Multiple Roles for the Endocannabinoid System During the Earliest Stages of Life: Pre- and Postnatal Development
Abstract
The endocannabinoid system, including its receptors (CB1 and CB2), endogenous ligands (‘endocannabinoids’), synthesising and degrading enzymes, as well as transporter molecules, has been detected from the earliest stages of embryonic development and throughout pre- and postnatal development. In addition, the endocannabinoids, notably 2-arachidonyl glycerol, are also present in maternal milk. During three distinct developmental stages (i.e. embryonic implantation, prenatal brain development and postnatal suckling), the endocannabinoid system appears to play an essential role for development and survival. Thus, during early pregnancy, successful embryonic passage through the oviduct and implantation into the uterus both require critical enzymatic control of optimal anandamide levels at the appropriate times and sites. During foetal life, the cannabinoid CB1 receptor plays a major role in brain development, regulating neural progenitor differentiation into neurones and glia and guiding axonal migration and synaptogenesis. Postnatally, CB1 receptor blockade interferes with the initiation of milk suckling in mouse pups, by inducing oral motor weakness, which exposes a critical role for CB1 receptors in the initiation of milk suckling by neonates, possibly by interfering with innervation of the tongue muscles. Manipulating the endocannabinoid system by pre- and/or postnatal administration of cannabinoids or maternal marijuana consumption, has significant, yet subtle effects on the offspring. Thus, alterations in the dopamine, GABA and endocannabioid systems have been reported while enhanced drug seeking behaviour and impaired executive (prefrontal cortical) function have also been observed. The relatively mild nature of the disruptive effects of prenatal cannabinoids may be understood in the framework of the intricate timing requirements and frequently biphasic effects of the (endo)cannabinoids. In conclusion, the endocannabinoid system plays several key roles in pre- and postnatal development. Future studies should further clarify the mechanisms involved and provide a better understanding of the adverse effects of prenatal exposure, in order to design strategies for the treatment of conditions such as infertility, mental retardation and failure-to-thrive.
Almost 20 years ago, a specific receptor for the major active ingredient of the Cannabis sativa (marijuana) plant was described (1). This first cannabinoid (CB) receptor, later denoted CB1, paved the way for the discovery of its first endogenous ligand, anandamide (1, 2). Subsequently, the endocannabinoids and their receptors, which together are termed the ‘ECBR’ system (endocannabinoid-CB-receptor system), have been observed in almost every brain structure and organ system and have been shown to participate in a large number of physiological functions (1, 3).
The ECBR system is actively present from the earliest stages of ontogenetic development, during fertilisation and in the pre-implantation embryo. In this review, the essential role of the ECBR system in three crucial stages of development is described: (i) embryonic (pre)implantation; (ii) prenatal neural development and (iii) newborn suckling (Table 1) (4, 5).
| Crucial role for the ECBR system in development | References |
|---|---|
| Prenatal development: | |
| Implantation | (6–13, 15) |
| Brain development | (17, 21–30) |
| Neonatal development: suckling | (3, 4, 16, 33) |
| Manipulating the endocannabinoid system:Prenatal marihuana/Δ9-THC has permanent effects on neurochemistry, brain function (fMRI) and behaviour in the offspring | (40–50) |
- ECBR, endocannabinoid-CB-receptor system; , THC, Δ9-tetrahydrocannabinol; fMRI, magnetic resonance imaging.
Prenatal development
The pre-implantation embryo and implantation: critical role for the ECBR system
The ECBR system has been detected in virtually all components of the reproductive system and at virtually all stages of fertilisation and development (6, 7). Thus, cannabinoid receptors and anandamide have been detected in sperm cells from sea urchins as well as humans and other species. Anandamide may be released from sea urchin egg cells, where it is postulated to modulate the rate of fertilisation by the sperm. This modulation is accomplished by inhibition of the acrosome reaction (7). Anandamide is also synthesised in rodent testis, uterus and oviduct and has been found in human uterus and seminal fluid, whereas the anandamide degrading enzyme fatty acid amide hydrolase (FAAH) (1) has been detected in the uterus (7). Furthermore, CB1 and CB2 receptors have been described in the pre-implantation mouse embryo (8), with the CB1 receptor being present at higher concentrations than those detected in the brain (8). CB1 receptors are also present in the uterus (6). These observations led to the discovery that cannabinoids and endocannabinoids arrest the development of two-cell embryos into blastocytes. Subsequent studies with selective receptor antagonists indicated that the cannabinoid-induced embryonic growth arrest is mediated by CB1 and not by CB2 receptors (9). Furthermore, endocannabinoids are found in reproductive tissue. Thus, anandamide levels in the uterus are very high and anandamide binding to CB1 receptors on blastocytes would conversely lead to cell death (6). Therefore, for implantation to take place, uterine anandamide levels have to be lowered on the day and at the site of implantation. Failing to do so prevents implantation of the embryo (6, 9). Thus, reducing anandamide levels at the implantation site is a critical condition for implantation to occur and hence determine survival of the embryo. On the other hand, anandamide binding to CB1 receptors on the inter-implantation sites of the uterine epithelium inhibits gap junctions and facilitates the necessary uterine changes required for normal gestation (6).
