Neurophysiological techniques to assess pain in animals
Abstract
Neurophysiological techniques are widely applied to animals, both in the search as a monitor for adequacy of anaesthesia, and studies to assess the efficacy of analgesic agents. Laboratory animals have been extensively used in models to investigate pain in man. However a substantial number of studies have also used neurophysiological techniques to increase knowledge of pain in specific animal species, with the aim of improving animal welfare. This review provides an overview of neurophysiological techniques involving the brain that have been used in the assessment of pain in animals. An explanation of the methodology of EEG recording, with particular emphasis on veterinary studies, is given. Neurophysiological models developed to assess pain in different species are described, and their relevance to advancements in animal welfare or best clinical practice indicated.
Introduction
Over the last decade the importance of effective recognition and management of pain has been increasingly recognized in veterinary practice. Among others specialists in veterinary anaesthesia have been advocating the importance of analgesia for many years, however more recently this has extended into general practice. The increased demand for knowledge about pain management from veterinarians has resulted in initiation of the International Veterinary Academy of Pain Management, a multi-disciplinary organization that seeks to promote the acquisition and dissemination of knowledge related to pain in animals. The veterinary profession has also adopted the IASP definition of pain (in man) to define pain in animals (Paul-Murphy et al., 2004): ‘Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (Merksey & Bogduk, 1994). This move signifies universal recognition that pain in man and animals is similar emphasizing the need for provision of effective analgesia.
Despite these positive advances in attitudes towards pain management in animals, surveys carried out in small animal veterinary practice indicate that pain is ‘under treated’ in small animal patients (Lascelles et al., 1995; Dohoo & Dohoo, 1996; Capner et al., 1999; Hugonnard et al., 2004; Williams et al., 2005). The low use of analgesics despite acceptance that animals are in pain after surgery was ascribed to difficulties in recognizing pain, lack of knowledge of appropriate use of analgesics and fear of side effects. A parallel study carried out among equine practitioners in the UK (Price et al., 2002) identified similar barriers to the optimal management of pain in horses. Similar findings have been reported by the laboratory animal research community (Roughan & Flecknell, 2003).
Pain recognition remains a fundamental hurdle to provision of effective pain management. There is currently no gold standard method to quantify pain perception in either man or animals. There is presently limited knowledge of pain behaviours in many animal species (Price et al., 2003), and it is recognized that normal pain behaviour is altered by hospitalization and observation (Paul-Murphy et al., 2004). Confounding effects of anaesthetics and other drugs given peri-operatively limit validity of behaviour as an assessment tool. Neither can current techniques of assessment acknowledge the importance of subjectivity in pain. As assessors we are forced to make a judgment on whether the animal is in pain, a situation far removed from the ‘self-reporting’ utilized in verbal human beings. Improved techniques to assess pain in animals would benefit pain research studies and that could also translate into better pain management in clinical practice.
Involvement of the cerebral cortex in pain processing was doubted for many decades. The historical finding that electrical stimulation of the cerebral cortex rarely elicited painful sensations was taken as evidence against the participation of the cerebral cortex in pain processing (Kenshalo & Willis, 1991). However the combination of data from neurophysiological experiments in animals and recent functional neuroimaging studies in man have conclusively demonstrated the involvement of widely distributed cerebral areas in pain processing (Schnitzler & Ploner, 2000).
In man, neurophysiological techniques have become increasingly important in studies to elucidate ascending pain pathways and cortical representation of pain. Techniques that afford a direct window on the function of the central nervous system, such as electroencephalography (EEG) and magnetoencephalography (MEG) provide a unique insight into pain processing and how activation of nociceptive pathways results in pain perception in a conscious individual. Even though non-invasive functional imaging techniques such as positron emission tomography and functional magnetic resonance imagine are becoming more widely accessible, the spatiotemporal resolution of EEG and MEG remains superior (Kakigi et al., 2005). Somatosensory evoked potentials (SEPs), particularly laser evoked potentials (LEPs) have proved useful tools to investigate pain perception associated with noxious thermal or electrical stimuli applied to the skin (Kakigi et al., 2000).
Neurophysiological techniques have been widely evaluated in veterinary species, both as devices to measure depth of anaesthesia and to assess pain. Changes in EEG variables as a marker of nociception (in anaesthetized animals) or pain (in conscious animals) have probably received most attention. Many of these studies have been carried out in horses (Miller et al., 1995b; Otto et al., 1996; Murrell et al., 2003, 2005b; Haga & Dolvik, 2005). This may reflect the relatively high complication rate associated with equine anaesthesia compared with small animals (Johnston et al., 1995; Brodbelt, 2005). Other techniques applied to pain evaluation in animals include measurement of evoked potentials (SEPs and auditory evoked potentials) and the Bispectral Index (BIS).
