Use of hepatic protein biomarkers for tracing the exposure of veal calves to illegal growth-promoters: investigations on experimental samples and preliminary application under field conditions
Because of the short production cycle, veal calves represent a bovine category particularly at risk to be treated with growth-promoters (GPs). In the EU, the use of hormonal growth promoters is banned (Council Directives 96/22 and 96/23 of the European Commission), but they are permitted for use in beef cattle in some third countries like the USA (US Food and Drug Administration, 2004). In the EU Member States a very limited number of non-compliant results for GPs, such as sexual steroids, β-agonists, and corticosteroids, were found in 2005 in bovines. The actual prevalence remained below 0.3% of the analyzed samples (Serratosa et al., 2006). In contrast to these reassuring figures, the results of both the histological screening tests performed in target tissues of regularly slaughtered calves (Biolatti et al., 2003), and the analyses of seized black market preparations (De Brabander et al., 2004) suggest that the treatment of veal calves with a large array of GPs is far from being abandoned. In an attempt to tackle this illegal practice more effectively, a number of assays based on the biological effects of the different GPs are being developed (Nebbia, 2006) with the aim to replace histological tests, which are relatively unspecific and time consuming. The search for biomarkers of the exposure to illegal GPs could also be critical to enhance the efficacy of HACCP procedures aimed at improving food quality and safety which are routinely set up especially by large-scale retail chains.
Recent genomic and proteomic approaches towards the identification of banned GPs use have attracted the interest of many researchers. For instance, significant changes in the expression of selected androgen-responsive genes were consistently observed in prostate samples from veal calves experimentally treated with a combination of 17β-oestradiol benzoate and 17β-boldenone undecylenate or testosterone enantate (Toffolatti et al., 2006). In addition, the up-regulation of reticulocalbin (RCN), and the down-regulation of adenosine kinase (AK) were consistently detected in hepatic subfractions obtained from veal calves administered with a combination of 17β-oestradiol benzoate, clenbuterol, and dexamethasone sodium phosphate for growth-promoting purposes (Gardini et al., 2006). More recently, a likely post-translational modification of apolipoprotein A1 has been detected in plasma of veal calves administered with a combination of the anabolic steroids boldione and boldenone (Draisci et al., 2007). Data generated from these proteomic studies should be validated, and possibly transferred to field conditions, by analyzing the expression of the selected biomarkers using Western blot techniques. Therefore, it was the aim of the present study to investigate the changes in RCN and AK expression by means of Western Blotting in veal calf liver tissue subfractions from both experimentally treated, and regularly slaughtered individuals.
Experimental samples originated from a trial lasting 55 days, in which 22 male crossbred Friesian calves were randomly divided into two groups, and either not treated (n = 11) or administered (n = 11) one of the most frequently employed GP combinations. This combination consisted of 17β-oestradiol benzoate (10 mg i.m. for three times at d = 0, d = 17, and d = 34, respectively), clenbuterol (20 μg/kg b.w./day, dissolved in milk, from d = 15 to d = 55), and dexamethasone sodium phosphate (4 mg/day for six consecutive days and 5 mg/day for further 6 days, dissolved in milk, from d = 34 to d = 46). Six individuals from either group were sacrificed 24 h after the last clenbuterol treatment (trial 1), whereas the other animals (five treated plus five controls, trial 2) were sacrificed on d 83, i.e. after a 27-day wash-out period. Liver samples were collected from all animals, washed in chilled isotonic KCl and put on ice until further processing. In addition, 38 liver specimens, free from macroscopic alterations, were collected from clinically healthy veal calves slaughtered in three different abattoirs (denoted A, B, and C) located in the Region of Piedmont (Northern Italy). Hepatic microsomal and cytosolic subfractions were isolated as described earlier (Nebbia et al., 2003), and the protein content of the samples was quantified with the method described by Lowry et al. (1951). Aliquots corresponding to 100 μg protein were electrophoretically resolved on 12% SDS-polyacrylamide gel (Laemmli, 1970) and transferred onto a nitrocellulose membrane. Immunoblottings were performed with a rabbit polyclonal antiserum against rat AK (diluted 1:1000) kindly provided by Professor Tadeusz Pawelczyk (University of Gdansk, Poland) or a rabbit anti-human RCN-1 antibody (Bethyl Laboratories, Montgomery, TX) diluted 1:1000. As secondary antibody an anti-rabbit antibody linked to horseradish peroxidase was used. Stained bands were detected by chemiluminescence (ECL kit, GE Helthcare, Piscataway, NJ), scanned with GS-800 Calibrated Densitometer, and evaluated with Quantity One software version 4.5.2. (Bio-Rad Laboratories, Hercules, CA, USA). AK or RCN expression in control veal calves (trial 1 and 21 samples) was used to compare relative amounts of AK or RCN from experimentally treated or regularly slaughtered animals. Due to the relatively high variability of data, results were expressed in arbitrary units as medians ± SD; the Mann-Whitney U-test was used to compare changes in protein expression between control and treated animals, and the level of significance was set at P < 0.05.
