Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line
Summary
Calcitonin gene-related peptide (CGRP) is widely distributed and plays important roles in a wide array of biological functions. It is enriched in primary sensory neurons and hence involved in nociception and neurogenic inflammation. Recent studies have shown that CGRP can be produced by immune cells such as monocytes/macrophages following inflammatory stimulation, suggesting a role in innate immunity. However, it is unclear how CGRP is up-regulated in macrophages and if it plays a role in macrophage functions such as the production of cytokines and chemokines. Using enzyme-linked immunosorbent assay (ELISA) and multiplex ELISA, lipopolysaccharide (LPS) was found to induce CGRP in the RAW 264.7 macrophage cell line. LPS-induced inflammatory mediators such as nerve growth factor (NGF), interleukin-1β (IL-1β), IL-6, prostaglandin E2 (PGE2) and nuclear factor-κB (NF-κB) signalling are involved in inducing CGRP, whereas the NGF receptor trkA and CGRP receptor signalling pathways are unexpectedly involved in suppressing LPS-induced CGRP, which leads to the fine-tune regulation of CGRP release. Exogenous CGRP and CGRP receptor antagonists, in a concentration-dependent manner, stimulated, inhibited or had no effect on basal or LPS-induced release of monocyte chemoattractant protein-1, IL-1β, IL-6, tumour necrosis factor-α and IL-10 in RAW macrophages. The ligand-concentration-dependent regulation of the production of inflammatory mediators by CGRP receptor signalling is a novel mechanism underlying the stimulating and suppressing role of CGRP in immune and inflammatory responses. Together, our data suggest that monocytes/macrophages are an important source of CGRP. Inflammation-induced CGRP has a positive or negative reciprocal effect on the production of other pro- and anti-inflammatory mediators. Thereby CGRP plays both facilitating and suppressing roles in immune and inflammatory responses.
Abbreviations:
-
- CGRP
-
- calcitonin gene-related peptide
-
- CLR
-
- calcitonin-like receptor
-
- COX2
-
- cyclo-oxygenase 2
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- IL-10
-
- interleukin-10
-
- IL-1β
-
- interleukin-1β
-
- IL-6
-
- interleukin-6
-
- LPS
-
- lipopolysaccharide
-
- MCP-1
-
- monocyte chemoattractant protein-1
-
- NF-κB
-
- nuclear factor-κB
-
- NGF
-
- nerve growth factor
-
- PGE2
-
- prostaglandin E2
-
- RAMP1
-
- receptor-activity-modifying protein 1
-
- TNFα
-
- tumour necrosis factor-α
-
- trkA
-
- tyrosine receptor kinase A
Introduction
Calcitonin gene-related peptide (CGRP) is a peptide derived from the alternative splicing of the calcitonin gene.1 It is widely distributed in both central and peripheral nervous systems and exerts a wide array of biological effects.2–4 In peripheral tissues, CGRP is particularly enriched in primary sensory neurons5 and plays an important role in nociception and neurogenic inflammation.6 CGRP has recently been shown to be produced by immune cells such as human B lymphocytes7,8 and monocytes,9 suggesting its role in the modulation of immune responses and inflammation. We have shown that CGRP is up-regulated in invading macrophages in injured rat sciatic nerves and, through an autocrine or paracrine mechanism it contributes to the up-regulation of the pro-inflammatory cytokine interleukin-6 (IL-6) in invading macrophages.10 In rat peritoneal macrophages, the endotoxin lipopolysaccharide (LPS) increased CGRP contents in a concentration-dependent manner.10 These data suggest that CGRP is produced by macrophages following inflammatory stimulation and its up-regulation in macrophages affects the functions of invading macrophages, hence influencing the outcome of inflammation.
Monocytes/macrophages are the main effector cells of the immune system and play an essential role in host defence mechanisms against infectious micro-organisms and tumour cells. By secreting numerous biologically active molecules, macrophages are involved not only in the regulation of the secondary immune response, but also in the process of inflammation and tissue repair. A growing body of evidence suggests that CGRP plays an important role in regulating the functions of macrophages, including the production of inflammation-related chemokines and cytokines. It is therefore important to understand how CGRP is up-regulated in macrophages during the inflammatory response and which functions of macrophages are modulated by CGRP. To address these issues, we used the RAW 264.7 murine macrophage cell line to obtain a large quantity of homogeneous macrophages and LPS as a prototype of inflammatory stimulus to examine the possible factors that can induce CGRP in RAW macrophages. The first aim of the present study was to determine whether LPS could induce CGRP in the RAW macrophage cell line.
Lipopolysaccharide has been reported to up-regulate the expression of inflammatory mediators such as IL-1β, tumour necrosis factor-α (TNFα), IL-6, nerve growth factor (NGF), inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2)/prostaglandin E2 (PGE2) in RAW264.7 macrophages.11–13 The nuclear factor-κB (NF-κB) signalling pathway is involved in LPS-induced production of inflammatory mediators in this cell line.12,14 As a transcription factor, NF-κB plays a key role in the transcriptional regulation of genes of numerous inflammatory mediators including iNOS, COX2, IL-1β, IL-6 and TNFα.15 Among the inflammatory mediators, NGF,7,9 IL-1β,16,17 IL-618 and TNFα19 have been shown to induce CGRP in human B lymphocytes, monocytes, sensory neurons and various other cell types. Hence, the second aim of this study was to investigate the inflammatory mediators likely to be involved in LPS-induced CGRP in RAW macrophages and whether LPS-induced CGRP is mediated through the NF-κB signalling pathway.
