Imbalance of MMP-2 and MMP-9 expression versus TIMP-1 and TIMP-2 reflects increased invasiveness of human testicular germ cell tumours
Summary
The histological classification of testicular germ cell tumours (TGCTs) to seminoma or non-seminomatous germ cell tumours is at present the main criterion for the clinical outcome and selection of the treatment strategy. In view of the need to identify novel prognostic biomarkers for TGCTs, we investigated the expression of the matrix metalloproteinases MMP-2 and MMP-9 in testicular tumour tissues and cell lines of both seminoma and non-seminoma origin. Immunohistochemistry and zymography analysis of tumoural tissues showed significantly higher levels of MMP-2 and MMP-9 compared with normal testis with the active forms detected only in the tumour tissues. Three cell lines representative of the different tumour types, JKT-1 seminoma, NCCIT teratocarcinoma and NTERA2/D1 embryonal carcinoma were also evaluated for their expression of these MMPs using qPCR and zymography and for their invasive properties. The more invasive non-seminomatous teratocarcinoma and embryonal cells expressed considerably more MMP-2 and MMP-9 compared with seminoma cells exhibiting lower invasiveness. Furthermore, an inverse relation was observed between invasiveness and the expression of endogenous inhibitors TIMP-1 and TIMP-2. The MMP inhibitor Marimastat inhibited invasion in all cell lines, the highest inhibition was observed in the more invasive NTERA2/D1 and NCCIT cells, which presented the highest ratio of MMP-2 and MMP-9 vs. TIMP-1 and TIMP-2. These results highlight the importance of MMP-2 and MMP-9 in the invasiveness of testicular tumours and suggest that their levels, vs. those of TIMP-1 and TIMP-2, may represent potential biomarkers for testicular malignancy.
Introduction
Testicular germ cell tumours (TGCTs), although relatively rare are the most common malignancy in men between 15 and 45 years of age and represent 95% of testicular neoplasias (Gashaw et al., 2005). The incidence of testicular tumours has been increasing over the past decades (Peng et al., 2009; Vidrine et al., 2010). Although TGCTs have become one of the most curable solid neoplasms, because of the advantage of diagnostic and therapeutic methods, the prognosis of highly advanced cases with bulky metastatic lesions is still generally poor. Histologically, the TGCTs can be classified as seminomas germ cell tumours, which originate from undifferentiated germ cells, and non-seminomatous (NSGCTs), which are arise from undifferentiated (embryonal carcinoma) and differentiated multipotent cells (Diez-Torre et al., 2004). The histopathological diagnosis, especially the classification to seminoma or NSGCTs is the most important criterion for the selection of the treatment strategy, as NSGCTs are generally more aggressive. Patients with stage-I NSGCTs develop occult metastases at a rate of 20–33% (Sogani et al., 1984; Freedman et al., 1987; Thompson et al., 1988; Foster & Roth, 1998). It has been demonstrated that the percentage of embryonal carcinoma and the presence of vascular invasion can provide reliable prognostic indicators to identify patients at high risk for occult retroperitoneal disease (Heidenreich et al., 1998). In these patients with clinical stage I NSGCTs other biological markers have not yet been shown to be of prognostic significance. A recent study demonstrated that the presence of vascular invasion was associated with gain of a region in chromosome 17 (17q12) and more specifically with the expression of inflammatory cytokine CCL2 in NSGCTs of stage I (Gilbert et al., 2011). Hence, it is important to evaluate novel markers for the development and prognosis of TGCTs (Bray et al., 2006).
Tumour microenvironment plays an important role in tumour progression. We have previously demonstrated significant alterations in the composition of matrix-secreted proteoglycans in testicular tumours, which correlated with the metastatic potential of the tumours (Labropoulou et al., 2006). Matrix metalloproteinases (MMPs) are crucial mediators of the alterations observed in the microenvironment during cancer progression. The MMPs constitute a family of zinc-dependent endopeptidases requiring proteolytic cleavage to become active. These enzymes regulate a variety of physiological processes and signalling events, like extracellular matrix degradation, cell migration, invasion, proliferation and angiogenesis (Visse & Nagase, 2003; Kessenbrock et al., 2010; Gialeli et al., 2011; Hadler-Olsen et al., 2011).
