HAART and the heart: changes in coronary risk factors and implications for coronary risk in men starting antiretroviral therapy
Abstract.
Objectives. To estimate changes in coronary risk factors and their implications for coronary heart disease (CHD) rates in men starting highly active antiretroviral therapy (HAART).
Methods. Men participating in the Swiss HIV Cohort Study with measurements of coronary risk factors both before and up to 3 years after starting HAART were identified. Fractional polynomial regression was used to graph associations between risk factors and time on HAART. Mean risk factor changes associated with starting HAART were estimated using multilevel models. A prognostic model was used to predict corresponding CHD rate ratios.
Results. Of 556 eligible men, 259 (47%) started a nonnucleoside reverse transcriptase inhibitor (NNRTI) and 297 a protease inhibitor (PI) based regimen. Levels of most risk factors increased sharply during the first 3 months on HAART, then more slowly. Increases were greater with PI- than NNRTI-based HAART for total cholesterol (1.18 vs. 0.98 mmol L−1), systolic blood pressure (3.6 vs. 0 mmHg) and BMI (1.04 vs. 0.55 kg m2) but not HDL cholesterol (0.24 vs. 0.32 mmol L−1) or glucose (1.02 vs. 1.03 mmol L−1). Predicted CHD rate ratios were 1.40 (95% CI 1.13–1.75) and 1.17 (0.95–1.47) for PI- and NNRTI-based HAART respectively.
Conclusions. Coronary heart disease rates will increase in a majority of patients starting HAART: however the increases corresponding to typical changes in risk factors are relatively modest and could be offset by lifestyle changes.
Introduction
The introduction of highly active antiretroviral therapy (HAART) has led to substantial reductions in HIV- and AIDS-related morbidity and mortality of HIV infected patients [1–3]. Cure of the infection cannot, however, be achieved with current regimens and toxicities and other adverse effects have become increasingly important.[4] In addition to hepatitis, pancreatitis, neuropathy and gastrointestinal symptoms there is concern about metabolic complications, including hypercholesterolemia, hypertriglyceridemia, insulin resistance, impaired glucose tolerance and type 2 diabetes [5, 6].
These metabolic effects of HAART can be expected to increase the risk of coronary heart disease (CHD), however their clinical significance is a matter of ongoing debate [7, 8]. In the present study we estimated the effect of HAART on lipid levels, blood pressure and BMI by using measurements made before and after initiation of HAART in HIV-1 infected men participating in the Swiss HIV Cohort Study (SHCS) [1]. The effect of these changes in risk factors on rates of CHD was then estimated using a prognostic model for the risk of CHD. This model was specifically designed to include risk factors affected by starting HAART, and was developed using data from five large long-term cardiovascular cohort studies conducted in HIV-uninfected men.
Methods
The Swiss HIV Cohort Study
The Swiss HIV Cohort Study (SHCS) is a prospective study of HIV infected adults seen at the outpatient clinics of the Swiss University Hospitals in Basle, Berne, Geneva, Lausanne and Zurich, the Cantonal Hospitals in Lugano and St Gallen, and by affiliated private physicians [1, 9]. It has been approved by local ethics committees, and written informed consent is obtained at registration. Follow-up visits are scheduled every 6 month. Between September 1988 and November 2005, a total of 13 810 persons were enrolled in the SHCS. Measurements on cardiovascular risk factors have been collected systematically since 2000.
Estimation of the effect of HAART on coronary risk factors
We examined levels of cardiovascular risk factors in HIV-1 infected men participating in the SHCS before and after initiation of HAART, defined as treatment with a combination of three or more antiretroviral drugs, at least one of which was either a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI). Women were not included because too few female patients had measurements of cardiovascular risk factors. We identified male patients who had not previously been exposed to antiretroviral drugs and had at least one set of risk factor measurements (total cholesterol, HDL cholesterol, glucose, systolic blood pressure (SBP), BMI and triglyceride) both in the 6-month period before and in the 36-month period after starting HAART. Values of glucose and triglyceride were log transformed in all analyses: 30% of measurements were taken in the fasting state. Measurements from 6 month before to 3 years after starting HAART were included in analyses, provided they preceded any change of regimen or cessation of HAART. Men on any insulin or lipid-lowering, anti-diabetic or anti-hypertensive drugs at initiation of HAART were excluded from analyses. If a patient was prescribed any of these drugs after starting HAART, their subsequent measurements were excluded, because the inclusion of such measurements would bias estimates of metabolic changes associated with HAART.