As NGF is able to induce CGRP expression in lymphocytes and monocytes,7,9 NGF tyrosine receptor kinase A (trkA) receptor signalling is also possibly involved in inducing CGRP in RAW macrophages. We have reported earlier that components of the CGRP receptor complex such as the calcitonin receptor-like receptor (CLR) and CGRP receptor activity modifying protein (RAMP1) are enriched in invading macrophages.10 In trigeminal ganglion cultures, CGRP was shown to induce its own gene expression and RAMP1 is able to enhance CGRP receptor activity.20 It would be of interest to establish if CGRP receptor signalling exerts an effect on LPS-induced CGRP in RAW macrophages. The third aim of our study was therefore to determine whether trkA and CGRP receptor signalling pathways are involved in LPS-induced CGRP.
In the literature, the role of CGRP in the production of pro- and anti-inflammatory chemokines and cytokines is controversial. Depending on the cell type and concentration, CGRP can either facilitate or suppress the production of these molecules.21–23 The fourth aim of this study was, using exogenous CGRP and CGRP receptor antagonists, to establish the possible role of CGRP receptor signalling in basal and LPS-induced pro-inflammatory chemokines such as the monocyte chemoattractant protein-1 (MCP-1), pro-inflammatory cytokines as IL-1β, IL-6 and TNFα, and the anti-inflammatory cytokine IL-10 in the RAW macrophage cell line.
In the present study we used an in vitro model of murine macrophage cell line culture and LPS as a prototype of inflammatory stimuli. Various inflammatory mediators such as PGE2 and CGRP; neutralizing antisera against NGF p75 receptor, trkA, RAMP1, CLR, IL-1β and IL-6; inhibitors of COX2, inhibitor of IκB, transcription and protein synthesis; peptide and non-peptide CGRP antagonists were used to determine their role in LPS-induced CGRP and other inflammatory mediators.
Materials and methods
Materials
RAW 264.7 macrophages were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Bacterial LPS (extracted from Escherichia coli, 90H4012) was purchased from Sigma (St Louis, MO). Mouse neutralizing antisera against IL-1β, IL-6 and NGF receptor chimera were purchased from R&D Systems (Minneapolis, MN). A neutralizing antiserum against NGF receptor trkA was obtained from Chemicon Inc. (Temecula, CA). Dulbecco’s modified Eagle’s minimum essential medium (DMEM), penicillin/streptomycin, heat inactivated fetal bovine serum (FBS) were obtained from Invitrogen Canada Inc. (Burlington, ON, Canada). Prostaglandin E2 and a selective COX2 inhibitor, NS-398, were purchased from Cayman Chemical Inc. (Ann Arbor, MN). Human CGRP and a CGRP1 receptor antagonist CGRP8-37 were gifts from Dr A. Fournier, Institut National de la Recherche Scientifique-Santé, Pointe Claire, QC, Canada.24 Non-peptide CGRP antagonist BIBN4096BS is a gift from Dr H. Doods, Boehringer Ingelheim, Germany.25 Goat antisera raised against CLR and RAMP1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antisera raised against CLR and RAMP1 were generous gifts from Dr N.W. Bunnett, University of California at San Francisco, CA. The characterization of both antisera was reported recently.26 An inhibitor of transcription of messenger RNA (mRNA), actinomycin-D and an inhibitor of protein synthesis, cycloheximide, were purchased from BioMol International, L.P. (Plymouth Meeting, PA). Extraction-free CGRP enzyme-linked immunosorbent assay (ELISA) Kits were purchased from Bachem (Torrance, CA).
Culture of RAW 264.7 macrophage cell line
RAW 264.7 macrophages were cultured and maintained in DMEM containing penicillin/streptomycin (1 : 200) and 10% heat-inactivated FBS in a 37° incubator with 5% CO2 and 95% air. Cells were seeded at the density of 3 × 105 to 5 × 105/ml. Passages of 5–20 were used for the treatments. Lipopolysaccharide (1–1000 μg/ml) was used to treat cells for 3, 6, 12, 24 and 48 hr. Neutralizing IL-1β antiserum (1 and 10 ng/ml), IL-6 antiserum (1 and 10 ng/ml), NGF receptor chimera (1·5 and 5 μg/ml), selective COX2 inhibitor NS-398 (10 and 20 μm), neutralizing antisera against NGF receptor trkA (1 : 1000), CLR antiserum (1 : 500 and 1 : 1000), RAMP1 (1 : 500 and 1 : 1000), PGE2 (1–30 μm), actinomycin-D (1 μm) and cycloheximide (1 μm) were used alone or in co-treatment with LPS (1 μg/ml). The PGE2 and NS-398 were dissolved in ethanol and prepared as 10-mm stock solutions. Co-treatments lasted for 24 hr. Culture media were collected and stored at −80° until further analysis. All treatments were performed in triplicate and each experiment was repeated at least three times.
ELISA of CGRP
Following treatment, culture media were collected in pyrogen-free Eppendorf tubes and frozen at − 80° or underwent ELISA immediately. An extraction-free CGRP ELISA Kit was used. All procedures were performed according to the manufacturer’s instructions and the microplate was read using a microplate reader (Molecular Devices, Sunnyvale, CA). The detection range for CGRP was 0–10 ng/ml. Each treatment was performed in triplicate for each experiment. The mean value of CGRP released in culture medium following treatments was compared statistically among groups.
