Natural chaperonin of the hyperthermophilic archaeum, Thermococcus strain KS-1: a hetero-oligomeric chaperonin with variable subunit composition
Abstract
To study the difference in expression of the chaperonin α- and β-subunits in Thermococcus strain KS-1 (T. KS-1), we measured their intracellular contents at various growth temperatures using subunit-specific antibodies. The β-subunit was significantly more abundant with increasing temperature (maximum at 93°C), whereas the α-subunit was not. Native PAGE with Western blot analysis indicated that the natural chaperonins in the crude extracts of T. KS-1 cells grown between 65°C and 95°C migrate as single bands with different mobility. The recombinant α- and β-subunit homo-oligomers migrated differently from each other and from natural chaperonins. Immunoprecipitation also showed that the natural chaperonin was the hetero-oligomer. These results indicate that chaperonin in T. KS-1 formed a hetero-oligomer with variable subunit composition, and that the β-subunit may be adapted to a higher temperature than the α-subunit. T. KS-1 probably changes its chaperonin subunit composition to acclimatize to the ambient temperature.
Introduction
Molecular chaperones are a class of proteins that assist the proper folding of other proteins in cells (Gething and Sambrook, 1992). Chaperonin is one such molecular chaperone and is classified into two groups, I and II (Kim et al., 1994). Chaperonins of eubacteria, chloroplasts and mitochondria belong to group I, this type of chaperonin (GroEL) being composed of one kind of subunit. GroEL forms a double-ringed, toroidal homo-oligomer with sevenfold rotational symmetry and is capped with the chaperonin cofactor GroES. The group II chaperonins are found in archaea and in the cytosol of eukaryotes. They are weakly homologous to the group I chaperonins and are thought to function without a GroES-like cofactor (for a review, see Gutsche et al., 1999). The eukaryotic chaperonin containing t-complex peptide 1 (CCT) consists of eight or nine kinds of subunit and also forms a double-ringed hetero-oligomer with eight- or ninefold rotational symmetry (for a review, see Kubota et al., 1995). The expression of these subunits is tightly co-regulated to maintain their constant ratio, and the subunit arrangement in the oligomer is fixed (Kubota et al., 1999).
The chaperonins of most archaea, except those of some methanogens and Pyrococcus species that are composed of a single species of subunit, consist of two homologous subunits (Archibald et al., 1999). The most archaeal chaperonins form an eight- or ninefold symmetrical hetero-oligomer (Archibald et al., 1999). The subunit stoichiometry of chaperonins from Thermoplasma acidophilum and Sulfolobus solfataricus has been reported to be 1:1 and 1:2 respectively (Knapp et al., 1994; Ditzel et al., 1998; Ellis et al., 1998). In Pyrodictium occultum and Sulfolobus shibatae, both the chaperonin subunits are accumulated in response to heat shock (Phipps et al., 1991; Trent et al., 1991; Kagawa et al., 1995). In these archaea, it is thought that natural chaperonin forms a hetero-oligomer of fixed subunit composition, and that the expression of the subunits is co-regulated by the growth temperature.
The chaperonin in hyperthermophiles is thought to contribute to their thermotolerance (Phipps et al., 1991; Trent et al., 1991). Although the natural archaeal chaperonins have weak ATPase activity (Phipps et al., 1991; Trent et al., 1991; Guagliardi et al., 1994; Waldmann et al., 1995; Andräet al., 1998) and ability to bind the denatured proteins (Trent et al., 1991; Guagliardi et al., 1994; Waldmann et al., 1995), ATP-dependent protein refolding activity has been shown in only a few archaeal chaperonins, e.g. the natural chaperonin of S. solfataricus (Guagliardi et al., 1994; 1995), recombinant chaperonin from Methanococcus thermolithotrophicus (Furutani et al., 1998) and recombinant chaperonin homo-oligomers from Thermococcus strain KS-1 [T. KS-1; Yoshida et al., 1997; Yoshida et al. (2000) Corrigendum. J Mol Biol 299: 1399–1400]. We have shown previously that the α- and β-subunits of the chaperonin of T. KS-1 have highly homologous amino acid sequences and form homo-oligomers with eightfold double-ring toroidal structure [Yoshida et al., 1997; Yoshida et al. (2000) Corrigendum. J Mol Biol 299: 1399–1400]. These homo-oligomers exhibited ATP-dependent protein-refolding activity. Despite their high homology, their protein-refolding activities and the temperature dependence of their ATPase activities were different [Yoshida et al., 1997; Yoshida et al. (2000) Corrigendum. J Mol Biol 299: 1399–1400]. To understand the roles of these subunits and their functional difference in vivo, we examined the effect of growth temperature on the cellular contents of these subunits in natural chaperonin of T. KS-1 cells.
