A review of causal factors and control measures for bloat in farmed salmonids with a suggested mechanism for the development of the condition
Abstract
A mechanism for the development of bloat in salmonids is proposed and explained in terms of the physiology of digestion and osmoregulation in fish. Understanding the causal factors for bloat enables control measures to be identified. In farmed salmonids, the chyme produced during the digestion of nutrient-rich pelleted foods that rapidly disintegrate in the stomach will be a potent activator of a negative feedback mechanism (enterogastric control), which slows stomach emptying to protect the small intestine from nutrient overload. In saline environments salmonids continuously drink sea water to replace fluid lost across the gills. Fluid loss is increased during periods of stress caused by factors such as low oxygen levels, elevated temperature or high salinity. When ingestion of nutrient-rich food results in prolonged activation of enterogastric control, slowed stomach emptying leads to decreased absorption of water, thirst and increased drinking. This further exacerbates stomach distention. The proposed mechanism for the development of bloat is supported by on-farm experience where measures to control bloat include reducing food intake, altering the composition of the diet and using appropriate strategies to reduce stress.
Introduction
A syndrome in which the abdomen of salmonids is abnormally distended by an enlarged, water-filled stomach has been reported from Canada (Hicks 1989), Norway (Staurnes, Andorsdottir & Sundby 1990), New Zealand (Lumsden, Clark, Hawthorn, Minamikawa, Fenwick, Haycock & Wybourne 2002) and Chile (P. Bustos, personal communication) (Fig. 1). The syndrome has been variously termed bloat, water-filled stomach and gastric dilation and air sacculitis (Lumsden et al. 2002). The condition is seen in salmonids reared in sea water and fed fishmeal-based pelleted rations. While it has been reported rarely in Atlantic salmon, Salmo salar L., salmonid species of the genus Oncorhynchus [rainbow trout, Oncorhynchus mykiss (Walbaum), chinook salmon, Oncorhynchus tshawytscha (Walbaum), coho salmon, Oncorhynchus kisutch (Walbaum)] are more susceptible to bloat (Hicks 1989; Staurnes et al. 1990; Lumsden et al. 2002; P. Bustos, personal communication).

Bloat in a seacage-reared chinook salmon.
Stomachs of bloated fish can become severely distended and in such cases the thin-walled flaccid stomach does not contract when the contents are removed. In bloat-affected rainbow trout, the mean weight of stomach contents relative to body weight was 4.6% for normal fish, 10.8% for moderately affected and 30.7% for markedly affected fish (Staurnes et al. 1990). Similar relative increases in stomach volumes have been seen in bloat-affected chinook salmon populations in New Zealand (C. D. Anderson, personal observations) (Fig. 2).
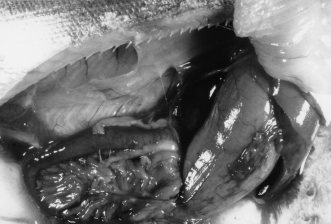
Abdominal contents of a sea-cage-reared chinook salmon (a different individual from fish in Fig. 1). Note the congestion of serosal blood vessels of the moderately distended liquid-filled stomach.
The distended stomachs of bloat-affected salmonids contain an excessive amount of liquid and variable amounts of food and oil. During bloat outbreaks a large amount of oil may accumulate on the surface of the rearing cage in situations where wave action and cage construction favour this (Staurnes et al. 1990; Lumsden et al. 2002).
In some bloat episodes, especially in chinook salmon in New Zealand, the swim bladder may also be distended with viscous cloudy fluid and variable amounts of food particles and dietary oil. The luminal contents of affected swim bladders are usually contaminated with a mixed microbial flora and in chronic cases the swim bladder wall may become severely congested and inflamed. Stomach and swim bladder dilation may occur either singly or together in affected fish (Lumsden et al. 2002).
Bloat appears to develop over several months. The thinning of the abdominal wall musculature, absence of contraction of the stomach wall and reduction in abdominal fat are consistent with prolonged distention of the abdomen, reduced energy intake and stress (Staurnes et al. 1990; Lumsden et al. 2002). Excessive stomach distention and fluid in the swim bladder have been reported from chinook salmon smolts soon after entry to sea water (Hicks 1989; Lumsden et al. 2002). However, in New Zealand, advanced stages of bloat often do not become prevalent until the salmon are approaching harvest, some 12–18 months after transfer to sea water.