Traditional analgesiometric tests to assess the efficacy and potency of analgesics in laboratory animals measure only antinociceptive effects. They use either thermal [e.g. tail-flick or hot-plate test (D'Amour & Smith, 1941; Woolfe & MacDonald, 1944)], mechanical [e.g. paw pressure test (Morgan et al., 2006)], or electrical (Swedburg, 1994) stimuli. Although this type of test is relatively simple to execute, these brief noxious stimuli cannot be equated to clinical pain. Therefore making assessments about analgesic drugs based solely on phasic noxious stimuli may not be appropriate. In contrast, neurophysiological techniques can measure the cortical response to a complex pain stimulus and provide information about pain perception. These studies can also be carried out under anaesthesia, providing a uniquely ethical pain model (Miller et al., 1995a; Otto et al., 1996; Murrell et al., 2003, 2005b; Johnson et al., 2005a,b). Neurophysiological techniques have also been applied to assess the pharmacodynamics of centrally acting drugs such as opioids and sedatives (Bovill et al., 1983; Scott et al., 1991; Cox et al., 1998; Johnson et al., 2003).
We intend to provide an overview of neurophysiological techniques involving the brain that have been used in the assessment of pain in animals (Table 1). Electroencephalographic recording is essential to these techniques, therefore an explanation of the methodology of EEG recording is given. Neurophysiological models developed to assess pain in different species will be described, and their relevance to advancements in animal welfare or clinical best practice indicated.
| Neurophysiological technique | Advantages | Disadvantages |
|---|---|---|
| Raw electroencephalogram | Flexible – the user can manipulate the recording equipment in order to optimize signal qualityAll EEG data are retained allowing comprehensive detailed analysis The data are recorded continuously in real time rather than provision of discontinuous data points | Large quantity of data are generated which can hinder interpretation Data requires complex mathematical processing after collection (e.g. Fast Fourier transformation), therefore it cannot be immediately interpretedA basic understanding of EEG recording is required |
| Bispectral index | Data output (a single number) is easy to interpret and generated in real timeBispectral monitor is easy to use, does not require prior knowledge of EEG recording techniquesMay have potential as a clinical monitoring tool in the future | Derived from EEG and other data collected during anaesthesia of human patientsNot validated in animalsRaw EEG data are summarized to generate a single number. EEG data cannot be retrieved |
| Somatosensory evoked potentials (SEP) | Evoked potentials generated from high intensity noxious stimuli provide specific information on neural processing of noxious stimuliTargeted placement of electrodes at specific intra-cerebral loci allows the brain structures involved in the neural processing of pain to be identifiedThe SEP waveform is relatively easy to interpret | Can only provide information about neural processing of somatic stimuliThe stimuli used to generate the SEP should only stimulate nociceptive fibres, or the SEP will include non-nociceptive componentsSignal averaging is required to generate an SEP, therefore noxious stimuli must be repeatedly appliedThe amplitude of the SEP signal is small compared with the EEG or electrical noise. Some skill is required to generate an SEP with an acceptable signal-to-noise ratio |
The electroencephalogram
What is the EEG?
The EEG is the electrical activity recorded from electrodes placed at various locations on the scalp (human) or head (other species). It consists of the summated electrical activity of populations of neurones together with a contribution from the glial cells. Neurones are excitable cells with intrinsic electrical properties that result in the production of electrical and magnetic fields. These fields may be recorded at a distance from their sources and are termed ‘far-field potentials’. Fields recorded a short distance from their source they are termed ‘near-field’ or local field potentials. Activity recorded from the surface of the cortex is described as the electrocorticogram (ECoG), whilst electrical activity recorded from the scalp is the EEG. The EEG and ECoG are both far-field potentials.
When an action potential travelling along a nerve fibre reaches a synapse it generates a postsynaptic potential, an alteration in the membrane potential of the postsynaptic neuron. An excitatory neurone generates an excitatory postsynaptic potential (EPSP) and an inhibitory neurone generates an inhibitory postsynaptic potential (IPSP). Postsynaptic potentials interact with each other and if there is sufficient summation of these potentials, the axon hillock reaches threshold and an action potential is generated in the postsynaptic neurone. This process propagates forward transmission of nerve impulses. Action potentials are too small and transient in terms of current flow to affect the extracellular environment, but the generation of EPSPs and IPSPs in postsynaptic neurones results in the creation of active current sinks and sources respectively in the extracellular fluid immediately adjacent to the cell membrane of the postsynaptic neurone (the direction of current flow is defined by the direction along which positive charges are transported). Current sinks and sources can be simply defined as local electrical currents that flow from a location where they cannot be detected into a location where they can be detected (current source) or vice versa (current sink). Glial cells adjacent to current sinks and sources contribute to their formation and maintenance as the changes in electrical potential alter the Nernst equation for that region of cell membrane and so lead to localized alterations in the concentrations of intracellular and extracellular ions and glial cell membrane potentials (Silva, 2004).
