Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial
See Appendix
Abstract.
Objective. To assess the prophylactic efficacy of aspirin and a high-dose antioxidant vitamin combination in patients with peripheral arterial disease (PAD) in terms of reduction of the risk of a first vascular event (myocardial infarction, stroke, vascular death) and critical limb ischaemia.
Design. Randomized, placebo-controlled, double-blind clinical trial with 2 × 2 factorial design.
Setting. Thirty-seven European angiology/vascular medicine units.
Subjects. A total of 366 outpatients with stage I-II PAD documented by angiography or ultrasound, with ankle/brachial index <0.85 or toe index <0.6; 210 patients completed the follow-up.
Interventions. Four treatment groups: (i) oral aspirin (100 mg daily), (ii) oral antioxidant vitamins (600 mg vitamin E, 250 mg vitamin C and 20 mg β-carotene daily), (iii) both or (iv) neither, given for 2 years.
Main outcome measure. Major vascular events (cardiovascular death, myocardial infarction or stroke) and critical leg ischaemia.
Results. Seven of 185 patients allocated aspirin and 20 of 181 allocated placebo suffered a major vascular event (risk reduction 64%, P = 0.022); five and eight patients, respectively, suffered critical leg ischaemia (total 12 vs. 28, P = 0.014). There was no evidence that antioxidant vitamins were beneficial (16/185 vs. 11/181 vascular events). Neither treatment was associated with any significant increase in adverse events. Inclusion of this trial in a meta-analysis of other randomized trials of anti-platelet therapy in PAD makes the overall results highly significant (P < 0.001) and suggests that low-dose aspirin reduces the incidence of vascular events by 26%.
Conclusions. For the first time direct evidence shows that low-dose aspirin should routinely be considered for PAD patients, including those with concomitant type 2 diabetes.
Introduction
Peripheral arterial disease (PAD) can cause critical limb ischaemia, which may result in amputation, and is a strong risk factor for major vascular events, such as myocardial infarction, ischaemic stroke and death [1–3]. In 1994, a meta-analysis of 145 randomized trials, 42 of which involved PAD, showed that allocation to antiplatelet therapy reduces serious vascular events (nonfatal myocardial infarction, nonfatal stroke and vascular mortality) by about one quarter in a wide range of patients at high risk of occlusive vascular disease [4] . Most of the trials had been conducted with medium-dose aspirin (75–325 mg daily) and in 1997 the US FDA approved this for a range of conditions [5]. However, as most of the antiplatelet trials in PAD involved agents other than aspirin, the FDA did not approve aspirin for patients with PAD [5]. The meta-analysis was updated in 2002, but still no randomized clinical trials evaluating the efficacy of aspirin exclusively in PAD patients were found [6]. Consequently, the currently widespread practice of prescribing aspirin as an antiplatelet agent for the prevention of vascular events in PAD is not supported by adequate clinical evidence.
Experimental data show that high levels of antioxidant vitamins are associated with protection against LDL oxidation, which could augment atherogenesis [7, 8]. Epidemiological studies have indicated an inverse correlation between vitamin C and/or vitamin E levels and the prevalence of peripheral vascular disease and coronary heart disease [9–11]. At the time the study was designed one randomized, double-blind, placebo-controlled clinical trial (CHAOS) had suggested that vitamin E was effective in reducing nonfatal myocardial infarction in patients with angiographically proven coronary atherosclerosis and the ASAP trial had suggested that vitamin E + vitamin C supplements slow down common carotid atherosclerosis in men [12, 13]. Additional clinical evidence was not available.
We report the result of a randomized, double-blind clinical trial in PAD comparing daily low-dose aspirin (100 mg), high-dose antioxidant vitamin combination (20 mg β-carotene, 250 mg vitamin C and 600 mg vitamin E), both aspirin and antioxidant vitamins, and in the last group only placebo according to a 2 × 2 factorial design.
Methods
Study design
The study was a randomized, double-blind, controlled clinical trial. To obtain data on the efficacy of both aspirin and antioxidant vitamins we used a 2 × 2 factorial design, comparing aspirin versus nonaspirin and vitamins versus nonvitamins. Patients were allocated to the following four treatments: active aspirin and placebo antioxidant vitamins, active high-dose antioxidant vitamins and placebo aspirin, both or neither. Patients entered a run-in period, during which their eligibility and willingness to collaborate were assessed, stable control of any diabetes was achieved and suggestions on life habits was given. Patients were then to be randomized and followed up regularly every 3 months for up to 2 years (actual follow-up mean ± SD 20.7 ± 6.4 months).