Multiplex ELISA of MCP-1, IL-1β, TNFα, IL-6 and IL-10
The RAW 264.7 macrophages were maintained in DMEM containing penicillin/streptomycin (1 : 200) and 10% FBS. Cells were seeded at a density of 3 × 106 to 5 × 106/ml in 24-well culture plates. Passages of 5–20 were used for the following treatments. Vehicle, LPS (1 μg/ml), CGRP (1, 10 and 100 nm), CGRP8-37 (0·1, 1 and 10 μm) and BIBN4096BS (0·01, 0·1 and 1 μm) were used to treat cells for 24 hr. Culture media were collected and stored at − 80°. All samples were assayed for MCP-1, IL-1β, IL-6, TNFα and IL-10 according to the manufacturer`s instructions using Mouse Cytokine Lincoplex Kits (Linco Diagnostic Services Inc., St Charles, MO). Each treatment was repeated at least three times. The mean and SEM were determined for each treatment and compared statistically among groups.
Statistical analysis
Each treatment was performed in triplicate in each session of experiments. The mean value of each treatment was determined and compared statistically using one-way analysis of variance with post-hoc Dunnett’s multiple comparison method to compare more than two groups, e.g. vehicle versus treatments or LPS versus co-treatments. The significance level was set at P < 0·05.
Results
Activation of toll-like receptor-4 induced CGRP synthesis in RAW macrophages and possible regulatory mechanisms
Following treatments with LPS, CGRP release from cultured RAW 264.7 macrophages was measured using ELISA. At concentrations of 0·1 and 1 μg/ml LPS significantly increased CGRP release from cultured RAW 264·7 macrophages (Fig. 1a, P < 0·05 or < 0·01). Co-treatment of LPS with an inhibitor of protein synthesis, cycloheximide (1 μm), or with an inhibitor of mRNA transcription, actinomycin-D, abolished the LPS-induced CGRP release (Fig. 1a), suggesting that mRNA transcription and new protein synthesis are involved in the effect of LPS on CGRP release. The LPS-induced CGRP release from RAW macrophages was time-dependent, with LPS (1 μg/ml) treatment for 3 hr being ineffective whereas treatments for 6, 12, 24 and 48 hr induced significant increases (Fig. 1b, P < 0·05 or < 0·01). The LPS induces the maximum release of CGRP from RAW macrophages 24 hr after treatment.

Lipopolysaccharide (LPS) concentration- and time- dependently increased calcitonin gene-related peptide (CGRP) release from RAW macrophages. (a) LPS concentration- dependently increased CGRP release from RAW 264.7 macrophages. * indicates P < 0·05 or < 0·01, LPS versus vehicle. Co-treatment of LPS (1 μg/ml) with 1 μm cycloheximide (CHX) or 1 μm actinomycin-D (AMD) blocked LPS-induced CGRP release. (b) 1 μg/ml LPS time- dependently increased CGRP release. * indicates P < 0·05 or < 0·01, LPS versus vehicle. The time-point for LPS to induce the maximum CGRP release was 24 hr. Mean ± SEM, n = 3.
To explore whether NGF, IL-1β, IL-6 and COX2-derived PGE2 are involved in LPS-induced CGRP release, we used co-treatment of LPS with a NGF sequester (NGF receptor Fc chimera), neutralizing antisera against IL-1β or IL-6, and a selective COX2 inhibitor (NS-398). Co-treatment of LPS with the NGF receptor Fc chimera (1·5 and 5 μg/ml) significantly suppressed LPS-induced CGRP release (Fig. 2a, P < 0·05). When co-treated with LPS, neutralizing antisera against IL-1β (1 and 10 ng/ml) or IL-6 (1 and 10 ng/ml) significantly suppressed LPS-induced CGRP release (Fig. 2a, P < 0·001). The selective COX2 inhibitor NS-398 (10 and 20 μm) also significantly suppressed LPS-induced CGRP release (Fig. 3a, P < 0·05). Moreover, 10, 20 and 30 μm exogenous PGE2 on its own significantly increased CGRP release from RAW macrophages compared with vehicle treatment (Fig. 3b, P < 0·05) whereas 1 μm PGE2 had no effects. Exogenous PGE2 also significantly enhanced LPS-induced CGRP release (Fig. 3b, P < 0·05). Co-treatment of PGE2 with the transcription inhibitor actinomycin-D (1 μm) or the inhibitor of protein synthesis, cycloheximide (1 μm), abolished PGE2-induced CGRP release from RAW macrophages, suggesting that PGE2 induces CGRP in RAW macrophages at both gene and protein levels.
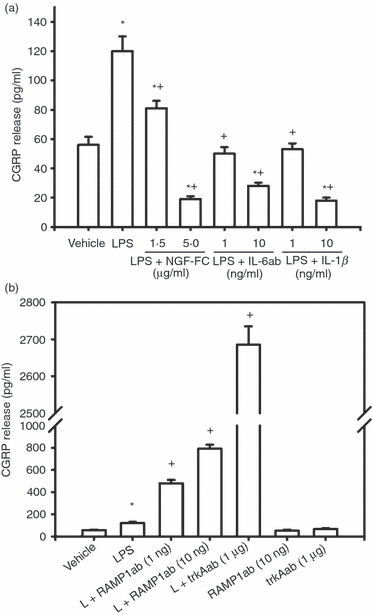
Possible inflammatory mediators involved in lipopolysaccharide (LPS) -induced calcitonin gene-related peptide (CGRP) release from RAW macrophages. LPS (1 μg/ml) significantly increased CGRP release from RAW macrophages (a, b, *P < 0·001). (a) Co-treatment of LPS with 1·5 and 5 μg/ml nerve growth factor (NGF) receptor Fc chimera (sequestering NGF), 1 and 10 ng/ml interleukin-6 (IL-6) antiserum or 1 and 10 ng/ml IL-1β antiserum significantly suppressed LPS-induced CGRP release. Higher concentrations of NGF receptor Fc chimera, IL-6 and IL-1β further suppressed CGRP release to lower levels than vehicle. * indicates P < 0·05 or P < 0·001, antisera + LPS versus vehicle. + indicates P < 0·001, antisera + LPS versus LPS. (b) Co-treatment of LPS (L) with receptor-activity-modifying protein 1 (RAMP1) antiserum (1 and 10 ng/ml) or tyrosine receptor kinase A (trkA) receptor antiserum (1 μg/ml) significantly enhanced LPS-induced CGRP release compared with LPS treatment (+P < 0·001). RAMP1 and trkA antisera by themselves had no effect. Mean ± SEM, n = 3.
