Hypercholesterolaemia impairs monocyte function in CAD patients
Abstract.
Objectives. Hypercholesterolaemia (HC) impairs arteriogenesis, i.e. collateral artery growth. Monocytes are crucial mediators of arteriogenesis. We investigated the impact of the cardiovascular risk factor HC on ligand-induced monocyte chemotaxis.
Subjects. The migratory response of monocytes towards the arteriogenic ligands vascular endothelial growth factor-A (VEGF-A) and monocyte chemoattractant protein-1 (MCP-1) in hypercholesterolaemic coronary artery disease (CAD) patients (n = 14), hypercholesterolaemic controls (n = 8) and age-matched healthy controls (n = 19) was analysed. Furthermore, the serum VEGF-A level was determined in all individuals.
Results. VEGF-A-induced monocyte chemotaxis was severely impaired in hypercholesterolaemic CAD patients when compared with age-matched healthy controls (P < 0.001). The same was true for the migratory response towards MCP-1 (P < 0.001). VEGF-A- and MCP-1-induced monocyte chemotaxis of hypercholesterolaemic controls was also decreased in comparison with the healthy control group, but not as severe as observed in the hypercholesterolaemic CAD patients. VEGF-A serum levels did not differ between the three study groups.
Conclusions. Hypercholesterolaemia severely impairs monocyte function in hypercholesterolaemic CAD patients. Monocyte dysfunction is probably connected to impaired collateral artery growth. The duration of the cardiovascular risk factor HC seems to influence the extent of monocyte dysfunction, as there exists a continuum of diminished monocyte chemotaxis in the three study groups. Further trials are warranted in order to determine whether statins can reverse the negative influence of HC on cell function.
DEAR SIR,
One of the major cardiovascular risk factors is hypercholesterolaemia (HC), which greatly contributes to the high mortality and morbidity of coronary artery disease (CAD) worldwide. Besides promoting atherosclerosis progression, HC negatively influences collateral artery development [1]. Arteriogenesis, i.e. the growth of collateral arteries, is regarded as one of the most important mechanisms to enhance regional perfusion in the chronically ischaemic myocardium. Increased chemoattraction of monocytes and tissue-resident macrophages [2, 3], who serve as a bioreactor and reservoir of various growth factors, towards jeopardized tissue leads to an accelerated arteriogenic response. In contrast, impaired arteriogenesis in HC is associated with a reduced monocyte/macrophage influx during this process [4]. Vascular endothelial growth factor (VEGF-A) is capable of mediating monocyte chemotaxis to places of regional ischaemia [5]. Likewise, the chemokine monocyte chemoattractant protein-1 (MCP-1), which is regarded as one of the most potent arteriogenic factors, attracts monocytes [6]. Previously, our group established an ex vivo assay for the assessment of the migratory potential of monocytes [7].
We investigated the migratory response of monocytes towards VEGF-A and MCP-1 from CAD patients with HC as single cardiovascular risk factor. Their chemotaxis values were compared with the results obtained from healthy, age-matched controls. In addition, we assessed VEGF-A serum levels in all individuals. Fourteen patients [nine men, five women; mean age (±SD) 65 years (±11)] with angiographically proven CAD and HC [either untreated (LDL-C ≥ 4.2 mmol L−1) or already treated with lipid-modifying medication] were included in the study. Exclusion criteria were diabetes mellitus, smoking, uncontrolled arterial hypertension, active infections, malignancies, nephropathy or a recent acute coronary syndrome (<12 weeks). Age-matched, healthy volunteers were recruited as a control group. Actually not planned, eight recruited healthy individuals [five men, three women; 58 (±8) years] suffered from previously unknown HC. This subgroup was separately analysed from the healthy controls [n = 19; eight men, 11 women; age 61 (±9) years]. Informed consent was obtained from patients and volunteers according to the requirements of the local ethical committee in Ulm. Monocyte isolation and chemotaxis analysis was performed as previously described by our group [7]. In brief, monocytes were isolated using Ficoll/Percoll density centrifugation. Monocyte chemotaxis was quantified using a 48-well micro-chemotaxis chamber (Neuropore, Gaithersburg, MD, USA). Cells were allowed to migrate for a total of 3 h. Cell migration was stimulated with either VEGF-A165 (1 ng mL−1; Chiron, Emeryville, CA, USA) or MCP-1 (10 ng mL−1; PeproTech, Rocky Hill, NJ, USA). Random migration of unstimulated monocytes diluted in DMEM (chemokinesis) served as control and was referred to as 100% migration. VEGF-A serum concentrations were measured with an immunoradiometric assay using two different monoclonal antibodies (Genentech, South SanFrancisco, CA, USA) binding to two different epitopes of VEGF-A, as previously described [7]. The sensitivity of the assay was 20 pg mL−1. Values are expressed as mean (±SD) unless otherwise stated. Kruskal–Wallis test and Mann–Whitney U-test with Bonferroni correction were used for nonparametric comparisons. Analyses were performed using spss (version 12.0; Chicago, IL, USA). A two-tailed P < 0.05 was considered significant.
