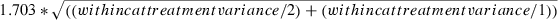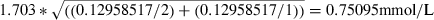Glargine and protamine zinc insulin have a longer duration of action and result in lower mean daily glucose concentrations than lente insulin in healthy cats
Abstract
The pharmacological effects of glargine, protamine zinc (PZI), and lente insulins were evaluated in nine healthy cats. A 3-way crossover study was performed and plasma concentrations of insulin and glucose were determined for 24 h after a single subcutaneous injection of each insulin at 3-day intervals.
Time to onset of action did not differ between insulins. Mean time to first nadir glucose was longer for glargine (14 h) relative to PZI (4 h) and lente (5 h). PZI was biphasic in action with nadirs at 4 and 14 h with the second nadir occurring at a similar time to glargine. Nadir glucose did not differ significantly between insulin types. The duration of action was similar for glargine and PZI and was longer than that for lente insulin. Mean daily glucose after glargine and PZI were also similar and were lower than after lente insulin.
Time to reach peak insulin did not differ between insulin types. Time to return to baseline insulin level for PZI was longer than glargine but did not differ significantly from lente.
In conclusion, healthy cats injected subcutaneously with glargine, compared to those injected with lente insulin, have a later glucose nadir and longer duration of action. Glargine and PZI had similar durations of action in study cats but a larger study is required to obtain precise comparisons of duration of action.
Introduction
Diabetes mellitus is the second most common endocrinopathy in cats and occurs with moderate frequency (Panciera et al., 1990; Gerber et al., 1994; Rand, 1997; Baral et al., 2003). Treatment options include dietary management, oral hypoglycemic agents, and/or insulin therapy. Despite advances in dietary management and oral hypoglycemic agents, insulin therapy still provides the most effective and reliable means of achieving glycemic control in diabetic cats.
While the insulins currently available for treating feline diabetes often result in resolution of clinical signs, treatment with them is less than ideal for several reasons. Neutral protamine Hagedorn (NPH, also known as isophane insulin) and lente insulins have a relatively short duration of action in cats, and when administered twice daily, most cats have marked hyperglycemia (>18 mmol/L) for several hours prior to each insulin injection. Lente insulin is the most commonly used type of insulin in Australia and the United Kingdom (UK). Protamine zinc insulin (PZI) is the longest-acting insulin available for veterinary use. PZI was withdrawn from the human market in 1991, but is commercially available for veterinary use in the UK and the United States of America (USA).
Glargine is a synthetic insulin analogue with a very long duration of action. It is produced using recombinant DNA technology utilizing Eschericia coli. Designed for once daily administration, it is marketed for use in humans as a very long-acting and ‘peakless’ insulin with respect to its glucose lowering effect. Glargine gained approval from the United States Food and Drug Administration in April 2000, for use in treating type 1 and type 2 diabetes in humans. The pharmacokinetics and pharmacodynamics of glargine have not yet been reported in healthy or diabetic cats. The availability of a longer acting, relatively peakless insulin would potentially provide more effective glycemic control and result in improved treatment for diabetes in cats.
The aim of this study was to investigate the pharmacokinetic and pharmacodynamic properties of glargine and compare them with those of PZI and lente insulin.
Materials and methods
The study was performed as a 3-way crossover design in which all nine cats received each of the three insulins (glargine, PZI, and lente) at 72 h intervals. Plasma insulin and glucose concentrations were measured for 24 h in each cat after receiving each type of insulin.
Animals
Nine adult neutered cats (five male, four female) that were clinically healthy and had ideal body condition scores (3 on a 1–5 scale) were obtained from the School of Veterinary Science at The University of Queensland. The study was approved by The University of Queensland Animal Ethics Committee and the cats were rehomed after the trial.
All cats were housed together for 3 weeks prior to the commencement of data collection. During the last week of this period, cats were housed in individual cages. Throughout the trial, cats were fed an extruded dry feline maintenance diet (approximate caloric distribution: protein 17%, carbohydrate 16%, and fat 67%). Cats were fed once daily in the morning and uneaten food was left in the cages overnight, except prior to a test day. Prior to a test day, uneaten food was removed from the cages after the morning feed, 18 h prior to insulin administration, and food was then withheld until the conclusion of the 24 h testing period.