Of particular importance in tumour cell invasion are MMP-2 (gelatinase A) and MMP-9 (gelatinase B). These enzymes have three repeats of a type-II fibronectin domain inserted in the catalytic domain, which interact with collagens and gelatin. Up-regulation of these enzymes has been positively correlated with higher incidence of metastases in different cancer types and is considered as a valuable prognostic factor (Visse & Nagase, 2003; Kessenbrock et al., 2010; Gialeli et al., 2011; Hadler-Olsen et al., 2011). Their activity is regulated at various levels, including transcription, secretion, activation and inhibition by tissue inhibitors of metalloproteinases (TIMPs) (Brew & Nagase, 2010; Hadler-Olsen et al., 2011). TIMP-1 and TIMP-2 can inhibit the proteolytic activity of MMP-9 and MMP-2 by forming non-covalent 1 : 1 stoichiometric enzyme-inhibitor complexes through strong interactions with the haemopexin-like domain of the enzymes. It has been suggested that the balance between MMPs and TIMPs in vivo determines the extent of ongoing proteolysis in the peritumoural microenvironment and the disruption of this balance in cancer may be a factor in the progression of tumours to more malignant phenotypes (Brew & Nagase, 2010; Kessenbrock et al., 2010; Gialeli et al., 2011).
Little is known about the mechanisms and factors potentially involved in the invasiveness of TGCTs. Although the over-expression of MMP-2 and MMP-9 is known to correlate with tumour progression and metastasis in several malignancies (Vaisanen et al., 1999; Farias et al., 2000; Inoue et al., 2002; Ranuncolo et al., 2002, 2003; Talvensaari-Mattila et al., 2003; Trudel et al., 2003; Juuti et al., 2006; Liu et al., 2010), no such studies are available on TGCTs or testicular tumour cell lines. The aim of this study was to examine the expression of MMP-2 and MMP-9 and their inhibitors, TIMP-1 and TIMP-2 in tissues from testicular tumours and in several tumour germ cell lines and to correlate with tumour cell aggressiveness.
Material and methods
Patients and tissue samples
Primary tumours were obtained at surgery from nine patients with GCTs (five with seminoma and four with NSGCTs). Six control healthy testicular tissues were taken from autopsies. All tissue samples were frozen immediately after their collection and used for tissue extraction. For immunohistochemical studies, 43 tissue samples (18 seminomas and 25 NSGCTs) were selected from the archives of the Pathology Department at the University Hospital of Patras. None of the patients had received prior chemotherapy or irradiation. The study was performed in accordance with the precepts established by the Helsinki Declaration, approved by Ethic Committee of Patras University Hospital and patients were enrolled after giving written consent. All data were analysed anonymously. After an initial review of all available haematoxylin – eosin stained slides of surgical specimens, serial sections from a representative paraffin block of each case were immunostained.
Tissue extraction
Tissue samples were minced into small pieces and homogenized with a Polytron homogenizer in 50 mm Tris-HCl, pH 7.5, containing 10 mm CaCl2, 2 m KCl and proteinases inhibitors (1 mm phenylmethanesulfonyl fluoride, 10 mm N-ethylmaleimide and 10 mm benzamidine-HCl) using 3 mL of buffer per gram of tissue on ice. After stirring for 1 h at 4 °C, the homogenates were centrifuged and the protein content of the samples was determined.
Cell lines and culture
The human seminoma cell line JKT-1 was established as previously described and was cultured for up to 40 passages to avoid a drift (Bouskine et al., 2010). The molecular signature of JKT-1 cells used in our study was similar to that described previously (Bouskine et al., 2010) concerning the expression of seminoma markers: placenta alkaline phosphatase (PLAP), NANOG, OCT3/4, AP2γ and HIWI (Fig. S1).
Human non-seminomatous cell lines: embryonal carcinoma NTERA2 /D1 and teratocarcinoma cell NCCIT were purchased from American Type Culture Collection (ATCC, Manassas, VA). The NTERA2 /D1 cell line was cultured in DMEM supplemented with 1 mm sodium pyruvate and 10% heat-inactivated foetal calf serum (HI-FCS), whereas JKT-1 cell line was cultured in DMEM supplemented with 2 mm sodium pyruvate and 10% HI-FCS. The NCCIT cell line was cultured in RPMI 1640 supplemented with 10 mm HEPES, 1 mm sodium pyruvate, 4.5 g /L glucose and 10% HI-FCS. All culture media contained 100 IU/mL penicillin and 100 IU/mL streptomycin. The cells were cultured on either regular dishes or matrix-coated dishes obtained by pre-incubation for 30 min on ice with 0.1 mg /mL of type-I collagen or with 0.1 mg /mL of MatrigelTM (Biocoat, BD Biosciences, San Jose, CA, USA). Cells were also treated with 10 ng /mL of TGF-β in serum-free medium, for 24 h at 37 °C. Culture medium was collected and protein concentration was determined.