Results
Western blot analysis of the chaperonin subunits in Thermococcus strain KS-1 cells grown at various temperatures
The Western blot analysis showed that the anti-α- and anti-β-subunit antibodies were specific to the corresponding subunits, respectively, and had little cross-reactivity with the other proteins in T. KS-1 (Fig. 1A). The anti-αβ-subunit antibody that had been raised against the recombinant α-subunit reacted with either of the subunits (data not shown). The total soluble proteins in the extracts of T. KS-1 cells grown at various temperatures were applied to the SDS–PAGE and stained by Coomassie brilliant blue R-250 (CBB). The intensities of most protein bands were about the same, whereas that of the band corresponding to the chaperonin was increased with increasing growth temperature (Fig. 1B). The chaperonin subunits in the extracts of T. KS-1 cells were detected by Western blot analysis using these antibodies (Fig. 1C). The band that stained with anti-αβ-subunit antibody was also increased with increasing temperature. The cellular content of the α-subunit was lower at higher temperature with the minimum at 90–93°C. However, that of the β-subunit increased with increasing temperature up to 93°C (Fig. 1C). At 95°C, near the maximum growth temperature for T. KS-1, its level decreased again.

Cellular contents of the Thermococcus chaperonin subunits and effect of growth temperature.
A. Specificity of antibodies against the C-terminal regions of the α- and β-subunits of Thermococcus KS-1 chaperonin. Purified homo-oligomers of the recombinant chaperonin subunit (0.1 µg) and a cell extract (5 µg) of Thermococcus strain KS-1 grown at 90°C were applied to SDS–PAGE on 13% polyacrylamide gel and then transferred to a PVDF membrane. The chaperonin subunits were stained with Coomassie brilliant blue R-250 (left) or immunologically detected with the anti-α- (middle) and anti-β-subunit antibodies (right). α, recombinant α-chaperonin subunit; β, recombinant β-chaperonin subunit; Ext, cell extract of Thermococcus strain KS-1.
B. SDS–PAGE analysis of the cell extracts of Thermococcus strain KS-1 grown at different temperatures. Thermococcus cells were cultured at 65°C, 70°C, 75°C, 80°C, 85°C, 90°C, 93°C and 95°C. Soluble proteins of the cultured cells (10 µg) were loaded on to 13% SDS polyacrylamide gel and stained with Coomassie brilliant blue R-250. The arrowhead shows the band corresponding to the chaperonin.
C. Western blot analysis of the chaperonin subunits in Thermococcus strain KS-1 cells grown at different temperatures. Soluble protein of the cultured cells (10 µg) was loaded onto 13% polyacrylamide gel for an SDS–PAGE analysis and blotted on to a PVDF membrane. The chaperonin subunits were detected with the anti-α- (top), anti-β- (middle) and anti-αβ-subunit antibodies (bottom).