Patient population
The study involved outpatients with symptomatic (claudicant) or asymptomatic PAD documented by angiography or ultrasound, who had one ankle/brachial index (ABI) <0.85 or one toe index <0.6. Patients were referred either by the GP or by the hospital ER doctor for a diagnostic work-up. Diabetics could be included, provided metabolic control was stable (HbA1c) according to their diabetologist. Only patients who accepted randomization (i.e. continuation after the run-in period) were included in the study. The main exclusion criteria were: Fontaine stage III or IV peripheral vascular disease; life expectancy <24 months; vascular surgery or angioplasty in the last 3 months; pregnancy or lactation; contraindication to aspirin; major cardiovascular events requiring antiplatelet therapy; participation in another clinical trial; uncooperative patients; treatment with drugs that interfere with hemostasis, such as anticoagulants, antiplatelet agents and prostanoids, peripheral vasodilators, aspirin and/or supplementary vitamins that could not be discontinued or had to be introduced.
Treatment
Patients were given placebo during a 2-month run-in period. Subsequently those who had been compliant and were willing to continue were allocated to one of four treatment groups: (i) aspirin (100 mg daily), (ii) antioxidant vitamins (600 mg vitamin E, 250 mg vitamin C and 20 mg β-carotene daily), (iii) both or (iv) neither. Treatment consisted of one tablet (aspirin or matching placebo) and two capsules (vitamins or matching placebo) to be taken after the evening meal.
Compliance was assessed by recording the number of returned tablets and capsules at each visit, asking the patients the reasons for any discrepancies and asking whether they had taken any additional aspirin or vitamins.
End-points
The primary end-point was the combined incidence of fatal and nonfatal vascular events (myocardial infarction, stroke and pulmonary embolism) and critical leg ischaemia. The safety end-point was the incidence of bleeding.
Critical leg ischaemia was defined as ischaemic rest pain for more than 2 weeks, ulcers or gangrene with systolic ankle pressure ≤60 mmHg and/or toe pressure ≤30 mmHg, or, in patients with pain alone, systolic ankle pressure ≤40 mmHg and/or toe pressure ≤30 mmHg)13; vascular death as any death that could not be definitely ascribed to a nonvascular cause, such as cancer; nonfatal myocardial infarction as a suspected event with two or more of the following criteria: characteristic ischaemic chest pain lasting ≥ 20 min, creatine kinase (CK), creatine kinase MB (CK-MB), lactic dehydrogenase (LDH) or aspartate aminotransferase (AST) greater than or equal to twice the normal upper limit (in the absence of another explanation), new 40 ms Q-waves in at least two adjacent leads or new dominant R-wave in V1; haemorrhagic stroke as primary intracranial haemorrhage (intracerebral, subarachnoid or subdural) documented by computerized tomography (CT) or magnetic resonance imaging (MRI) findings; ischaemic strokeas any other acute neurological vascular event with focal signs lasting >24 h if in a new location or accompanied by new CT or MRI findings (or more than 7 days if it was the worsening of a previous deficit); vascular event as a myocardial infarction, stroke or vascular death.
Adverse events
Information on adverse events (AEs) was collected by the investigator by direct questioning, performing a physical examination and reviewing the results of laboratory tests during visits.
An AE was any adverse change from the patient's pretreatment condition, including intercurrent illness, which occurred during the course of the study after treatment had started, whether or not considered related to treatment.
Adverse events were classified as serious if they resulted in death, were life-threatening or produced a permanent or substantial disability, or resulted in hospitalization or prolongation of hospitalization or were cancer, a congenital abnormality or the result of an overdose. All serious AEs and study end-points were reviewed once a year by a Safety Committee.
Clinical AEs were classified as mild if they caused discomfort, but no disruption of normal daily activity; moderate if they caused discomfort sufficient to reduce or affect normal daily activity; severe if they were incapacitating with inability to work or perform daily activity. Abnormalities in laboratory tests were classified as mild if they required only a repetition of the test; moderate if they required follow-up, including an extra visit before the next scheduled one and/or referral; severe if they required immediate action, possibly hospitalization.