Zymographic analyses
Gelatin zymography was performed essentially as described (Bourd-Boittin et al., 2005). Serum-free conditioned media or tissue extracts corresponding to the same cell number and /or containing equal amounts of protein were subjected to SDS-PAGE in 10% polyacrylamide SDS gels, containing 0.1% (w/v) gelatin. Known molecular weight protein standards and samples containing a mixture of MMP-2 and MMP-9 used as positive control were also applied. After electrophoresis, the gels were washed with 2.5% Triton-X-100 to remove SDS and then incubated at 37 °C for 20 h in 10 mm Tris-HCl, pH 7.5, containing 150 mm NaCl and 5 mm CaCl2. The gels were stained with 0.25% (w/v) Coomassie blue G-250 and then destained in 20% methanol containing 10% acetic acid. Areas of protease activity were detected as clear bands against the blue gelatin background. Experiments performed in the presence of 10 mm EDTA in the incubation buffer resulted in the abolishment of all zones of gelatin digestion, confirming that the enzymes are metalloproteinases. The amount of protein loaded onto the gels was within the linearity of the enzymatic activity; quantification and comparison of the gelatinolytic activity (relative intensity of lysis bands) of MMP-9 and MMP-2 were performed by densitometry analysis using the Scion Image software.
Gelatin reversed zymography, for the analysis of TIMPs, was performed on 13% polyacrylamide gels, containing 0.1% (w/v) gelatin and 20 ng /mL recombinant MMP-2 activated by treatment with 1 mm aminophenylmercurie acetate (APMA) for 30 min at 37 °C. Conditioned media, adjusted to the same cell number were subjected to SDS-PAGE and gels were treated as described for gelatin zymography. Samples containing a mixture of TIMP-1 and TIMP-2 were also applied as positive control. The presence of TIMPs was visualized as Coomassie blue stained bands.
Immunohistochemical analysis of MMP-2 and MMP-9 in tumour tissues
Tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Serial 5-μm sections were deparaffinized with xylene and dehydrated with 98% ethanol. Antigen retrieval was performed using microwave oven in 10 mm citric acid buffer (pH 6.0). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 5 min at room temperature. Non-specific protein binding of the antibodies was blocked by incubation with 3% normal swine serum in PBS for 20 min at room temperature. Slides were incubated with anti-MMP-9 rabbit polyclonal antibody (AB19016; Chemicon International Inc. Temecula, CA, USA) or anti-MMP-2 rabbit polyclonal antibody (AB809; Chemicon International Inc.) diluted 1 : 200 in PBS containing 1% normal swine serum for 1 h, followed by 30 min incubation with biotinylated anti-rabbit antibody diluted 1 : 200 and the avidin-biotin-peroxidase technique (Dako Co., Copenhagen, Denmark). The staining was developed with 3,3-diaminobenzidine (DAB)/hydrogen peroxide for 5 min at room temperature and slides were counterstained with haematoxylin. A positive tissue control from breast cancer and a negative reagent control (without primary antibody) were run in parallel. The level of immunoreactivity in cancer cells was expressed by scoring the percentage of positive cells into three groups: strong staining >30% of tumour cells stained; low staining 10–30% of tumour cells stained; and negative staining <10% of positive tumour cells. Three independent researchers randomly evaluated the specimens using this method.
Fluorescence immunocytochemistry
Cells were seeded on glass coverslips coated with 0.1 mg/mL of collagen type I or 0.1 mg /mL of matrigel and grown for 24 h in complete medium. Then cells were washed with PBS and fixed for 10 min with 4% paraformaldeyde, containing 0.5% glutaraldeyde. After fixation, blocking was performed with 3% BSA in PBS. Cells were incubated with the rabbit polyclonal anti-MMP-9 antibody (diluted 1 : 100) (Ab38898; Abcam, Cambridge, MA, USA) or the mouse monoclonal anti-MMP-2 antibody (diluted 1 : 50) (gift from Pr. R. Fridman, Wayne State University, Detroit, USA), for 2 h at room temperature, followed by two washes with PBS and incubation for 1 h at room temperature with the Alexa Fluor 488-donkey anti-mouse antibody (A21202; Invitrogen, Molecular Probes, Carlsbad, CA, USA) or the Alexa Fluor 594-donkey anti-rabbit antibody (A21207; Invitrogen, Molecular Probes). For F-actin, cells were incubated with phalloidin-TRITC (P1951; Sigma, St. Louis, MO, USA) for 5 min. Cells were washed with PBS and incubated with DAPI (Invitrogen, Molecular Probes) for 5 min. Stained cells were mounted under coverslips in Mowiol buffer (Mowiol 4.88; Calbiochem, San Diego, CA, USA). Fluorescence microscopy was performed with a Labophot fluorescence microscopy (Nikon, Tokyo, Japan).