The mean LDL-C level (±SD) measured 3.8 mmol L−1 (±0.6) in age-matched, healthy volunteers (n = 19), 3.0 mmol L−1 (±0.7) in CAD patients treated with a low-dose statin (i.e. in most cases simvastatin 10 mg day−1 or equivalent) (n = 9), 4.7 mmol L−1 (±1.2) in CAD patients without a statin (n = 5) and 4.4 mmol L−1 (±0.8) in volunteers with an uncomplicated HC (n = 8). The mean VEGF-A-stimulated migration of monocytes (±SD) from CAD patients with HC was significantly decreased compared with age-matched, healthy controls [105% (±11.7) vs. 139% (±22.3); P < 0.001; Fig. 1a]. Likewise, when stimulated with MCP-1, the migratory response of monocytes from hypercholesterolaemic CAD patients was significantly impaired compared with healthy controls [110% (±11.4) vs. 139% (±21); P < 0.001; Fig. 1b]. Interestingly, the migratory response of controls with uncomplicated HC was decreased compared with healthy controls, but not as severely as in the CAD group. Their chemotaxis towards VEGF-A scored 125% (±11.4) and towards MCP-1 121% (±11.1). There was a significant (P < 0.001) difference of VEGF-A-induced migratory response between CAD patients with HC and controls with HC. We could not detect any clear influence of statin treatment on monocyte chemotaxis in CAD patients. VEGF-A serum levels did not significantly differ between CAD patients [246 pg mL−1 (±182)], controls with previously unrecognized HC [194 pg mL−1 (±85)] and healthy volunteers [290 pg mL−1 (±289)].
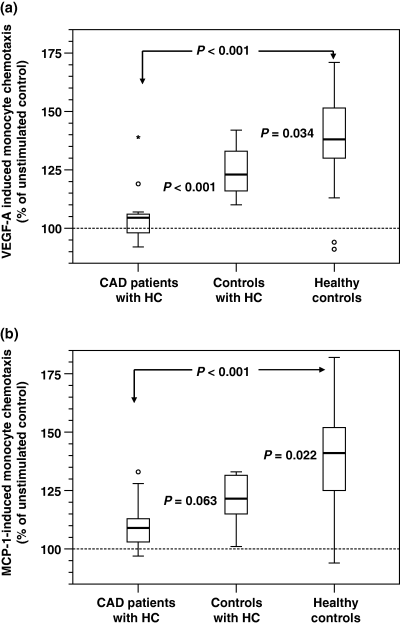
Monocyte chemotaxis analysis in hypercholesterolaemic coronary artery disease patients (n = 14), hypercholesterolaemic controls (n = 8) and healthy controls (n = 19). Monocytes were isolated from peripheral blood samples and stimulated with either vascular endothelial growth factor-A (VEGF-A) (1 ng mL−1; a) or monocyte chemoattractant protein-1 (MCP-1) (10 ng mL−1; b) using a 48-well micro-chemotaxis chamber. Data are presented as box plots displaying medians, upper and lower quartiles, minimal and maximal values (° = extremes; * = outliers).
In the present study, we show for the first time that the cardiovascular risk factor HC severely impairs monocyte chemotaxis when stimulated with either the growth factor VEGF-A or the chemokine MCP-1. In the presence of HC, monocytes partially loose their ability to migrate towards these different arteriogenic molecules. There seems to be a continuum with regard to the functional impairment between healthy monocytes, monocytes from previously unrecognized hypercholesterolaemic individuals and monocytes from patients with diagnosed atherosclerotic disease. As monocytes are a key component in the process of arteriogenesis [2], assessment of their migratory response might have a predictive value partially reflecting a patients’ ability to develop functional collaterals in an ischaemic heart or in the peripheral circulation. Thus, chemotaxis might be regarded as a novel functional cellular parameter to monitor cellular integrity under the influence of the cardiovascular risk factor HC. Interestingly, the impaired chemotactic response is unrelated to VEGF levels in these patients, as we could not detect any difference in serum values of the three study groups. These data seem to be contradictory to the literature [8]. Nevertheless, we have to take into account that LDL-C levels in our CAD patients and controls were only moderately elevated, our patients had only one single, isolated cardiovascular risk factor and part of our patients took a low-dose statin, which could decrease VEGF-A levels [8]. In the present study, we cannot find a clear difference in the chemotactic response of monocytes from CAD patients treated with or without a low-dose statin. However, the subgroup of patients treated with a statin was small and this trial was not designed to address this question in a prospective, randomized fashion. It will be an important task of future studies to determine whether statin treatment can reverse impaired monocyte chemotaxis.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
The study was supported in part by Deutsche Forschungsgemeinschaft (SFB451/B1 and Heisenberg scholarship Wa734/5-1 to J.W. and GRK460 to F.S.C.) and by a Marie Curie Fellowship to F.S.C.




