Pharmacokinetics and milk penetration of orbifloxacin after intravenous and intramuscular injections to dromedary lactating camels (Camelus dromedaries)
Orbifloxacin is a third-generation fluoroquinolone that has been developed especially for use in veterinary medicine. It exhibits bactericidal activity against numerous aerobic gram-negative and gram-positive bacteria with a high degree of therapeutic efficacy, even when multiple pathogens are present (Wolfson & Hooper, 1989; Ihrke et al., 1999). In Japan, intramuscular administration of this drug has been shown to be effective and safe for the treatment of gastrointestinal and respiratory infections in cattle and swine (Nakamura, 1995). Mastitis has both an extreme zoonotic and economic importance and it is the cause of multiple hazardous effects on human health and animal production. The incidence of clinical mastitis in the female camel had a higher percentage rate (17.34%) (Hegazy et al., 2004). Intramammary infections in dairy camels are mainly of bacterial origin. Most of the published papers about mastitis treatments report clinical observations or recommendations adapted from results obtained in cows. In facts, there are no published data on the pharmacokinetics and milk penetrations of fluoroquinolones especially orbifloxacin in lactating camels to expect their use for treatment of mastitis. However, the wide application of fluoroquinolones represents a potential hazard to consumers because of persistence of their residues in milk. The increase demands on camel milk and its products necessitate knowledge of milk residues of different antibacterials in lactating camels.
The potential value of orbifloxacin in the camel is indicated by previous studies describing its clinical efficacy and pharmacokinetics in a variety of animals (Davis et al., 2006; Marín et al., 2007). There have been some inadequate published data on the pharmacokinetics of other fluoroquinolones in camels (Aliabadi et al., 2003; Laraje et al., 2006; Abd El-Aty et al., 2007). Consequently, this report describes the plasma disposition kinetics, absolute bioavailability and milk penetration of orbifloxacin in healthy lactating dromedary camels following IV and IM administrations of a single dose of the drug at a dose rate of 2.5 mg/kg b.w.
Orbifloxacin was obtained as a powder from Schering-Plough, Kenilworth, New Jersey, USA and reconstituted in sterile saline to a final concentration of 5% prior to administration. Mueller–Hinton Agar was purchased from Alkan Medical Division, Dokki, Giza, Egypt. All other chemicals were of analytical grade.
Six healthy female lactating camels (Camelus dromedarius, one humped camel), 5–7 years old ranging in body weight from 375 to 490 kg were used in this experiment. The camels were in optimal nutritional condition, were fed high quality Lucerne (alfalfa) hay once daily and water was allowed ad libitum. The camels were milked twice daily and determined to be healthy on the basis of clinical examination. The Advisory Committee constituted by the Faculty of Veterinary Medicine, Cairo University, approved the experimental protocol.
All animals received orbifloxacin by the i.v. and i.m. routes according to a crossover scheme (3 × 3). Orbifloxacin was administered intravenously via the left jugular vein at a dosage of 2.5 mg/kg b.w. as a bolus. For the i.m. administration, the injection site was located into the left gluteal muscles. Neither pain nor irritation was observed at the site of injection at any time after treatment. The experiment was followed by a washout period of 15 days and the animals were permuted for the second experiment.
Venous whole blood samples (5 mL each) were taken by jugular venepuncture into 10 mL heparinized vacutainers (Becton Dickinson). The sampling times were 0 (blank sample), 0.16, 0.33, 0.5, 0.75, 1, 2, 4, 6, 8, 10, 12, 18, 24, 30, 36 and 48 h after treatment. All the blood samples were centrifuged at 3000 g for 15 min to separate the plasma. The plasma samples were frozen at −20 °C until analysed.
Milk samples were collected in vials from each camel after complete milking of the gland, immediately prior to each treatment, and at 0.5, 1, 2, 4, 6, 8, 10, 12, 18, 24, 30, 36, 48 and 72 h postdosing. At each time point, a milk sample was collected by hand. Plasma and milk samples were analysed within 6 h of obtainment.
Quantitation of orbifloxacin in plasma and milk samples was accomplished by a modified agar diffusion bioassay method previously reported by Bennett et al. (1966), using Klebsiella pneumoniae ATCC 10031 as the reference organism (Heinen, 2002). The microbiological assay does not separate the parent compound from the active metabolites. Nevertheless, it measures the total activity which could be more useful for pharmacodynamic evaluations than high performance liquid chromatography (HPLC) methods (McKellar et al., 1999).