Sample size
Meta-analyses of previous anti-platelet trials in this and other conditions indicated that an annual event rate of 10% might be reduced by about a quarter. To have an 80% chance of detecting this, at P < 0.05, the plan was to follow 2000 randomized patients for a mean of 2 years, unless the data monitoring committee intervened. Eventually, however, nontrial aspirin use for concomitant diseases grew, the randomization rate diminished, and recruitment was therefore stopped.
Statistical methods
The main comparisons involve ‘intention-to-treat’ analyses of time to first vascular event (myocardial infarction, stroke, vascular death) or critical limb ischaemia.
Continuous variables such as age and ABI were described using appropriate statistics (mean, standard deviation), whereas for categorical variables relative and absolute frequencies were used.
Time to event was defined as the time from randomization to first vascular or non vascular event or death from any cause; patients known to be alive and without disease at the time of analysis were censored at their last follow-up date. Cox's proportional hazards model was used to compare time to event in the treatment groups: active aspirin versus nonaspirin stratified by vitamin allocation, and active vitamins versus nonvitamins stratified by aspirin allocation.
Results are expressed as hazard ratio (HR) and its 95% confidence interval (95% CI). P < 0.05 was considered as statistically significant.
All the patients who took at least one dose of study medication were included in the safety analysis and all patients who had taken at least one dose of study medication and who had a baseline assessment were included in the intention to treat analysis. The final statistical analysis was carried out in Oxford, using SAS, SPSS and JMP SAS programs.
Study organization
The study was an independent trial, designed, initiated and co-ordinated solely by the Research Center on Vascular Disease of the University of Milan. Thirty-seven European angiology centres (which subsequently established a broader network of European researchers and clinicians operating in the field of angiology, VAS – Vascular- Independent Research and education – European organization) took part in it.
The randomization list was prepared by an independent organization (MCM office), which also prepared a series of sealed envelopes, each with the code break of one patient. Investigators were given a copy of the sealed envelopes related to the randomization numbers allocated to their centre, to be opened in an emergency and to be returned at the end of the trial.
The 100 mg aspirin tablets used and matching placebo were purchased from Sofar Italia (Sofar S.p.A., Milan, Italy). The raw material for the vitamin preparation was given by BASF free of charge and prepared by an external service (Pharmavite Natura Made, Pharmavite, Valencia, CA, USA), also free of charge. The bottles were packaged and numbered according to a randomization list balanced in blocks of four by MCM. The study was monitored exclusively by the co-ordinating centre.
All end-points were validated by a Central Validation Committee and all safety data were reviewed once a year by an external Safety Committee. The study complied with the Declaration of Helsinki, the European Good Clinical Practice Guidelines and any local legislation, and was conducted under the auspices of the International Union of Angiology, International Diabetes Foundation (Europe), St Vincent Declaration's Initiative, WHO EUROPE (Quality of Care and Technologies Unit) and WHO EUROPE (Outcomes Indicators Quality Project).
It was approved by the competent Ethics Committee of all participating centres before initiation of the trial. All patients gave their informed consent in writing before they underwent any study-related procedures. The CLIPS Ethics and Guarantor Committee provided a preliminary ethical evaluation of the study and monitored it to ensure the maintenance of absence of any conflict of interest and of the independence stated.
All kinds of support have been listed in the Acknowledgments section. This paper communicates the results of the study to all sponsors.
Results
A total of 366 PAD patients were randomized; of these, 76% were suffering from type 2 diabetes and were homogeneously distributed amongst the four treatment groups (Table 1a). Of these, 113 (31%) discontinued participation for reasons other than a vascular event, usually not long after randomization (61 completed poorly); 11 (3%) patients died (carcinoma n = 2, major vascular events n = 9) and 32 (9%) experienced a nonfatal major vascular event that resulted in withdrawal. The remaining 210 patients completed the follow-up. There were no important differences amongst the four treatment groups in baseline features and in compliance (Table 1a). Overall, 7 of 185 (4%) patients given aspirin and 20 of 181 (11%) patients given nonaspirin suffered major vascular events (2 vs. 11 myocardial infarction, 4 vs. 7 stroke, 1 vs. 2 pulmonary embolus), indicating a risk reduction of 64% (P = 0.016, HR = 0.35, 95% CI 0.15–0.82 ). The difference became slightly more pronounced when the five versus eight cases of critical limb ischaemia were added (total 12 vs. 28, P = 0.013, HR 0.42, 95% CI 0.21–0.83; Table 2a). There were no significant differences between the group given aspirin alone and the group given aspirin + vitamins (Table 1b).