Bloat may occur throughout the year but is more prevalent in summer in New Zealand (Lumsden et al. 2002) and in winter in Norway (Staurnes et al. 1990; Rorvik, Skjervold, Fjaera & Steien 2000).
An increase in blood plasma osmolarity has been reported in moderate and markedly affected rainbow trout reared in cold water in Norway (Staurnes et al. 1990; Rorvik et al. 2000), but only in advanced cases of bloat in chinook salmon in New Zealand (Lumsden et al. 2002). In Norway, increased prevalence of bloat has been associated with increasing sea water salinity (Staurnes et al. 1990).
Ingestion of histamine and other biogenic amines has been reported to induce gastric distention with inflammation and ulceration of the mucosa in rainbow trout (Watanabe, Takeuchi, Satoh, Toyama & Okuzumi 1987; Fairgrieve, Myers, Hardy & Dong 1994). However, ulceration and inflammation of the stomach mucosa or wall has not been reported from bloat-affected rainbow trout or chinook salmon (Hicks 1989; Staurnes et al. 1990; Lumsden et al. 2002).
A mild increase in mortality rate has been associated with a high prevalence of bloat in rainbow trout and chinook salmon (Staurnes et al. 1990; Lumsden et al. 2002). Processing difficulties and economic losses have also been experienced, with leakage of oil from bloat-affected fish at harvest, microbial contamination of fillets with swim bladder contents during processing, and down-grading of affected fish due to a thin abdominal wall.
The mechanism for the development of bloat has not been defined in detail in published literature, although there is some agreement on the likely contributing factors. The aim of this study is to propose a mechanism for the development of bloat in salmonids, explained in terms of the physiology of digestion and osmoregulation in fish. Understanding this mechanism enables strategies for the prevention and management of bloat to be identified.
Digestive physiology
Research has shown that there is a remarkable similarity between the gastrointestinal tract physiology of fish and that present in other well studied vertebrates including humans (Smith 1980; Mazeaud & Mazeaud 1981; Holmgren, Grove & Fletcher 1983; Nilsson & Holmgren 1993; De Silva & Anderson 1995; Tortora & Grabowski 1996). The existence of similar enterogastric controls in salmon to other domestic livestock and man is supported by findings reported in the literature, and by observations made during episodes of bloat in chinook salmon in New Zealand.
The main functions of the stomach are storage, initial chemical digestion and controlled delivery of chyme to the small intestine. In rainbow trout, cod and flatfish, food entering the stomach initially causes rapid relaxation (Smith 1980). Subsequent distention to accommodate the meal proceeds at a slower pace. The presence of food in the stomach stimulates increased gastric motility and secretion of digestive juices (Nilsson & Holmgren 1993). In fish, the amount of acid and pepsin produced is roughly proportional to the amount of stomach distention (Smith 1980). Contractions of the stomach wall triturate food and, with the aid of digestive juices, reduce the food to a thin liquid (chyme) which is able to pass through the normally partially open pyloric sphincter (Fig. 3).
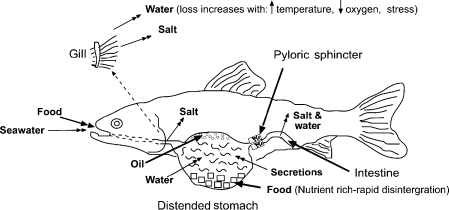
Diagram showing the factors implicated in the development of bloat.
In mammals, the presence of food and the products of digestion in the stomach stimulate gastric hormone release and autonomic nerve impulses that act to widen the lumen of the pyloric sphincter. Gastric contractions are then able to push partially digested food through the pyloric sphincter into the intestine (Tortora & Grabowski 1996).
The rate of gastric emptying in fish varies with temperature, season, activity, body size, satiety, food type and metabolic rate. There appear to be contradictory findings on the effect of meal size on the rate of gastric emptying (Smith 1980; De Silva & Anderson 1995).
Enterogastric control over the delivery of chyme to the small intestine
The flow of chyme into the proximal small intestine is under both nervous control (the enterogastric reflex) and hormonal control. Receptors in the small intestinal mucosa and intestinal wall monitor the quantity and quality of the chyme, responding to factors such as excessive distention, acidity, osmolarity and high oil levels (De Silva & Anderson 1995). This information is relayed to the stomach via nervous impulses and hormone secretions, which act on the gastric muscles and mucosa to reduce peristaltic activity and gastric secretions and slow gastric emptying. In cod, it has been shown that gastric acid secretion is inhibited when the intestine is perfused with either an acidic solution or high salinity sea water, while secretion is normal when the intestine is perfused with water of a salinity of 50% or 33% of sea water (Holstein 1979a,b).