Invasion assay
The invasiveness of the cell lines was assessed by using a modified Boyden chamber assay. Cell culture transwell inserts (8 μm; Falcon, Franklin Lakes, NJ, USA) were coated for 3 h with 33 μg /cm2 of growth factor-reduced MatrigelTM (Biocoat, BD Biosciences, San Jose, CA, USA) at 37 °C. The chambers were air-dried for 18 h, and the MatrigelTM barrier was then reconstituted by adding 500 μL DMEM serum free for 3 h at 37 °C. The inserts were placed into 24-well dishes containing in the lower chamber 750 μL of medium supplemented with 10% FCS as chemoattractant. Cells (5 × 104) were plated onto the MatrigelTM–coated inserts in 500 μL of medium supplemented with 0.1% FCS. When used, the MMP inhibitors: 10 μm Marimastat (British Biotechnology, Oxford, UK), 2.5 μg/mL recTIMP-1 or 2.5 μg /mL recTIMP-2 were added together with the cells. After 18 h of incubation, cells on the upper surface of the filter were mechanically removed with a cotton swab, and those which migrated underneath the surface were fixed, stained with Diff-QuickTM (Dade Behring, Dudingen, Switzerland) and analysed using Image J software.
RNA isolation and qPCR
Total RNA was extracted from the cells by Trizol Reagent (Invitrogen, Cergy Pontoise, France). The quality of the RNA was analysed by electrophoresis on the agarose gels stained with ethidium bromide. Reverse transcription of 1 μg of RNA was performed using M-MLV reverse transcriptase and random hexamers according to the manufacturer’s protocol (Invitrogen). To evaluate the expression levels of MMP-9, MMP-2, TIMP-1 and TIMP-2 normalized to the housekeeping β2 microglobulin (β2M) gene transcript, real-time quantitative PCR was performed using Perfect Master Mix Probe (AnyGenes, Creteil, France) on LightCycler 2.0 (Roche Diagnostics, Meylan, France). Selected sets of primers and labelled probes (Eurogentec, Biosense, Italy) were used for MMP-9 (upper primer: 5′-CATTCAGGGAGACGCCCA-3′, lower primer: 5′-AACCACGACGCCCTTGC-3′, probe: 5′-TTCGACGATGACGAGTTGTGGTCCCT-3′), MMP-2 (upper primer: 5′-CCGTCGCCCATCATCAA-3′, lower primer: 5′-AGGTATTGCACTGCCAAC TCTTT-3′, probe: 5′-CGATGTCGCCCCCAAAACGGA-3′), TIMP-1 (upper primer: 5′-GAGAACCCACCATGGCCC-3′, lower primer: 5′-TATCAGCCACAGCAACAACAGG-3′, probe: 5′- CTTTGAGCCCCTGGCTTCTGGCA-3′), TIMP-2 (upper primer: 5′-CCACCCAGAAGAAGAGCCTG-3′, lower primer: 5′-CAGCGCGTGATCTTGCAC-3′, probe 5′-CCACAGGTACCAGATGGGCTGCG-3′) and β2 microglobulin (upper primer: 5′-CGCTCCGTGGCCTTAGC-3′, lower primer: 5′-GAGTACGCTGGATAGCCTCCA-3′, probe: 5′-TGCTCGCGCTACTCTCTCTTTCTG-3′). Gene expression levels were determined using standard calibration curves prepared from gene-specific PCR products cloned using the TOPO II TA cloning kit (Invitrogen). All of the experiments were performed in triplicate.
Statistical analysis
Data are expressed as mean ± SE. Student’s t-test was used to compare differences in the various experiments.
Results
MMP-2 and MMP-9 are highly expressed in human testicular tumours
The expression of MMP-2 and MMP-9 was examined in tumoural and normal testicular tissues. Tissue extracts containing equal amount of proteins were subjected to gelatin zymography. Results in Fig. 1 show that although in normal testicular tissues the levels of MMP-2 were low and those of MMP-9 were hardly detected, their levels were markedly higher in the TGCTs samples. Quantification of the MMP-2 and MMP-9 bands showed 1.7-times higher levels for MMP-2 and 37-times for MMP-9 in TGCTs as compared with normal testes (Table 1). More importantly, the active forms of MMP-2 and MMP-9 were detected only in the testicular tumours (Fig. 1 and Table 1).
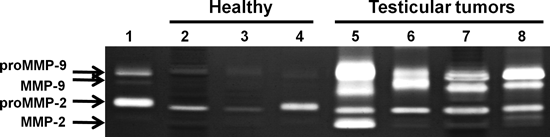
Up-regulation of MMP-2 and MMP-9 in human testicular tumours. Representative gelatin zymography analysis of tissue extracts from normal testes (lanes 2–4) and TGCTs (lanes 5–8). Standards of proMMP-9/MMP-9 and proMMP-2 were applied to lane 1. Lanes 2 and 3: normal testes, lane 4: normal testicular tissue adjacent to seminoma presented in lane 8, lane 5: immature teratoma, lane 6: mixed-type TGCT (seminoma, embryonal carcinoma and teratoma), lanes 7 and 8: seminomas.
| Relative densitometric units | ||
|---|---|---|
| Healthy testes (n = 6) | TGCTs (n = 9) | |
| pro-MMP-9 | 335 ± 160 | 12261 ± 4580a |
| MMP-9 | ND | 6730 ± 1010a |
| pro-MMP-2 | 1920 ± 740 | 3292 ± 320a |
| MMP-2 | ND | 2315 ± 2040a |
- Quantification was performed measuring relative intensity of lysis bands in zymographs. ND, not detected. Values represent mean values ± SE. aStatistically significant differences compared with healthy testes (p < 0.05). Experiments were performed in triplicates.