Insulin
The insulins tested were porcine lente insulin 40 U/mL (Caninsulin, Intervet, Boxmeer, Netherlands), protamine zinc insulin 40 U/mL (PZI-VET, IDEXX Pharmaceuticals, Westbrooke, ME, USA) and glargine 100 U/mL (Lantus®, Aventis Pharmaceuticals, Frankfurt, Germany).
Procedure
At least 48 h prior to the first test day, the cats were anesthetized with propofol (Diprivan, Zeneca Limited, Macclesfield, UK) and an 18 g polyurethane central venous catheter (Cook Veterinary Products, Brisbane, Australia) was placed in one jugular vein to facilitate blood sampling. Patency was maintained by twice daily flushing with heparinized saline (20 U/mL heparin in 0.9% NaCl).
Each cat was assigned to one of three treatment sequences using an incomplete counter-balanced design, with each group receiving a different type of insulin on each testing day in the allocated sequential order. Insulin was administered in the dorsal thoracic area by subcutaneous injection at a dose of 0.5 U/kg body weight (rounded to the nearest half unit). Food was withheld for 10 h prior to and for 24 h after insulin administration.
Blood samples were collected via jugular catheter before (−30 and −5 min) and 15, 30, 45, 60, 90 min and 2, 4, 6, 8, 10, 12, 14, 16, 20 and 24 h after administration of each insulin. Blood samples were placed into EDTA tubes on ice immediately after collection, and centrifuged within 1 h to prevent in vitro glycolysis (Christopher & O’Neill, 2000). The resultant plasma was stored at −70 °C until assayed. Remaining red cells were aseptically washed with saline, re-suspended in saline, and auto transfused to maintain red cell mass (Appleton et al., 2001).
Sample analysis
Plasma glucose concentrations were determined using a YSI stat 2300 glucose analyzer (YSI Inc, Yellow Springs, OH, USA). Plasma insulin concentrations were measured using a commercially available radioimmunoassay kit (Phadeseph Insulin RIA; Pharmacia and Upjohn Diagnostics AB, Uppsala, Sweden) validated for measurement of feline insulin (Lutz & Rand, 1993).
Onset of action and duration of action
Onset of insulin action was defined for each cat-treatment combination as the time from treatment (0 h) to the first timepoint when blood glucose concentration was significantly lower than baseline (P < 0.05). Duration of insulin action was defined as the time from treatment to the first estimated timepoint after the last glucose nadir when blood glucose concentration was not significantly different from baseline (P > 0.05). Many of these timepoints fell between 16 and 24 h where sampling occurred every 4 h, so interpolation was used to more accurately estimate duration of action. Baseline glucose and insulin concentrations were estimated for each cat-treatment combination as the average of concentrations at −30 and −5 min.
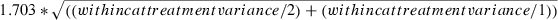
where 1.703 = the critical value of the t-distribution at the 0.10 level of significance with a two-tailed test and 27 degrees of freedom. (See Appendix for details of calculations.)
Because the distribution of insulin concentrations was markedly right skewed, concentrations at −30 and −5 min were log transformed before estimation of the 90 percent range of differences as described for glucose. The resulting estimate was then back-transformed. Because the difference between two log-transformed values is equivalent to the ratio between the back-transformed values, the back-transformed 90 percent range of differences was interpreted on a multiplicative rather than additive scale. Thus, time to return to baseline for insulin was then estimated for each cat as the time to the first sampling after peak where the observed plasma insulin concentration was less than baseline multiplied by the back-transformed 90 percent range of differences, which was 1.44.
Comparisons between insulin types
Area under the curve for glucose concentration was calculated for each cat-treatment combination using the linear trapezoidal method (Rowland & Tozer, 1989). For insulin, area above baseline from time 0 until return to baseline for each cat-treatment combination was calculated using the linear trapezoidal method but no statistical comparisons were made between insulin types. For all variables, effects of insulin type were assessed using analysis of variance after accounting for treatment sequence, cat within treatment sequence, day, insulin type, and the interaction of day by insulin type. The distribution of times to peak insulin concentration were markedly right skewed so were log transformed before analysis. Because all three pairwise comparisons were a priori in nature, pairwise comparisons were performed for all variables regardless of overall P-value, using the overall analysis of variance residual mean square and degrees of freedom.