Thus, there is a ‘negative feedback’ mechanism operating to protect the intestinal tract from excessive stretching, mucosal damage and nutrient overload. This combined nervous and hormonal feedback mechanism between the intestine and stomach is referred to in this study as ‘enterogastric control’.
These enterogastric feedback mechanisms also increase the muscular tone, contracting down the pyloric sphincter and slowing the flow of chyme into the intestine. Once digestion and absorption have reduced the volume and nutrient concentration of the chyme in the small intestine, receptor activation and negative feedback reduces, and the pyloric sphincter relaxes, allowing the flow of chyme from the stomach to the intestine to increase.
During periods of slowed gastric emptying, gastric digestive secretion is reduced. In addition, there is increased time for contact between the acid secretions and the food. Both these factors act to reduce the acidity of the chyme. It is also probable that water will be osmotically drawn from blood into the stomach, diluting the chyme and reducing its osmolarity (Nilsson & Holmgren 1993; Wilson, Gilmour, Henry & Wood 1996) (Fig. 3).
In time, gastric motility and secretions build up again and the chyme becomes better digested – less irritant and more nutrient-dilute. In addition, fish will continue to drink sea water that mixes with the stomach contents, further diluting the chyme. As digestion proceeds and the chyme becomes progressively more dilute and better digested, it is able to pass into the intestine without triggering the enterogastric control mechanism so strongly.
Fluid flows and gastric volume
For salmon reared in sea water, fluid enters the stomach from three sources (Fig. 3).
Drinking
Sea water passing over the gills osmotically draws water from blood that perfuses the gills. This water is replaced by the fish drinking sea water. In rainbow trout, the normal rate of drinking is approximately 70 mL per 24 h per kg fish at 11 °C (Wilson et al. 1996).
Fluid from blood
In rainbow trout, some of the Na+ and Cl− content of imbibed sea water may be absorbed during its passage through oesophagus, and sea water will be further diluted by water osmotically drawn from the blood vessels of the stomach wall – this being approximately 70 mL per 24 h per kg fish (Parmelee & Renfro 1983; Evans 1993; Wilson et al. 1996).
Gastric secretions
During digestion there is also water movement from blood into the gastrointestinal tract as digestive secretions. In humans, this is estimated at approximately 30 mL gastric secretions per 24 h per kg body weight (Tortora & Grabowski 1996).
Thus for a salmonid in sea water, approximately 170 mL water per kg fish (or approximately three times the imbibed volume), enters the stomach and is passed into the small intestine over 24 h (Evans 1993).
Salt and water from the imbibed sea water and gastric sources are absorbed in the intestine and the salt excreted via the gills (Fig. 3). Under normal circumstances 90% of the imbibed salt and 70–85% of the imbibed water are absorbed. However, if gastric emptying is slowed, less water will reach the intestine for absorption. Fish will become thirsty and drink more.
In addition to the fluid inflow to the stomach a 1 kg salmon may eat approximately 12 g day−1. The mean stomach volume of normal marine-reared rainbow trout fed commercial pellets is approximately 50 mL kg−1 fish (Staurnes et al. 1990).
Swim bladder
The volume of the swim bladder of a fish in sea water is held at approximately 5% of the body volume so the fish maintains neutral buoyancy (Alexander 1993). In salmonids, the swim bladder is connected to the lower oesophagus by the pneumatic duct. The entrance to this duct is effectively within the stomach (Yasutake & Wales 1983).
The salmon is able to expel air from the swim bladder when it wants to dive rapidly to escape from predators. When danger has passed, the fish rises to the surface and swallows air into the stomach. The air is then forced through the pneumatic duct to reinflate the swim bladder.
Swim bladder inflation is likely to be a complex reflex action that is under conscious and unconscious nervous control. The reflex action would involve the coordination of involuntary and voluntary muscles, relaxation of the pneumatic duct sphincter and constriction of the lower oesophageal sphincter. The latter sphincters lie in close proximity to each other.
Extreme distention of the stomach in a fish suffering bloat will put abnormal strain on all the stomach structures, including those of the lower oesophagus and pneumatic duct. This is likely to adversely affect the normal functioning and control of these structures allowing the leakage of contaminated gastric liquids into the swim bladder.