As most neurones are elongated cells, these changes in extracellular potential summate along the length of the cell membrane to give the neurone an overall electrical vector (a vector is a characteristic that has both magnitude and direction). A constantly varying dipole (positive at one end, negative at the other) configuration is formed along the length of the neuronal cell body. In certain parts of the central nervous system, notably laminae III and V of the cerebral cortex, neurones lie in a sheet-like configuration and are aligned with their dendritic processes and axon hillocks on opposite sides of the layer of cells. Under these circumstances, the electrical vectors of individual neurones will summate to give an overall vector for a region of cerebral cortex. The EEG is the far-field potential of this vector as recorded between two electrodes and represents the overall activity of a region of cerebral cortex. These summated far-field potentials can be relatively large (of the order of tens of millivolts), especially if the activity of the neuronal cells is phase locked or synchronous. For this reason, the principle generators of the EEG are thought to be the pyramidal neurones of the cortex, which are lined up perpendicular to the cortical surface and form layers of neurones in a palisade (Speckmann & Elgar, 1987).
The EEG reflects electrical activity in cortical neurones but does not provide direct information on lower centres of the brain, such as the brain stem and thalamus. However these centres have a strong regulatory influence on cortical function, particularly during periods of unconsciousness and general anaesthesia, thus the EEG is believed to indirectly reflect activity in these centres in addition to that of the cerebral cortex (Simons et al., 1989).
EEG recording techniques
The electrical activity of the brain can be recorded intracerebrally, from the cortex or dura (ECoG) or the surface of the scalp (EEG), however the principle of EEG recording is similar for all locations. In essence, the recording apparatus must comprise (i) electrodes, (ii) signal recording amplifiers and (iii) a recording system.
Electrodes
Electrodes make the connection between the conducting fluid of the tissue and the input circuit of the amplifier. For accurate recordings there should be minimal distortion of the signal at this interface.
When recording EEG in human beings the electrodes are usually placed on the surface of the scalp. Typical electrodes consist of multiple fine chlorided silver wires fixed in a rigid plastic cup. The plastic cup is fixed to the scalp with an adhesive and filled with conducting gel through a hole in the top. Electrode contact with the tissue is therefore made via an electrolytic bridge so that gel in contact with the electrode is not disturbed by scalp movement, reducing the chance of electrical artefact.
A variety of different electrodes have been used to record the EEG in animals. Johnson and Taylor (1997, 1998) and Johnson et al. (1999) used stainless steel needle electrodes placed subdermally in horses, a technique that has been adopted by others (Murrell et al., 2003, 2005a). Haga et al. (1999, 2001) and Haga and Dolvik (2005) used commercially available self-adhesive silver chloride electrodes applied to the skin surface in both pig and horse studies. Silver chloride needle electrodes placed subdermally have been used in dogs (Murrell et al., 2004). Subdermal needle type electrodes have the advantage that the conducting electrode tip is bathed directly in tissue fluid, improving the interface between the generator and recording system. When using adhesive electrodes on the skin it is important to first shave and degrease the skin, for example with diethyl ether. Electrode movement against the surrounding tissue during recording must be avoided to prevent artefact, and can be a significant source of noise during recording in awake animals.
Impedance is the total opposition that a circuit presents to alternating current and the impedance between the electrode and tissue should be small compared with the input impedance of the amplifier. This will minimize loss of EEG signal. Any metal in contact with an ionized liquid exhibits a steady electrical potential, which in the context of an EEG electrode is termed the electrode potential. Although the potentials per se do not distort the fluctuating voltage recorded by the electrodes they add a fixed value to it. If the steady potential arising at each of the two electrodes connected to an amplifier is of the same value the design of the differential amplifier will ensure that the microvolt neurophysiological signals will still be recorded accurately. Electrode potentials are unavoidable, but minimizing differences in this potential between pairs of recording electrodes will reduce distortion of the EEG signal (Cooper et al., 1974). Electrodes should therefore be made of the same material of high purity and surface contamination should be avoided.