Times for blood glucose to return to baseline were compared between insulin types using Kaplan–Meier survival analysis (Kaplan & Meier, 1958) with stratification by cat. P-values were calculated using the log rank test. Analysis of variance for calculating the 90 percent range of differences and statistical analyses of outcome variables were performed using Stata version 9.2 (Statacorp, College Station, TX, USA).
Results
Glucose variables
Time to onset of action did not vary between insulin types (P = 0.311) (Table 1; Fig. 1). Time to return to baseline differed significantly (P = 0.003) between insulin types (Fig. 2). Times to return to baseline following treatment with glargine or PZI did not differ between types (P = 1.000), but were significantly longer following treatment with lente (P = 0.005 and 0.034, respectively). Following treatment with glargine and PZI, blood glucose concentration failed to return to baseline within 24 h in five of nine cats, compared with only one of nine cats following treatment with lente (Table 1; Fig. 2). Times to return to baseline glucose concentration after treatment with glargine or PZI were much more variable than following treatment with lente (Fig. 2).
| Glargine | PZI | Lente | ||
|---|---|---|---|---|
| Onset of action (h) | 1.8 ± 0.8a (0.25–8) | 0.8 ± 0.2a (0.25–2) | 1.1 ± 0.2a (0.25–4) | P = 0.311 |
| Time to first nadir (h) | 14 ± 1.9 b (10–24) | 3.7 ± 0.8a (1.5–8) | 4.7 ± 0.8a (2–10) | P = 0.001 |
| Time to last nadir (h) | 14 ± 1.9a (10–24) | 14 ± 1.9a (6–24) | 4.7 ± 0.8 b (2–10) | P = 0.003 |
| Glucose concentration at nadir (mmol/L) | 1.7 ± 0.3a (0.8–3.9) | 1.4 ± 0.1a (1.0–2.1) | 1.9 ± 0.3a (1.1–3.9) | P = 0.426 |
| Increase in glucose conc. after nadir (mmol/L) | 2.5 ± 1.3b (1.1–4.3) | 3.2 ± 0.3ab (1.5–5.0) | 3.7 ± 0.5a (1.7–5.6) | P = 0.026 |
| Duration of action | ||||
| Minimum mean (h)* | 22.2 ± 1.8a (12->24) | 20.9 ± 2.4a (9->24) | 10.0 ± 2.2 b (5->24) | P = 0.003† |
| Proportion of cats not returned to baseline glucose conc. by 24 h [number of 9 cats] | 0.55 [5] | 0.55 [5] | 0.11 [1] | |
| Mean daily glucose conc. (mmol/L) | 2.9 ± 0.2a (2.2–4.4) | 3.0 ± 0.2a (2.4–3.8) | 3.9 ± 0.3 b (2.7–5.6) | P = 0.022 |
| Mean 0–12hr glucose conc. (mmol/L) | 2.8 ± 0.3a (1.2–4.5) | 2.8 ± 0.2a (2.3–4.1) | 3.5 ± 0.4a (2.3–5.5) | P = 0.262 |
| Mean 12–24hr glucose conc. (mmol/L) | 3.0 ± 0.2a (1.8–4.2) | 3.1 ± 0.2a (2.3–4.2) | 4.3 ± 0.3 b (3.2–5.6) | P = 0.003 |
| Peak insulin conc. (μU/mL) | 33.7 ± 3.1b (17–50) | 79.6 ± 13.2a (30–167) | 73.4 ± 9.8a (30–125) | ‡ |
| Time to peak insulin conc. (h) | 2.1 ± 0.5a (0.5–4) | 3.4 ± 1a (0.75–10) | 2.9 ± 0.8a (0.5–6) | P = 0.663 |
| Time for insulin to return to baseline (h) | 6.7 ± 1.3a (0.6–13.0) | 10.5 ± 1.3 b (5.3–16.4) | 8.4 ± 0.5ab (5.6–10.2) | P = 0.021 |
| Area under insulin curve above baseline to return to baseline (μU·h/mL) | 80.2 ± 20.5b (3–197) | 258.5 ± 28.9a (107–349) | 220 ± 19.9a (128–298) | ‡ |
- *Mean was calculated by assuming that cats which had not returned to baseline by 24 h returned at 26 h.