Factors contributing to the development of bloat in farmed salmonids
Physical properties of the diet
The diet of farmed salmon and their wild counterparts varies markedly.
Gastric digestion of, for example, a meal of sprats will be very slow when compared with the digestion of a meal of commercial pellets. Salmon swallow their food whole and the intact skin and fibrous tissues of the internal organs of the sprats will resist the penetration of digestive juices. The sprat tissues will be slowly solubilized from the limited areas progressively exposed during digestion and trituration in the stomach (Smith 1980).
Because the digestible components of a sprat meal are exposed gradually, there will be sufficient digestive juices to ensure the chyme that is produced is well-digested and nutrient-dilute. In this situation, the enterogastric feedback control mechanism will not be strongly activated.
Chyme produced during digestion of pelleted foods that rapidly disintegrate in the stomach will contain considerably higher concentrations of nutrients and will be less well digested (Jobling 1986). The rapid release of fine food particles (such as fishmeal and flour) will expose a large volume of finely-divided food to a limited volume of digestive juice. The chyme produced under these conditions is likely to be a potent activator of enterogastric feedback controls because it is concentrated and is likely to contain poorly digested material.
Salmon pellets manufactured by the extrusion process have superior binding properties to steam-pressed pellets (De Silva & Anderson 1995). These binding characteristics act to slow the disintegration and digestion, as shown in a comparative study of extruded and steam-pressed pelleted diets in rainbow trout held in fresh water at 15.6 °C where the stomach was 50% evacuated after 9 h for an extruded pellet and after just 5 h for a steam-pressed pellet diet (Hilton, Cho & Slinger 1981). Experience with coho salmon in Chile has shown that bloat may be seen when the food type is changed from extruded to steam-pelleted rations, but not in reverse (P. Bustos, personal communication).
Lipid content of the diet
Lipid is the most slowly digested and absorbed constituent of salmon rations. Increasing the amount of oil in the food will increase the activation of receptors in the intestine, contributing to delayed gastric emptying and stomach distention.
Large volumes of separated oil in bloated stomachs of farmed salmonids has been reported from New Zealand and elsewhere (Staurnes et al. 1990; Lumsden et al. 2002). Separation of oil from pelleted food in the stomach is likely to occur when peristaltic activity has become very minimal or ceased (Fig. 3). Reduced peristaltic activity would be expected where the muscle wall has been severely over-stretched causing damage to the musculature and associated structures.
When enterogastric control is lifted in less severely bloated salmon in which the stomach muscles are still functional, the separated oil will be mixed with the other contents before these are progressively evacuated into the intestine. However, it has been observed in bloated chinook salmon in New Zealand and in rainbow trout in Norway that oil can accumulate in the stomach over a number of days (Austreng, Storebakken & Asgard 1987; Staurnes et al. 1990; Lumsden et al. 2002).
The accumulation of dietary oil on the surface of net-pens has been attributed to release of oil from uneaten food and leakage of food oil from fish stomachs. Surface oil accumulation appears to be more marked in bloat-affected populations (Staurnes et al. 1990; Lumsden et al. 2002). Food oil on the water surface of sea-cages in Norway was more common in cages holding rainbow trout that were affected by bloat than those holding unaffected Atlantic salmon, although both species were of a similar size and were being fed the same food (Staurnes et al. 1990).
In Nova Scotia, Canada, surface oil accumulation and bloat in rainbow trout reared in sea water have been controlled by reducing the dietary oil level to 17% and holding the trout in oxygen-rich water. These measures controlled surface oil within a few days (P. Drinnan, personal communication). Reducing the level of dietary oil would have increased the rate of gastric emptying, reducing distention and oil accumulation in the stomach and hence oil leakage.
Food intake
Wild salmon eat less frequently than farmed fish and hence not only is natural food digested more slowly, but there is also likely to be more time for the digestive tract to digest a meal, empty out and rest, before the next patch of prey is located. Farmed salmon, on the other hand, are fed a number of meals spread throughout daylight hours. Thus, the stomachs of farmed salmon will be almost constantly digesting food, and with little or no time to empty and rest it is likely they will be moderately enlarged compared with those of their wild counterparts.
The appetite of fish appears to vary inversely with stomach fullness (Smith 1980). Farmed fish eat until stretch receptors in the stomach wall register that the stomach is full. However, if the food is nutrient rich, then an excessive volume of imbibed water will further distend the stomach. Repeated gastric over-distention of the stomach at each meal will gradually enlarge the stomach allowing larger meals to be eaten before stretch receptors begin suppressing appetite (Rigaud, Trostler, Rozen, Vallot & Apfelbaum 1995). This increasing meal size will prolong gastric emptying time and increase the rate at which bloat develops.