The small neurophysiological potentials resulting from the electrical activity of the brain cause tiny currents to flow through the electrodes and input circuit, allowing this activity to be recorded. A metal interface that allows this to happen unhindered is said to be reversible. Pure silver electrodes coated with a layer of silver chloride are commonly chosen for recording the surface EEG (Geddes, 1972). Chloriding the silver metal results in a reversible electrode that has a low resistance to low frequency potentials that constitute the EEG. Other metals such as stainless steel are nonreversible . Nonreversible electrodes can block steady biological potentials and attenuate low frequency components of the neurophysiological signal.
Many metals, including chlorided silver, cause inflammatory reactions when implanted in brain tissue for periods longer than 1–2 days (Cooper & Crow, 1966). Electrodes required to be left in situ for longer periods can be made from tungsten, platinum, iridium or stainless steel, all of which are relatively inert compared with silver. Although stainless steel is nonreversible resulting in poor recording characteristics, it is readily available and causes little tissue reaction.
Sites of electrode placement. The International 10-20 system of electrode placement is the most widely used method to describe the location of scalp EEG electrodes in man (Jasper, 1958). The electrodes are placed using nasion (the point between the forehead and nose), inion (the bump at the back of the skull), right and left pre-auricular points as reference points. Distances between these reference points are measured with calipers and the electrodes are placed at fixed percentages of these distances. The common interval is normally 10 or 20% of a scalp measurement, hence the nomenclature ‘10-20’ system. Scalp locations are designated by an abbreviation using letters that refer to the cerebral regions and numbers for relative locations. Even numbers are right-sided locations; odd numbers are left-sided locations. Low numbers are closer to the midline; high numbers are more lateral. Mid-sagittal positions are designated with a z, e.g. Cz. An enhanced system of closely spaced electrodes is used when more precise recording is needed.
Inter-species differences between the shape and size of the cranium prohibit development of a standard system of electrode placement for recording the surface EEG across species. The relatively small cranium size in most animals compared with man also limits the total number of electrode positions that can be usefully recorded. In contrast to the 32 channels routinely used in man, studies in animals are often restricted to one or two channels. This impedes the ability to relate EEG data to specific cortical areas and therefore localize sources of the EEG recorded from the scalp surface. The site of electrode placement will influence the EEG, and may account for differences between EEG data reported in studies from different groups. It is not uncommon for animal studies to describe electrode placement using the human 10-20 International system (Morris et al., 1997; Jang et al., 2004). This nomenclature is undesirable because of species differences in the anatomy of the cranium relative to the underlying structures of the brain. It is more accurate to describe the site of electrode placement relative to identifiable landmarks on the head of a given species.
A stereotaxic atlas is available for many laboratory animal species to allow accurate placement of intracerebral electrodes for recording from precise brain loci. Bregma, a clearly identifiable landmark on the dorsal surface of the exposed skull is used as the reference point for electrode placement in specific sites. These invasive experimental studies are commonly carried out in mouse and rat models and a wealth of studies have investigated the functional neurophysiology of different brain structures in these species.
Electrode montages. Each EEG channel records the difference in electrical potential between two recording electrodes. Channels may either record voltage differences between adjacent electrode sites (bipolar recording) or between various electrode sites and a common reference electrode (referential recording). In bipolar recording both electrodes are located over cerebrally active tissue and so contributions from each electrode will appear in two adjacent channels, once as the ‘active or exploring’ and once as the ‘reference’ electrode. Referential recording is more commonly used in veterinary studies (Morris et al., 1997; Johnson & Taylor, 1998; Haga et al., 1999; Murrell et al., 2003). In these montages the potential at each (active) electrode is measured relative to a standard reference potential, arbitrarily assigned a value of zero. The referential electrode is usually placed some distance from the source of EEG generators, but electrodes placed too far from the brain may not be practical because prominent ECG and movement artefacts will be recorded. A point on the head approximating the rostral margin of the brain is often chosen to site the reference electrode. The reference and active electrodes may also be described as non-inverting and inverting electrodes respectively. Studies investigating changes in the EEG with nociception in horses have used three or four electrodes to generate one or two channels of EEG. Many of these studies have used the electrode configuration described by Mayhew and Washbourne (1990), with the active electrode over the right zygomatic process, the reference electrode over the parietal suture rostral to the divergence of the temporal muscles from the midline and the ground electrode caudal to the poll (Johnson & Taylor, 1997, 1998; Johnson et al., 1999; Murrell et al., 2003, 2005a). Others have used similar configurations with the active electrode(s) also placed over the temporal region of the brain (Ekström et al., 1993). Studies in dogs have utilized an active electrode placed caudal to the lateral canthus of the eye, corresponding to the temporal region of the brain (Carrasco-Jimenez et al., 2004). The sites of electrode placement and type of montage should be considered during critical appraisal of EEG investigations in animals. An understanding of electrode montage terminology is essential in order to be able to correctly interpret the data presented.