Enzyme-linked immunosorbent assay (ELISA) of the α- and β-subunits of chaperonin in Thermococcus strain KS-1 cells grown at various temperatures
As the binding efficiency of these antibodies to the corresponding chaperonin subunits was different, the amount of each expressed subunit could not be quantified precisely by the Western blot analysis. We thus determined the amounts of the α- and β-subunits of natural chaperonin in the cell extracts of T. KS-1 by ELISA (Fig. 2). The α-subunit content in the soluble proteins was in the range from 9.6 ± 1.1 ng (mean ± SD) of chaperonin (µg of total soluble protein)−1 at 80–90°C to 5.4 ± 1.8 ng (mean ± SD) of chaperonin (µg of total soluble protein)−1 at 93–95°C, whereas that of the β-subunit increased with growth temperature from 6.5 ± 2.5 ng (mean ± SD) of chaperonin (µg of total soluble protein)−1 at 80°C to the maximum of 60 ± 20 ng (mean ± SD) of chaperonin (µg of total soluble protein)−1 at 93°C. However, at 95°C, it decreased to 20 ng of chaperonin (µg of total soluble protein)−1. The concentrations of total chaperonin, as the sum of both subunits, in the soluble protein fraction at 80°C and 93°C were ≈ 15 ng and 65 ng of chaperonin (µg of total soluble protein)−1 respectively. The percentage of total chaperonin in the soluble proteins increased from 1.5% at 80°C to 6.5% at 93°C. The ratio of β/α changed significantly with the growth temperature; when the cells were grown at 80°C, β/α was about 0.6, but it reached 15 for the cells grown at 93°C. The intracellular contents of the chaperonin subunits probably reflect the expression of the chaperonin subunits. These results suggest that the expression of the α- and β-subunits of chaperonin is differently regulated, and that only the expression of the β-chaperonin subunit is increased at temperatures higher than the optimum. However, it is not clear from these results whether the T. KS-1 chaperonin subunits form the homo- or hetero-oligomer in vivo.
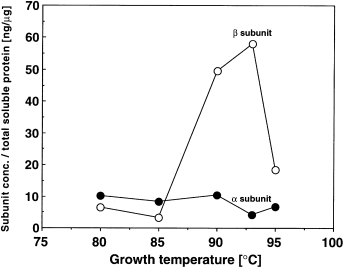
Intracellular concentrations of the chaperonin subunits in Thermococcus strain KS-1 cells grown at various temperatures. Thermococcus cells were cultured at 80°C, 85°C, 90°C, 93°C and 95°C, and the resulting cells were lysed by sonication. After removing the membrane by centrifugation, the soluble fraction was diluted in PBS and then analysed by ELISA to determine the concentrations of chaperonin subunits in the soluble proteins.
Native PAGE of natural chaperonin from Thermococcus strain KS-1 grown at different temperatures with Western blot analysis
The native PAGE/Western analysis revealed that the mobility of the recombinant α- and β-subunit homo-oligomers was different. They both migrated as multiple bands (Fig. 3), with the recombinant α-subunit homo-oligomer migrating a little faster than the β-subunit homo-oligomer. However, natural chaperonins of cell extracts from T. KS-1 cells grown at various temperatures that were detected by anti-αβ antibody apparently migrated as a single band with different mobility (Fig. 3). The Western blot analysis by anti-α- or -β-subunit antibody showed that the bands contained both α- and β-subunits with different stoichiometry (Fig. 3). No other band was detected with anti-αβ-, -α- or -β-subunit antibodies. These results indicate that the natural chaperonin existed as a hetero-oligomer with a variable β/α ratio.
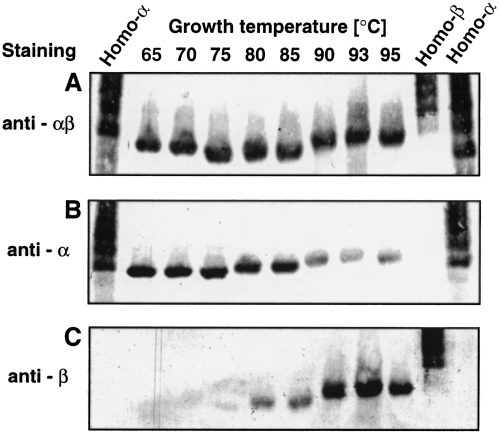
Native PAGE analysis of the cell extracts of Thermococcus strain KS-1 grown at different temperatures. The soluble cell extracts of Thermococcus strain KS-1 grown at 65°C, 70°C, 75°C, 80°C, 85°C, 90°C, 93°C and 95°C were subjected to a native PAGE analysis on 3–7% linear gradient polyacrylamide gel and then transferred to a PVDF membrane. The chaperonin subunits were immunologically detected with the anti-αβ- (A), anti-α- (B) and anti-β-subunit antibodies (C).
