Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice
Summary
Sleep fragmentation is present in numerous sleep pathologies and constitutes a major feature of patients with obstructive sleep apnea. A prevalence of metabolic syndrome, diabetes and obesity has been shown to be associated to obstructive sleep apnea. While sleep fragmentation has been shown to impact sleep homeostasis, its specific effects on metabolic variables are only beginning to emerge. In this context, it is important to develop realistic animal models that would account for chronic metabolic effects of sleep fragmentation. We developed a 14-day model of instrumental sleep fragmentation in mice, and show an impact on both brain-specific and general metabolism. We first report that sleep fragmentation increases food intake without affecting body weight. This imbalance was accompanied by the inability to adequately decrease brain temperature during fragmented sleep. In addition, we report that sleep-fragmented mice develop glucose intolerance. We also observe that sleep fragmentation slightly increases the circadian peak level of glucocorticoids, a factor that may be involved in the observed metabolic effects. Our results confirm that poor-quality sleep with sustained sleep fragmentation has similar effects on general metabolism as actual sleep loss. Altogether, these results strongly suggest that sleep fragmentation is an aggravating factor for the development of metabolic dysfunctions that may be relevant for sleep disorders such as obstructive sleep apnea.
Introduction
Poor sleep quality generally consists of sleep fragmentation (SF) and may result from different sleep pathologies, such as sleep-related movement disorders, narcolepsy, obstructive sleep apneas (OSA) or from other conditions not directly related to sleep, such as chronic pain. SF is a core feature of OSA that represents a highly prevalent sleep disorder worldwide affecting 3–7% of men and 2–5% of women in the active population (Young et al., 2002). A growing body of epidemiological evidence has unraveled the link between OSA, sleep curtailment or poor-quality sleep and dramatic metabolic impairments such as obesity and type 2 diabetes (Attal and Chanson, 2010; Spiegel et al., 2009). A few nights of restricted sleep are sufficient to induce an increase in appetite and a general glucose metabolism imbalance in healthy young subjects (Spiegel et al., 2004). Importantly, not only curtailment of the time spent asleep but also a loss of sleep quality leads to glucose metabolism impairment. As a matter of fact, suppression of slow-wave sleep (SWS) during 3 nights (Tasali and Ip, 2008) or 2 days of enforced SF (Stamatakis and Punjabi, 2010) induce impairments in glucose metabolism and hormone levels in humans. Furthermore, impairments in glucose homeostasis have been also reported in rats following 8 days of sleep disturbances (Barf et al., 2010). In spite of these results, the specific role of SF in these mechanisms remains poorly understood. This justifies the necessity to use realistic rodent models aiming at establishing independent effects of SF. Most of the animal models developed focused on the cognitive impact of short-term SF (Guzman-Marin et al., 2007; Tartar et al., 2006) and do not reflect the most common clinical presentation, which consists of SF over long periods of time.
In this context, we developed an instrumental model of chronic SF in mice that exhibit sleep perturbations similar to those observed in human pathology (Baud et al., 2010). In the present study, we investigate the effects of SF for 14 days on metabolic parameters such as brain and body temperature, food intake, glucose metabolism and hormone levels.
Materials and Methods
SF device (CaResS)
A homemade rotating device named ‘CaResS’ (French acronym for Cage pour la Restriction de Sommeil) was developed to perform automatic instrumental SF on mice (Petit et al., 2006). Specific attention was paid to develop a system that maximally diminished stressful stimulations for the animal. This is in contrast with systems using water surrounding (Rechtschaffen et al., 2002), limited space for movements (McGuire et al., 2008) or human intervention. This device consists of a cylindrical Plexiglas cage (diameter 30 cm) with inner dividing walls and a mobile circular floor activated by two lateral electrical motors. The floor is covered with sawdust, and the animal has free access to water and food. Rotation of the floor (1.5 rpm) is triggered by a software and invariably shifts the mouse against dividing walls to wake it up, wherever it is lying down.
Animals and experimental design
Adult male C57BL6 mice (7–9 weeks old, 22–25 g; Charles River, France) were single housed in the CaResS device under a 12 h light : 12 h dark cycle (lights on at 05:00 hours, ZT0) in standard conditions (temperature at 23 ± 1 °C). After 7 days of habituation, mice were randomly divided into three groups for a 1-day or 14-day protocol beginning and finishing at night onset (17:00 hours, ZT12): (i) the sleep-fragmented group (F), where SF was enforced at a rate of 60 arousals h−1 activating CaResS 20 s min−1; (ii) the quiet control group (QC) corresponding to mice left undisturbed in the CaResS device; and (iii) the motor control group (MC), where mice underwent enforced locomotion 40 min h−1 during the active period only (17:00–05:00 hours; ZT12–ZT0) to match locomotor activity of the F group while minimally interfering with sleep. Accordingly, the total amount of cage rotation for both MC and F groups was 8 h day−1.