Amplifiers and digitizers
There are two main kinds of devices used for recording signals, analogue and digital. Analogue devices like chart recorders feature a pen moving up and down over a paper chart, moved along at a certain rate. Digital devices, more correctly analogue to digital (A to D) converters, record the signal on a computer. Most physiological signals are now recorded using A to D converters. These offer advantages of cost, accuracy and ease of data extraction for further analysis over analogue systems. There are, however, a number of features of digital data recording that must be understood if such systems are to be used accurately.
Analogue to digital converters resolve the value of the signal at instantaneous points in time as numerical values. These numbers along with the times at which they were recorded are stored in a computer file. When a signal is digitized, its value is recorded at specific times, but no value is recorded between these times. The digitized signal is considered to have an indefinite value between sampling times. If the sampling interval is sufficiently short then all the information in the signal will be recorded. It is even possible to calculate the value of the original signal between sampling times and so reconstruct the signal. If the sampling interval is increased, there will come a point where the signal is suddenly no longer recorded in enough detail to reconstruct the values between sampling times. At this point, the higher frequency components are not completely lost, rather they are represented by lower frequencies which contaminate the whole of the recording. The recorded signal can look substantially different to the original and is to all intents and purposes useless. This phenomenon is known as aliasing. Aliasing is the most common reason for digitized data recordings to be meaningless. Once aliasing has occurred there is no way to recover the original signal.
In order to prevent a recording from being contaminated by aliasing it is important to ensure that there is no activity in the signal above a certain frequency limit and that the sampling rate is high enough to record accurately at the limit frequency. All activity above the limit frequency should be removed using a low pass filter applied to the signal before it is digitized. The Nyquist frequency is the lowest possible sampling frequency at which all the information of a signal will be accurately recorded. In order to prevent aliasing, the signal must be sampled at least twice as fast as the highest frequency present in the signal. Once the highest frequency in the signal is known, the Nyquist frequency is calculated and the sampling rate is set faster than this frequency.
Analysis of EEG data
A significant body of data are generated by even short periods of EEG recording, therefore powerful computers are required to process these data in order to facilitate interpretation. Fast Fourier transformation (FFT) is commonly used to quantify information contained within the raw EEG signal. FFT is often carried out ‘off-line’ at the end of experiments and it is a mathematical process that changes the raw EEG signal from the time domain to the frequency domain, generating a power spectrum (Fig. 1). Simple descriptors can be derived from the power spectrum, including median and spectral edge frequencies and total power. EEG changes during nociception are frequently reported as percentage changes in these descriptors compared with an unstimulated baseline period (Otto et al., 1996; Haga et al., 2001; Murrell et al., 2003, 2005a; Haga & Dolvik, 2005). Commonly a change in the level of ‘synchronization’ of the EEG is described. De-synchronization is characterized as increased high frequency activity and decreased power in low frequency bands of the EEG, and is often associated with increased level of arousal. Conversely synchronization refers to an EEG pattern of high amplitude, low frequency activity.
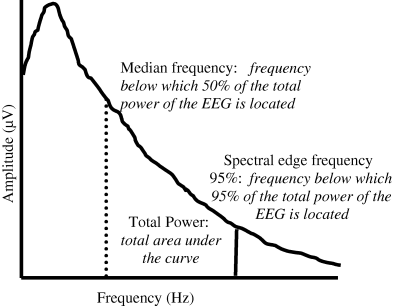
Schematic representation of an EEG power spectrum. The dashed line represents median frequency, the solid line represents spectral edge frequency 95%.
In human studies the frequency of EEG activity has been denoted by delta (0–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and beta (>12 Hz), and the relative amount of activity in each frequency band is reported. These frequency bands also have functionality associated with them, for example in man EEG alpha and theta oscillations have been suggested to reflect cognitive memory and performance (Klimesch, 1999). This method has been adopted by some authors investigating EEG in animals (Ekström et al., 1993; Miller et al., 1995a; Otto et al., 1996; Ong et al., 1997; Haga & Dolvik, 2005). The division of the EEG power spectrum into these frequency bands in animals is completely arbitrary. It maybe inappropriate to classify the EEG frequency spectrum similarly across species because false functionality assumptions might be inferred.