The local anandamide concentration appears to be fine-tuned by varying levels of tissue FAAH. It has been previously demonstrated that FAAH mRNA is present at pre-implantation sites, within implanting embryos, and at the uterine implantation site (9, 10), where it inversely correlates with anandamide levels. Maccarone et al. (11) reported FAAH down-regulation in the uterus of pregnant and pseudopregnant mice during the implantation period and, in clinical studies, this group showed that FAAH concentrations in lymphocytes of women who miscarried were lower than lymphocyte FAAH concentrations from women who gave birth (12).
More recently, a further site where anandamide is involved in the control of successful gestation has been demonstrated. Increasing levels of the anandamide synthesising enzyme N-acylphopshatidylethanolamine-selective phospholipase D, together with decreasing FAAH levels throughout the oviduct, was shown to be essential for successful transport of the pre-implantation embryo and a successful subsequent outcome in terms of an unimpaired pregnancy (13). Taken together, this evidence suggests that the control of anandamide levels by respective enzymes and possibly a specific anandamide membrane transporter, is responsible for creating optimal local anandamide concentrations at specific sites directly involved in embryonic implantation and development (6).
In view of the multiple and critical roles of FAAH in pregnancy outcome, FAAH-like compounds could be developed as, suggested previously, as a novel approach to treat infertility (6, 14). Recently, the endocannabinoid 2-arachidonoyl glycerol (2-AG) was identified in uterus and blastocytes and appears to have a similar role to anandamide in embryonic implantation (15). Interestingly, uterine 2-AG levels, similar to those measured in maternal milk (16) and the developing brain (17), occur at approximately 1000-fold higher levels than anandamide (15).
Therefore, perhaps a more general therapeutic approach (i.e. manipulating multiple components of the ECBR system simultaneously) should be explored.
The adipocyte-secreted hormone leptin has a role in regulating energy homeostasis, which involves an inhibitory influence on the endocannabinoid system (18). Leptin also acts to promote fertility by stimulating embryonic development (19). Leptin defective ob/ob mice are infertile. Investigating the cause of this infertility, we previously reported that levels of both anandamide and 2-AG were elevated in the uteri of ob/ob mice with respect to wild-type littermates due to reduced FAAH activity in the case of anandamide and reduced monoacylglycerol lipase and enhanced diacylglycerol lipase activity in the case of 2-AG. The process mediating endocannabinoid cellular uptake was also impaired in ob/ob mice, whereas the levels of cannabinoid and anandamide receptors were not modified. Leptin reversed these effects in the ob/ob mice (14). Despite the oppositional interaction between the endocannabinoid and leptin systems at the biochemical level, in vivo infertility in ob/ob mice was not restored by chronic administration of the CB1 receptor antagonist SR141716 (SR141716), and the endocannabinoid reuptake inhibitor OMDM-1 did not interfere with fertility in wild-type females (14). Thus, the in vivo interaction between the leptin and ECBR systems that regulates fertility should be studied more closely.
Taken together, the complex yet essential role of the ECBR system in fertilisation and implantation may explain the reported association between early miscarriage and marijuana smoking (6) and the reduced success rate of in vitro fertilisation in male and female marijuana consumers (20).
Neural development: fundamental role for the ECBR system
Cannabinoid CB1 and CB2 receptor mRNA is first identified in embryonic rat brain at approximately day 11 of gestation (21). Postnatally, a gradual increase in CB1 receptor mRNA (22) and in the density of CB1 receptors has been measured (23) in the whole brain. Similar developmental patterns of CB1 receptors were found during human pre- and postnatal development with CB1 receptors first detected at week 14 of gestation in the human embryo (24). A progressive increase in the concentration of CB1 receptors was found in the frontal cortex, hippocampus, basal ganglia and cerebellum between the fetal period and adulthood (25). Interestingly, during the 20th week of gestation, a selective expression of CB1 receptors was recorded in the limbic area of the hippocampus and the basal nuclear group of the amygdala, as compared to a more homogeneous expression of CB1 receptor mRNA in the adult human brain (26), suggesting a role for CB1 receptors in the development of emotional processing.