- † P-value calculated using Kaplan–Meier survival analysis.
- ‡Means reported for descriptive purposes only.
- Data reported as mean ± SEM. Values in parentheses indicate range. Bolding highlights means that differ significantly (P < 0.05) from other means in the same row; means with different superscripts are significantly different from each other.
- PZI, protamine zinc insulin.
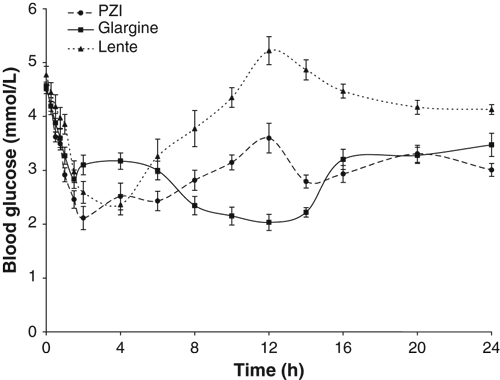
24 h Mean blood glucose concentration curve (mean ± SE) after subcutaneous administration (0.5 U/kg) of glargine, PZI or lente insulins in a 3-way cross over design in nine healthy cats.
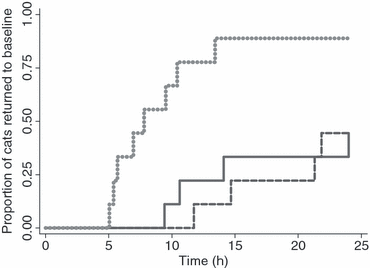
Cumulative proportions of cats returned to baseline blood glucose concentrations by time after subcutaneous administration (0.5 U/kg) of glargine (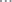 ), PZI (
), PZI ( ) or lente (
) or lente ( ) insulins in a 3-way cross over design in nine healthy cats.
) insulins in a 3-way cross over design in nine healthy cats.
Mean time to reach the first nadir glucose concentration was 14 h for glargine, which was significantly longer than that for PZI (4 h; P = 0.001) or lente insulin (5 h; P = 0.001) (Table 1; Fig. 1). After PZI treatment the blood glucose concentration had a biphasic curve, with nadirs for the average curve occurring, on average, at 4 and 14 h after insulin administration. Mean time to second or last nadir was similar for glargine and PZI (both 14 h; P = 1.000) which were both later than the lente nadir (5 h; P = 0.003 for each pairwise comparison). Insulin type was not associated with nadir glucose concentration (P = 0.426). The maximal increase in glucose concentration after first nadir was significantly less for glargine (mean increase of 2.5 mmol/L) than for lente insulin (mean increase of 3.7 mmol/L; P = 0.008). There was a numerical trend that approached significance for this value to be less for glargine than for PZI (mean increase of 3.2 mmol/L; P = 0.094) (Table 1; Fig. 1).
The mean daily glucose concentrations were similar for glargine (2.9 mmol/L) and PZI (3.0 mmol/L; P = 0.886), and both were less than lente (3.9 mmol/L; P = 0.013 and P = 0.017, respectively) because of the shorter duration of action of lente (Table 1; Fig. 1). When the first 12 h after insulin administration were compared, there was no significant difference (P = 0.262) in mean glucose concentration between the three types of insulin (Table 1). Mean glucose concentrations from 12 to 24 h were similar for glargine (3 mmol/L) and PZI (3.1 mmol/L; P = 0.858), but both were significantly lower than lente (4.3 mmol/L; P = 0.002 and P = 0.003, respectively) (Table 1).