A linear relationship existed between the zone of inhibition and the logarithm of orbifloxacin concentrations in plasma and milk with a correlation coefficient of 0.988 and 0.978, respectively. The limit of quantification for the plasma and milk was 0.05 μg/mL. The mean percentage recovery of orbifloxacin (measured by comparing zone of inhibitions of the spiked samples with external standards in phosphate buffer saline) from plasma and milk was 95 and 91%, respectively. The intra-assay variation coefficients were <5.3 for plasma and <3.7% for milk. The interassay variation coefficients were <6.4 for plasma and 4.5% for milk.
The extent of protein binding was determined in vitro according to the method described previously by Craig and Suh (1991). This method was based on the diffusion of free antibiotic into the agar medium. The differences in the diameters of the inhibition zones between the solutions of the drugs in the buffer and plasma samples were then calculated.
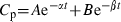
The extent of drug penetration from the blood into the milk was expressed as the ratios of AUCmilk/AUCplasma and Cmax-milk/Cmax-plasma (Ziv et al., 1995).
Pharmacodynamic efficacy of orbifloxacin was determined by calculating the Cmax/minimum inhibitory concentration (MIC) and AUC24/MIC ratios following i.m. administration using the respective mean MIC value for susceptible Escherichia coli, Klebsiella pneumoniae, Salmonella sp. and Pasteurella sp. (0.12 μg/mL) according to Haines et al., 2001.
The mean plasma pharmacokinetic variables for orbifloxacin were statistically compared by nonparametric analysis, using the Mann–Whitney test and instant version 3.00 (GraphPad Software, San Diego, CA, USA). Means were considered significantly different at P < 0.05 and P < 0.001.
Clinical examination of all animal before and after each trial did not reveal any abnormalities. No local or systemic adverse reaction to orbifloxacin occurred after i.v. or i.m. administration. The mean plasma and milk concentration–time profiles of orbifloxacin following single i.v. and i.m. administrations of 2.5 mg/kg b.w. are presented graphically in Fig. 1. Mean ± SD values of pharmacokinetic parameters estimated from the curve fitting are shown in Table 1. There were significant differences between i.v. and i.m. route of administration for the distribution and elimination rate constant, distribution and elimination half-lives. The kinetic values of milk following i.v. and i.m. administration are listed in Table 2. A one-compartment open model with first order absorption best described the milk concentration–time profiles following i.m. administration of orbifloxacin to lactating camel. Following i.m. administration of the drug, the Cmax/MIC and AUC0-24/MIC ratios were 16 and 87 h, respectively. The study revealed that plasma orbifloxacin concentrations vs. time decreased in a bi-exponential manner following i.v. injection, demonstrating the presence of distribution and elimination phases and justifying the use of two-compartment open model. This finding is in agreement with that reported for orbifloxacin in lactating goats by Marín et al. (2007). Conversely, three-compartment model was reported for danofloxacin and moxifloxacin in camels by Aliabadi et al., 2003 and Abd El-Aty et al., 2007; respectively. Plasma concentration profiles showed a rapid initial distributive phase, followed by a slower β-phase with an estimated mean terminal elimination half-life of 5.74 ± 1.16 h. This finding was shorter to that recorded in male camels (12.56 h) (Abd El-Aty et al., 2007). In this respect, Petracca et al. (1993) studied the influence of lactation on marbofloxacin pharmacokinetics in sows. They found that antimicrobial secretion in milk contributed greatly to marbofloxacin elimination and significantly shortend its plasma elimination half-life to its half in nonlactating animals.