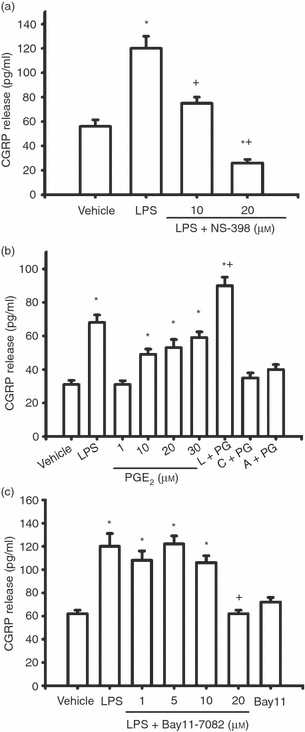
Cyclooxygenase 2 (COX2) -derived prostaglandin E2 (PGE2) and transcription factor nuclear factor-κB (NF-κB) are involved in lipopolysaccharide (LPS) -induced calcitonin gene-related peptide (CGRP) release from RAW macrophages. LPS (1 μg/ml) significantly increased CGRP release from RAW macrophages (a–c, *P < 0·001). (a) Co-treatment of LPS with the selective COX2 inhibitor, NS-398, significantly suppressed LPS-induced CGRP release. * indicates P < 0·05 or < 0·001, NS-398 + LPS or LPS versus vehicle. + indicates P < 0·05 or < 0·001, NS-398 + LPS versus LPS. (b) PGE2 concentration- dependently induced CGRP release or enhanced LPS (L) -induced CGRP release. * indicates P < 0·05 or < 0·001, LPS, PGE2 or PGE2 + LPS versus vehicle. + indicates P < 0·05, PGE2 + LPS versus LPS. Co-treatment of 30 μm PGE2 (PG) with 1 μm cycloheximide (c) or 1 μm actinomycin-D (a) blocked PGE2-induced CGRP release. (c) Bay11-7082 concentration dependently suppressed LPS-induced CGRP release. * indicates P < 0·05, Bay11-7082 + LPS versus vehicle. + indicates P < 0·01, Bay11-7082 + LPS versus LPS. Mean ± SEM, n = 3.
To explore whether NF-κB is involved in LPS-induced CGRP release, we used Bay 11-7082, an inhibitor of IκB phosphorylation, a process known to release NF-κB from binding to IκB and to facilitate the nuclear translocation of NF-κB. Bay 11-7082 suppressed LPS-induced CGRP release concentration-dependently (Fig. 3c, P < 0·05), but had no effects on CGRP release by itself.
Unexpectedly, co-treatment of LPS with a neutralizing antiserum against the CGRP receptor component RAMP1 or NGF trkA receptor dramatically enhanced LPS-induced CGRP release from RAW macrophages (Fig. 2b, P < 0·001). Treatments with RAMP1 antiserum or trkA antiserum alone had no effects on CGRP release. Either co-treated with LPS or by itself, an antiserum against CGRP receptor component CLR (1 : 500 to 1 : 1000) did not induce any significant change in CGRP release compared with vehicle (not shown). Two commercially available antisera against CLR and RAMP1 (Santa Cruz Biotechnology) induced similar effects on CGRP release when co-treated with LPS or alone (not shown).
The effects of CGRP and CGRP receptor antagonists on basal and LPS-induced release of chemokines and cytokines in RAW macrophages
We explored next whether exogenous CGRP is able to affect basal and LPS-induced release of pro-inflammatory and anti-inflammatory chemokines and cytokines and whether LPS-induced endogenous CGRP is involved in the release of these chemokines and cytokines. At a concentration of 1 μg/ml, LPS significantly increased the release of MCP-1, IL-1β, IL-6, TNFα and IL-10 from cultured RAW macrophages (4, 5, P < 0·001).
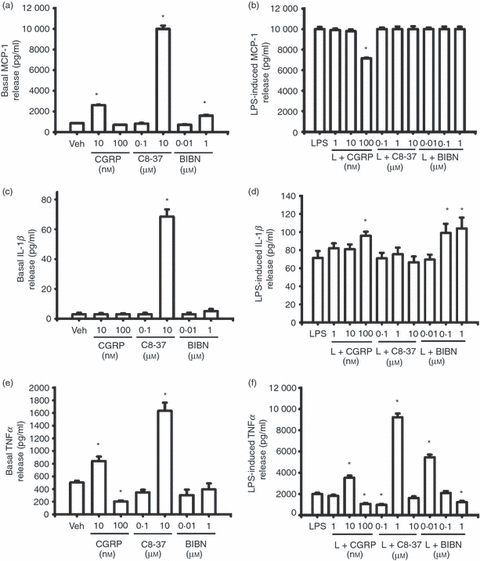
Effects of exogenous calcitonin gene-related peptide (CGRP), CGRP8-37 and BIBN4096BS on basal and lipopolysaccharide (LPS) -induced release of monocyte chemotactic protein 1 (MCP-1), interleukin-1β (IL-1β) and tumour necrosis factor-α (TNFα) from RAW 264.7 macrophages. Compared with vehicle treatment, LPS (1 μg/ml) significantly increased MCP-1 (b), IL-1β (d) and TNFα (c) release from RAW macrophages (P < 0·001). (a) and (b) show the effect of CGRP, CGRP8-37 and BIBN4096BS on basal and LPS-induced MCP-1 release. * indicates P < 0·05, P < 0·01 or < 0·001, treatment versus vehicle (a) or treatment versus LPS (b). (c) and (d) show the effect of CGRP, CGRP8-37 and BIBN4096BS on basal and LPS-induced IL-1β release. * indicates P < 0·05 or < 0·001, treatment versus vehicle (c) or treatment versus LPS (d). (e) and (f) show the effect of CGRP, CGRO8-37 and BIBN4096BS on basal and LPS-induced TNFα release. * indicates P < 0·05, P < 0·01 or < 0·001, treatment versus vehicle (e) or treatment versus LPS (f). Mean ± SEM, n = 3.