Insulin variables
The time to reach maximal insulin concentration was not affected by insulin type (P = 0.663). Mean time to return to baseline insulin level for PZI (11 h) was longer than glargine (7 h; P = 0.007) but did not differ significantly from lente (8 h; P = 0.089) at the P = 0.05 level (Table 1). The peak insulin concentration and the area under the 24 h insulin concentration curve for each insulin are reported for descriptive purposes only in Table 1.
Discussion
This is the first report of the pharmacokinetics and pharmacodynamics of glargine in domestic animals. The most important finding from this study is that in healthy cats glargine had a very long duration of action, which was similar to that of PZI and significantly longer than that of lente insulin. The resultant glucose concentrations were still below baseline at 24 h after insulin administration in five of nine cats treated with glargine and with PZI. In contrast, glucose concentrations in eight of nine cats treated with lente had returned to baseline by 14 h. It remains to be investigated if the variability in return to baseline glucose concentration seen in healthy cats treated with glargine or PZI also occurs in diabetic cats. In our study, the findings for lente and PZI were similar to those reported in studies of diabetic cats, suggesting that the results associated with glargine administration in healthy cats are relevant to diabetic cats (Moise & Riemers, 1983; Martin & Rand, 2001).
The blood glucose lowering effect and duration of action of glargine in healthy cats are similar to data in diabetic humans (Owens et al., 2000). Glargine is marketed for human-use as a very long-acting and ‘peakless’ insulin. This lack of peak is in relation to glargine’s glucose utilization rate, which is determined by the amount of intravenous glucose required to maintain a constant plasma glucose concentration after subcutaneous injection of insulin, and indicates insulin activity. In our study, there were definite peaks and nadirs in glucose and insulin concentrations for all three insulins, but the glucose utilization rate was not studied.
Studies in humans with type 1 diabetes have shown patients treated with glargine achieve superior glycemic control with fewer hypoglycemic episodes compared with patients treated with NPH insulin (Fulcher et al., 2005). Humans with type 2 diabetes treated with glargine achieved effective glycemic control with a reduced incidence of hypoglycemia compared with those treated with NPH insulin, although glycemic control, as measured by glycated hemoglobin, was not different between patients treated with different insulins (Fonseca et al., 2004). The use of glargine in diabetic cats may reduce the risk of hypoglycemia and needs to be evaluated.
Peak plasma insulin concentration and area under the 24 h insulin concentration curve for each insulin type were not statistically compared because the insulin assay was not validated for each insulin. Insulin concentrations measured for glargine may have been artifactually low because glargine is reported to have lower cross-reactivity than other insulins to the antibodies used in insulin assays designed to detect human insulin (Lepore et al., 2000). This was not done in our study because endogenous and exogenous insulin could not be differentiated from total insulin measured and the assay was only validated for the measurement of feline insulin. Although insulin concentrations returned to baseline earlier for glargine than for PZI, the duration of glucose lowering effect was similar for the two insulins. The apparent earlier return of insulin concentration to baseline for glargine may be artifactual, reflecting the difficulty of measuring glargine because of lower cross-reactivity with the antibody. The magnitude of this artifact may be compounded by low blood insulin concentrations. Alternatively, insulin concentrations measured in the blood may not be a good indicator of the duration of insulin action because the target sites are extravascular in the interstitium.
After injection, glargine forms hexomeric micro-precipitates in the subcutaneous tissue, which gradually break down. This slow release of glargine into the systemic circulation produces the sustained action. Importantly, the formation of micro-precipitates is dependant on the interaction of the acidic insulin (pH = 4) and the relatively neutral subcutaneous tissues (pH = 7). It is for this reason that glargine should not be mixed or diluted before administration.