Endocannabinoids are also present from early development. During the fetal period, anandamide is present at much lower (almost 1000-fold) concentrations than 2-AG (27). Moreover, the developmental pattern differs between the two endocannabinoids. Thus, whereas concentrations of anandamide gradually increase throughout development until adult levels are reached (28) (Fig. 1a), fetal levels of 2-AG are similar to those observed in young and in adult brains although, in rats, a considerable peak value is observed on the first day after birth (28) (Fig. 1b).
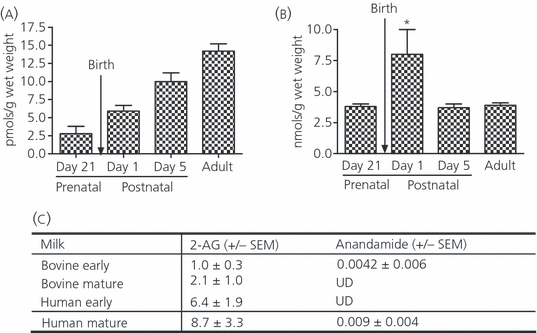
A remarkable aspect of CB1 receptor localisation in the developing brain is its transient appearance on neural fibre tracts (white matter areas). Thus, in human fetal brain, the highest cannabinoid receptor binding densities (measured by [3H]CP55940 autoradiography) appear in the pyramidal tract, brachium conjunctivum and subventricular germinative (proliferative) zones (25), as well as in the corpus callosum, stria terminalis, anterior commissure, midbrain and cerebral cortex (17, 27, 29). In these same areas, WIN55,212-2 (a cannabinoid receptor agonist)-stimulated [35S]GTPγS binding (a measure of receptor activation) was also at its highest, indicating that these CB1 receptors were functional (25).
The abundance of the endocannabinoids and their receptors in the developing nervous system and in these ‘atypical’ regions suggests that the ECBR system is a likely candidate involved in the regulation of the structural and functional maturation of the nervous system. Indeed, a fundamental role for the ECBR system in various aspects of neural development, including neurogenesis, glia formation, neuronal migration and axonal elongation, has now been demonstrated (5, 17). Very recently, CB1 receptors were observed to be enriched in the axonal growth cones of GABAergic interneuroness in rodent cortex toward the end of gestation, suggesting that endocannabinoids in the developing brain function as axon guidance cues and are involved in synaptogenesis (30).
Postnatal development
Critical role for cannabinoid CB1 receptors in suckling and milk intake
Weanling offspring of undernourished dams displayed lower body weights and anandamide levels compared to controls, whereas 2-AG concentrations were unchanged (31). Given the dependence of pups on maternal fatty acid precursor supply for the production of long chain polyunsaturated fatty acids, together with a previous observation that dietary supplementation with essential fatty acids increased concentrations of anandamide but not of 2-AG in piglets (32), it was hypothesised that a decrease in hypothalamic anandamide concentrations in offspring induced by maternal undernutrition may result from a disruption in the supply of essential fatty acids from the maternal blood and/or from the milk (31).
We previously reported the presence of endocannabinoids in bovine as well as human milk; 2AG was present in at least 100–1000-fold higher concentrations than anandamide (16) (Fig. 1c). This observation, together with the high levels of CB1 receptor mRNA and 2-AG that have been observed on the first day of life in structures including the hypothalamic ventromedial nucleus (28), which is associated with feeding behaviour, suggested that pup brain-derived 2-AG comprises a major stimulus for the newborn to initiate milk ingestion immediately after birth. Indeed, in a series of studies performed in neonatal mice, we have demonstrated that CB1 receptor activation is critically important for the initiation of the suckling response. Thus, when the CB1 receptor antagonist SR141716 is injected in newborn mice, milk ingestion and subsequent growth is dramatically compromised in most pups (75–100%) and death follows within days after antagonist administration (16). The antagonist must be administered within 24 h of birth in order to obtain the full effect: injections on day 2 result in a 50% death rate; SR141716 administration on day 5 has no effect at all on pup growth and survival (33). In order to determine whether the proximity to birth, rather than the developmental stage of the pup is critical for the impaired suckling induced by SR141716, we injected the antagonist into newborn precocial mice (Egyptian Spiny mice, Acomys cahirinus), which are born with open eyes and the ability to walk, run and ingest solid food. Our data show that SR141716 also significantly delayed development in these pups (H. Fride, M. Matan, S. Steinberg, unpublished data).