| Vitamins alone | Placebo | Aspirin alone | Vitamins + aspirin | |
|---|---|---|---|---|
| (a) | ||||
| Patients | 91 | 90 | 91 | 94 |
| Male, n (%) | 70 (76.9) | 74 (82.2) | 67 (73.6) | 71 (75.5) |
| Age (years ± SD) | 66.6 ± 8.3 | 65.6 ± 8.9 | 64.2 ± 9.4 | 67.6 ± 7.6 |
| Claudication, n (%) | 65 (72.2) | 71 (78.9) | 71 (78.0) | 76 (80.8) |
| Asymptomatic, n (%) | 25 (27.8) | 19 (21.1) | 20 (22.0) | 18 (19.2) |
| Diabetic, n (%) | 68 (74.7) | 66 (73.3) | 71 (78.0) | 72 (76.6) |
| Hypertensive, n (%) | 63 (69.2) | 55 (61.1) | 51 (56.0) | 56 (59.6) |
| ABI (mean ± SD) | 0.65 ± 0.22 | 0.63 ± 0.23 | 0.65 ± 0.22 | 0.61 ± 0.20 |
| Current/ex/never-smokers, n (% of total smokers) | 24/44/23 (73.6) | 18/58/14 (80.0) | 26/52/12 (71.1) | 27/45/22 (71.3) |
| Discontinued treatment, n (%)a | 24 (26.4) | 24 (26.7) | 34 (37.4) | 31 (33.0) |
| (b) | ||||
| Patients | 91 | 90 | 91 | 94 |
| Stroke (nonfatal plus fatal) | 1 + 1 | 5 + 0 | 0 | 2 + 2 |
| Myocardial infarction (nonfatal plus fatal) | 7 + 1 | 2 + 1 | 0 + 1 | 0 + 1 |
| Pulmonary embolus (nonfatal plus fatal) | 0 | 1 + 1 | 0 | 1 + 0 |
| Vascular death | 2 | 2 | 1 | 4 |
| Nonvascular deathb | 0 | 0 | 1 | 1 |
| Critical limb ischaemia | 4 | 4 | 2 | 3 |
| Vascular event | 10 | 10 | 1 | 6 |
| Vascular event or critical limb ischaemia | 14 | 14 | 3 | 9 |
| Bleeding | 0 | 0 | 3 | 1 |
- aDiscontinued trial treatment prematurely (but without having suffered a vascular event or critical limb ischaemia) and not followed further. bBoth patients died of cancer.
| Aspirin (n = 185) | Nonaspirin (n = 181) | P-valuea | HR (95% CI) | |
|---|---|---|---|---|
| Stroke nonfatal plus fatal | 2+2 | 6+1 | 0.33 | 0.54 (0.16–1.85) |
| Myocardial infarction nonfatal plus fatal | 0+2 | 9+2 | 0.03 | 0.18 (0.04–0.83) |
| Pulmonary embolus nonfatal plus fatal | 1+0 | 1+1 | 0.57 | 0.50 (0.05–5.54) |
| Vascular death | 5 | 4 | 0.78 | 1.21 (0.32–4.52) |
| Nonvascular deathb | 2 | 0 | 0.99 | – |
| Vascular event | 7 | 20 | 0.02 | 0.35 (0.15–0.82) |
| Vascular event or critical limb ischaemia | 12 | 28 | 0.01 | 0.42 (0.21–0.83) |
| Bleeding | 4 | 0 | 0.99 | – |
- –, Statistics could not be calculated because of the lack of events in the second group. aAspirin versus nonaspirin groups P = 0.074 (chi-quadro = 3.18). bBoth nonvascular deaths were from cancer.