Cellular distribution of MMP-2 and MMP-9 in testicular tumours and cell lines
Immunohistochemical staining of tumoural tissues was then performed to identify the cellular source of MMP-2 and MMP-9 in TGCTs. In normal testes, cytoplasmic and membranous staining for MMP-2 was identified in the germ cells within normal seminiferous tubules, whereas cytoplasmic staining was also observed in stromal cells in the interstitial connective tissue (Fig. 2a). MMP-9 staining was much weaker than MMP-2 in the germ cells and none could be observed in the interstitial connective tissue in the interlobular septa surrounding the seminiferous tubules (Fig. 2b). Tumour cells in seminoma (Fig. 2c and d), embryonal carcinoma (Fig. 2e and f) and teratocarcinoma (Fig. 2g and h) exhibited strong staining for both MMP-2 and MMP-9, which appeared mainly cytoplasmic and perinuclear although in some cases, membranous staining for both MMP-2 and MMP-9 could be detected. Strong staining for MMP-2 was found in 16/18 seminomas and 24/25 NSGCTs (Table 2). MMP-9 was highly expressed in 13/18 of seminomas and 18/25 of NSGCTs (Table 2). These data clearly suggest that the tumour cells express most of the MMP-2 and MMP-9 in TGCTs.
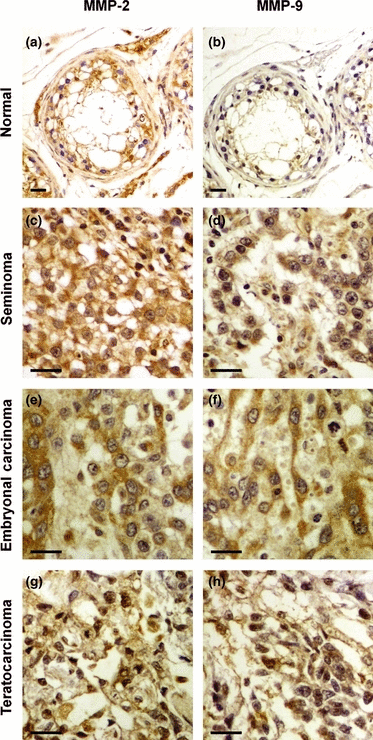
MMP-2 and MMP-9 are highly expressed by tumour cells in TGCTs. Immunohistochemical staining for MMP-2 (a, c, e and g) and MMP-9 (b, d, f and h) in normal testicular tissue (a and b), seminoma stage II (c and d), embryonal carcinoma stage II (e and f) and embryonal/teratoma (teratocarcinoma) stage II (g and h). Bars, 25 μm.
| Histological type | MMP-2 positive tumour cells | MMP-9 positive tumour cells | ||||
|---|---|---|---|---|---|---|
| <10% | 10–30% | >30% | <10% | 10–30% | >30% | |
| Seminoma | 0 | 2 | 16 | 1 | 4 | 13 |
| NSGCTs | 0 | 1 | 24 | 2 | 5 | 18 |
The tumoural expression of MMP-2 and MMP-9 was also demonstrated by immunocytochemistry in TGCT cell lines representative of the three testicular tumour types JKT-1 seminoma, NTERA2/D1 embryonal carcinoma and NCCIT teratocarcinoma (Fig. 3). Staining for both MMP-2 and MMP-9 was more intense in the embryonal carcinoma (Fig. 3d and e) and the teratocarinoma cells (Fig. 3g and h) than in the seminoma cells (Fig. 3a and b). In accordance with the immunohistochemical staining of the tumoural tissues, MMP-2 and MMP-9 staining in the tumour cell lines was predominantly cytoplasmic.
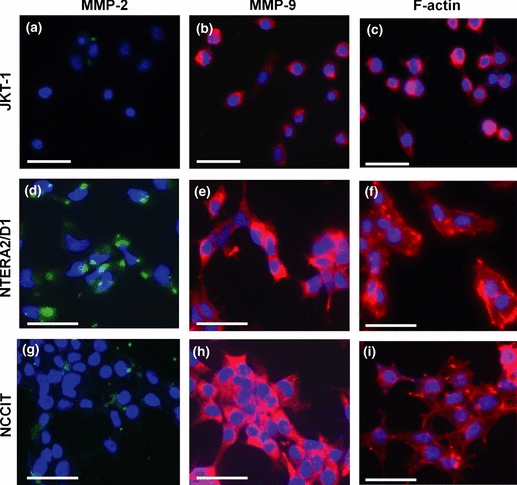
Subcellular localization of MMP-2 and MMP-9 in testicular tumour cell lines. Cellular localization of MMP-2 (green) (a, d and g), MMP-9 (red) (b, e and h) and F-actin (red) (c, f and i) in JKT-1 (a–c), NTERA2/D1 (d–f) and NCCIT (g–i) tumour cell lines. Nuclear counterstaining was performed with DAPI (blue). Bars, 25 μm.