Patterns of risk factor changes were initially analysed by using fractional polynomial regression [10] to graph the shape of the association between risk factor levels and time on HAART. Separate curves were fitted for patients on PI- and NNRTI-based HAART. For each risk factor, we fitted univariate models using fractional polynomials of degree 1 and 2 with all possible combinations of powers selected from the set {−2, −1, −0.5, 0 (log transformation), 0.5, 1, 2, 3 } and compared models by calculating differences in log-likelihoods. When polynomials that differed by 1 degree were compared, differences in log likelihood were referred to a chi-squared distribution with two degrees of freedom. The pre-HAART value of each risk factor was assumed to be the same as the latest measurement before initiation. Sensitivity analyses examined whether the shape of curves varied with age (<40 or ≥40). Predicted mean values at 0, 1, 2 and 3 years after starting HAART were calculated from the best-fitting models.
We used multivariate multilevel models, fitted using MlwiN [11], to estimate mean HAART-associated changes in risk factors allowing for both within-patient correlations in risk factor levels and for correlations between the levels of different risk factors over time. These analyses included all risk factor measurements in the 6 month period prior to HAART, together with post-HAART measurements from 3 month to 3 years after starting therapy (thus excluding the period when levels were changing most rapidly). These models assumed (based on observed patterns) that the mean change in risk factor levels associated with starting HAART does not vary greatly from 3 month after starting HAART. They incorporated between person random effects for both pre-HAART mean and post-HAART change for each risk factor as well as observation (measurement) level random effects. Further details are available from the authors. Regression coefficients and covariance parameters were excluded if their Wald P-value was >0.2 in the full model. The fit of the reduced models was compared with that of the full models.
The predicted effect of mean changes in CHD risk factors need not equal the mean effect of the specific changes observed in individuals starting HAART. Further, the distribution of predicted changes in CHD rates is of interest, as clinical decisions on preventative treatment tend to be based on threshold levels of CHD risk factors. Therefore we used a simulation exercise to model the multivariate distribution of the CHD risk factor changes, which could then be used to predict the distribution of changes in CHD risk factors associated with starting HAART. The multivariate normal distributions defined by the parameters of the multivariate multilevel models were used as input to a simulation exercise in which risk factor changes for 10 000 patients were randomly drawn, separately for PI- and NNRTI-based HAART. Percentiles of this distribution were used to estimate 95% prediction intervals, defined as the range within which the central 95% of the distribution of individual patients’ changes in each risk factor falls.
Estimation of change in coronary risk in HIV-1 infected patients starting HAART
We used a prognostic model for CHD, which was developed to apply to HIV-1 positive patients starting HAART. Briefly, data from five observational cohort studies, (the Framingham Offspring Study [12], British Regional Heart Study (BRHS) [13], Caerphilly and Speedwell Studies [14, 15] and Whitehall II Study [16]), were used to construct a model for rates of CHD in men aged 40–70 without prevalent CHD and followed for up to 10 years. The outcome was CHD events, defined as myocardial infarction (MI) or death from CHD (ICD9 codes 410–414). The model is based on the Gompertz distribution with age as the underlying time variable and baseline hazards estimated separately in each cohort. The variables considered for inclusion in the model were the six variables listed earlier, together with age and smoking. The parameter estimates and their standard errors from the prognostic model are provided in the appendix. The prognostic model was used to derive CHD rate ratios corresponding to the changes in risk factors observed in the SHCS, for PI- and NNRTI-based HAART separately. Glucose intolerance was modeled in three categories: normal (glucose ≤ 5.5 mmol L−1), impaired glucose tolerance (glucose > 5.5 to ≤7 mmol L−1), and diabetic (glucose > 7 mmol L−1 or a diagnosis of diabetes). Triglycerides were not prognostic in the multivariable model. The sets of changes in risk factors sampled during the simulation exercise were used to derive predicted distributions of rate ratios in patients starting PI- and NNRTI-based HAART.