As mentioned above, a proteomic analysis of 2DE gels of liver preparations from trial 1 treated veal calves had revealed a more than 50% decrease in the intensity of the spot corresponding to AK, and a more than three-fold rise in the intensity of the spot corresponding to RCN, in cytosolic and microsomal hepatic subfractions, respectively; in that study (Gardini et al., 2006), immunoblottings indicated a good cross reactivity between anti-rat AK antibody and the corresponding bovine protein, and confirmed the results obtained in 2DE-gels. However, only the cytosolic fractions were tested in those experiments. Therefore, the first aim of this study was to examine the expression of RCN in microsomal fractions from trial 1 calves and to assess the effect of treatment withdrawal (trial 2) on the expression of both protein biomarkers. Microsomal proteins from treated calves (trial 1) cross reacted with anti-human RCN antibodies, resulting in a visible band of about 45 kDa. The intensity of this band was, on average, two and a half times higher than that of the control animals (Fig. 1a), thereby matching the results obtained in 2DE gels. By contrast, no significant differences either in RCN (Fig. 1b) or in AK expression (data not shown) could be observed between control and treated animals from trial 2, in which animals were slaughtered 27 days after the treatment was discontinued. Taken together, these results provide evidence that the described changes in liver protein expression reflect the biological response to the administration of the GP combination adopted in this study.
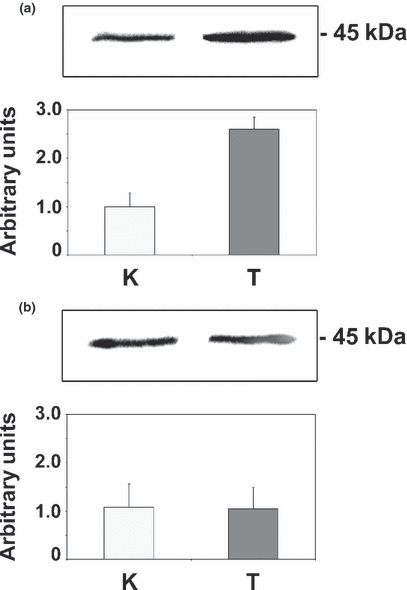
Representative immunoblotting experiments and densitometric analysis of reticulocalbin (RCN) levels in control (K; n = 6) and experimentally treated (T; n = 6) veal calves. Data are expressed as medians ± SD. (a) Trial 1 – RCN expression 1 day after the last treatment (Mann-Whitney U-test statistical analysis: P = 0.0095); (b) Trial 2 – RCN expression 27 days after the last treatment (Mann-Whitney U-test statistical analysis: P = 0.826).