The EEG as a tool to assess pain in animals
Studies investigating EEG changes during noxious stimulation
The EEG has been extensively investigated as a monitor of anaesthetic depth in man (Rampil & Matteo, 1987; Long et al., 1989; White & Boyle, 1989; Drummond et al., 1991; Schwender et al., 1996), essentially as a tool to prevent inadequate anaesthesia which could result in intra-operative awareness (Jones, 1994). A large number of studies have been carried out to investigate the effect of a clinical nociceptive stimulus on the EEG of anaesthetized patients and have produced varying findings. Some have identified de-synchronization associated with nociceptive stimulation (Rampil & Matteo, 1987; Wilder-Smith et al., 1995; de Beer et al., 1996; Schwender et al., 1996). Other studies have failed to identify EEG changes (White & Boyle, 1989; Dwyer et al., 1994; Schraag et al., 1998), or have reported synchronization (Kochs et al., 1994; Kiyama & Takeda, 1997; Kiyama & Tsuzaki, 1997).
A number of studies have also evaluated EEG as a potential monitor of anaesthetic depth in animals, and many of these have been carried out in horses. These studies have sought changes in the EEG which can be correlated to nociception, and therefore provide an indication of inadequate anaesthesia. The minimal anaesthesia model developed by Johnson and co-workers (Murrell et al., 2003; Johnson et al., 2005a; McGregor, 2005; Woodbury et al., 2005) was first applied to investigate the effects of a surgical noxious stimulus on the EEG of horses anaesthetized with halothane (Murrell et al., 2003). This study identified de-synchronization in the EEG during castration, resulting in an increase in median frequency (F50), and decrease in total power. Others have also identified a shift towards higher frequency activity during surgical stimulation in horses (Ekström et al., 1993; Miller et al., 1995a; Otto et al., 1996, 1998). However, similar to studies carried out in man, this finding is not universal, and other studies failed to identify EEG changes during application of a surgical nociceptive stimulus (Haga & Dolvik, 2005). The variability in the results of clinical EEG studies of nociception suggests that the EEG is not a sensitive indicator of nociception, and that in order to capture EEG changes during stimulation a number of experimental conditions must be standardized. These include the depth of anaesthesia, anaesthetic agents administered and severity of noxious stimulation. Murrell et al. (2003) administered anaesthetic agents without recognized antinociceptive action, and used emasculation of the spermatic cord which is recognized to be a potent noxious stimulus (Thornton & Waterman-Pearson, 1999). Each animal also acted as its own control, such that individual variability in the EEG did not influence the results. This is in contrast to a cohort of control animals that did not receive surgery, a design that has been used in other studies (Miller et al., 1995a; Otto et al., 1996).
The minimal anaesthesia model has been applied to other species, such as deer, sheep and rats, and similar de-synchronization changes in the EEG during noxious stimulation were observed (Slyvester et al., 2002; Johnson et al., 2005a; McGregor, 2005; Woodbury et al., 2005).
Analysis of the EEG effects of a noxious stimulus can be a useful means of investigating analgesia for painful manipulations in applied veterinary research. In the minimal anaesthesia model, general anaesthesia is administered to a stable plane such that the animals are unconscious, but still able to demonstrate EEG responses to noxious stimulation. A specified noxious stimulus is carried out, such as velvet antler removal in deer, and changes in EEG variables used to compare the degree of cortical response. Compared with more traditional approaches to studies of potentially painful manipulations, this model has the advantage that, provided analgesics are administered before recovery from anaesthesia, a negative control group can be included without compromising the welfare of animals used in the study. It can also be used to compare the effectiveness of different analgesic regimens (Murrell et al., 2002; Haga & Ranheim, 2005; Johnson et al., 2005a). This model is particularly useful in animal species such as deer that do not demonstrate clearly recognizable pain-related behaviour (Wilson & Stafford, 2002).
Halothane is chosen as the maintenance anaesthetic agent for use in the minimal anaesthesia model. It is not considered to have antinociceptive properties (England & Jones, 1992) and appears to cause less depression of the cortex at concentrations sufficient to provide a surgical plane of anaesthesia compared with isoflurane, sevoflurane and methoxyflurane (Johnson & Taylor, 1998; Antunes et al., 2003; Murrell et al., 2005b). Choice of anaesthetic agent may partly account for the differences in results between EEG nociceptive studies carried out in both man and animals. This is exemplified by the studies of Haga and co-workers. This group did not identify EEG changes during castration in horses or application of noxious stimuli to pigs when animals were anaesthetized with isoflurane (Haga et al., 1999; Haga & Dolvik, 2002, 2005). When this model was applied to investigate EEG changes during castration in piglets and animals were anaesthetized with halothane characteristic de-synchronization changes in the EEG were found (Haga & Ranheim, 2005).