Phalloidin-TRITC staining of actin cytoskeleton on the tumour cells seeded on matrigel showed the formation of fillopodia and lamellipodia, consistent of the migratory phenotype, in NTERA2/D1 (Fig. 3f) and NCCIT cells (Fig. 3i), but not in seminoma cells (Fig. 3c). Similar results were obtained when cells were seeded on collagen type I.
Imbalance of MMP-2 and MMP-9 vs. TIMP-1 and TIMP-2 expression in testicular tumour cell lines
The proteolytic potential of MMP-2 and MMP-9 is determined by the balance between their expression levels vs. that of TIMP-1 and TIMP-2. The expression of MMP-2 and MMP-9 as well as that of TIMP-1 and TIMP-2 was investigated in the testicular tumour cell lines JKT-1, NTERA2/D1 and NCCIT using both zymography/reverse zymography and qPCR.
Cells were cultured either on regular dishes or on collagen type I or matrigel pre-coated dishes and serum-free conditioned media were subjected to gelatin zymography (Fig. 4a). Secreted pro-MMP-2 and pro-MMP-9 were identified in all cell lines, but were more prominent in the embryonal carcinoma cells NTERA2/D1, where the active form of MMP-2 was also apparent. Neither the expression levels nor the expression pattern of the MMPs seemed to be affected by the matrix on which the cells were grown (Fig. 4a). In view of the increased expression of TGFβ in testicular neoplasms (Cardillo et al., 1998), a growth factor known to regulate MMPs’ expression, we examined its effect on the expression of MMP-2 and MMP-9 in testicular tumour cells. However, in all three cell lines, no significant change in the expression of these MMPs could be observed when cells were treated with 10 ng/mL TGFβ (data not shown).
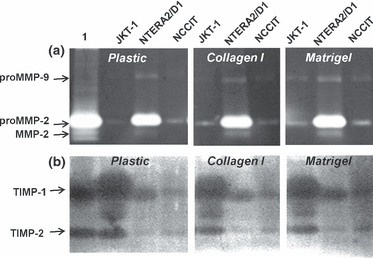
Protein levels of matrix metalloproteinases (MMPs) and TIMPs in testicular tumour cell lines. (a) Gelatin zymography and (b) reverse gelatin zymography analyses of culture media derived from seminoma (JKT-1), embryonal carcinoma (NTERA2/D1) and teratocarcinoma (NCCIT) cells cultured on plastic, collagen type I and matrigel. Lane 1 represents standard recombinant pro-MMP-2/MMP-2 (a) or a mixture of TIMP-1 / TIMP-2 (b).
Reverse zymography analysis of serum-free conditioned medium shows that both TIMP-1 and TIMP-2 are highly expressed in the seminoma cells and are markedly lower in NTERA2/D1 and NCCIT cells (Fig. 4b). The lowest level of TIMP-1 was identified in NCCIT cells. No differences were noticed when cells were cultured on either collagen type I or on matrigel (Fig. 4b).
The gene expression of MMP-2, MMP-9, TIMP-1 and TIMP-2 was then investigated in these testicular tumour cell lines by qPCR. Results show that the non-seminomatous tumour cell lines (NTERA2/D1 and NCCIT) expressed significantly higher levels of mRNA coding for MMP-2 (Fig. 5a) and MMP-9 (Fig. 5b) than the seminoma JKT-1 cells. Mean ratios of the MMP gene transcript relative to β2M were 175.4 ± 12.2 for MMP-2 and 2.7 ± 0.5 for MMP-9 in NTERA2/D1 embryonal carcinoma; 3.3 ± 0.1 for MMP-2 and 2.1 ± 0.2 for MMP-9 in NCCIT teratocarcinoma and 0.3 ± 0.04 for MMP-2 and 0.41 ± 0.01 for MMP-9 in JKT-1 seminoma cells. These data showed 585- and 11-times higher levels for MMP-2 and 6.5- and 5-times for MMP-9 in NTERA2/D1 and NCCIT, respectively, compared with JKT-1.