Results
Trends in CHD risk factors after starting therapy
Since 2000, 2114 naive patients in the SHCS started HAART. Of these, 724 were female, 117 had not attended a follow-up clinic, 670 did not have risk factor measurements both before starting HAART and during follow-up and 47 received CHD or diabetes medication from HAART initiation, leaving 556 men included in analyses. Compared with all men followed up during the study period, men who have sex with men were slightly over represented in our study sample, and patients with a history of intravenous drug use were slightly less likely to be included. The median (IQR) age at start of HAART was 38 years (33–45 years) and 50% of patients were current smokers. Two hundred and ninety-seven (53%) patients started PI-based HAART, of whom 34% received nelfinavir (NFV), 48% ritonavir boosted lopinavir, 8% ritonavir boosted indinavir and 8% either ritonavir or another ritonavir boosted regimen, and 259 (47%) started NNRTI-based HAART, of whom 92% received efavirenz and 8% nevirapine. Four hundred and twelve patients (74%) started HAART regimens containing zidovudine and lamivudine; no other NRTI combination was taken by more than 6% of patients. Within 3 years of starting therapy, 49% of patients initiating with PI- and 36% with NNRTI-based HAART changed regimen. However, only 15% changed from PI- to NNRTI-based HAART and 8% vice versa. The numbers of men with risk factor measurements were 297, 196 and 116 <1, 1–1.99 and 2–2.99 years after starting PI-based HAART. Corresponding numbers for NNRTI-based HAART were 259, 171 and 90. During the 3 years of follow up, subjects dropped out of the study population for the following reasons: 22 (4%) patients died, 49 (9%) started nonantiretroviral drugs which potentially might have affected their CHD risk factor measurements, 134 (24%) changed type of antiretroviral regimen and 174 (31%) had less than 2 years of follow up because they started HAART within 2 years of the close of the database.
Median (IQR) baseline risk factor levels were: cholesterol 4.0 mmol L−1 (3.4–4.7), HDL cholesterol 0.91 mmol L−1 (0.74–1.12), glucose 4.9 mmol L−1 (4.4–5.3), SBP 120 mmHg (110–130), BMI 22.6 kg m−2 (20.6–24.6) and triglyceride 1.3 mmol L−1 (0.8–1.9). The median CD4 cell count before starting HAART was 190 cells μL−1 (98–277), median log HIV-1 RNA was 5.05 log copies mL−1 (4.61–5.46) and 127 patients (23%) had AIDS.
Figure 1 shows graphs of mean risk factor levels over time from initiation of HAART, estimated from the best-fitting fractional polynomial models. The levels of most risk factors increased sharply during the first 3 month on HAART, then more slowly from 3 month to 3 years. Changes in triglyceride were smaller for NNRTI- than for PI-based HAART, changes in cholesterol were large for both regimens, and changes in HDL cholesterol were greater for NNRTI- than for PI-based HAART. Increases in glucose were small for both regimens, but confidence intervals were wide. There was a small increase in SBP in those on PI-based HAART, but no evidence of an increase in patients on NNRTI-based HAART. The increase in BMI was slightly smaller for those on NNRTI- than PI-based HAART. The differences between changes in risk factors on NNRTI- versus PI-based HAART were greatest for HDL cholesterol and triglycerides.
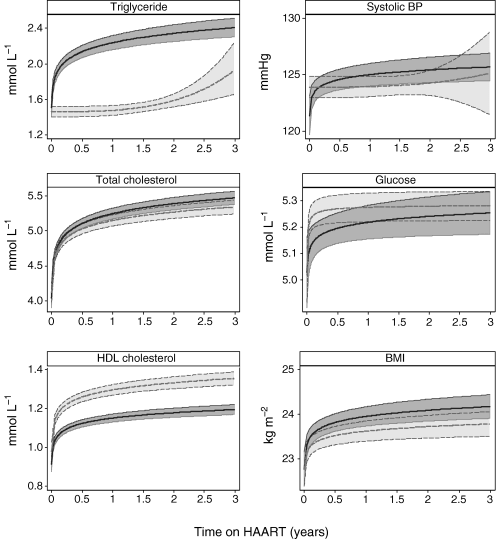
Graphs showing estimated mean risk factor levels at initiation of HAART and up to 3 years afterwards, for patients starting PI-based regimens (solid lines) and NNRTI-based regimens (dashed lines). Estimates were derived using fractional polynomial regression: shaded areas indicate 95% confidence intervals.