Immunoprecipitation of the chaperonin of Thermococcus strain KS-1 grown at different temperatures
To examine whether the natural chaperonins form the hetero-oligomer, natural chaperonin was purified from large-scale cultured cells grown at 80°C and 90°C. The cells grown at 90°C were subjected to heat shock at 93°C for 20 min. The ratios of β/α in these purified native chaperonins from the cells grown at 80°C and 90°C were determined to be ≈ 0.45 and 8.5, respectively, by ELISA. The recombinant homo-oligomers were precipitated with the corresponding anti-α- or anti-β-subunit antibodies respectively, (Fig. 4, lanes 1 and 2). In the mixture of the two homo-oligomers, the respective antibodies recognized only their corresponding homo-oligomers and did not cross-react with the non-targeted homo-oligomer (Fig. 4, lane 5). This indicates that the subunit-specific antibody recognized its target subunit in the oligomers and immunoprecipitated only the oligomers containing the target subunit. The purified natural chaperonins obtained from cells grown at 80°C and 90°C were immunoprecipitated with the anti-α- or anti-β-subunit antibodies (Fig. 4, lanes 3 and 4). In either of the resulting precipitates, both the α- and the β-subunits were detected (Fig. 4B and C, lanes 3 and 4,). This result also indicates that the natural chaperonin contained a hetero-oligomer.
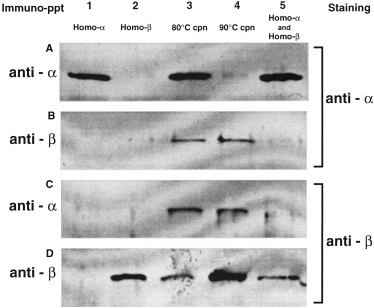
Immunoprecipitation of the purified natural and recombinant chaperonins by the anti-α- and anti-β-subunit antibodies. The recombinant chaperonin homo-oligomers and natural chaperonins were immunoprecipitated with the anti-α-subunit antibody (A and C) and anti-β-subunit antibody (B and D). The supernatant (4 µl) was analysed by SDS–PAGE with 13% polyacrylamide gel. After the SDS–PAGE analysis, the chaperonins were detected immunologically with the anti-α- subunit antibody (A and B) and anti-β-subunit antibody (C and D). Lane 1, recombinant α-subunit homo-oligomer; lane 2, recombinant β-subunit homo-oligomer; lane 3, purified natural chaperonin from the T. KS-1 cells grown at 80°C; lane 4, purified natural chaperonin from the T. KS-1 cells grown at 90°C; lane 5, mixture of the recombinant α- and β-subunit homo-oligomers.
Discussion
It has been reported that both the α- and the β-subunits of chaperonin accumulate in response to a high growth temperature and/or heat shock in archaea such as S. shibatae (Trent et al., 1991; Kagawa et al., 1995), P. occultum (Phipps et al., 1991), Haloferax volcanii (Kuo et al., 1997) and Archaeoglobus fulgidus (Emmerhoff et al., 1998). The ratio of the α- to β-subunit is constant at different growth temperatures, and their expression is thought to be co-regulated (Phipps et al., 1991; Trent et al., 1991; Kagawa et al., 1995). In T. KS-1, however, we found that the expression of the β-subunit of chaperonin increased with increasing temperature up to 93°C, whereas the α-subunit was expressed more at a lower temperature (1, 3). These results suggest that the gene expression of the α- and β-subunits of T. KS-1 chaperonin is differently regulated, and that only the β-subunit is a thermally inducible heat shock protein. In S. shibatae, H. volcanii and A. fulgidus, the two chaperonin subunit genes shared significant sequence conservation in the 5′ flanking regions, in which the archaeal consensus TATA promoter-like sequence is included (Kagawa et al., 1995; Kuo et al., 1997; Emmerhoff et al., 1998). In both the T. KS-1 α- and β-subunit genes, the TATA promoter-like sequence has also been found 60 bp and 100 bp upstream of the initiation codon (Yoshida et al., 1997). However, the base sequences between these promoter-like sequences and the initiation codons were significantly different in these subunit genes. Although no consensus heat-inducible regulatory sequence element has been reported in archaea (Thompson and Daniels, 1998), this dissimilarity may reflect the difference in their expression.