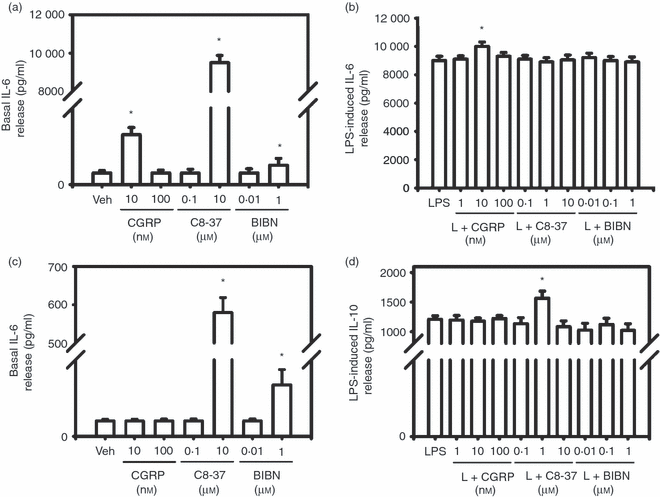
Effects of exogenous calcitonin gene-related peptide (CGRP), CGRP8-37 and BIBN4096BS on basal and lipopolysaccharide (LPS) -induced release of interleukin-6 (IL-6) and IL-10 from RAW 264.7 macrophages. Compared with vehicle treatment, LPS (1 μg/ml) significantly increased IL-6 (b) and IL-10 (d) release from RAW macrophages (P < 0·001). (a) and (b) show the effect of CGRP, CGRP8-37 and BIBN4096BS on basal and LPS-induced IL-6 release. * indicates P < 0·05 P < 0·01 or < 0·001, treatment versus vehicle (a) or treatment versus LPS (b). (c) and (d) show the effect of CGRP, CGRP8-37 and BIBN4096BS on basal and LPS-induced IL-10 release. * indicates P < 0·05, P < 0·01 or P < 0·001, treatment versus vehicle (c) or treatment versus LPS (d). Mean ± SEM, n = 3.
Compared to vehicle, 10 nm CGRP significantly increased basal MCP-1 release (Fig. 4a, P < 0·01), an event reversed by 10 nm CGRP8-37 (not shown), whereas 100 nm had no effect. At the lower concentrations, both CGRP8-37 (0·1 μm) and BIBN4096BS (0·01 μm) by themselves had no effects on basal MCP-1 release from RAW cells (Fig. 4b,c). A higher concentration of CGRP8-37 (10 μm) or BIBN4096BS (1 μm) significantly increased basal MCP-1 release (Fig. 4a, P < 0·05 or P < 0·001). When co-treated with LPS, 1 and 10 nm CGRP had no effects on LPS-induced MCP-1 whereas 100 nm CGRP dramatically suppressed LPS-induced MCP-1 release (Fig. 4b, P < 0·05). To determine if endogenous CGRP induced by LPS in RAW macrophages is involved in LPS-induced release of MCP-1, both peptide CGRP receptor antagonist CGRP8-37 and non-peptide antagonist BIBN4096BS were used with LPS to co-treat RAW macrophages. Either CGRP8-37 or BIBN9069BS at all concentrations had no effect on LPS-induced MCP-1 release (Fig. 4b).
Compared with vehicle treatment, both low and high concentrations of CGRP by itself had no effect on basal IL-1β release from RAW macrophages (Fig. 4c). The higher concentration of CGRP8-37 alone significantly increased basal IL-1β release (Fig. 4c, P < 0·001) but the lower concentration had not effect. BIBN4096BS at either low or high concentration by itself had no effects on basal IL-1β release (Fig. 4c). When co-treated with LPS, 100 nm CGRP significantly enhanced LPS-induced IL-1β release (Fig. 4d, P < 0·05) although the lower concentrations had no effect. CGRP8-37 at all concentrations had no effect on LPS-induced IL-1β release (Fig. 4d). Although 0·1 and 1 μm BIBN4096BS significantly enhanced LPS-induced IL-1β release (Fig. 4d, P < 0·05), treatment with 0·01 μm BIBN4096BS was ineffective.