It is possible that with excessive distention of the stomach, as would occur in the later stages of bloat, the stretch receptors in the stomach wall become damaged. Without normal feedback from these receptors indicating stomach fullness, fish would continue to eat until the nutrient levels in blood rise as the first part of the meal is absorbed (Holmgren et al. 1983).
Over-distention of the stomach, as seen in fish affected with bloat, will be painful. Pain stimuli acting through the parasympathetic nervous system will slow digestion and the rate of gastric emptying, which in turn will further increase the gastric distention (Mazeaud & Mazeaud 1981). Pain would generally be expected to reduce appetite but not in this situation where the pain occurs after the meal has been consumed.
In Canada and Chile, bloat in chinook and coho salmon and rainbow trout has been controlled by reducing the food intake or starving bloat-affected fish for 2–5 days (Hicks 1989; P. Bustos, personal communication). This would allow time for a stomach, that was not excessively stretched, to empty of food and fluid and the repair of damaged tissue to commence. In Chile, starvation has been followed by a reduction in the feeding rate, with the size of the reduction depending on the severity and prevalence of bloat (P. Bustos, personal communication). A reduction in feeding rate will reduce the length of time enterogastric controls are active and acting to restrict the passage of water into the small intestine.
Osmotic stress
Bloat episodes have only been reported in farmed salmonids being reared in saline environments (Hicks 1989; Staurnes et al. 1990; Lumsden et al. 2002; P. Bustos, personal communication) and it appears to increase in prevalence with increasing water salinity (Staurnes et al. 1990). In saline environments salmonids drink to replace water lost osmotically across the gills. The more saline the rearing water, the greater the fluid loss and water intake.
A very low prevalence of excessive gastric distention has been reported in chinook salmon reared in fresh water and being fed nutrient-rich steam pelleted food (Lumsden et al. 2002). This could be explained by heavy feeding of salmon that have been selected for rapid growth and perhaps indirectly for a high food intake. Rainbow trout drink fresh water with a dry pellet meal. Ingested water amounted to 25–35% of the stomach fluid 1 h after a meal (Kristiansen & Rankin 2001) and this, together with digestive secretions and prolonged activation of enterogastric controls, is likely to produce excessive stomach distention in gross-feeders, even in fresh water.
Oxygen requirement and water temperature
In New Zealand, bloat has historically been more prevalent in summer and in the Marlborough Sounds, where water is warmer than at the more southerly salmon farming locations (Lumsden et al. 2002).
Increased temperature raises the fish's metabolic rate and hence its oxygen requirement. Thus, in summer, a greater area of the gill is perfused with blood, and the flow of water over the gills (respiration rate) is increased to satisfy oxygen needs. In these circumstances, there is more osmotic loss of water from the gills and fish drink more sea water to maintain body fluid levels (Evans 1993) (Fig. 3). During periods of restricted chyme flow from the stomach this increased water intake will add to stomach distention.
Salmon reared in sea-cages are dependent on the flow of oxygen-rich sea water through the cage. Any reduction in dissolved oxygen resulting from factors such as rapid weed growth fouling the net, or recirculation of ‘used water’ from neighbouring cages, will result in increased perfusion of the gills and increased drinking. In rainbow trout being farmed in Nova Scotia, Canada, in a saline lagoon with limited tidal exchange and low oxygen levels, bloat was controlled in the height of summer by raising the rearing nets to retain the fish in water containing more oxygen (P. Drinnan, personal communication).
Low water temperatures, in combination with salinities approaching full strength sea water, probably create osmoregulatory problems for all salmonids (Finstad, Staurnes & Reite 1988). During winter in Norway Atlantic salmon migrate to brackish water and even swim upstream in the coldest periods (Berg 1964 cited by Finstad et al. 1988). In brown trout reared in fresh water, growth ceased at temperatures below 4 °C (Finstad et al. 1988). This lack of growth is probably due to the combined effects of reduced food intake and severe inhibition of metabolism at very low temperatures (Elliott 1981). In their study of osmoregulation of freshwater-acclimatized rainbow trout transferred to diluted sea water (26‰) at either 8 or 1 °C, Finstad et al. (1988) found the initial increase in plasma concentrations of Na+, Cl+ and Mg+ was not reduced and stabilized in the fish held at 1 °C.