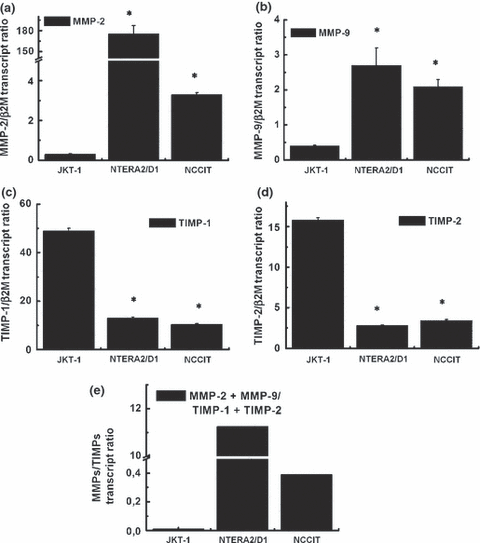
Expression of matrix metalloproteinases (MMPs) and TIMPs in testicular tumour cell lines. qPCR analysis of MMP-2 (a), MMP-9 (b), TIMP-1 (c) and TIMP-2 (d) gene expression in JKT-1, NTERA2/D1 and NCCIT tumour cell lines. (*) Statistically significant differences (p < 0.01) compared with the expression level in JKT-1 cells. (e) Ratio of expression levels of MMP-2 and MMP-9 vs. TIMP-1 and TIMP-2 in the three cell lines.
Transcript ratio of TIMP-1 (Fig. 5c) and TIMP-2 (Fig. 5d) was much higher in the JKT-1 (49.3 ± 1.1 for TIMP-1 and 15.8 ± 0.3 for TIMP-2) compared with that of NTERA2/D1 (13 ± 0.5 for TIMP-1 and 2.8 ± 0.02 for TIMP-2) and NCCIT (10.4 ± 0.3 for TIMP-1 and 3.4 ± 0.02 for TIMP-2).
Collectively, these data revealed imbalance in the MMP-2 and MMP-9 vs. TIMP-1 and TIMP-2 gene expression, with NTERA2/D1 and NCCIT cells exhibiting higher ratios of MMP-2 + MMP-9/TIMP-1 + TIMP-2 (11.3 and 0.39 respectively) compared with JKT-1 cells (0.01) (Fig. 5e).
Invasion potential of testicular tumour cells
When examined for their ability to invade through Matrigel, the non-seminomatous cells, which show higher MMP-2 and MMP-9 vs. TIMP-1 and TIMP-2 ratios, were found to be more invasive with NCCIT showing the highest rate of invasion compared with NTERA2 / D1. The seminoma JKT-1 cells were the least invasive (Fig. 6). The addition of TIMP-1, TIMP-2 or Marimastat, a pharmacological inhibitor of MMPs that has been developed as a potential antitumour drug and predominantly targets MMP-2 and MMP-9, inhibited the invasion of all testicular tumour cell lines (Fig. 6). This indicates that MMP-2 and MMP-9 play a key role in invasiveness of testicular tumour cells. The inhibition was significantly higher in embryonal carcinoma (NTERA2 / D1) compared with NCCIT suggesting that MMP-2 and MMP-9 may contribute to a higher extent in the invasiveness of NTERA2/D1 cells (Fig. 6). It is noteworthy that these two cell types exhibited a higher ratio of MMP-2 and MMP-9 vs. TIMP-1 and TIMP-2. Accordingly, modifying this ratio by the addition of recombinant TIMPs had a greater inhibitory effect on the invasiveness of these cells.
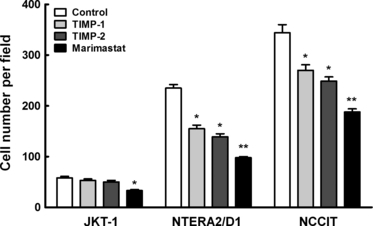
Invasiveness of testicular tumour cell lines is regulated by matrix metalloproteinases inhibitor. Quantification of testicular tumour cells invaded through matrigel in the absence or presence of 2.5 μg/mL TIMP-1, 2.5 μg/mL TIMP-2 or 10 μm marimastat. Statistically significant differences (*p < 0.05, **p < 0.01) compared with cells invaded in the absence of any agent. Experiments were performed in triplicates.
Discussion
The importance of MMP-2 and MMP-9 in tumour biology has been concluded from the correlation of their expression with tumour progression (Vaisanen et al., 1999; Farias et al., 2000; Inoue et al., 2002; Ranuncolo et al., 2002, 2003; Mitropoulou et al., 2003; Talvensaari-Mattila et al., 2003; Trudel et al., 2003; Juuti et al., 2006; Kousidou et al., 2008; Liu et al., 2010). Their role in the invasive capacity of testicular tumour cells has not previously been reported in either in vitro or in situ studies. In a recent study, MMP-2/TIMP-2 ratio in the serum of patients with TGCTs was reported to be higher in patients than in controls, thus implicating MMP-2 in this type of malignancy (Diamantopoulos et al., 2010). Another study reported the up-regulation of both MMP-2 and MMP-9 in experimental teratocarcinoma models and their implication in neoangiogenesis (Silvan et al., 2010).