The present data from native PAGE with the Western blot analysis and immunoprecipitation indicate that natural chaperonin of T. KS-1 existed as a hetero-oligomer of α- and β-subunits (3, 4). The single bands of different mobility that were detected by native PAGE of natural chaperonins of the cell extract from cells grown at various temperatures indicate that the subunit composition in the hetero-oligomer changed with the growth temperature. In group II chaperonins of archaeal or eukaryotic cells, the subunit composition and subunit arrangement of the hetero-oligomer have been believed to be fixed (Gutsche et al., 1999). However, the present data indicate that the subunit composition of chaperonin in T. KS-1 changed with the growth temperature. The β/α subunit ratio of the purified T. KS-1 chaperonin was 0.5–0.6 at 80°C, but 8.5–15 at 90°C. If the natural chaperonin hetero-oligomer was a single species, six and 14 or 15 of the 16 subunits in the hetero-oligomer could have been the β-subunit at 80°C and 90–93°C respectively. The subunit arrangement in the oligomer may thus also be changeable. The data showing that the β-subunit content increased at a higher growth temperature suggest that it is more thermo-adapted than the α-subunit (1-3). The optimum temperature for ATPase activity of the β-subunit was higher than that of the α-subunit [Yoshida et al. (2000) Corrigendum. J Mol Biol 299: 1399–1400]. This fact also supports the view that the β-subunit is adapted to a higher temperature than the α-subunit. On the other hand, the α-subunit may be adapted to a lower temperature.
At 50°C, which is lower than the optimum growth temperature of T. KS-1, the ATP-dependent protein-refolding activity of the α-subunit homo-oligomer was reported to be significantly higher than that of the β-subunit homo-oligomer [Yoshida et al. (2000) Corrigendum. J Mol Biol 299: 1399–1400]. At higher temperatures such as 90°C, the β-subunit may have higher protein-refolding activity than that of the α-subunit. At higher temperatures in vivo, the natural chaperonin hetero-oligomer with a higher β-subunit composition may function more efficiently than natural chaperonin with a higher α-subunit composition.
The hyperthermophilic archaea belonging to Pyrococcus, which is closely related to the Thermococcus genus, have only one chaperonin subunit gene in the genome [Kawarabayashi et al. (1998); Genome projects of Pyrococcus furiosus (URL: http://combdna.umbi.umd.edu/bags.html) and Pyrococcus abyssi (URL: http://www.genoscope.cns.fr/cgi-bin/Pab.cgi)]. The deduced amino acid sequences of these chaperonin subunits are more similar to the β-subunit of T. KS-1 chaperonin (88–90% identity) than to its α-subunit (79–80%). The optimal growth temperature of the Pyrococcus species is higher than that of the Thermococcus species (Gonzalez et al., 1998). This is consistent with the hypothesis that the β-subunit is adapted to a higher temperature than the α-subunit. The α-subunit may be the prototype from which the β-subunit has evolved, because it has the GGM repeat sequence that is conserved in group I chaperonin (McLennan et al., 1993; Brocchieri and Karlin, 2000) and in some chaperonins of archaea belonging to Euryarchaeota (Kuo et al., 1997; Yoshida et al., 1997; Furutani et al., 1998). The α-subunit might have been lost after the divergence of the Pyrococcus species from the Thermococcus species.