At a lower concentration, exogenous CGRP (10 nm) by itself significantly increased TNFα release (Fig. 4e, P < 0·05), an event reversed by 10 nm CGRP8-37 (not shown). However, the higher concentration of CGRP (100 nm) significantly suppressed basal TNFα release from RAW macrophages (Fig. 4e, P < 0·05). When used alone, 0·1 μm CGRP8-37 had no effect on basal TNFα release whereas 10 μm CGRP8-37 significantly increased basal TNFα release compared with vehicle treatment (Fig. 4e, P < 0·05). When used alone, 0·01 and 1 μm BIBN4096BS had no effects on basal TNFα release (Fig. 4e). When used in co-treatment with LPS, 1 nm CGRP had no effect on TNFα release whereas 10 nm CGRP induced a significant increase (Fig. 4f, P < 0·001). In contrast, 100 nm CGRP markedly suppressed LPS-induced TNFα release (Fig. 4f, P < 0·05). CGRP8-37 (100 nm) significantly suppressed LPS-induced TNFα release (Fig. 4f, P < 0·05) wherease 1 μm CGRP8-37 significantly enhanced LPS-induced TNFα release (Fig. 4f, P < 0·001). However, 10 μm CGRP8-37 had no effect on LPS-induced TNFα release. At a lower concentration, BIBN4096BS (0·01 μm) significantly enhanced LPS-induced TNFα release (Fig. 4f, P < 0·001). At concentrations of 0·1 and 1 μm, BIBN4096BS had no effect or significantly reduced LPS-induced TNFα release, respectively (Fig. 4f, P < 0·05).
Compared with vehicle, 10 nm CGRP significantly increased basal IL-6 release (Fig. 5a, P < 0·05), an effect reversed by 10 nm CGRP8-37 (not shown) while 100 nm CGRP had no effect. When treated alone, 0·1 μm CGRP8-37 had no effect while 10 μm CGRP8-37 significantly increased basal IL-6 release (Fig. 5a, P < 0·001). At the lower concentration, 0·01 μm BIBN4096BS had no effect on basal IL-6 release while 1 μm BIBN4096BS significantly increased the release (Fig. 5a, P < 0·05). Compared with LPS treatment, only 10 nm CGRP significantly enhanced LPS-induced IL-6 release (Fig. 5b, P < 0·05) whereas 1 and 100 nm CGRP had no effects. Neither CGRP8-37 nor BIBN4096BS at all concentrations had any effect on LPS induced IL-6 release (Fig. 5b).
Either alone or co-treated with LPS, 1, 10 and 100 nm CGRP had no effect on basal or LPS-induced IL-10 release from RAW macrophages (Fig. 5c,d). When treated alone, 0·1 μm CGRP8-37 had no effect on basal IL-10 release whereas 10 μm CGRP8-37 significantly increased basal release of IL-10 from RAW cells (Fig. 5c, P < 0·001). When treated alone, 0·01 μm BIBN4096BS had no effect while 1 μm BIBN4096BS significantly increased basal release of IL-10 from RAW macrophages (Fig. 5c, P < 0·01). At concentrations of 0·1 and 10 μm, CGRP8-37 had no effect on LPS-induced IL-10 release whereas 1 μm CGRP8-37 significantly enhanced LPS-induced IL-10 release (Fig. 5d, P < 0·05). At all concentrations, BIBN4096BS had no effect on LPS-induced IL-10 release (Fig. 5d).
Discussion
LPS induces CGRP release in RAW macrophages
In the present study, we demonstrated that LPS, in a concentration- and time-dependent manner, increased CGRP release from RAW 264.7 macrophages. The LPS-induced CGRP release was blocked by the inhibitors of transcription and protein synthesis, suggesting that the effect of LPS occurs at both transcription and translation levels. The finding that LPS can induce CGRP release in RAW macrophages is consistent with earlier reports showing that LPS facilitates the production of CGRP in cultured rat peritoneal macrophages10 and in human monocytes.9 Interestingly, serum CGRP levels are dramatically increased in patients suffering from septic shock and have been considered as a predictive indicator of lethal outcome.27 Accordingly, monocytes/macrophages should be considered as an important source of increased levels of CGRP in serum during sepsis and in inflamed tissues (in addition to CGRP containing sensory nerve terminals innervating inflamed tissues and blood vessels). Increased CGRP levels in inflamed tissues play an important role in neurogenic inflammation as well as in immune responses initiated by immune cells.2 Based on the literature, the role of CGRP in the development of immune and inflammatory responses could be either facilitating or suppressing depending on the dynamics of immune and inflammatory process. Concentration-dependent regulation of the production of pro-inflammatory and anti-inflammatory mediators by CGRP might underlie the positive or negative role of CGRP in immune and inflammatory responses (see discussion below).
Possible mediators involved in modulating LPS-induced CGRP in macrophages
In the present study, we explored further the inflammatory mediators that are possibly involved in LPS-induced CGRP synthesis in RAW macrophages. We found that the NGF sequester (NGF receptor Fc chimera) is able to suppress LPS-induced CGRP release from RAW macrophages, suggesting a role for this neurotrophin in the up-regulation of CGRP induced by LPS. This hypothesis is consistent with previous reports showing that NGF is involved in LPS-induced synthesis of CGRP in human B lymphocytes and monocytes.7,9 Moreover, NGF and its receptors are induced in human monocytes28 and rat microglia29 following LPS treatments.
As shown earlier,11–13 and in the current study as well, LPS (1 μg/ml) dramatically increased the release of IL-1β and IL-6 from RAW macrophages. It has previously been shown that IL-1β acts as a potent inducer of CGRP in various types of cells16,17 and IL-6 facilitates the release of CGRP from nociceptive sensory terminals in the skin.18 We observed here that neutralizing antisera against IL-1β and IL-6 are able to suppress LPS-induced CGRP release, suggesting that these two cytokines can regulate the synthesis of CGRP in RAW macrophages. Although here we did not explore the role of TNFα in LPS-induced CGRP release, this cytokine is also likely to be involved because it has been shown to stimulate the synthesis of CGRP in trigeminal ganglion neuron cultures.19 Exogenous CGRP enhanced LPS-induced release of IL-1β, IL-6 and TNFα concentration-dependently (the present study). Accordingly, the three cytokines and CGRP may have reciprocal facilitating effects on their synthesis. Such a mechanism would enable the rapid establishment of networks of inflammatory mediators required during inflammatory responses.