Bispectral index
The BIS monitor (Aspect Medical Systems Inc., Newton, MA, USA) was developed in the search for a more reliable and simpler index for monitoring depth of anaesthesia in man. The simplicity of the BIS monitor makes it attractive to clinicians as a means to assess depth of anaesthesia and nociception. In contrast to the raw EEG signal, which requires complex processing and interpretation, the output of the BIS monitor is a single number. The BIS was derived from bifrontal EEG recordings from more than 5000 human subjects anaesthetized with different balanced anaesthetic protocols used in clinical practice (Sigi & Chamoun, 1994). These waveforms were analysed according to the burst suppression ratio, the relative alpha/beta ratio and biocoherence of the EEG. Biocoherence describes the phase coupling relationship between individual waves; a high value implies a common generator and may be associated with moderate sedation. Using a multivariate regression model, scientists from Aspect Medical Systems have transformed the relative contributions of each feature into a linear numeric index (BIS), ranging from 0 (isoelectric EEG) to 100 (fully awake). In man, although BIS may still predict somatic and autonomic responses to noxious stimuli better than some other EEG measures, the correlation between BIS and sedation is significantly greater than the correlation between BIS and indicators of nociception (Vernon et al., 1995; Leslie et al., 1996).
There has been an explosion of human studies investigating the usefulness of the BIS monitor for predicting awareness during anaesthesia, with varying results (Liu et al., 1997; Struys et al., 1998; Driessen et al., 1999; Drummond, 2000; Ekman et al., 2004). The BIS monitor has also been investigated in animals as a potential monitor of anaesthetic depth and nociception (Haga et al., 1999; Greene et al., 2002, 2003; Haga & Dolvik, 2002; Muir et al., 2003; Carrasco-Jimenez et al., 2004; Lamont et al., 2004). Although some of these studies have found that BIS tends to decrease with increasing depth of anaesthesia, similar to the trend in man, it is important to emphasize that the BIS is empirically derived from human data making it less than completely reliable for use in other species.
Evoked potentials
Evoked potentials are fragments of electrical activity time-locked to a specific sensory stimulus. The EEG recorded during application of a stimulus contains both EEG activity directly related to the sensory processing of the stimulus, and on-going background EEG activity related to other neural processes. Making the assumptions that the EEG activity related to the stimulus is the same every time the stimulus is presented, and that the background EEG varies randomly, the electrical activity specific to the stimulus can be extracted by signal averaging the EEG over a set of responses. Signal averaging tends the background EEG to a flat line leaving only the stimulus-related electrical activity, the evoked potential.
An evoked potential is represented by a voltage/time plot and appears as a series of positive and negative waves (usually called latencies) characterized by their time of onset after stimulation (latency) and amplitude. A particular latency represents the time the stimulus takes to travel through the neural pathway to the generator of that latency. Early waves are believed to correspond with synaptic connections early in the neural pathway consisting of simple, sequential connections and the primary processing of sensory information. Later waves are believed to reflect a summation of diverse synchronous activity in the brain arising from multiple diffuse projections of the neural pathways. These are analogous to the higher processing of sensory information (Bromm, 1984). The amplitude of latencies is considered to reflect the size or degree of activity of individual neural generators in the processing pathway.
Evoked potential studies investigating pain usually utilize SEPs. These are evoked by short stimulation of peripheral somatosensory fibres. SEPs evoked by high intensity stimulation represent neural processing of noxious stimuli (Bromm & Lorenz, 1998; Stienen et al., 2003, 2004). Recording SEPs from specific brain loci using intracerebral electrodes can be used to elucidate the contribution of specific structures to pain processing (Wang et al., 2004). Neural processing of noxious stimuli is also affected by anaesthetic drugs, so that drug-induced changes in SEP waveforms are considered to be related to an altered nociceptive state (Bromm & Scharein, 1983; Bromm et al., 1992; Kochs et al., 1996). Therefore SEPs are potential indicators of analgesic efficacy during general anaesthesia.
In order to selectively activate the nociceptive system, the stimulus should predominantly activate Aδ and C fibres (Gasser & Erlanger, 1929) with no or only low concurrent activation of other sensory modalities. Electrical, mechanical and thermal stimuli fulfil these requirements but they all have shortcomings. Electrical stimuli are easy to control and are therefore most commonly used to generate SEPs in animal and human studies. However an electrical stimulus bypasses the generator compartment of nerve terminals and will also usually stimulate all peripheral afferent fibres, including Aβ fibres that are not normally involved in nociception. Mechanical stimuli delivered with needles are also used in analgesiometry studies but are largely unsuited to SEP studies. Mechanoreceptors are activated in addition to nociceptors and conventional mechano-stimulators do not provide the fast and precisely controlled stimuli required for the study of time-locked events such as evoked potentials. Conventional heat stimulation using radiant heat from a light bulb or heat conducted from a contact thermode is also unsuitable to elicit a reliable SEP. The rate of increase in cutaneous temperature is too slow and neuronal responses to sequential stimuli cannot be considered to be exactly synchronous.