The optimum growth temperature for Thermococcus strain KS-1 is 85°C with a growth temperature range between 60°C and 97°C (Hoaki et al., 1994). This strain has been isolated from a sandy sea floor with a steep temperature gradient from 80°C at the surface to 106°C at a 30 cm depth in the sediment (Hoaki et al., 1995). Hot sea water was seeping from the deeper bottom to the surface. The hyperthermophiles in the sediment may move with the interstitial water, and their surrounding temperature may fluctuate. In such a habitat, hyperthermophiles must adapt to a relatively wide range of temperature. A hetero-oligomeric chaperonin that is composed of two kinds of subunit with different temperature ranges may be advantageous for a hyperthermophile living in an environment with fluctuating temperature or in various habitats with different temperatures.
Experimental procedures
Expression of the recombinant chaperonin subunits
The Thermococcus strain KS-1 chaperonin subunits were expressed in Escherichia coli strain BL21 (DE3) cells with the expression vector pK1Eα2 or pK1Eβ[Yoshida et al., 1997; Yoshida et al. (2000) Corrigendum. J Mol Biol 299: 1399–1400]. They were grown aerobically overnight at 37°C in 2× YT medium supplemented with 100 µg ml−1 ampicillin or 75 µg ml−1 kanamycin.
Culture conditions for Thermococcus strain KS-1
Thermococcus strain KS-1 was grown anaerobically as described previously with slight modifications (Hoaki et al., 1994). The medium contained 75% natural sea water, 5 ml l−1 Wolf's trace element solution (Wolin et al., 1963), 1.89 g l−1 yeast extract, 1.89 g l−1 peptone, 1.35 g l−1 cystine, ≈ 50 mg l−1 S0 and 0.6 mg l−1 resazulin. For the Western blot analysis and ELISA, 50 ml of an overnight culture of T. KS-1 at 90°C was inoculated into 1000 ml of the above-mentioned medium and incubated at 65–95°C in an electric oven. The incubation period for each growth temperature was 3 days at 65°C, 2 days at 70–75°C or overnight at 80–95°C. These are referred to as small-scale cultures. The cells were harvested by centrifugation at 6510 g for 20 min. For the immunoprecipitation analysis, 50 ml of the overnight culture at 90°C was inoculated into 1000 ml of the same medium and incubated for 10 h at 90°C, before being transferred to 20 l of the same medium in 30 l fermenters and incubated overnight at 80°C or 90°C (Hoaki et al., 1994). This was a large-scale culture. The cells grown at 90°C were subjected to heat shock at 93°C for 20 min before being harvested. This temperature change took ≈ 2 min. After cultivation, the large-scale culture was immediately cooled to room temperature by circulated water for ≈ 10 min and then pooled in plastic containers in an ice-chilled water bath, before the cells were harvested with a continuous centrifuge (Kokusan) at 10 000 g.
Antibodies and Western blot analysis
Chaperonin subunit-specific rabbit polyclonal antibodies were raised against the C-terminal peptides of the α- and β-subunits of Thermococcus strain KS-1 chaperonin [Yoshida et al., 1997; Yoshida et al. (2000) Corrigendum. J Mol Biol 299: 1399–1400]. Sixteen-amino-acid peptides, C-G-G-G-M-P-G-G-M-G-G-M-D-M-G-M and C-E-G-G-K-G-G-T-E-D-F-G-S-D-L-D, were synthesized for the C-terminal regions of the α- and β-subunits respectively. They were conjugated to keyhole limpet haemocyanin and injected into rabbits. The subunit-specific antibodies against the α- and β-subunits are called the anti-α- and anti-β-subunit antibodies respectively. A rabbit polyclonal antibody against the recombinant α-subunit homo-oligomer was also raised and is called the anti-αβ antibody. The IgG fractions were purified with an affinity column (Hitrap rProtein A; Amersham Pharmacia Biotech). T. KS-1 cells from a small-scale culture were suspended in a 50 mM HEPES–NaOH buffer (pH 6.8) containing 25 mM MgCl2 and disrupted with a Branson type 250 sonicator. After centrifugation at 55 000 g for 10 min, the supernatant containing ≈ 10 µg of proteins was loaded on to 13% polyacrylamide gel for the SDS–PAGE analysis. The supernatant containing 25 µg of proteins was also applied to 3–7% gradient polyacrylamide gel without SDS for the native PAGE analysis. The electrophoresed proteins were transferred to a polyvinylidine difluoride (PVDF) membrane using a semi-dry blotter (Nippon Eido). After blocking the non-specific protein binding with Block Ace (Dainippon Pharmaceutical), the membrane was incubated in phosphate-buffered saline (PBS) containing one of the antibodies and then incubated again with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Bio-Rad Laboratories). The bound antibodies were detected by incubation with 0.2 mg ml−1 diaminobenzidine in a 50 mM Tris-HCl buffer (pH 7.2) containing 0.1% H2O2.
