythrocytes were fixed with 4% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.2, treated with osmium tetroxide, dehydrated and embedded in epoxy resin as described previously (Urban et al., 1999). Thin sections were stained with uranyl acetate and lead citrate before examination in a Joel 1200EX transmission electron microscope.
Acknowledgements
This work was supported by the National Health and Medical Research Council, Australia. Expert technical assistance was provided by Ms Emma Fox. We thank Dr Sue Kyes for supplying cDNA. D.J.P.F. is supported by an equipment grant form the Wellcome Trust.