Table 1 shows the mean of each risk factor at 0, 1, 2 and 3 years after starting HAART, estimated from the fractional polynomial models, for those on PI- and NNRTI-based HAART. There was little evidence that mean changes in risk factors varied with age, or according to pre-HAART CD4 count or whether the patient had clinical AIDS before starting HAART (data not shown). Examination of model residuals showed that changes in risk factors post-HAART were approximately normally distributed, with no evidence of any outlying group of individuals.
| Risk factor | Years from start of HAART | PI-based HAART n = 297 patients | NNRTI-based HAART n = 259 patients |
|---|---|---|---|
| Total cholesterol (mmol L−1) | n = 1439 obs. | n = 1045 obs. | |
| 0 | 4.04 (3.90–4.19) | 4.12 (3.98–4.26) | |
| 1 | 5.25 (5.17–5.33) | 5.15 (5.07–5.23) | |
| 2 | 5.39 (5.30–5.48) | 5.27 (5.18–5.36) | |
| 3 | 5.47 (5.38–5.57) | 5.34 (5.24–5.44) | |
| HDL cholesterol (mmol L−1) | n = 1375 obs. | n = 989 obs. | |
| 0 | 0.91 (0.88–0.95) | 0.98 (0.93–1.03) | |
| 1 | 1.15 (1.13–1.17) | 1.30 (1.27–1.32) | |
| 2 | 1.18 (1.15–1.20) | 1.33 (1.30–1.36) | |
| 3 | 1.19 (1.17–1.22) | 1.35 (1.32–1.39) | |
| Glucose (mmol L−1) | n = 2029 obs. | n = 1185 obs. | |
| 0 | 5.03 (4.89–5.16) | 5.02 (4.92–5.12) | |
| 1 | 5.22 (5.15–5.28) | 5.27 (5.22–5.33) | |
| 2 | 5.24 (5.17–5.31) | 5.28 (5.22–5.33) | |
| 3 | 5.25 (5.17–5.33) | 5.28 (5.23–5.34) | |
| Systolic BP (mmHg) | n = 1152 obs. | n = 980 obs. | |
| 0 | 121.4 (119.7–123.2) | 123.9 (123.0–124.9) | |
| 1 | 125.0 (124.0–126.0) | 124.0 (123.1–124.9) | |
| 2 | 125.4 (124.3–126.6) | 124.3 (123.2–125.4) | |
| 3 | 125.7 (124.5–126.9) | 125.1 (121.5–127.8) | |
| BMI (kg m−2) | n = 1168 obs. | n = 981 obs. | |
| 0 | 22.8 (22.4–23.2) | 22.8 (22.4–23.2) | |
| 1 | 24.0 (23.7–24.2) | 23.6 (23.4–23.9) | |
| 2 | 24.1 (23.9–24.3) | 23.7 (23.5–24.0) | |
| 3 | 24.2 (23.9–24.4) | 23.7 (23.5–24.1) | |
| Triglyceride (mmol L−1) | n = 1433 obs. | n = 1042 obs. | |
| 0 | 1.51 (1.41–1.62) | 1.46 (1.41–1.52) | |
| 1 | 2.24 (2.16–2.32) | 1.48 (1.42–1.53) | |
| 2 | 2.34 (2.25–2.44) | 1.59 (1.52–1.66) | |
| 3 | 2.41 (2.31–2.52) | 1.92 (1.66–2.23) |
- Obs., observations; HAART, highly active antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Mean long-term changes and within-person correlations in risk factors
Table 2 shows estimated mean HAART-associated changes in the CHD risk factors for the period 3 month to 3 years after initiation of therapy, together with 95% prediction intervals derived by simulating 10 000 patients from the multivariate distributions defined by the parameters of the multilevel models. Mean differences in cholesterol, SBP, BMI and log triglyceride were greater for those on PI-based HAART. In contrast, the mean difference in HDL cholesterol was greater for those on NNRTI-based HAART (0.32 compared with 0.24 mmol L−1). The rise in glucose was similar for PI- and NNRTI-based HAART (1.02 vs.1.03 mmol L−1). There was no change in SBP for NNRTI-based HAART and therefore SBP was omitted from the model for NNRTI-based HAART.