Adenosine kinase is a cytosolic enzyme participating in the regulation of adenosine and adenine nucleotide concentrations in several tissues and involved in the purine salvage pathway as well as in glucose metabolism (Lavoinne et al., 1987). Although its detailed function remains largely unknown, RCN is thought to play a role in the normal behaviour and life of cells and its overexpression has been related to tumorigenesis, tumor invasiveness, and drug resistance (Fukuda et al., 2007). Both proteins are expressed in Bos taurus (Gardini et al., 2006), but little information is available about the AK and RC functions and turnover in the bovine species.
The second goal of this work was to perform a preliminary test on a panel of hepatic subfractions from veal calves commercially slaughtered in three different abattoirs as to the presence and the extent of expression of both biomarkers with respect to ‘negative’ and ‘positive’ controls (control and experimentally treated veal calves from trial 1, respectively) (Fig. 2). Concerning AK, a general trend towards a decrease in protein expression was observed in samples from the three abattoirs, but most notably in samples from abattoir C, which approached the values recorded in positive controls from trial 1. Median values of RCN expression showed limited variations with respect to negative controls in samples collected in abattoir A or B; by contrast, values even slightly higher than those measured in positive controls from trial 1 were detected in specimens from abattoir C. These findings suggest that in liver samples collected in abattoir C, which receives calves from small breeding units and conveys carcasses to small butcher chains, the expression of the two proposed biomarkers closely resembles the values displayed by experimentally treated animals. In contrast to what observed in samples from trial 1, when liver microsomes from veal calves treated with 17β-oestradiol only were probed with anti-human RCN antibodies and compared with matched controls, a very weak band was visible (Nebbia, unpublished results). Results from the present study therefore indicate that the observed down-regulation of AK and the up-regulation of RCN in samples from both experimentally treated and commercially slaughtered veal calves may be linked to β-agonist- and/or dexamethasone exposure. Experiments performed in veal calves are in progress to get further insight into the role of the single GP classes in determining the observed changes in liver protein expression.
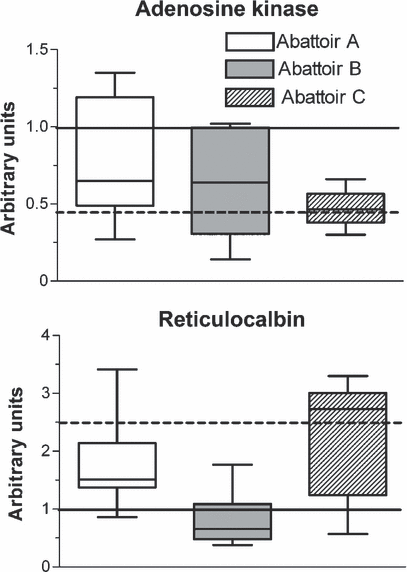
Analysis of adenosine kinase (AK) or reticulocalbin (RCN) expression in commercially slaughtered veal calves collected in three different abattoirs: A (n = 17), B (n = 12), C (n = 9). Samples were analysed by Western blotting and densitometric analysis, and the relative amount of proteins was quantified with respect to control and experimentally treated animals. Data presented are expressed as medians ± SD, i.e. (——) median of control veal calves; (−−−−) median of experimentally treated veal calves.
The small number of samples and the as yet unknown roles of bovine hepatic AK and RCN limit the specificity of this assay, as it can not be excluded that other causes like for example certain physiopathological processes or some feed ingredients (Brambilla & De Filippis, 2005) could exert the same alterations. Further studies involving larger numbers of field samples, and coupling the described Western blot analysis with at least one other biological test (e.g. the histological screening on target organs) are warranted to confirm these preliminary results.
Acknowledgments
This work was supported by a grant from Regione Piemonte - Progetti di ricerca sanitaria finalizzata: “L’approccio proteomico come metodo alternativo per evidenziare trattamenti con anabolizzanti in bovini da carne” and Regione Piemonte CIPE - Progetti di ricerca scientifica applicata 2004: “Individuazione di biomarcatori dei trattamenti illeciti nei bovini da carne con indagini di proteomica e genomica”.