Three sets of experiments were performed to obtain the results. In a first set of experiments, electroencephalogram (EEG)/electromyogram (EMG) recordings were performed at D1, D7 and D14. In a second set of experiments, brain and body temperature as well as locomotor activity were recorded at D1, D2, D7 and D14, and the urine of each animal was harvested between D9 and D10 for glucocorticoids (GC) determination. In a third set of experiments, animals underwent an intraperitoneal glucose tolerance test (ipGTT) at D10. Food consumption and animal weight measurements were performed during the first and second sets of experiments. This experimental design is summarized in Fig. 1a. All experiments were performed under the approval of the Veterinary Service of the Canton de Vaud.
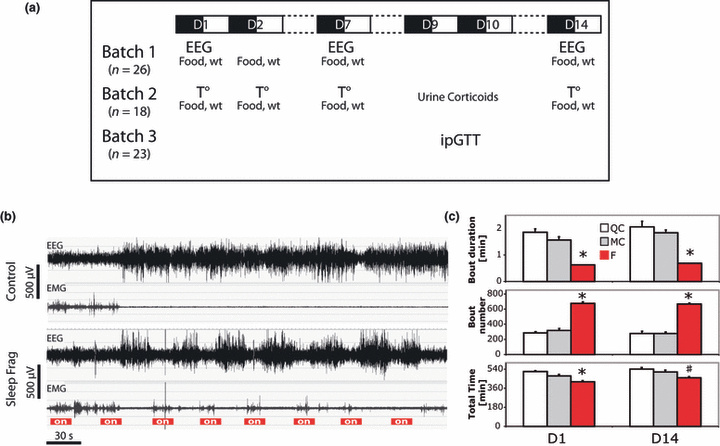
Protocol summary and SF. (a) Summary of the experiments performed with the different cohorts of animals. (b) Representative examples of EEG and parallel EMG recordings in a sleep-fragmented mouse and a control at the beginning of the rest phase. Note the repetitive arousals (> 4 s of desynchronization of the EEG) and simultaneous EMG activation during SF compared with rare spontaneous desynchronization in control. (c) SWS bout mean duration over 24 h, bouts number over 24 h and cumulated sleep time over 24 h (mean ± SEM, n for QC = 6, MC = 9, F = 11). *F is different from QC and MC; #F is different from QC only. Note that SF leads to a reduction of the duration of individual episodes, an increase in their number and a minimal sleep restriction. EEG, electroencephalogram; EMG, electromyogram; F, sleep-fragmented group; ipGTT, intraperitoneal glucose tolerance test; MC, motor control group; QC, quiet control group.
Food intake and weight
From the beginning of habituation to the end of the experiment, mice were fed with normal diet for rodents (ref.3436; Kliba-Nafag, Switzerland). Pellet weight and mice weight were measured daily before dark onset (ZT11; Fig. 1a). Food intake was measured as the difference in pellets weight over 24 h, taking into account the crumbs that were collected in a small receptacle placed under the food distributor in order to avoid food spillage bias.
EEG recording and scoring
Twenty-six mice (F, n = 11; MC, n = 9; QC, n = 6) were implanted for EEG and EMG recordings 3 weeks before the experiment. Briefly, anesthesia was induced with isoflurane 4%, and maintained with a cocktail of Xylasine 8% and Ketamine 9% (10 μL g−1 i.p.). The skull was drilled to insert two gold-covered screws used as EEG electrodes over the frontal (Bregma +1.5 mm and 1 mm laterally) and parietal cortex (Bregma −3 mm and 1 mm laterally), respectively. Two additional screws were inserted for implant anchorage. Two gold wire electrodes were placed into the nuchal muscles for EMG recording. The EEG and EMG electrodes were then soldered to a connector and covered with dental cement (Paladur, Le Mont-sur-Lausanne, Switzerland). Then, a swivel connected-wire was plugged to this connector to allow free moving of the animal. The EEG/EMG signals were recorded and digitalized with an Embla A10 amplifier (EMBLA, Amsterdam,The Netherlands) sampled at 100 Hz and filtered between 0.5 and 50 Hz. Using the Somnologica software (Medcare), 4-s epochs of the EEG/EMG were visually scored in three vigilance states [wakefulness, SWS and paradoxical sleep (PS)] according to classical criteria (Tobler et al., 1997). Finally, vigilance states parameters were averaged on the 12-h light and dark periods. Except during the temperature recordings, locomotor activity was recorded using infra-red sensors placed over the cage. These sensors detect all movements of the animal barycenter longer than ≈1 cm. Sampling frequency was set at 1 Hz. Data were collected through a homemade interface and transferred to a computer.