The present study suggests a determining role for MMP-2 and MMP-9 in the invasive phenotype of testicular tumours. This is based on the significantly higher levels of MMP-2 and MMP-9 that we observed in the different types of human testicular tumoural tissues, seminoma, embryonal carcinoma and teratocarcinoma, compared with normal testes. In vitro studies on three cell lines corresponding to these tumour types have also shown that the more invasive cells derived from NSGCTs, such as embryonal carcinoma NTERA2/D1 and teratocarcinoma NCCIT, expressed more MMP-2 and MMP-9 and less TIMP-1 and TIMP-2 than the less invasive seminoma JKT-1 cells. Hence, the proteolytic potential, reflected by the balance of expression of MMP-2 and MMP-9 vs. that of TIMP-1 and TIMP-2, correlates with their invasive capacity in vitro. The importance of the MMP/TIMP ratio in the invasive characteristics of these cells was demonstrated by the greater inhibition of invasion observed in the more invasive non-seminoma cells after rec-TIMPs addition. Furthermore, it is consistent with the clinical characteristics associated with these testicular tumours. Indeed, patients with NSGCTs have worst prognosis, their tumours are more aggressive than seminomas at diagnosis and the proportion of metastatic to localized tumours is often higher for non-seminomas than seminomas (Diez-Torre et al., 2004; Bray et al., 2006). Furthermore, the detection of the active forms of MMP-2 and MMP-9 in the zymogram of the tumour extracts but not in the extract of normal testes is a further manifest for their action within the tumour in vivo. The presence of the active enzyme in the tumour also suggests lower TIMP levels as TIMPs can also inhibit MMP activation, in particular that of MMP-2 which depends on the activity of other MMPs such as the membrane type MMP (MT1-MMP). The nature of MMP-2 or MMP-9 substrates or the mechanism by which these MMPs may promote TGCTs invasion remains to be elucidated.
Several factors were implicated in the regulation of the different MMPs or TIMPs, including mainly cytokines and growth factors. Sustained TGF-β signalling was shown to significantly increase MMP-9 expression in prostate and head and neck cancer cells (Wick et al., 2001; Diez-Torre et al., 2004; Bray et al., 2006; Sinpitaksakul et al., 2008), and MMP-2 expression in pancreatic, bladder and prostate cancer (Sehgal & Thompson, 1999; Ellenrieder et al., 2001; Dehnavi et al., 2009). However, in our study TGF-β treatment of cells had no significant effect on their level of expression in any of the cell lines. In addition, culturing the cells on collagen type I or on matrigel, which contains predominantly laminin, had no effect on their expression either. EMMPRIN/CD147, which is known to induce certain MMPs and is over-expressed in most cancer tissues may represent a potential regulatory factor in TGCTs, although it is normally without effect on TIMP expression (Huet et al., 2008).
The role of TIMPs in tumour biology appears more enigmatic. Their role in the inhibition of MMPs and hence in tumour invasiveness is accepted as a general rule. However, several studies have also shown a positive correlation of TIMPs with tumour progression in various cancer types such as head and neck squamous carcinoma (Ruokolainen et al., 2005), colorectal (Inagaki et al., 2010), prostate (Oh et al., 2011), ovarian (Rauvala et al., 2005), lung (Gouyer et al., 2005), oral (Katayama et al., 2004) and bladder (Grignon et al., 1996) cancers. This may suggest that TIMPs have other roles independent of MMP inhibition. However, the inverse correlation that we observed between TIMP-1 and TIMP-2 levels and the invasion potential in TGCTs suggests that their roles in this case may be primarily to inhibit MMP activity, at least in cell culture conditions. This is in agreement with the greater inhibition of invasion obtained with the rec-TIMPs or with the MMP inhibitor marimastat in the cells containing less TIMP-1 and TIMP-2 and more MMP-2 and MMP-9 (NTERA2/D1 and NCCIT).
The selection of treatment strategy for patients with testicular tumours is at present based on histopathological criteria, which permit the distinction between seminomas and the more aggressive NSGCTs. The higher MMP levels expressed by the more aggressive NSGCTs cell lines suggest that these enzymes and their proteolytic potential may provide additional biological markers of prognostic significance. Such molecules, which are crucial effectors for invasion and metastatic potential may also be useful in future for the pharmacological targeting of the disease.
Acknowledgements
This research has been co-financed by the European Union (European Regional Development Fund – ERDF) and Greek national funds through the Operational Programme ‘Regional Operational Programme’ of the National Strategic Reference Framework (NSRF) – Research Funding Programme: Support for research, technology and innovation actions in Region of Western Greece.
Conflict of interest
No conflict of interest exists for any of the above named authors contributing to this work.
Prior publication
No part of this work has been previously published.