| Risk factor | Mean difference (95% CI)a | 95% prediction intervalb | Predicted rate ratio of CHD eventc |
|---|---|---|---|
| PI-based HAART (n = 297) | |||
| Total cholesterol (mmol L−1) | 1.19 (1.04–1.33) | −0.83 to 3.20 | 1.37 |
| HDL cholesterol (mmol L−1) | 0.24 (0.20–0.27) | −0.20 to 0.67 | 0.83 |
| Log glucose (mmol L−1) | 0.02 (0.00–0.04) | −0.22 to 0.27 | 1.15 |
| SBP (mmHg) | 3.6 (1.9–5.2) | −9.5 to 16.1 | 1.05 |
| BMI (kg m−2) | 1.14 (0.91–1.38) | −2.12 to 4.37 | 1.02 |
| Log triglyceride (log mmol L−1) | 0.37 (0.30–0.44) | −0.36 to 1.09 | 1.00 |
| NNRTI-based HAART (n = 259) | |||
| Total cholesterol (mmol L−1) | 1.00 (0.86–1.32) | −0.87 to 2.76 | 1.31 |
| HDL cholesterol (mmol L−1) | 0.32 (0.28–0.36) | −0.13 to 0.77 | 0.78 |
| Log glucose (mmol L−1) | 0.03 (0.02–0.04) | −0.03 to 0.10 | 1.15 |
| SBP (mmHg) | 0 | 0 | 1 |
| BMI (kg m−2) | 0.67 (0.49–0.86) | −1.15 to 2.47 | 1.01 |
| Log triglyceride (log mmol L−1) | 0.09 (0.02–0.15) | −0.62 to 0.79 | 1.00 |
- CHD, coronary heart disease; HAART, highly active antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SBP, systolic blood pressure. aMean differences and 95% confidence intervals (CI) were estimated using multivariate multilevel models. b95% prediction intervals were estimated by simulating from the parameters of the multivariate normal distribution defined by the multilevel models. cPredicted CHD rate ratios corresponding to the estimated mean differences, derived from the CHD prognostic model.
Table 3 shows estimated mean differences in risk factor levels for the three most common third drugs used in HAART regimens. Because the large majority of NNRTI-based regimens used efavirenz, results for that drug (237 patients) are broadly similar to those for all NNRTIs displayed in Table 2. Mean differences in total cholesterol, HDL cholesterol, BMI and log triglyceride were somewhat greater for lopinavir-based regimens than for nelfinavir-based regimens.
| Risk factor | Nelfinavir (n = 101) | Mean difference (95% CI)a Lopinavir (n = 142) | Efavirenz (n = 237) |
|---|---|---|---|
| Total cholesterol (mmol L−1) | 1.14 (0.91–1.37) | 1.31 (1.09–1.53) | 0.99 (0.84–1.14) |
| HDL cholesterol (mmol L−1) | 0.19 (0.13–0.25) | 0.29 (0.24–0.34) | 0.32 (0.28–0.36) |
| Log glucose (log mmol L−1) | 0.04 (0.02–0.06) | 0.03 (−0.01–0.07) | 0.03 (0.02–0.04) |
| SBP (mmHg) | 3.9 (1.3–6.5) | 3.8 (1.5–6.1) | −0.1 (−1.81–1.61) |
| BMI (kg m−2) | 1.05 (0.59–1.51) | 1.23 (0.93–1.53) | 0.66 (0.47–0.85) |
| Log triglyceride (log mmol L−1) | 0.30 (0.20–0.40) | 0.47 (0.37–0.57) | 0.09 (0.02–0.16) |
- SBP, systolic blood pressure. aMean differences and 95% confidence intervals (CI) were estimated using multivariate multilevel models.
Change in coronary risk in HIV-1 infected patients starting HAART
The final multilevel model for NNRTI-based HAART omitted SBP as there was no evidence of changes after initiation of HAART. For PI-based HAART, the final model included all risk factors. The right hand column of Table 2 shows the predicted rate ratios (RR) corresponding to the mean change in each risk factor, based on the CHD prognostic model. Because triglycerides were not independently prognostic for CHD, the prognostic model does not include this factor and so the corresponding predicted rate ratios are 1. The greatest contribution to the rate ratio is from increases in cholesterol, which were 1.19 and 1.00 mmol L−1 for PI- and NNRTI-based HAART respectively, leading to estimated RR of 1.37 and 1.31 respectively. However, these effects are offset to a large extent by the effect of the increases in HDL cholesterol: RR 0.83 and 0.78 respectively for PI- and NNRTI-based HAART. For comparison, the effect of being a cigarette smoker compared with a nonsmoker is to multiply CHD rates by 2.0.
Based on the observed mean changes in risk factors, predicted CHD rate ratios associated with starting HAART based on the prognostic model were 1.25 (95% CI 1.10–1.43) and 1.06 (95% CI 0.92–1.21) for PI- and NNRTI-based HAART respectively. From the simulation analyses, the median rate ratios were 1.40 (95% CI 1.13–1.75) and 1.17 (95% CI 0.95–1.47) for PI- and NNRTI-based HAART respectively. Corresponding 95% prediction intervals, which give the range of rate ratios within which 95% of future patients are predicted to lie, were 0.75–2.66 and 0.70–1.93 for PI- and NNRTI-based HAART respectively. Note that median rate ratios modeled at the level of individual patients were greater than rate ratios corresponding to the population mean changes in risk factor levels.