ELISA detection of the chaperonin subunits in Thermococcus strain KS-1 cells
The contents of the chaperonin subunits in T. KS-1 cells were determined by ELISA. After coating with the rabbit anti-αβ antibody at 37°C for 2 h, a 96-well microtitre plate was treated overnight with 100% Block Ace at 4°C. The wells were then washed with Tris-buffered saline (25 mM Tris-HCl, pH. 7.4, 137 mM NaCl and 2.68 mM KCl) containing 0.05% Tween 20, filled with several concentrations of the soluble fraction of the cell extract diluted with 25% Block Ace in PBS and incubated at 30°C for 2 h. The wells were washed with Tris-buffered saline containing 0.05% Tween 20 and incubated with the horseradish peroxidase-conjugated anti-α- and anti-β-subunit antibodies that had been prepared according to the method of Nakane (1975). After removing the free antibodies by washing with the same buffer, the bound conjugated subunit-specific antibodies were detected by their peroxidase activity. Colour was developed with ABTS [2,2′-azido-di(3-ethyl-benzthiazoline-6-sulphonate)], a peroxidase substrate (Kirkegaard and Perry Laboratories), and the absorbance was measured at 405 nm. The concentrations of the α- and β-subunits in the cell extract were each determined using calibrated standard curves that had been obtained from purified recombinant T. KS-1 chaperonin α- and β-subunit homo-oligomers. These standard curves were not influenced by other E. coli soluble proteins.
Purification of the recombinant α- and β-subunit homo-oligomers of Thermococcus strain KS-1
Both recombinant α- and β-homo-oligomers were purified as described previously with slight modifications (Yoshida et al., 1997). Cells of E. coli BL21(DE3) harbouring expression vector pK1Eα2 or pK1Eβ for the α- and β-subunits, respectively, were suspended in 200 ml of 50 mM HEPES–NaOH pH 7.5 and lysed by sonication at 4°C for 20 min before dithiothreitol (DTT) was added to yield 1 mM. After removing the cell debris by centrifugation at 10 000 g for 60 min, MgCl2 and glycerol were added at 25 mM and 5% (v/v) respectively. This cell extract was heated at 70°C for 30 min. After centrifugation at 10 000 g for 60 min to remove denatured proteins, the supernatant was applied to a DEAE-Toyopearl column (Tosoh) equilibrated with 50 mM HEPES–NaOH, pH 7.5, 25 mM MgCl2, 1 mM DTT and 5% (v/v) glycerol. The proteins were eluted with a 0–500 mM NaCl linear gradient in the same buffer. Fractions containing the homo-oligomers were collected and dialysed against 50 mM HEPES–NaOH, pH 7.5, and 25 mM MgCl2. The dialysed sample was loaded onto a Q-Sepharose HP column (Amersham Pharmacia Biotech) equilibrated with 50 mM HEPES–NaOH, pH 7.5, and 25 mM MgCl2 and then eluted with a 0–500 mM NaCl linear gradient in the same buffer. The fractions containing the homo-oligomers were pooled and concentrated by ultrafiltration (Centriprep 50; Amicon). The recombinant α- and β-subunit homo-oligomers were then purified with a gel filtration column (G3000SWXL; Tosoh) equilibrated with 50 mM Tris-HCl, pH 7.2, 25 mM MgCl2 and 100 mM NaCl.