In bloated Norwegian rainbow trout, blood Na+ and Cl− concentration was elevated (Staurnes et al. 1990). Elevation of the salt level in blood was most probably the result of the trout being reared in close to full strength sea water (33‰) and impairment of metabolism in the cold water (5.5 °C) which prevented the excretion of sufficient salt and/or the absorption of enough sea water to maintain osmotic homeostasis. The increased salt content of blood would induce thirst and increase the intake of sea water. The combination of increased drinking and prolonged activation of enterogastric controls, brought about by ingestion of nutrient-rich food, is likely to result in over-distention of the stomach.
Although reduced food intake at low temperatures would tend to shorten the period over which enterogastric controls restrict gastric emptying, this will be counter-balanced by a reduced rate of digestion and gastric emptying (Smith 1980; Jobling 1981).
Handling and predation
When fish are crowded in nets or suffer predation the release of stress hormones results in an increase in the area of perfused gill and hence fluid loss and drinking (Mazeaud & Mazeaud 1981; Perry & McDonald 1993).
Genetic selection
Genetic selection of Atlantic salmon in Norway has resulted in an increase in growth rate of 15–20% per generation (Storebakken 2002). High growth rate is likely to be linked to increased food consumption stemming from selection of individuals that have dominant and aggressive feeding characteristics (Speare 1998). Thus, fish selected for growth rate are likely to eat more and, because they are fed nutrient-rich diets, may be more prone to developing bloat. The rainbow trout affected by bloat in Norway in 1990 had been selected for increased rate of growth (Staurnes et al. 1990).
Biogenic amines
Feeding of fishmeal containing histamine and other biogenic amines has been associated with gastric distention and in some cases gastric ulceration in rainbow trout (Watanabe et al. 1987; Fairgrieve et al. 1994). Diets containing histamine and its derivative gizzerosine have been shown to increase gastric acid secretion in Atlantic cod and poultry by stimulating H-2 receptors of gastric parietal cells (Holstein 1976; Masumura, Sugahara, Noguchi, Mori & Naito 1985).
Feeding of casein and fishmeal-based diets supplemented with histamine over a period of 12 weeks induced gastric distention in rainbow trout (Fairgrieve et al. 1994). The stomachs of these rainbow trout were distended by partially digested food and a thin, mucus-like fluid. There was no inflammation or ulceration of the mucosa or muscularis.
The development of gastric distention in these rainbow trout can be explained by acidic chyme causing prolonged activation of enterogastric controls and progressive stretching of the stomach due to accumulation of excessive amounts of fluid during the digestion of each meal.
Microbial contamination of the swim bladder
One would expect to find only very low levels of microbes in the swim bladder of a healthy salmon. Microbes are normally present in imbibed water and on the mucosa of the lower oesophagus. The pneumatic duct provides a route along which these microbes can be carried, especially during inflation.
It is possible that small amounts of stomach liquids will at times enter the swim bladder of a salmon, carrying with it microbes that have not been killed by stomach acid. It appears that healthy salmon in a farmed environment are in most cases able to control infrequent, low-level microbial contamination of the swim bladder. The microbes and small particles (e.g. small fat droplets) present in these incursions will be broken down and carried by inflammatory cells into the bloodstream. Small numbers of microbes may also enter the bloodstream from the swim bladder, but in an otherwise healthy fish, these would be dealt with by the immune system.
However, where gastric distention is extreme, leading to frequent and/or heavy contamination of the swim bladder, the inflammatory response may be overwhelmed. If the immune system is suppressed (e.g. in stress), or the number of invaders becomes too great, septicaemia may develop and the fish will die.
Conclusion
The syndrome of bloat appears to have a common pathogenesis in Norwegian rainbow trout, Nova Scotia rainbow trout, New Zealand chinook salmon and probably Chilean rainbow trout and coho salmon. This involves a combination of a failure of osmotic regulation and nutrient overloading of the intestine.
Farmed salmon fed nutrient-rich rations are likely to develop some degree of permanent stomach distention. Once this has occurred, factors such as changes in the composition or properties of the food, alteration of the feeding regime, increased stress (e.g. from handling or predation) or environmental changes (e.g. increased water temperatures, low water oxygen levels) may lead to the development of bloat.
Farm experience has shown that bloat in salmonids can be controlled by reducing food intake, altering the composition of the diet and using appropriate strategies to reduce stress caused by factors such as low oxygen levels, elevated temperature or high salinity.