The porcine lente used in the study was a 40 U/mL insulin-zinc suspension, and is registered for veterinary use (Caninsulin, Intervet). It is classified as an intermediate-acting insulin, is commonly used to treat diabetic cats in Australia, the UK, and Canada, and has been recently released in the USA for use in dogs (Vetsulin, Intervet). Its onset, duration of action, and time to nadir in this study in healthy cats were similar to those reported in diabetic cats (Martin & Rand, 2001). Despite twice daily administration of lente insulin in diabetic cats, there is a period of 2–6 h every 12 h where there is minimal exogenous insulin activity, resulting in marked hyperglycemia twice daily in most cats (Martin & Rand, 2001). The potency of lente and rate of fall of blood glucose concentration likely induces counter-regulatory mechanisms. Our study in healthy cats showed lente administration resulted in a rapid and over-exaggerated increase in blood glucose concentration following the glucose nadir. This might explain the short duration of action of lente insulin in diabetic cats compared to human patients. The shorter duration of action of lente compared to PZI and glargine makes treatment with it less likely to resolve clinical signs of diabetes mellitus.
Protamine zinc insulin is an insulin-zinc suspension containing 40 U/mL of insulin. The fish protein protamine is added to reduce absorption and increase the duration of action. It is regarded as long-acting, and has the longest duration of action of all the insulins approved for veterinary use. In this study the PZI onset and duration of action were similar to that previously reported for PZI in normal (Broussard & Peterson, 1994) and diabetic cats (Moise & Riemers, 1983). PZI was found to have a biphasic glucose curve with nadirs occurring at a mean of 4 and 14 h. The time to reach first nadir glucose concentration for PZI was significantly shorter than for glargine, but time to last nadir was similar to glargine (14 h). This biphasic curve of PZI has also been reported in diabetic cats (Moise & Riemers, 1983) and healthy cats (Broussard & Peterson, 1994). The cause of this biphasic glucose curve for PZI remains unclear, but since the 24 h insulin concentration curve was not biphasic (Fig. 3), it may reflect mild counter-regulatory mechanisms activated in the first few hours after injection (Fig. 1) rather than biphasic insulin absorption. The biphasic effect on glucose concentrations resulting in two glucose nadirs may present problems monitoring diabetic cats treated with PZI unless there is frequent sampling, for example every 2 h over 14 h or more during serial blood glucose curves (Fig. 1).
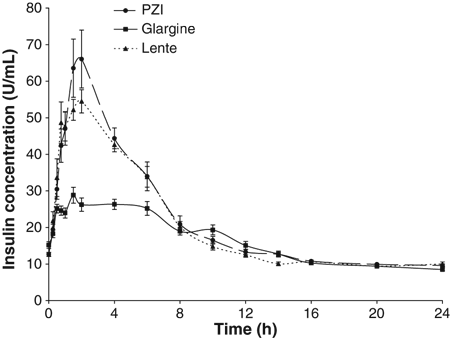
24 h Mean plasma insulin concentration (mean ± SE) after subcutaneous administration (0.5 U/kg) of glargine, PZI and lente insulins in a 3-way cross over design in nine healthy cats.
The longer duration of action of glargine and PZI compared to lente may improve glycemic control in diabetic cats currently treated with lente or NPH insulins. If blood glucose concentration in diabetic cats is maintained below the renal threshold most of the day, resolution of clinical signs associated with glycosuria (such as polyuria and polydipsia) should occur. Lower blood glucose concentration would also facilitate recovery of beta cells from glucose toxicity. Glucose toxicity describes the phenomena of reduced insulin secretion secondary to chronic hyperglycemia. Initially the loss of insulin secretion is reversible, but later there is permanent loss of beta cells (Link, 2001). This is likely to have most impact in newly diagnosed diabetic cats, because if glucose toxicity is resolved early, beta cells may become functional again. The increased endogenous insulin secretion would be evident as a reduced or absent requirement for exogenous insulin administration. Based on our study in healthy cats, it is expected that treatment of diabetic cats with PZI or glargine would result in better glycemic control and possibly higher remission rates than lente insulin.
A study in diabetic cats has shown that glargine administered once daily had similar glycemic control to lente insulin dosed twice daily (Weaver et al., 2006). A further clinical trial in diabetic cats is required to determine whether glycemic control is improved with glargine administered twice-daily vs. once daily.
Some cats require small doses of insulin (<2 U/dose) and dose rate errors due to administration of incorrect volumes may be more likely when using glargine (100 U/mL) compared with veterinary-use 40 U/mL insulin preparations such as PZI and lente, because of the smaller injection volumes with glargine. As previously discussed, glargine should not be mixed or diluted. However, this concern about dose rate errors associated with small injection volumes may be addressed with the availability of insulin syringes designed for 100 U/mL insulin with 0.5 unit gradations.