A selective COX2 inhibitor NS-398 was also able to suppress LPS-induced CGRP release, suggesting a role for COX2-derived prostanoids in our model. These data are consistent with a prior ex vivo study showing that LPS- and IL-1β-induced CGRP release from rat trachea is a prostanoid-dependent phenomenon.30 Moreover, LPS was shown to induce the up-regulation of COX2/PGE2 in RAW macrophages.31 The effects of a brief (10 min) treatment with PGE2 on CGRP release from dorsal root ganglion cultures have been reported before.32,33 We observed here that longer PGE2 treatment (24 hr) induced or enhanced LPS-stimulated CGRP release from RAW macrophages. As PGE2-induced CGRP release was blocked by the co-treatment with actinomycin-D or cycloheximide, de novo mRNA transcription and protein synthesis are most likely involved. These findings suggest that long-term PGE2 treatment may not only increase the release of CGRP, but also its transcription and synthesis in RAW macrophages. However, the PGE2 EP receptor subtype(s) involved here, as well as downstream signal transduction pathways, requires further studies.
In parallel with previous reports showing that NF-κB is involved in LPS-induced production of inflammatory mediators in monocytes/macrophages,12,34 co-treatment of LPS with an inhibitor of IκB phosphorylation suppressed LPS-induced CGRP release. This finding suggests that the NFκB signalling pathway is involved in LPS-induced CGRP synthesis in RAW macrophages. Our data are comparable to those in a previous report showing that NF-κB plays a role in IL-1β-induced CGRP secretion from human alveolar epithelial cells.16 However, how NF-κB mediates LPS-induced synthesis of CGRP has yet to be fully established.
Unexpectedly, we found that CGRP receptor accessory protein RAMP1 and NGF/trkA receptor signalling were negatively involved in LPS-induced CGRP synthesis. The CGRP receptor is a rather unique G protein-coupled receptor, because it shares a seven trans-membrane domain protein, CLR, with adrenomedullin (AM, a peptide member in the CGRP superfamily) and also requires accessory protein RAMP1 to be functional. The RAMPs are essential accessory proteins to chaperone CLR to the cell surface, which determines the receptor specificity.35 RAMP1 enables CLR to form CGRP receptor while RAMP2 and RAMP3 enable CLR to form AM1 and AM2 receptors,36 respectively. To our surprise, neutralizing antisera against either CGRP/RAMP1 or NGF/trkA receptor dramatically enhanced LPS-induced CGRP release, suggesting that RAMP1 and trkA exert negative feedback effects on the synthesis of CGRP. Neutralizing trkA or RAMP1 antiserum on their own had no effects on basal CGRP release from RAW macrophages, suggesting that the negative feedback action of trkA or RAMP1 occurs only when NGF or CGRP is up-regulated by inflammatory stimuli. Accordingly, when NGF or CGRP is increased, activation of RAMP1 or trkA receptor signalling can exert an inhibitory action on CGRP synthesis in RAW macrophages. This hypothesis is supported by a recent report showing that levels of serum CGRP in homozygous RAMP1-deficient mice were dramatically and transiently increased following peritoneal LPS challenge.37 RAMP1 by itself may have a fine tuning inhibitory effect on the synthesis of CGRP. Future studies to investigate LPS-induced CGRP synthesis in monocytes/macrophages of RAMP1 over-expressing transgenic mice20 and knockout mice37 should verify this hypothesis.
Role of CGRP receptor signalling in basal and LPS-induced pro-inflammatory and anti-inflammatory chemokines and cytokines
In the present study, we have used exogenous CGRP, peptide CGRP receptor antagonist CGRP8-37 and non-peptide CGRP receptor antagonist BIBN4096BS, to establish the possible role of CGRP receptor signalling in basal and LPS-induced pro-inflammatory and anti-inflammatory chemokines and cytokines in the RAW 264.7 macrophage cell line. The affinities of αCGRP, CGRP8-37 and BIBN4096BS to bind human CGRP receptors have been well established, with the affinities BIBN4096BS (Ki = 14·4 ± 6·3 pm) > αCGRP (Ki = 31·7 ± 1·6 pm) > CGRP8-37 (Ki = 3·6 ± 0·7 nm), respectively.25 Hence, the physiological concentrations for both CGRP and BIBN4096BS are within nm range25 whereas for CGRP8-37, it is within the μm range.38 We used the physiological range of concentrations of the antagonists in the current study. The mechanisms underlying the blocking activities of both antagonists on CGRP receptors are rather different. Since CGRP8-37 peptide includes all but the first seven amino acids at the C-terminal of CGRP, it works as a competitive antagonist to block the binding of full-length CGRP to its receptor. In contrast, the specific affinities of BIBN4096BS depend on its interaction with the RAMP1 subunit of CGRP receptor.39
From the literature, the role of CGRP in the induction of pro-inflammatory and anti-inflammatory chemokines and cytokines is controversial.21–23 In these studies, depending on the cell type and concentration, CGRP exhibits either stimulating or suppressing effect on the production of MCP-1, IL-1β, TNFα, IL-6 and IL-10. Consistently, CGRP receptor signalling in the current study also demonstrates positive or negative effects on basal and LPS-induced release of these inflammatory mediators depending on the concentration of CGRP and CGRP receptor antagonists. Generally speaking, a lower concentration of CGRP seems to facilitate the basal release of MCP-1, TNFα and IL-6 but had no effect on the basal release of IL-1β and IL-10. The facilitating effects were blocked by a lower concentration of CGRP8-37 (10 nm), suggesting that CGRP receptor mediates the effect. In contrast, a higher concentration of CGRP suppressed basal TNFα release but had no effect on others.