Temperature probes implantation and data analysis
Abdominal temperature was recorded by telemetry, whereas brain temperature was recorded using a wire-connected thermistor. Eighteen mice (n = 6 per group) were implanted 3 weeks before the experiment. Activity of these mice was determined by the same telemetry system used for temperature recordings. Briefly, anesthesia was induced with isoflurane 4%, and maintained with a cocktail of Xylasine 8% and Ketamine 9% (10 μL g−1, i.p.). The abdominal wall was incised longitudinally to insert a transmitter allowing temperature measurement and movement detection (TA10TA-F20; DSI, St Paul, MN, USA), and sutured back. Immediately afterwards, the scalp was incised longitudinally and a thermistor (Ge NTC Catheter Thermistors, 0.25 × 0.3 × 0.7 mm3; Adsem, Mountain View, CA, USA) soldered to a connector was inserted between the skull and the dura over the parietal cortex (Bregma −2.0 to −2.4 mm and 1.3 mm laterally). The implant was stuck to the bone with resin (Relyx, 3M, Rüsschlikon, Switzerland) and covered with dental cement.
Brain and abdominal signals were recorded during a baseline day and throughout the SF protocol. Abdominal temperature along with actimetry was sampled at 1 Hz using telemetric recordings by an antenna placed under the CaResS device (PhysioTelTM F series, DSI). Brain temperature was digitalized through Embla A10 amplifier (Medcare), recorded at a 100-Hz sampling frequency, analysed with the Somnologica© software (ResMed Schweiz, Basel, Switzerland) and re-sampled at 1 Hz thereafter. At the end of the experiment, each cortical thermistor was calibrated by putting it in a water bath of known temperature.
To analyse data, abdominal and brain temperatures were aligned with the corresponding actimetry recording, and separated by temperature recorded during active epochs (actimetry > 0) and rest epochs (actimetry = 0), also called ‘rest temperature’. Because rest epochs were less present during the dark period, brain and abdominal temperatures were averaged over three periods corresponding to equivalent rest time and plotted along the x-axis as a function of their mean time of occurrence. During the light phase, where rest epochs were more frequent, rest temperature was separated in six parts, averaged and plotted similarly. Moreover, to reduce the inter-individual variability in absolute values, each brain and abdominal rest temperature was normalized by the mean of abdominal temperature during the light phase before group averaging (for each individual: brain T° or abdominal T°– mean abdominal T° over 12 h light). Examples of individual daily variations of brain and abdominal temperatures at D0, D2 and D14 are shown in Fig. 3a–c. Corresponding mean values per group are shown in Fig. 3d–f. In another display of the results (Fig. 4), the difference in abdominal and brain rest temperatures was calculated minute per minute before averaging group values over 24 h (for each individual and for each minute: delta T° = brain T° − abdominal T°). All calculations were made using MATLAB (The Mathworks, Natick, MA, USA).
Corticosterone dosage
To reduce stress induced by repetitive blood sampling, corticosterone was quantified in urines according to the method described by Touma et al. (2003). In this study, excretion of 3H-corticosterone peaks 2 h following i.p. injection, suggesting a comparable delay between plasma and urine corticosterone levels. Using a similar protocol, urines from QC, MC and F groups were harvested at six different time points to establish a circadian rhythm. Briefly, urine was collected from mice restrained manually during 5–8 s. Because this procedure was slightly stressful, samplings were spaced every 8 h to cover six time points over 48 h in the middle of the protocol (D9–D10). Urine of five-eight mice was necessary to get a given time point. Corticosterone was extracted from urine samples with dichloromethane and assayed using an enzyme immunoassay kit (ASSAY DESIGNS, Enzo Life Sciences, Lausen, Switzerland) according to manufacturer’s instruction. In parallel, creatinine was quantified by a hospital laboratory (CHUV, Lausanne, Switzerland) equipped with routine creatinine dosage system. Results are expressed as the quantity of corticosterone per quantity of creatinine (μg g−1).
ipGTT
An ipGTT was performed in the middle of the protocol (D10) in another set of mice (n for QC = 8, MC = 7, F = 8). Sawdust was changed at the beginning of the dark phase of the previous day (17:00 hours, ZT12) and mice were fasted overnight. On the following morning (08:00 hours, ZT3), a glucose load (1.5 g kg−1) was administered i.p. Blood glucose was measured using a glucometer (Accu-Check, Aviva, Roche Diagnostics, Rotkreuz, Switzerland) at −30, −15, 0, +15, +30, +45, +60, +90, +120 and +150 min before or after glucose injection. The first concentration was measured in a small drop of blood obtained from a small tail incision. Subsequent drops of blood were obtained by gently removing the clot without new incision, because only a small quantity of blood (≈1 μL) is required to measure the blood glucose.
Statistical analysis
After checking for an independent group effect by a two-way anova with repeated measures, days of the protocol were considered independently. For the vigilance states, the post hoc differences between groups in quantities of sleep were analysed using a Bonferroni correction. For the ipGTT and GC circadian profile, a two-way anova was performed using MATLAB, and group means were compared using Tukey’s post hoc test. All other statistics were done using either parametric one-way anova or Kruskal–Wallis anova according to the result of Bartlett’s test of variance. When group differences were significant (P < 0.05), post hoc tests for multiple comparisons were performed using Tukey’s test. For all tests, significance was accepted when P < 0.05. All values are expressed as mean ± standard error to the mean (mean ± SEM).