Somatosensory evoked potential research of noxious stimuli has been revolutionized by the development of laser stimulation, termed LEPs (Kakigi et al., 2005). The laser can be used as a powerful heat source to the skin, providing a noxious stimulus that is specific, controllable, safe and reproducible. A laser beam does not actually contact the skin, therefore only nociceptive receptors are activated (Kakigi et al., 1989).
The vast majority of SEP studies in animals have been carried out in laboratory animals, either to investigate the functional neuroanatomy of pain pathways or the mechanism of action of analgesic drugs (Gordon et al., 1987). However SEPs have been recorded in clinically normal dogs sedated with acepromazine using electrical stimulation of the sciatic nerve as the noxious stimulus (Kornegay et al., 1981). The recorded SEP has a similar morphology to those recorded from humans, the waveform is composed of a series of negative (N) and positive (P) peaks. Peaks N14, P20 and N43 (14, 20 and 43 represent latency in ms) were consistently identified. Bertens (1986, 1988) used SEP monitoring to investigate the antinociceptive effects of pentobarbital, thiopental and fentanyl in dogs.
Carefully controlled laboratory studies in human beings have shown that amplitudes of late SEPs elicited by short noxious stimuli are correlated to the intensity of pain sensation (Bromm & Scharein, 1982; Chudler & Dong, 1983; Miltner et al., 1989; Kochs et al., 1996). Therefore the SEP paradigm can be used to quantify the efficacy of analgesics. Relating changes in the SEP waveform to pain perception inherently relies on the ability of human beings to verbally report pain. Until recently it has been impossible to correlate changes in SEPs with pain perception in animals, imposing a limitation on the usefulness of the SEP paradigm to evaluate animal pain perception. However Stienen and co-workers (Stienen et al., 2003, 2004; Oostrom van et al., 2005) have developed a unique model to evaluate the relationship between SEPs and pain perception in rats. A technique to record SEPs in awake, freely moving rats was developed, using electrical stimulation of the tail (Stienen et al., 2003). The model was expanded to record the SEP from different surface brain loci (Stienen et al., 2004), and it was found that SEPs recorded from the primary somatosensory cortex were resistant to the effects of stimulus repetition and the administration of analgesic drugs, while those recorded from the Vertex were sensitive to both manipulations. Differences in the latencies of the SEP recorded from these loci were identified. Stienen et al. (2004) proposed that SEPs recorded from the somatosensory cortex represent nociception per se, or the lateral pain pathway. The Vertex SEP was more sensitive to sensorimotor gating and analgesic drugs, suggesting that it may result from activation of the medial pain pathway, which is considered to contribute to the process of distinguishing between relevant and irrelevant nociceptive stimuli (Treede et al., 1999), one of the earliest events in the conscious perception of pain (Vogt et al., 2005). Future studies using this model may enable the relationship between SEPs and pain perception in animals to be quantified for the first time.
Conclusions
Neurophysiological tools are widely used in man to assess pain, in both research and clinical settings. They play a key role in the search to elucidate the precise mechanisms and pathways of pain perception, and to investigate neuropathological states that lead to altered pain sensation. The ultimate objective of these studies was the development of tools that will in turn be used to assess and improve analgesia strategies, particularly in the management of chronic pain.
Neurophysiological techniques are also widely applied to animals, both in search of a monitor of adequacy of anaesthesia, and studies to assess the efficacy of analgesic agents. Laboratory animals have been extensively used in models to investigate pain in man. However a substantial number of studies have also used neurophysiological techniques to increase knowledge of pain in specific animal species, with the aim of improving animal welfare. The technical complexity of EEG recording techniques and data analysis currently limits widespread application in a clinical environment. Evidence to date suggests that EEG data collected in clinical studies, where there is inherent variability in the animals and severity and type of nociceptive stimulus, is not a very sensitive indicator of nociception or pain. However research studies, using neurophysiological techniques such as EEG and evoked potentials in controlled environments, are able to provide a unique window on pain processing in animals. Knowledge gained from studies using these techniques can be applied to clinical and husbandry situations leading to improvements in pain management in animals and ultimately animal welfare.