Contrary to the effect of CGRP, a higher concentration of the peptide antagonist CGRP8-37 significantly increased the basal release of all chemokines and cytokines examined, but the lower concentration had no effect at all. Non-peptide antagonist BIBN4096BS also manifested the same tendency. However, at higher concentration, it only significantly increased the basal release of MCP-1, IL-6 and IL-10 but had no effect on IL-1β and TNFα. Similar to CGRP8-37, a lower concentration of BIBN4096BS had no effect on the basal release of chemokines and cytokines. These data suggest that at steady state CGRP receptor signalling suppresses the basal release of these chemokines and cytokines in RAW macrophages. We have noticed that CGRP8-37 has a much stronger effect than BIBN4096BS on the basal release of these chemokines and cytokines. CGRP8-37 has been shown to bind both CGRP receptors (CLR/RAMP1) and AM2 receptors (CLR/RAMP3), whereas BIBN4096BS is more selective to CGRP receptor binding sites.40,41 Although it is unknown if AM receptors are present in RAW macrophages, CLR, RAMP1, RAMP2 and RAMP3 have been shown to exist in murine bone marrow macrophages.42 Adrenomedullin was also shown to exhibit both stimulating and inhibiting effects on the production of chemokines and cytokines in a macrophage cell line.43 It is therefore highly possible that some effects of CGRP8-37 on the basal release in the current study may be mediated through its action on AM2 receptors. BIBN4096BS has been shown to exhibit species affinity because it binds primate CGRP receptors with higher affinity (100 times) over binding rodent CGRP receptors.25,39 Alternatively, the discrepancy of the effects of CGRP8-37 and BIBN4096BS on the basal release here may also be interpreted as the lower affinity of BIBN4096BS in binding murine CGRP receptors in RAW macrophages.
Depending on its concentrations, exogenous CGRP was shown to either stimulate or inhibit LPS-induced cytokine production in macrophages in previous reports.23,44–46 In line with these studies, in a concentration-dependent manner, exogenous CGRP increased LPS-induced release of IL-1β, TNFα and IL-6, suppressed LPS-induced TNFα release or had no effect on LPS-induced IL-10 release. The effects of CGRP8-37 on CGRP or LPS-induced pro-inflammatory cytokines in primary macrophages and other cell types have been reported previously.10,45–47 Depending on concentrations, CGRP8-37 either potentiated or inhibited CGRP or LPS-induced cytokine production in these studies. Similarly, the effect of CGRP8-37 on LPS-induced chemokine and cytokine release in the current study is also concentration-dependent. It enhanced LPS-induced TNFα and IL-10 release, suppressed LPS-induced TNFα release or had no effect on LPS-induced release of MCP-1 and IL-6. Information regarding the effects of BIBN4096BS on CGRP or LPS-induced chemokines and cytokines is relatively scarce. We previously showed that 0·1 and 1 μm BIBN4096BS suppressed increased IL-6 levels in injured nerves as well as CGRP-induced IL-6 in injured nerve explants.10 Using the same concentrations here, BIBN4096BS potentiated LPS-induced IL-1β and TNFα release, inhibited LPS-induced TNFα release or had no effect on LPS-induced release of MCP-1 and IL-6. The discrepancy in the effects of CGRP8-37 and BIBN4096BS on LPS-induced release might also suggest that the two antagonists do not act only on the same CGRP receptors. Tha adrnomedullin receptors AM1 (CLR/RAMP2) and AM2 (CLR/RAMP3) may also be involved in CGRP8-37-exerted effects on LPS-induced release. It is also noticeable that LPS-induced MCP-1 and IL-6 release are not inhibited by both CGRP receptor antagonists whereas exogenous CGRP is able to increase the basal release of both, suggesting that CGRP-induced MCP-1 and IL-6 may involve other mediator(s) that are not induced through CGRP receptors. Alternatively, it is also possible that the concentration ranges of both antagonists are not within the optimal concentration window to affect LPS-induced MCP-1 and IL-6, an assumption further supporting the ligand-concentration-dependent regulation of chemokines and cytokines by CGRP receptor signalling.
It can be generalized here that CGRP receptor signalling, in a ligand-concentration-dependent manner, exerts either stimulating or inhibiting effects on basal and LPS-induced release of pro-inflammatory and anti-inflammatory chemokines and cytokines. Ligand-concentration-dependent modulation of chemokine and cytokine by CGRP receptor signalling is probably a novel mechanism underlying the pro-inflammatory and anti-inflammatory properties of CGRP receptor signalling in immune and inflammatory responses.
Concluding remarks
In the present study, we observed that LPS concentration- and time- dependently induced the production of CGRP from RAW macrophages. The LPS-induced NGF, IL-1β, IL-6, PGE2 and NF-κB signalling facilitates this event whereas NGF trkA receptor and CGRP RAMP1 exert a negative feedback on the release of CGRP. These results suggest a fine-tune regulation of CGRP production in macrophages by other inflammatory mediators during immune and inflammatory responses. On the other hand, through autocrine or paracrine pathways, CGRP receptor signalling can either promote or inhibit the production of pro- and anti-inflammatory chemokines and cytokines in macrophages. The ligand-concentration-dependent modulation of inflammatory mediators by CGRP receptor signalling is a novel mechanism underlying the pro- and anti-immune and inflammatory roles of CGRP. Taken together, these data demonstrate that monocytes/macrophages are an important source of CGRP, which has a reciprocal effect on the production of pro- and anti-inflammatory mediators.
Acknowledgements
This study was supported by grants from Canadian Institutes of Health Research to Weiya Ma and Remi Quirion. F. Vercauteren is the recipient of a FRSQ postdoctoral fellowship.
Disclosures
The authors declare no conflict of interest.