Results
SF and vigilance states quantities
We visually observed that full awakenings (usually ≥ 12–16 s) occurred on D1 when the animals were manifestly disturbed by the system and moved to find a place to sleep. Conversely, after some time, mice exhibited only a short period of EEG desynchronization (≥ 4–8 s) accompanied by minimal movements during device activation. Exemplary of the EEG/EMG signals in sleep-fragmented mice is shown in Fig. 1b.
During the first day of the protocol, SWS was restricted in the F group compared with both QC and MC (−12−19%, F = 15, df = 2, P < 0.001). On D14, SWS restriction in the F group was still present (−10 to 14%, F = 7.8, df = 2, P < 0.01), but post hoc testing revealed that the decrease in total sleep duration was only significant compared with the QC group and not with the MC group (Fig. 1c; Table 1). Similar results were obtained on D7, and Table 1 shows additional details for 12-h values and D7. PS restriction was present throughout the protocol, and was significant compared with both QC and MC (−59–66% on D1, F = 47, df = 2, P < 0.001; −18–24%, F = 9.1, df = 2, P = 0.001 on D14).
| D1 | D7 | D14 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| QC | MC | F | QC | MC | F | QC | MC | F | |
| SWS time | |||||||||
| D | 158 ± 10 | 101 ± 9‡ | 123 ± 7 | 156 ± 14 | 108 ± 6‡ | 139 ± 10 | 163 ± 10 | 121 ± 10‡ | 149 ± 9 |
| L | 359 ± 4 | 375 ± 9 | 297 ± 10* | 368 ± 15 | 376 ± 9 | 311 ± 8* | 377 ± 6 | 393 ± 10 | 309 ± 4* |
| Total | 517 ± 9 | 476 ± 13 | 420 ± 12* | 524 ± 16 | 484 ± 13 | 449 ± 15† | 540 ± 15 | 513 ± 18 | 458 ± 12† |
| PS time | |||||||||
| D | 20 ± 3 | 7 ± 2‡ | 1 ± 0.1* | 12 ± 3 | 7 ± 1 | 10 ± 1 | 11 ± 2 | 6 ± 2 | 13 ± 2 |
| L | 59 ± 4 | 60 ± 2 | 25 ± 3* | 59 ± 6 | 53 ± 3 | 35 ± 2* | 62 ± 5 | 61 ± 3 | 41 ± 2* |
| Total | 79 ± 6 | 66 ± 3‡ | 27 ± 3* | 70 ± 4 | 60 ± 3‡ | 46 ± 3* | 73 ± 3 | 66 ± 4 | 54 ± 2* |
| SWS bouts | |||||||||
| Number D | 104 ± 11 | 116 ± 15 | 220 ± 14* | 81 ± 15 | 102 ± 17 | 223 ± 15* | 120 ± 22 | 137 ± 20 | 253 ± 12* |
| Number L | 181 ± 12 | 202 ± 21 | 522 ± 20* | 138 ± 4 | 164 ± 6 | 488 ± 9* | 158 ± 8 | 162 ± 13 | 480 ± 5* |
| Duration D | 1.6 ± 0.2 | 1 ± 0.1‡ | 0.7 ± 0.01* | 2.2 ± 0.3 | 1.3 ± 0.2‡ | 0.8 ± 0.03* | 1.6 ± 0.3 | 1 ± 0.1‡ | 0.7 ± 0.02* |
| Duration L | 2 ± 0.1 | 2 ± 0.1 | 0.7 ± 0.01* | 2.7 ± 0.1 | 2.3 ± 0.1 | 0.8 ± 0.02* | 2.4 ± 0.1 | 2.5 ± 0.2 | 0.8 ± 0.01* |
- For each day (D1, D7 and D14), the SWS and PS time expressed in minutes during the dark period (D) and the light period (L) and summed over 24 h. Individual SWS bout mean duration and their number are also reported. Number of mice for QC (n = 6), MC (n = 9) and F (n = 11). Post hoc multiple comparisons using Bonferroni correction were calculated when the group effect of the two-way anova was positive (P < 0.05).
- F, sleep-fragmented group; MC, motor control group; PS, paradoxical sleep; QC quiet control group; SWS, slow-wave sleep.
- *F different from QC and MC; †F different from QC but not from MC; a: MC different from QC.
In the F group, a decrease in the length of individual SWS episodes (−58 and −62%, P < 0.001 on D1 and D14, respectively, see Table 1 for statistical values) was accompanied by an increase in the number of bouts compared with MC and QC groups (+110 and +150%, P < 0.001 on D1 and D14, respectively; Fig. 1c), confirming that SF was effective throughout the protocol.