There was no evidence that antioxidants were beneficial: 16/185 (9%) vs. 9/181 patients given vitamin compared with 11 of 181 (6%) patients given nonvitamin, suffered vascular events (P = 0.45, HR 1.45, 95% CI 0.62–2.91; Table 2b).
| Vitamins (n = 185) | Placebo (n = 181) | Significance | HR (95% CI) | |
|---|---|---|---|---|
| Stroke nonfatal plus fatal | 3 + 3 | 5 + 0 | 0.86 | 1.11 (0.34–3.63) |
| Myocardial infarction nonfatal plus fatal | 7 + 2 | 2 + 2 | 0.22 | 2.08 (0.64–6.67) |
| Pulmonary embolus nonfatal plus fatal | 1 + 0 | 1 + 1 | 0.54 | 0.47 (0.04–5.19) |
| Vascular death | 6 | 3 | 0.39 | 1.83 (0.47–7.32) |
| Nonvascular death | 1 | 1 | 0.90 | 0.84 (0.05–13.49) |
| Vascular event | 16 | 11 | 0.45 | 1.35 (0.62–2.90) |
| Vascular event or critical limb ischaemia | 23 | 17 | 0.45 | 1.27 (0.68–2.38) |
| Bleeding | 1 | 3 | 0.31 | 0.31 (0.03–3.00) |
Treatment with aspirin or with vitamins was not associated with any significant increase in AE leading to withdrawal (Tables 2a and 2b). In particular, treatment with aspirin was not associated with an important increase in episodes of bleeding or in gastrointestinal AE (Table 2a). The reported episodes of bleeding amongst the patients treated with aspirin were: melaena (n = 1), retinal haemorrhage (n = 2) and epistaxis (n = 1); three of the episodes were mild and one was moderate. The gastrointestinal events that led to withdrawal were: peptic ulcer (n = 2) and gastritis with aspirin, and nausea, acute diverticulitis and gastritis with placebo. The most common nonend-point cardiovascular event was angina/manifestation of coronary artery disease (n = 8); other nonend-point vascular events were transient ischaemic attacks (TIA) or documentation of cerebral ischaemia by MRI (n = 3), percutaneous transluminal angioplasty (PTA) limb (n = 3), thromboendarterectomy (n = 2), valvular cardiac surgery (n = 2), carotid stenosis (n = 2), heart failure, arterial hypertension, atrial flutter and percutaneous transluminal coronary angioplasty (PTCA).
Discussion
This study indicates that 100 mg aspirin daily reduces the risk of major vascular events in patients with PAD, whereas antioxidant vitamins do not.
To our knowledge, this is the first randomized, controlled clinical trial that provides direct evidence on the prophylactic use of aspirin in PAD patients. A significant reduction in the risk of major vascular events both with and without critical limb ischaemia was achieved, comparing aspirin versus nonaspirin treatment groups.
This finding is also important because many clinical trials on PAD have compared novel antiplatelet agents to aspirin; consequently, it provides validation of the reference drug used.
CLIPS provides, for the first time, directly randomized confirmation that low-dose aspirin can, like other effective antiplatelet regimens, prevent serious vascular events in such patients.
Finally, CLIPS confirms the efficacy of aspirin also in PAD patients with concomitant type 2 diabetes, representing a percentage higher than that found in other trials due to the inclusion of both asymptomatic and symptomatic patients.
CLIPS should also be considered together with the recently updated worldwide meta-analysis of 42 other antiplatelet trials in PAD (mean: 231 patients/trial), most of which involved drugs other than aspirin [6]. For the prevention of vascular events, revision of the meta-analysis to include CLIPS makes the estimated risk reduction only slightly greater (26% instead of 23%: Fig. 1), but makes the statistical significance substantially more pronounced (P < 0.001 instead of 0.004). After allowing for the effects of some noncompliance with allocation to active treatment (or to control in all trials), this 26% reduction suggests that actual use of low-dose aspirin would reduce the event rate by about one-fourth in a wide range of patients with PAD. Hence, low-dose aspirin (e.g. 75–150 mg daily) should routinely be considered for patients with a history of PAD, including type 2 diabetes, as their risk of a serious ischaemic vascular event is generally high even in the absence of any definite previous history of myocardial infarction or stroke.
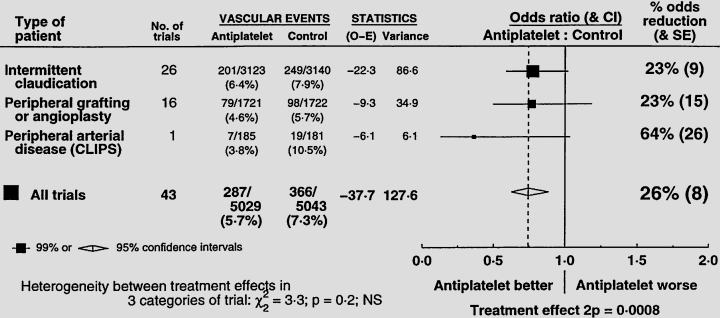
Updated meta-analysis of the randomized trials of antiplatelet therapy in peripheral arterial disease.