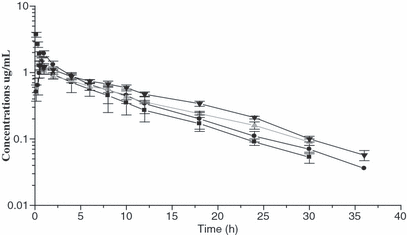
Mean ± SD of plasma (i.v., i.m.) and milk (i.v., i.m.) concentrations of orbifloxacin in camel after i.v. and i.m. administration of 2.5 mg/kg b.w. (n = 6).
| Parameters | Unit | i.v. | i.m. |
|---|---|---|---|
| α (kab) | h−1 | 3.16 ± 0.41 | 1.45 ± 0.25* |
| t 1/2α ( t 1/2ab) | h | 0.22 ± 0.02 | 0.51 ± 0.07* |
| β(kel) | h−1 | 0.13 ± 0.03 | 0.15 ± 0.06 |
| t 1/2β ( t 1/2el) | h | 5.74 ± 1.16 | 5.95 ± 1.21 |
| K 21 | h−1 | 0.74 ± 0.06 | – |
| K 12 | h−1 | 2.01 ± 0.19 | – |
| V dss | L / kg | 1.73 ± 0.31 | – |
| Cl tot | L / h / kg | 0.23 ± 0.02 | – |
| AUC | μg·h / mL | 11.21 ± 0.96 | 11.64 ± 1.34 |
| AUC 24 | μg·h / mL | 10.34 ± 1.21 | 10.45 ± 0.92 |
| MRT | h | 6.42 ± 1.14 | 7.54 ± 1.45 |
| C max | μg / mL | – | 1.93 ± 0.61 |
| T max | h | – | 1.52 ± 0.41 |
| F | % | – | 97.47 ± 11.32 |
- β, elimination rate constant; t1/2α, distribution half-life; t½ab, absorption half-life; t1/2β, elimination half-life; t½el, elimination half-life; K12 and K21, first-order rate constants for drug distribution between the central and peripheral compartments; Vdss, volume of distribution; Cltot, total body clearance; MRT, mean residence time; AUC, area under the curve from zero to infinity by the trapezoidal integral; Cmax, maximum plasma concentration; Tmax, time to peak concentration; F%, bioavailability.
- Values after i.m. administration were significantly different from corresponding values following i.v. administration (*P < 0.001).
| Parameters | Unit | i.v. | i.m. |
|---|---|---|---|
| C max | μg / mL | NA | 1.29 ± 0.42 |
| T max | h | NA | 1.26 ± 0.24 |
| t 1/2β ( t 1/2el) | h | 7.36 ± 0.81 | 8.47 ± 1.35 |
| AUC | μg·h / mL | 11.69 ± 3.51 | 13.65 ± 2.41 |
| C max milk/Cmax plasma | Ratio | NA | 0.69 ± 0.07 |
| AUC milk/AUCplasma | Ratio | 1.07 ± 0.12 | 1.21 ± 0.09* |
- C max, maximum milk concentration; Tmax, time to peak concentration; t½el, elimination half-life; AUC, area under the curve from zero to infinity by the trapezoidal integral; NA, not available.
- Values after i.m. administration were significantly different from corresponding values following i.v. administration (*P < 0.05).
Orbifloxacin exhibits a relatively high volume of distribution at steady-state (1.73 ± 0.31 L/kg) which exceeded the volume of body water of the camel, suggesting an extensive tissue distribution. This Vdss was in agreement with that of moxifloxacin in camels (1.78 L/kg; Abd El-Aty et al., 2007) but lower to that of danofloxacin in camels (2.53 L/kg; Aliabadi et al., 2003).
Following i.m. injection, the data were best represented by a one-compartment model and the estimated Cmax (1.93 ± 0.61 μg/mL) was reasonably similar to that reported for moxifloxacin in male camel (2.16 μg/mL) (Abd El-Aty et al., 2007) but slightly lower than that recorded for marbofloxacin in camels (2.5 μg/mL) (Laraje et al., 2006). The time of maximum concentration of orbifloxacin in the camel (tmax = 1.52 ± 0.41 h) was longer than that recorded for moxifloxacin in male camel (0.41 h) (Abd El-Aty et al., 2007). The MRT of orbifloxacin (7.54 ± 1.45 h) was similar to that recorded for moxifloxacin (7.29 h) (Abd El-Aty et al., 2007) and marbofloxacin (7.78 ± 0.49 h) (Laraje et al., 2006) following i.m. administration in camels. The mean terminal half-life of orbifloxacin (5.72 ± 1.17 h) was longer than those recorded in horse (3.42 h) (Davis et al., 2006) and goats (3.43 h) (Marín et al., 2007) indicating a slower elimination in camel than other species.