The incidence of CHD events subsequent to starting HAART was low in this cohort: during the follow up period one patient had a carotic endarterectomy, three had unspecified procedures for CHD, and four were diagnosed with diabetes. No patient experienced a myocardial infarction or died from CHD related causes.
Discussion
We examined changes in coronary heart disease (CHD) risk factors associated with starting HAART and estimated corresponding changes in CHD rates using a prognostic model. Total cholesterol, glucose, BMI and triglyceride increased after patients started HAART, with generally greater increases for PI-based than NNRTI-based HAART. In patients on NNRTI-based HAART, the predicted increase in CHD rates is partially offset by increases in HDL cholesterol. The changes in risk factors correspond to predicted median increases in CHD rates of 40% and 17% for PI- and NNRTI-based HAART, respectively.
Our analyses are based on comprehensive measurements of CHD risk factors made in a large, unselected group of patients. Analysing differences in risk factors within patients, rather than comparing different groups of patients as in previous studies [17–19] should reduce confounding by differences in lifestyle factors and risk behaviors. Predicted CHD rate ratios were based on a prognostic model developed using data from five major prospective cardiovascular cohort studies and tailored to the cardiovascular risk factors associated with HAART-associated metabolic disturbances, including BMI and blood glucose. The widely used Framingham or PROCAM risk equations [20, 21] include the presence of diabetes, but not BMI or blood glucose. Further, the Framingham risk equations reflect experience in past decades and will overestimate present levels of coronary risk [22]. The data from the cardiovascular cohorts used here are more recent, and include the Framingham Offspring Study, [12] rather than the original Framingham Heart Study.
There are important limitations to our approach. The wisdom, or otherwise, of extrapolating estimates of coronary risk derived from studies of non HIV-infected populations to HIV-infected patients with drug-induced metabolic complications remains to be determined. The prevalence of smoking was lower (32% vs. 50%) and age higher (median 50 vs. 38 years) in the cardiovascular cohorts than in the Swiss HIV Cohort. Subjects were predominantly white in both HIV and cardiovascular cohorts. Waist-hip ratio is a stronger predictor of CHD risk than BMI [23] but we were not able to include this variable, which is known to be affected by metabolic complications of HAART, as it was only available in one of the cardiovascular cohorts. The glucose measurements used in developing the prognostic model were all fasting measurements, but this was true of only a minority of the glucose measurements in SHCS patients. The measurement error resulting from nonfasting measurements will have reduced the precision with which mean changes after starting HAART are estimated. Furthermore, predicted rate ratios might be underestimated, because of measurement errors and short-term variability in CHD risk factors. Our study was too small to allow detailed analyses of individual drugs, but differences between individual drugs exist. For example, recent analyses of the 2NN trial [24] and the Swiss cohort [25] showed that amongst NNRTI-based regimens nevirapine produces a more favourable lipid profile than efavirenz. Underlying rates of CHD, allowing for risk factor levels, are higher in the UK and USA and estimated absolute CHD risks would be too high if the model were used to estimate CHD risks in Switzerland. However, relative effects (rate ratios) resulting from risk factor changes should be comparable across populations, given that the effects of CHD risk factors tend to be similar in different settings. Our estimates of the effect of starting HAART on CHD rates should therefore be applicable to many settings, although the decision to start preventive medication such as statins should ideally be based on absolute risk, and therefore on risk equations calibrated to the population from which a patient comes.
Several studies have recently examined the risk of CHD in HIV-1 infected populations, with conflicting results. Some were based on large numbers of patients, but lacked information on cardiovascular risk factors [26–29]. Possible underreporting of cardiovascular events in earlier years, when physicians were not yet aware of the metabolic complications associated with HAART, a duration of follow up that may have been too short for cardiovascular events to manifest themselves and retrospective collection of data are also important potential problems [7, 8]. The D : A : D study, a collaboration of prospective studies of HIV-infected patients, was designed to address this issue and included the collection of standardized data on cardiovascular risk factors and CHD outcomes. It found that rates of myocardial infarction increased by a factor of 1.26 per year of exposure to combination antiretroviral therapy [30].