In order to support and expand this research, we investigated the effects of VCHSR, a CB1 receptor antagonist, which, unlike the inverse agonist activity of SR141716, presumably only causes a neutral receptor blockade (34). The data obtained demonstrated that VCHSR has growth arresting effects, similar to those of SR141716 (35). Thus, the impaired suckling induced by neonatally administered SR141716 is not limited to this specific compound, but is apparently due to the cessation of an endocannabinoid ‘tone’ or the inhibition of constitutive CB1 receptor activity in the newborn (35).
Additional studies indicated that the dramatic effect of CB1 receptor blockade is dose-dependent and further supported a specific CB1 receptor-mediated effect. Thus, co-application of Δ9-tetrahydrocannabinol (THC) with SR141716 almost completely reversed the SR141716-induced growth failure (16). Furthermore, CB1 receptor-deficient mice displayed deficient milk suckling during the first days of life whereas, by day 3 of life, they had developed normal suckling behaviour. Their weight gain, however, remained significantly lower than the C57BL/6 control mice. Furthermore, as expected, the growth curve of CB1 receptor knockout mice was not affected by neonatal injections of the CB1 receptor antagonist. Conversely, survival rate and the initiation of the suckling response were significantly inhibited by the CB1 receptor blocker, suggesting the existence of an additional ‘CB3’ receptor, possibly up-regulated in CB1−/− knockout mice (33). The phenomenon appears to have a genetic component because its severity varied between the three strains of mice studied (Sabra, C57BL/6 and ICR, unpublished data).
In a further set of experiments, 2– to 11-day-old pups that had been injected with SR141716 or vehicle on day 1 of life were exposed to anaesthetised nursing dams. Although vehicle-injected pups all located the nipples and nursed from the dam on every testing day, the SR141716-injected pups approached the nipple but could not suckle, thus lacking the oral-motor strength to ingest milk through the nipple. However, we observed that, when exposed to a dish with a milk/cream mixture, which can be ingested by licking without the need for sucking, the SR141716-treated pups were able to ingest the same amount of milk as controls (E. Fride, H. Dahan, unpublished data). This series of experiments suggests that the SR141716-treated pups have severe oral-motor impairment. Interestingly, as described above, anandamide plays a fundamental role in axon guidance and synaptogenesis (30), whereas 2-AG was shown to be required for axonal growth (36). Moreover, blockade of CB1 receptors inhibited the axonal targeting of CB1 receptors by causing a sequestration of CB1 receptors on somatodendritic membranes in cultured hippocampal neuroness (37). Therefore, it is possible that neonatal CB1 receptor blockade interferes with CB1 trafficking to the synaptic region. If this were to occur on a suckling-relevant nerve, impaired milk suckling could be compromised. A highly relevant finding is that CB1 receptor activation participates in the modulation of glycinergic synaptic currents in hypoglossal motoneuroness of postnatal rats (38), whereas resection of the hypoglossal nerve in rat pups compromised milk suckling, resulting in 100% mortality (39). Thus, we speculate that when pups are treated with SR141716 at birth, incomplete synaptogenesis of the hypoglossal nerve may fail to adequately activate tongue movement (39), which is critical for sucking (38).
Based on the critical importance of the timing of CB1 receptor blockade and the abundance of 2-AG in maternal milk and in the postnatal brain as described above, we designed a working model (Fig. 2), which suggests that pup-derived 2-AG release at birth enables the first milk sucking session (via CB1 receptor activation). In the normal situation, pup derived 2-AG will be supplemented with milk-derived 2-AG, thus enabling CB1 receptor activation during the next nursing session. We hypothesise that blockade of CB1 receptors immediately after birth prevents the activation of the pups sucking apparatus by brain derived 2-AG, and therefore milk is not ingested and brain-derived 2-AG (now the sole source of 2-AG) levels remain too low to activate enough CB1 receptors required for sucking during the next nursing session. As a result, the neonate does not ingest sufficient milk for growth and survival.