Food intake, weight and locomotor activity
Food intake was similar in all three groups on D1 (anova, F = 1.5, df = 2, P > 0.05), but a slight significant increase was observed in the F group throughout the rest of the protocol (+14–29% on D2, F = 9.4, df = 2, P < 0.001; +13–29% on D7, F = 12.0, df = 2, P < 0.001; +10–18% on D14, F = 4.3, df = 2, P < 0.05; Fig. 2a and b). However, animals of the F group displayed no significant daily weight loss or gain compared with other groups throughout the protocol (D1–D14, anova, F < 1.3, df = 2, P > 0.05). Locomotion was increased in MC and F groups throughout the protocol (anovas on D1, F = 20.6, D2, F = 10.9, D7, F = 11.8, and on D14, F = 19.8, P < 0.001 and df = 2 throughout).
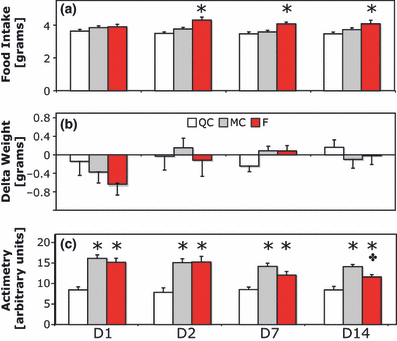
Food intake, weight change and actimetry during 14 days of SF. (a) Daily food intake measured in grams. Note the significant increase in food intake from D2 to D14 (n = 12, 13 and 11 for groups QC, MC and F, respectively, *P < 0.05). (b) Daily weight change. (c) Twenty-four hour means of locomotor activity (actimetry) recorded by infra-red sensors. Note the significant increase in activity in the F and MC groups (*P < 0.001), and also the difference between MC and F on D14 (+P < 0.05). F, sleep-fragmented group; MC, motor control group; QC quiet control group.
We cannot totally rule out that some ‘passive movements’ (i.e. movements of the animal related to the rotation of the cage floor without voluntary locomotor activity) were detected by our infra-red sensors located on the top of the cage. This could constitute an overestimation of the locomotor activity in MC and F animals. However, at the end of the first day, mice were habituated to the cage rotation and limited these ‘passive movements’ to a minimum. Indeed, direct observation of mice indicates that they prefer to lie close to the walls and move less but often. Moreover, because the total time of floor rotation per day was the same for MC and F groups, the fact that the two groups differed by the quantity of ‘passive movements’ is unlikely. On the contrary, the F group had similar (D1–D7, Tukey post hoc, P > 0.05) or even lower activity levels (D14, Tukey post hoc, P < 0.05) compared with the MC group (Fig. 2c), and the MC group exhibited an increased locomotor activity compared with QC (D1–D14, Tukey post hoc, P < 0.05), with a slight but not significant increase in food intake. These results strengthen the idea that the locomotor activity could not explain the higher level of energy expenditure observed exclusively in the F group.
Abdominal and cortical temperature
Parallel measurements of abdominal temperature, brain temperature and actimetry were performed. In control conditions, abdominal and brain temperature covaried tightly, and the largest variations were clearly correlated to locomotion. Temperature ranged from 35 °C at rest to 38 °C when highly active (Fig. 3a). We focused our analysis on ‘rest temperature’ when mice were either in quiet wake or asleep (shaded areas in Fig. 3a–c). Both rest temperatures exhibited daily variations, being higher during the dark phase and lower during the light phase (anova2 showed no group effect, but a circadian effect, F = 31.9, df = 8, P < 0.001; Fig. 3d).