The lack of efficacy of the antioxidant vitamins is consistent with the disappointing results of much larger multicentre trials published in recent years. No benefit was achieved with vitamin E in terms of reduction in cardiovascular or total mortality in the GISSI Prevenzione trial [14], which included 11 324 patients surviving recent myocardial infarction, given supplements of polyunsaturated fatty acids (PUFA), vitamin E (300 mg daily), both or neither for 3.5 years, or in terms of reduction in the incidence of myocardial infarction, stroke or cardiovascular death in the extension of the HOPE trial (HOPE-TOO) [15], in which 3994 patients were given either vitamin E (400 IU) or placebo for a mean period of 7 years. Moreover, long-term vitamin supplementation (600 mg vitamin E, 250 mg vitamin C and 20 mg β-carotene daily) failed to produce any significant difference in terms of 5-year vascular mortality or incidence of any type of vascular event in 20 536 UK adults with coronary disease, other occlusive arterial disease or diabetes included in the randomized, placebo-controlled MRC/BHF Heart Protection Study [16].
Conflict of interest
No conflict of interest was declared.
Acknowledgements
To all participating patients
Practical or financial support: Accuson; Aethra Telecommunications (videoconference facilities); Alitalia (airway tickets); BASF (raw material for vitamins); Bayer, Euromedia Link (logistics); Brunelleschi Hotel, Milan (hospitality); Cariplo Foundation; DHL (deliveries); Hewlett Packard (computers); IBM (computers); Knoll; MCM (independent randomization); Perimed AB; Ramada Hotels (hospitality); Pharmavite Natura Made (vitamin encapsulation); RAS Insurance (low price insurance); Roche; Schering Cardiovascular (printing charges); Servier; Telecom (telephone lines); Squibb.
Suggestions from: H. Boccalon (F), K. Breddin (D), Paoletti (I), C.Patrono (I), M. Verstraete (B).
Appendix
CLIPS group
Austria: E. Pilger, E. Pabst. G. Kostner (A). Belgium: J. C. Wautrecht. Germany: G. Baitsch, K. Breddin, C. Diehm, H. Podhaisky, B. M. Taute. Greece: E. J. Diamantoupolos. Italy: T. Aimar, G. Alari, S. Albano, N. Altamura, E. Arosio, M. Bortolon, R. Caccia, M. Carotta, G. Carzaniga, M. Catalano, P. Contini, A. Difolca, M. Dore, F. Fabris, F. Falaschi, B. Franchini, C. Frare, M. Frascisco, C. Giansante, E. Giona, M. Giubbolini, F. Lucarelli, E. Marchitelli, M. R. Marcialis, A. Martignoni, E. Melillo, M. Minola, D. Monetti, G. Nenci, M. Paolicelli, L. Pasqualini, E. Perilli, G. Pettinà, L. Poli, S. Radicchia, M. Rossi, G. Scandale, G. Scondotto, D. Sergi, C. Setacci, S. Spada, A. R. Todini, G. Vaudo, F. Verlato, A. Visonà, M. Zannoni. Poland: M. Bielawiec, A. Jawien, A. Meissner, J. Michalak, R. Nizankowski, M. Wandzilak. Romania: J. A. Avram, R. Avram, N. Hancu, A. Marian, I. Marian, M. Murariu, G. Negrisanu, D. Olinic, N. Olinic, R. Trambitas, I. Veresiu, I. Victoria. Slovenia: M. Kozak, A. Mavri. Spain: J. S. Bahì, P. S. Bahì. Sweden: J. Brunkwall. Switzerland: A. Gallino, F. Mahler.
Ethics and Guarantors Committee
A. Bollinger (CH), G. Born (UK), S. Coccheri (I), A. Gustafson (S) and P. Mantegazza (I).
Trial Management
M. Catalano (Chair) (I), G. Carzaniga (Trial Monitor) (I), A. Baxter (computing) (UK), M. Recchia, M. Cinquini, E. Rulli (Statistician) (I), E. Grande, Y. Stewart, (Secretaries) (I), F. De Renzo (Trial Administrator) (I), P. Cassani and F. Sori (legal supervision) (I).
Writing group
M. Catalano (I), G. Born (UK) and R. Peto (UK).