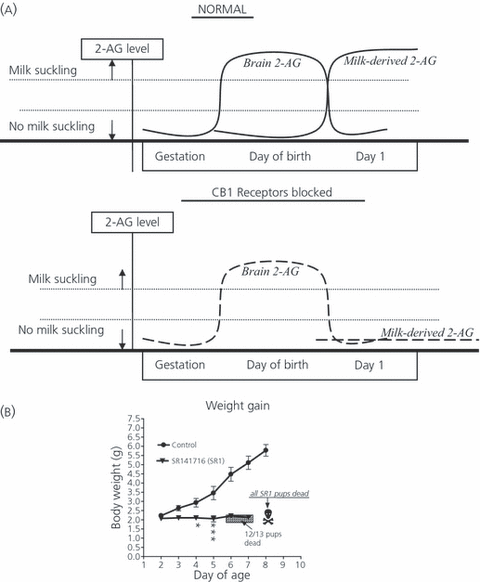
Working model for the origin and effects of 2-AG on milk suckling. In the normal situation (upper panel), high levels present in pup brain after birth induce suckling (by activating CB1 receptors on the hypoglossal nerve, see text), supplying additional 2-AG from the ingested milk. The milk- (and possibly brain)- derived 2-AG enable the up to suck milk from the nipple the next day. When CB1 receptors are blocked on the day of birth (lower panel), the pup-derived 2-AG cannot activate CB1 receptors so that the suckling apparatus is not functional. Hence, no milk is ingested. The next day, 2-AG is not present, or perhaps only present at low levels (produced by the pup brain only), and is not sufficient to activate suckling; thus, again, no 2-AG is provided via the milk and no further suckling will be possible. Thus milk sucking is never initiated.
Effects of developmental manipulation of the ECBR system on the offspring
In this short review, three developmental stages have been described in which the ECBR system plays a crucial role. It is therefore rather surprising that maternal exposure to external cannabinoids during pregnancy or the nursing period (due to marijuana smoking or administration of cannabinoids in animal experiments) exerts relatively subtle effects on the fetus and postnatal or adult offspring (4, 40–42). For example, at midgestation, human fetuses of marijuana smoking mothers had lower body weights than controls (43) whereas, in another study on midgestational fetuses of marijuana smoking mothers, a selective decrease in D2 dopamine receptors was observed in the amygdala, but no changes in CB1 mRNA and in dopamine D1 receptors were found in this structure. Neither were there any changes in the other structures studied: the striatum and hippocampus (44). Data from animal studies have shown that prenatal and postnatal exposure to Δ9-THC interfered with normal dopamine-dependent motor functions and the hypothalamic-pituitary-adrenal stress axis in the adult offspring (42). Furthermore, prenatal Δ9-THC administration to rats, facilitated morphine and heroin self administration in the female offspring (45, 46), whereas a number of brain areas, including the prefrontal cortex, amygdala and hippocampus, displayed altered concentrations of mu-opioid receptors (46); another study reported changes in preproenkephalin mRNA expression in the nucleus accumbens and amygdala of the offspring (45). Memory retention in the adult offspring in a passive avoidance task was disrupted by prenatal exposure to the synthetic cannabinoid WIN55,212. The memory impairment was correlated with a shortening of long-term potentiation and a reduction in extracellular glutamate in the hippocampus (47).
In an elegantly designed prospective study of the children of marijuana smoking mothers, Fried et al. (48) specifically demonstrated a subtle but significant impairment of higher cognitive (‘executive’) functioning, which is ascribed to changes in the prefrontal cortex. Importantly, in a study using functional magnetic resonance imaging, the same offspring, as young adults, displayed a bilateral increase in neural activity in the prefrontal cortex and elevated activity in the right premotor cortex (49).
We have performed experiments on the adult offspring of mice injected daily with Δ9-THC during the last week of gestation, with the aim of detecting changes in the ECBR system per se (41). Thus, we found both behavioural and biochemical evidence that the CB1 receptors were up-regulated in the brains of prenatally exposed offspring (41). These data are consistent with an overactive endogenous cannabinoid CB1 receptor system resulting, perhaps, in a greater vulnerability to the addictive potential of cannabis or other drugs (46). In support of this theory, offspring of marijuana smoking mothers were found to be more frequent marijuana users at the age of 14 years (50).
Concluding remarks
Endocannabinoids and their receptors are highly abundant components of the developing organism, as has been demonstrated in a number of species. This ECBR system not only controls its own proper functioning in the adult organism, but also critically impacts on the general development of the organism. We have highlighted an essential role for the ECBR system in embryonic implantation (and the preceding pre-implantation events) and in the development of the nervous system, regulating processes such as neurogenesis, axon guidance and synaptogenesis. Immediately after birth, cannabinoid CB1 receptor activation appears to play an essential role in the initiation of suckling, which is necessary for successful postnatal growth, development and thriving.
The dramatic role for the ECBR system in the control of various aspects of development has only come to light in recent years. Exposure to cannabinoids (e.g. by maternal marijuana consumption) has significant yet rather subtle deleterious effects on the offspring. The reason for this is not clear. However, it is possible that the dosage and timing of the exposure is important in determining the eventual outcome. Further studies need to be performed to further elucidate the precise mechanisms by which the ECBR system controls development and to utilise this knowledge for therapeutic benefit in conditions such as infertility, mental retardation and failure-to-thrive.
Conflicts of interest
The author has declared no conflicts of interests.