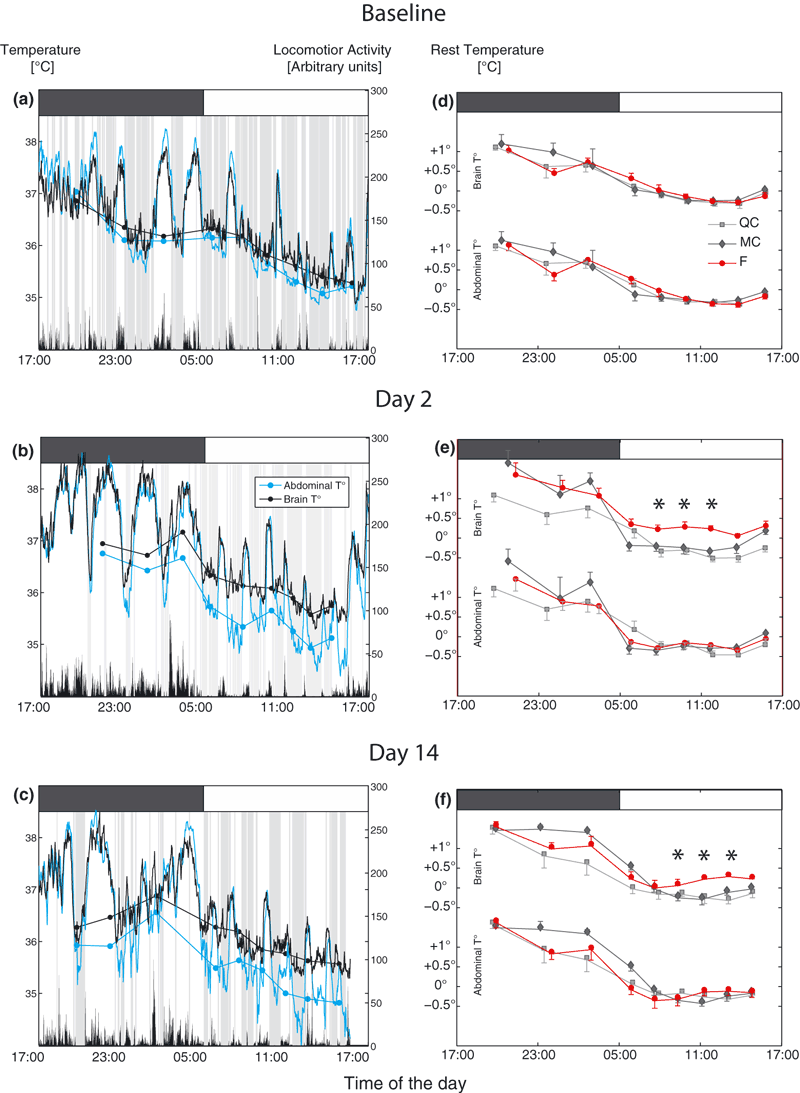
Abdominal and brain temperature recording in parallel with actimetry. (a–c) Examples of daily variations of the brain and abdominal curves for one mouse during baseline, and after 2 and 14 days of SF protocol. Black and blue curves are, respectively, brain and abdominal temperature minute by minute expressed in degrees Celsius (left y-axis). Black vertical bars just above the x-axis represent the levels of active motion over 1 min expressed in arbitrary units (right y-axis). Shaded areas represent rest (actimetry = 0). Dots and continuous lines represent the rest temperature averaged over thirds of the rest time during the dark phase and over sixths during the light phase, and plotted as a function of their time of mean occurrence (see Materials and methods). Gray and white boxes above the graphs represent daytime and nighttime, respectively. The x-axis represents the time of the day (in hours) and is the same on each panel. Note the dissociation of brain and abdominal temperature during rest phases on D2 and D14. (d–f) Averaged variations of brain and abdominal temperatures during the rest periods in each group throughout the light–dark cycle at D0, D2 and D14 (n = 6/group). Gray lines, black lines and red lines represent the QC, MC and F groups, respectively. Note that brain rest temperature remains higher for the F group during the light phase. F, sleep-fragmented group; MC, motor control group; QC quiet control group.
On D2 and D14, brain temperature showed a tendency to remain higher during the light phase in the F group (anova2 on D2, group effect, F = 17.2, df = 2, P < 0.001, no interaction anova2 on D14, group effect, F = 11.9, df = 2, P < 0.001 and an interaction, F = 2.2, df = 16, P < 0.01; see Fig 3d for post hoc significance), whereas abdominal temperature showed no significant difference between groups (anova2 on D2, group effect, F = 1.5, df = 2, P > 0.05; on D14, group effect, F = 3.0, df = 2, P > 0.05). Furthermore, from D2 to D14, individual differential rest temperature between brain and abdominal (‘delta temperature’) showed a significant gap of ∼0.4 °C in the F group compared with MC and QC groups (anova on D2, F = 13.9, df = 2, P < 0.001; on D7, F = 10.9, df = 2, P = 0.001; on D14, F = 15.6, df = 2, P < 0.001; Fig. 4).
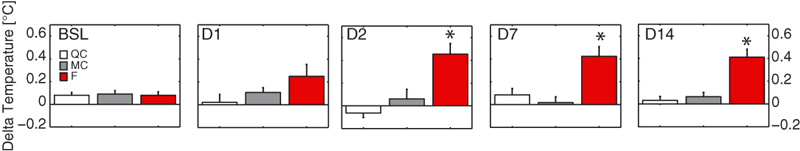
Evolution of delta temperature throughout the SF protocol. Abdominal T° was subtracted from brain T° minute per minute during rest phases and averaged over the 12 h of the rest phase. Values represented here are the mean per group. Note the significant dissociation between brain and abdominal temperature from D2 to D14 (white bars, gray bars and red bars correspond to QC, MC and F groups, respectively). F, sleep-fragmented group; MC, motor control group; QC quiet control group.