Estimating changes in the risk of CHD associated with HAART is problematic, primarily because of the difficulty in finding a suitable comparator population. Patients with HIV-1 infection who remain untreated will have less advanced disease and differ according to cardiovascular factors such as body mass index (BMI), levels of exercise or smoking. Comparisons with individuals not infected with HIV may also be problematic. The prevalence of risk factors such as smoking differs between HIV-infected and non infected populations [31] and HIV-infection itself may affect cardiovascular risk, either directly through a pro-inflammatory effect on endothelial cells [32] or indirectly by inducing lipid abnormalities [33]. Interestingly, a cross-sectional French study [34] that used the Framingham equation to compare estimated CHD risk in 223 male patients treated with PI-based HAART in France with uninfected men from the French arm of the population-based WHO-MONICA Project found a relative risk of 1.39 for treated men, similar to our estimate of 1.40.
By examining trends in CHD with time on HAART, the D : A : D study [30] avoided the choice of a comparator population but leaves open the possibility of confounding by changes in CHD risk factors associated with increased well-being of patients rather than with the direct effects of HAART. On the other hand, direct effects of HAART not captured by classical metabolic risk factors, or direct effects of HIV infection itself should be observed in studies such as D : A : D but are not accounted for in the approach presented here. In a modelling exercise based on the Framingham risk equation, data from the D : A : D study were used to investigate whether the observed increases in rates of CHD can be attributed to changes in conventional CHD risk factors [35]. The observed and predicted rates of myocardial infarction increased over time in a parallel fashion suggesting that the observed increase in risk of CHD is due to changes in conventional risk factors. Interpretation of results is hampered by a large amount of missing data (for example, smoking history was missing in 24% and HDL cholesterol in 40% of patients). Furthermore, the outcome used in the Framingham model differed from the definition of MI used in D : A : D, which included sudden death from unknown causes, but excluded silent infarctions. D : A : D also modeled population changes in risk factors rather than within-patient changes as we did in our study.
In conclusion, the study of changes in CHD risk in HIV-1 infected patients starting potent antiretroviral therapy is fraught with methodological difficulties. Our study avoided some of these problems, but introduced strong assumptions regarding the transferability of estimates of coronary risk from prospective studies of non HIV-infected populations to HIV-infected patients. This assumption requires testing in a validation study. The results suggest that CHD rates will increase in a majority of HIV-infected patients treated with HAART. However, these increases are generally modest, particularly for patients starting with NNRTI-based regimens, and could be offset by lifestyle changes, particularly smoking cessation.
Conflict of interest
J.A.C. Sterne has received travel grants and a research grant from GSK. M. May has received travel grants from GSK. H.C. Bucher has received travel grants, grants or honoraria from Abbott, BMS, GSK and MSD. B. Ledergerber has received travel grants from Roche, Abbott, BMS, GSK, MSD, and Aventis. H. Furrer has participated in advisory boards of Abbott, GSK, BMS, Roche, Gilead, and MSD. The institution of H. Furrer has received unrestricted educational grants from Abbott, GSK, BMS, Roche, Gilead, MSD, Boehringer-Ingelheim and Essex. M. Egger has received travel grants, grants, or honoraria from BMS, Boeringer-Ingelheim, and GSK.
Acknowledgements
The Swiss HIV Cohort Study is supported by the Swiss National Science Foundation. The analyses reported here were supported by UK Medical Research Council (MRC) grant G0100221. The University of Bristol Department of Social Medicine is the lead center of the MRC Health Services Research Collaboration. We are grateful to the participants of the Swiss HIV Cohort Study. The members of the study group are M. Battegay, E. Bernasconi, J. Böni, H. Bucher, Ph. Bürgisser, S. Cattacin, M. Cavassini, R. Dubs, M. Egger, L. Elzi, P. Erb, M. Fischer, M. Flepp, A. Fontana, P. Francioli (President of the SHCS, Centre Hospitalier Universitaire Vaudois, CH-1011-Lausanne), H. Furrer (Chairman of the Clinical and Laboratory Committee), M. Gorgievski, H. Günthard, B. Hirschel, I. Hösli, Ch. Kahlert, L. Kaiser, U. Karrer, O. Keiser, C. Kind, Th. Klimkait, B. Ledergerber, B. Martinez, N. Müller, D. Nadal, M. Opravil, F. Paccaud, G. Pantaleo, L. Perrin, J.-C. Piffaretti, M. Rickenbach (Head of Data Center), C. Rudin (Chairman of the Mother & Child Substudy), P. Schmid, D. Schultze, J. Schüpbach, R. Speck, P. Taffé, P. Tarr, A. Telenti, A. Trkola, P. Vernazza (Chairman of the Scientific Board), R. Weber, S. Yerly.