PZI and glargine in this study resulted in similar glucose lowering effects (based on both mean daily glucose concentrations and duration of insulin action) and both were superior to lente. However, glargine is not registered for use in cats in any country. Therefore, in countries where veterinarians are required by law to first use a veterinary product for treatment of diabetic cats, PZI should be used in preference to lente insulin. A clinical trial in diabetic cats comparing glargine, PZI and lente insulins is required to fully evaluate the potential of glargine for the treatment of feline diabetes mellitus.
In summary, this study in healthy cats showed that glargine has a longer duration of action than lente insulin and a similar duration of action to PZI but a larger study is required to obtain precise comparisons between PZI and glargine. A study in diabetic cats is required to evaluate glargine’s effectiveness and to determine whether it results in better treatment outcomes than current insulins.
Acknowledgments
The authors gratefully acknowledge the Australian Companion Animal Health Foundation for funding of this study, IDEXX Pharmaceuticals for supply of PZI insulin, Tad B. Coles, DVM of Overland Park, KS, for technical editorial assistance, and all the veterinary technicians from The University of Queensland Veterinary School.
Appendix
Appendix A–Calculation of 90 percent range of differences for baseline plasma glucose concentration
The 90 percent range of differences was calculated using an approach based on the method reported by Bland and Altman (1999) and using the t-distribution as recommended by Chinn (1990). Variance within cat treatment combination was estimated using the −30 and −5 min values for each of the 27 cat treatment combinations from the nine cats (Table A1).
| Cat number | Insulin type | Glucose at −30 min (mmol/L) | Glucose at −5 min (mmol/L) |
|---|---|---|---|
| 1 | 1 | 4.42 | 3.6 |
| 2 | 1 | 4.8 | 4.84 |
| 3 | 1 | 3.76 | 3.82 |
| 4 | 1 | 4.78 | 5.05 |
| 5 | 1 | 5.35 | 5.03 |
| 6 | 1 | 4.08 | 4.67 |
| 7 | 1 | 5.4 | 4.5 |
| 8 | 1 | 4.66 | 4.56 |
| 9 | 1 | 4.26 | 3.55 |
| 1 | 2 | 4.26 | 5.48 |
| 2 | 2 | 4.78 | 4.83 |
| 3 | 2 | 4.72 | 4.83 |
| 4 | 2 | 4.4 | 5 |
| 5 | 2 | 4.99 | 5.23 |
| 6 | 2 | 4.37 | 3.38 |
| 7 | 2 | 3.57 | 3.74 |
| 8 | 2 | 4.64 | 4.39 |
| 9 | 2 | 4.87 | 4.45 |
| 1 | 3 | 3.07 | 2.44 |
| 2 | 3 | 4.78 | 4.83 |
| 3 | 3 | 4.7 | 4.36 |
| 4 | 3 | 5.89 | 5.32 |
| 5 | 3 | 4.92 | 4.34 |
| 6 | 3 | 5.75 | 5.89 |
| 7 | 3 | 5.03 | 4.73 |
| 8 | 3 | 4.25 | 4.16 |
| 9 | 3 | 5.79 | 5.89 |
Variance within cat treatment combination was calculated as the residual mean square from analysis of variance after fitting cat and insulin type within cat (Table A2). Thus the estimate had 27 degrees of freedom (54 observations so 53 degrees of freedom less 8 degrees of freedom for the nine cats and 18 degrees of freedom for insulin type within cat.
| Source | Partial SS | df | MS | F | Prob > F |
|---|---|---|---|---|---|
| Cat | 6.4104822 | 8 | 0.801310277 | 6.18 | 0.0001 |
| Insulin_type|Cat | 17.0248661 | 18 | 0.945825895 | 7.30 | 0.0000 |
| Residual | 3.4987996 | 27 | 0.12958517 | ||
| Total | 26.9341479 | 53 | 0.50819147 |