GC measurements
To explore endocrine effects of SF that could possibly explain the need for increased energy intake, we measured hormones with catabolic actions. GC levels were quantified in urine at six time points over D9 and D10 to establish a circadian rhythm. A two-way anova of the results with experimental groups and Zeitgeber time (ZT2–ZT22) as factors showed a clear group effect (F = 14.7, df = 2, P < 0.001) and a clear time effect (F = 16.9, df = 5, P < 0.001) and also an interaction (F = 2.8, df = 10, P < 0.01), meaning that the SF effect depends on the time point of measurement. As expected, minimal levels of GC were observed during the resting period and a peak was reached at the beginning of the active period (Fig. 5). The 24-h rhythmic pattern was conserved in all three groups, but the F group showed increased GC levels during the active period at ZT14 (P = 0.01 versus QC and P = 0.09 versus MC, Tukey post hocanova) and ZT18 (P < 0.01 versus QC and P = 0.01 versus MC, Tukey post hocanova), and during the rest period at ZT6 (P = 0.01 versus QC and P = 0.03 versus MC, Tukey post hocanova). The MC group had a slightly higher peak of GC than the QC group at the beginning of the active period, although not significant. However, beyond ZT14 (19:00 hours), this transient increase returns to control value, indicating that continuous cage rotation is not further accompanied by stress. Of note is the fact that levels measured in urine are phase shifted by approximately 2 h compared with plasma values due to bladder accumulation of urine (Touma et al., 2003).
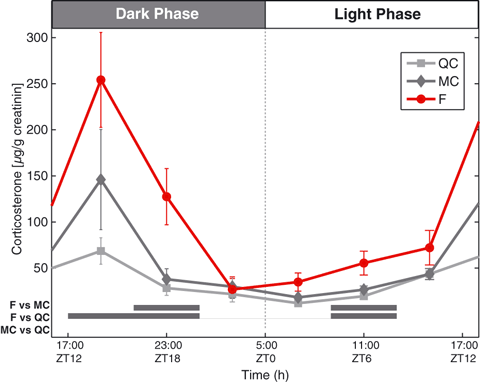
Daily corticosterone profile changes during long-term SF. Corticosterone circadian variations in the urine on D9 and D10 (see Materials and methods). Urinary corticosterone was normalized by creatinine for each sample before group averaging. Note the increase in the amplitude of the circadian variation for the F group. Horizontal gray bars: significance for Tukey post hoc test (P < 0.05). F, sleep-fragmented group; MC, motor control group; QC quiet control group.
To further evaluate stress, animals were visually inspected daily. They did not develop any apparent physical problem, such as skin and paws lesions or hair loss, as seen in protocols of long-term sleep deprivation (Rechtschaffen et al., 2002).
ipGTT
To evaluate the involvement of glucose metabolism in the observed hyperphagic–hypermetabolic state, we performed an ipGTT at D10 (Fig. 6). A two-way anova analysis of the result with group (QC, MC, F) and time point after injection as factors showed a clear group effect (F = 24.3, df = 2, P < 0.001), a clear time point effect (F = 162.2, df = 6, P = 0) and no interaction (F = 1.0, df = 12, P = 0.4). We observed that the glucose load resorption was much slower in the F group, with higher blood glucose levels at 45 min (P = 0.001 versus QC and MC, Tukey post hocanova), 60 min (P = 0.01 versus both), 90 min (P < 0.01 versus both) and 120 min (P < 0.05 versus both) after injection (Fig. 6a). The calculation of the area under the curve further confirmed glucose intolerance (+43 and +47% versus QC and MC, respectively, anova, F = 8.9, df = 2, P < 0.01; Fig. 6b).
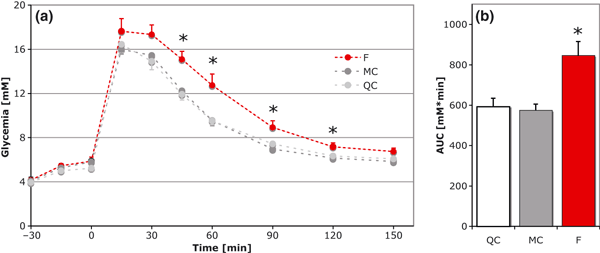
(a) ipGTT after overnight fasting (QC, n = 8, MC, n = 7 and F, n = 8, mean ± SEM). Injection at t = 0. Note the clear intolerance with higher values at t = 45 (P = 0.001), t = 60 (P = 0.01), t = 90 (P < 0.01) and t = 120 (P < 0.05). (b) Area under the curve (AUC) calculated for individual mice and averaged by groups. The F group had increased levels of blood glucose during the 2 h sampling (+43–47%, P < 0.01 compared with QC and MC). F, sleep-fragmented group; MC, motor control group; QC quiet control group.
Discussion
The hyperphagia observed in our study is in accordance with the hyperphagia frequently reported as an effect of sleep loss (Koban et al., 2008). Because hyperphagia persisted throughout our protocol despite a reduction in SWS and PS time that was relatively small and no longer significant towards the end of the experiment, we propose that not only sleep quantity but also sleep quality are important for food intake regulation in our model. In addition, hyperphagia was not accompanied by any weight increase (Fig. 2), suggesting that these sleep disturbances likely lead to an increase in energy expenditure. Using calorimetric measurements, others showed that PS deprivation in animals leads to increased metabolic rate over 24 h (Hipolide et al., 2006). More specifically, the loss of the quality of sleep during experimental SF in humans increases metabolic rate during sleep (Bonnet et al., 1991). The molecular pathways linking sleep disturbance and appetite regulation are not known in detail, but they may include circulating hormones such as leptin and ghrelin and neuropeptides such as orexin or Neuropeptide Y (Martins et al., 2010).