Appendix
Calculation of hazard ratio for CHD from the prognostic model
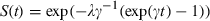
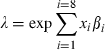
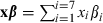 . The hazard ratio is then the exponentiated linear predictor RR = exp(xβ). For example, if it is assumed that SBP increases by 5 mmHg, total cholesterol by 2 mmol L−1, and BMI by 1 kg m2, then
. The hazard ratio is then the exponentiated linear predictor RR = exp(xβ). For example, if it is assumed that SBP increases by 5 mmHg, total cholesterol by 2 mmol L−1, and BMI by 1 kg m2, then
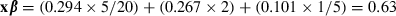
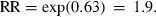
| Covariate label | Coefficient, β | SE | Reference value | |
|---|---|---|---|---|
| Linear predictor | ||||
| Systolic BP(per 20 mmHg) | X1 | 0.294 | 0.037 | 120 (divide by 20) |
| Total cholesterol (per mmol L−1) | X2 | 0.267 | 0.035 | 5 |
| HDL-cholesterol (per mmol L−1) | X3 | −0.779 | 0.148 | 1 |
| Log triglyceride [per log (mmol L−1)] | X4 | −0.003 | 0.084 | 0 (trig = 1 mmol L−1) |
| Glucose > 5.5 to ≤7 (mmol L−1) | X5a | 0.136 | 0.097 | Ref group glucose ≤ 5.5 mmol L−1 |
| Glucose > 7 (mmol L−1) or diabetes | X5b | 0.439 | 0.150 | |
| BMI (per 5 kg m−2) | X6 | 0.101 | 0.062 | 25 (divide by 5) |
| Ex-smoker | X7a | 0.044 | 0.130 | Ref group never smoker |
| Pipe or cigar smoker | X7b | 0.370 | 0.161 | |
| Current cigarette smoker | X7c | 0.712 | 0.087 | |
| Constant | X8 | −6.148 | 0.225 | 3% 10 year CHD risk |
| γ | ||||
| Shape parameter | ||||
| Constant | 0.067 | 0.007 | ||
| Age | t | 40 | ||
Multivariate multilevel model
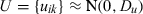
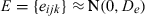
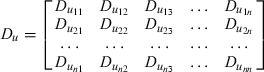
| Log triglyceride | Cholesterol | HDL cholesterol | Log glucose | SBP | BMI | |
|---|---|---|---|---|---|---|
| PI-based HAART | ||||||
| Log triglyceride | 1 | |||||
| Cholesterol | 0.53 | 1 | ||||
| HDL cholesterol | −0.063 | 0.29 | 1 | |||
| Log glucose | −0.005 | −0.14 | −0.074 | 1 | ||
| SBP | 0.09 | 0.32 | 0.29 | 0.05 | 1 | |
| BMI | 0.30 | 0.43 | 0.20 | 0.11 | 0.76 | 1 |
| NNRTI-based HAART | ||||||
| Log triglyceride | 1 | |||||
| Cholesterol | 0.34 | 1 | ||||
| HDL cholesterol | −0.25 | 0.31 | 1 | |||
| Log glucose | 0.00 | 0.00 | 0.00 | 1 | ||
| SBP | 0.13 | 0.05 | 0.03 | 0.00 | 1 | |
| BMI | 0.22 | 0.43 | 0.50 | 0.00 | 0.29 | 1 |
- HAART, highly active antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Table A2 below shows the estimated within-person correlations between changes in risk factors. As expected, difference in total cholesterol was positively correlated with difference in HDL cholesterol, triglyceride, SBP and BMI. For those on NNRTI-based HAART, there was no evidence of correlation between increase in glucose and the other risk factors. However, for those on PI-based HAART, an increase in glucose was associated with a decrease in both total and HDL cholesterol. Based on these patterns, the correlations of changes in glucose with all other variables were set to zero in the final model for NNRTI-based HAART. For PI-based HAART, the correlations of changes in glucose with each of triglyceride, total cholesterol, HDL cholesterol and SBP were set to zero, as were the correlations of changes in triglyceride with each of HDL cholesterol and SBP.