Purification of the natural chaperonin from Thermococcus strain KS-1 cells
T. KS-1 cells from the large-scale culture were suspended in a 50 mM HEPES–NaOH buffer, pH 7.5, containing 1 mM DTT, 5% (v/v) glycerol and 1 mM phenylmethylsulphonyl fluoride (PMSF). After sonicating at 4°C for 20 min, the soluble fraction was applied to a DEAE-Toyopearl column (Tosoh) that had been equilibrated with the 50 mM HEPES–NaOH buffer, pH 7.5, containing 25 mM MgCl2, 1 mM DTT and 5% (v/v) glycerol. The proteins were eluted with a linear gradient of 0–500 mM NaCl in the same buffer. The fractions containing chaperonin were collected and dialysed against the 50 mM HEPES–NaOH buffer, pH 7.5, with 25 mM MgCl2. The dialysed sample was loaded onto a Q-Sepharose HP column (Amersham Pharmacia Biotech) equilibrated with the 50 mM HEPES–NaOH buffer pH 7.5 containing 25 mM MgCl2 and eluted with a linear gradient of 0–500 mM NaCl in the same buffer. The chaperonin fractions were concentrated by ultrafiltration (Amicon Centriprep 50) and applied to a G3000SWXL gel filtration column (Tosoh) equilibrated with the 50 mM HEPES–NaOH buffer, pH 7.0, containing 25 mM MgCl2 and 100 mM NaCl. The chaperonin fractions were pooled and dialysed against the 50 mM HEPES–NaOH buffer, pH 7.5, containing 25 mM MgCl2 and applied to a Super Q column (Tosoh) equilibrated with 50 mM HEPES–NaOH, pH 7.5, and 25 mM MgCl2, before the proteins were eluted with a linear gradient of 0–500 mM NaCl in the same buffer.
Immunoprecipitation of chaperonin with the chaperonin subunit-specific antibody
The recombinant α- and β-subunit homo-oligomers, purified natural chaperonins from the cells cultured at 80°C and 90°C and a 1:1 mixture of the recombinant α- and β-subunit homo-oligomers (2 µg of each) were dissolved in 100 µl of buffer A (50 mM HEPES–KOH, pH 7.5, containing 50 mM MgCl2 and 300 mM KCl). After incubation with 20 µl of 3.6 mg ml−1 the anti-α antibody or 80 µl of 3.8 mg ml−1 the anti-β antibody at room temperature for 4 h, the sample was mixed with 10 µl of protein A/G bound resin (Immunocatcher; CytoSignal) and incubated while shaking at room temperature for 1 h. The resin particles were subsequently harvested by filtration and washed twice with buffer A. The collected resin particles were then incubated in 400 µl of an SDS–PAGE sample buffer for 15 min at room temperature. The supernatant (4 µl) was analysed by SDS–PAGE with a 13% polyacrylamide gel. The chaperonin subunits were detected by Western blotting, using the subunit-specific antibodies.
Other methods
Proteins were analysed by PAGE, on either 13% polyacrylamide gel in the presence of SDS (SDS–PAGE) after denaturation by incubation at boiling point in the sample buffer (Laemmli, 1970) or 3–7% linear gradient polyacrylamide gel without SDS (native PAGE). The gel samples were stained with CBB. The protein concentration was measured by the Bradford method with a protein assay kit (Bio-Rad Laboratories), using bovine serum albumin as the standard (Bradford, 1976).
Acknowledgements
We thank Dr H. Taguchi of Tokyo Institute of Technology for valuable discussions, and Ms C. Ohkami for technical assistance. We are grateful to Dr S. Kimura of Chiba University for native PAGE analysis. Dr M. Furutani is acknowledged for his critical reading of the manuscript. This work was supported by grant aid for scientific research on priority areas (11153206) from the Ministry of Education, Science, Sports and Culture of Japan, and by the ‘Biodesign Research Program’ from RIKEN to M. Yohda. This study was conducted at the Marine Biotechnology Institute of Japan as part of The Basic Knowledge Creation and Development program supported by the New Energy and Industrial Technology Development Organization of Japan.