Rat brain temperature decreases by ∼1 °C during rest periods (Franken et al., 1991). Here we report an uncoupling between abdominal and brain temperature decrease only during rest periods, where brain temperature remains at a higher level in the F group from D2 to D14. While sleep restriction is known as a factor increasing heat production, it cannot be the only factor in our experiments as temperature uncoupling was present in the F group at D7 and D14 without significant reduction of the SWS quantity compared with the MC group (Fig. 2; Table 1). So, we can hypothesize that the observed temperature uncoupling reflects processes taking place during SF, such as an increase in brain metabolism and/or a decrease in heat dissipation. It is indeed conceivable that the short duration of sleep episodes during SF maintains high cerebral metabolism. On the other hand, cerebral blood flow deregulation might also play a role in the observed effects as it is thought to be a critical regulator of brain temperature (Zhu et al., 2009). SF has been shown to be a prominent factor for the decrease in cerebral blood flow velocity and cerebrovasomotor reactivity observed in patients with sleep apneas (Hajak et al., 1996; Qureshi et al., 1999). Because sympathetic tone is also increased in patients with OSA (Somers et al., 1995), we can hypothesize that a direct neuronal impact of SF in brain circulation regulation might be responsible for the elevated brain temperature observed in our sleep-fragmented mice.
We next explored the impact of SF on peripheral glucose metabolism. We report that chronic SF induces an increase in the peak level of corticosterone without phase shifting. Because measures were done on D9 and D10, when mice were fully adapted to SF procedures, and compared with animals subjected to the same amount of device activation (MC), this effect is probably due to SF per se and not to an instrumental bias. Indeed, our ‘CaResS’ device was specifically designed to reduce the discomfort of mice during a chronic exposure to sleep disturbances by including sawdust, absence of ‘space-restriction’ and by avoiding any ‘human’ intervention. Moreover, the protocol of fragmentation is based on regular activation of the cage floor rotation, which can be considered as a predictable stimulus for the mice. According to recent considerations about the ‘stress’ concept (see Koolhaas et al., 2011), this constitutes an important issue to decrease stress. Although the major factor controlling GC levels is circadian time, arousals lead to a transient increase (Balbo et al., 2010). Thus, multiple arousals might be sufficient to induce sustained hypothalamo-pituitary axis activation and maintain corticosterone levels above normal. However, our chronic method does not induce a major stress response as observed in chronic total sleep deprivation experiments where GC might increase several fold (Rechtschaffen et al., 2002). Chronic exposure to high levels of GC is known to induce dyslipidemia, as well as insulin resistance and glucose intolerance (Van Raalte et al., 2009). However, the observed change in GC alone might be insufficient to fully explain the development of glucose intolerance in these mice (Karatsoreos et al., 2010). More probably, it could play a synergistic role with other factors, such as an increased sympathetic tone, possibly through the induction of insulin resistance. Such a mechanism has been shown in humans where only 2 days of SF led to an increase in cortisol and in sympathetic tone and to the development of insulin resistance (Stamatakis and Punjabi, 2010). Recently, Barf and collaborators showed that 8 days of sleep disturbances without sleep curtailment impaired glucose tolerance, attributing these effects to SF rather than sleep loss (Barf et al., 2010).
The OSA syndrome was shown to be an independent risk factor for the development of the metabolic syndrome (Coughlin et al., 2004). Our data underline the probable role of SF in this epidemiological relation and may explain the impact of OSA on GC regulation (Henley et al., 2009) and glucose intolerance (Pallayova et al., 2010). Through similar physiopathology, SF might also underlie the higher incidence of diabetes type 2 in narcolepsy (Honda et al., 1986). Supporting this point, SF displayed by a murine model of narcolepsy is thought to be responsible for the metabolic changes observed in these mice (Zhang et al., 2007). Altogether, these and our data indicate that SF represents an allostatic load on endocrine and autonomous systems that might, in the long term, lead to the development of diseases.
In conclusion, we developed a clinically relevant sleep disturbances model in mice where SF is the prominent factor. This model mimics important metabolic effects of SF observed in human experiments and stresses the fact that quality of sleep is of great importance when considering metabolic stability. While intermittent hypoxia was initially the favorite candidate to explain metabolic effects of OSA, our results suggest that SF might play a crucial role as well.
Acknowledgements
This work was supported by a Swiss National Science Foundation grant (3100AO-108336/1) to Pierre J. Magistretti. Pierre J. Magistretti is the recipient of the Asterion Foundation chair. Maxime O. Baud’s work was directly supported by a Swiss National Science Foundation personal MD-PhD grant (323600-119351/1). We thank Joël Gyger for his excellent technical assistance, and Dr Moisan M.-P. (INRA, Bordeaux), for her help in urinary glucocorticoids assay.
Conflict of Interest
The authors declare no conflict of interest.