The systemic bioavailability of orbifloxacin in lactating camels after i.m. administration was nearly complete (97.47 ± 11.32%). This value indicates the excellent absorption of the drug from that injection site and the absorption process was rapid with absorption half-life (t1/2ab) 0.51 ± 0.07 h. This higher value for bioavailability was lower than that recorded for danofloxacin in camels (114.5%) (Aliabadi et al., 2003) and orbifloxacin in lactating goats (105%) (Marín et al., 2007) On the contrary, our value was effectively higher than that reported after i.m. administration of marbofloxacin in camels (71.95%) (Laraje et al., 2006). In this study, plasma protein binding was 21–27% lower than the value reported for moxifloxacin in male camels (33–38%) (Abd El-Aty et al., 2007). Orbifloxacin penetration from the blood into the milk was rapid and showed high levels of concentrations in milk secretion. Fluoroquinolones exhibit concentration-dependent killing and it is therefore not necessary to maintain their concentrations above the MIC throughout the entire dosing period. Because of this, peak milk concentration is a very important parameter. In this study, the milk Cmax following i.m. dosing 1.29 ± 0.42 μg/mL was close to those reported for orbifloxacin in lactating goats (1.77 μg/mL) (Marín et al., 2007). Also, the AUCmilk/AUCplasma and Cmax-milk/Cmax-plasma ratios indicated a wide penetration of orbifloxacin from the bloodstream to the mammary gland of lactating camels following both routes of administration. The findings of Fernandez-Varon et al. (2006) and Carceles et al. (2007) for moxifloxacin in lactating goats support this observation.
Orbifloxacin, as with several other fluoroquinolones, is amphoteric with pKa range of 5.95–9.01. Passive diffusion across biological membranes is a function of fluoroquinolone lipophilicity relative to the pKa values of the two ionizable moieties. The good penetration of orbifloxacin from the blood into the camel’s milk at pH 6.5–6.7 was, therefore, predictable on the basis of the ion trap mechanism. Owing to the greater persistence of the drug in the milk relative to those in the plasma, the drug is trapped in the milk and demonstrated a AUCmilk/AUCplasma ratio > 1. These findings are in agreement with several reports of other fluoroquinolones in lactating animals (Abd El-Aty & Goudah, 2002; Fernandez-Varon et al., 2006; Carceles et al., 2007). Moreover, orbifloxacin could be a substrate of efflux proteins belonging to the superfamily of ATP-binding cassette transporters such as breast cancer resistance protein (BCRP), which are specialized drug transporters involved in drug passage from plasma into the milk. In fact it has been established that the expression of BCRP is highly up-regulated in the lactating mammary gland of ruminants (Jonker et al.,2005). Furthermore, it is highly expected that BCRP plays an important role in the active secretion of orbifloxacin into the milk, as was shown for enrofloxacin in ewes (Pulido et al., 2006) but this mechanism should be further evaluated.
From this data, orbifloxacin could have success against susceptible mastitic pathogens in camels after parenteral administration. Pharmacokinetic–pharmacodynamic indices have been used to predict optimum dosing strategies. For a concentration-dependent drug, such as orbifloxacin, successful treatment usually correlates with AUC0-24/MIC and Cmax/MIC, and high ratios of the latter have also been associated with a lower incidence of the development of resistance (Lode et al., 1998). There are no published studies involving camels that indicate which of these parameters may be the best predictor of clinical cure, or what the respective target ratios might be. Most experts agree that a Cmax/MIC of 8–10, or an AUC0-24/MIC of greater than 100–125 have been associated with a cure. Accordingly, our values are near the desired ratios and may be adequate for the successful treatment of pathogens with an MIC value of 0.12 μg/mL. Therefore, using the above surrogate markers, it was determined that when administered by the i.m. route at 2.5 mg/kg, orbifloxacin is likely to be effective against bacterial isolates with MIC of 0.12 μg/mL (Aliabadi et al., 2003). Consequently, orbifloxacin could be useful in the treatment of systemic severe infections and mastitis in camels as it exhibits high systemic availability and good distribution to the udder, after i.v. and i.m. administration. The presence of detectable orbifloxacin residues in milk indicates that milk should not be used for human consumption for 2 days after drug administration.




