The melanocortin receptor agonist NDP-MSH impairs the allostimulatory function of dendritic cells
Re-use of this article is permitted in accordance with the Terms and Conditions set out at http://wileyonlinelibrary.com/onlineopen#OnlineOpen
Summary
As α-melanocyte-stimulating hormone (α-MSH) is released by immunocompetent cells and has potent immunosuppressive properties, it was determined whether human dendritic cells (DCs) express the receptor for this hormone. Reverse transcription–polymerase chain reaction detected messenger RNA specific for all of the known melanocortin receptors in DCs. Mixed lymphocyte reactions also revealed that treatment with [Nle4, DPhe7]-α-MSH (NDP-MSH), a potent α-MSH analogue, significantly reduced the ability of DCs to stimulate allogeneic T cells. The expression of various cell surface adhesion, maturation and costimulatory molecules on DCs was also investigated. Although treatment with NDP-MSH did not alter the expression of CD83 and major histocompatibility complex class Ι and ΙΙ, the surface expression of CD86 (B7.2), intercellular adhesion molecule (ICAM-1/CD54) and CD1a was reduced. In summary, our data indicate that NDP-MSH inhibits the functional activity of DCs, possibly by down-regulating antigen-presenting and adhesion molecules and that these events may be mediated via the extracellular signal-regulated kinase 1 and 2 pathway.
Abbreviations:
-
- cAMP
-
- cyclic 3′, 5′-adenosine monophosphate
-
- ERK
-
- extracellular signal-related protein kinase
-
- GM-CSF
-
- granulocyte–macrophage colony-stimulating factor
-
- HDMEC
-
- human dermal microvascular endothelial cells
-
- ICAM-1
-
- intercellular adhesion molecule 1
-
- MC-Rs
-
- melanocortin receptors
-
- MLRs
-
- mixed lymphocyte reactions
-
- NDP-MSH
-
- [Nle4, DPhe7]-α-melanocyte-stimulating hormone
-
- PI-3 kinase
-
- phosphatidylinositol-3-kinase
-
- PKA
-
- protein kinase A
-
- POMC
-
- pro-opiomelanocortin
-
- RT-PCR
-
- reverse transcription–polymerase chain reaction
Introduction
Alpha-melanocyte-stimulating hormone (α-MSH) is a 13-amino-acid peptide that mediates a wide range of physical effects including the stimulation of melanocyte proliferation and melanin synthesis, thermoregulation, prolactin release and immunomodulation.1,2 It is derived by proteolytic cleavage from the precursor molecule pro-opiomelanocortin (POMC), as are other melanocortin hormones including adrenocorticotropic hormone, β-MSH and γ-MSH.1,3 The pituitary gland and other cell types such as monocytes, macrophages, epidermal cells, keratinocytes, Langerhans cells and melanocytes can produce POMC peptides.4,5
The hormone α-MSH exerts its function by binding to melanocortin receptor 1 (MC-1R). This receptor belongs to a family of receptors that bind with differing affinities to the melanocortin proteins. It contains seven membrane-spanning domains (typical of G-protein-coupled receptors) and couples to adenylate cyclase to generate cyclic 3′,5′-adenosine monophosphate (cAMP).2,5
Dendritic cells (DCs) are professional antigen-presenting cells that play an essential role in modulating responses by B and natural killer cells and T lymphocytes against pathogens and cancer cells.6 The unique ability of DCs to prime T cells is linked to the DC maturation state. When they reside in peripheral tissues, DCs are in an immature state, as reflected by high antigen uptake but low major histocompatibility complex (MHC) class ΙΙ and costimulatory molecule expression. The DCs become mature once they have captured antigen, down-regulated their endocytic activity and up-regulated co-stimulatory and MHC molecules to present antigen to naive T cells in the draining lymph nodes.7
There is evidence that α-MSH acts as a potent suppressor of the immune system by inducing interleukin-10 (IL-10) production in peripheral blood monocytes8 and down-regulating nitric oxide production in macrophages.5,9 As DCs play a key role in initiating the immune response, we investigated whether a potent α-MSH analogue, [Nle4, DPhe7]-MSH (NDP-MSH; Melanotan-Ι), had a direct impact on DC phenotype, maturation, differentiation and function. We report novel findings that human DCs express messenger RNA (mRNA) for all the known MC-R subtypes and that NDP-MSH significantly reduces the ability of DCs to stimulate allogeneic T cells in mixed lymphocyte reactions (MLRs). This effect may be exerted via the down-regulation of cell surface adhesion, antigen-presenting and co-stimulatory molecules [intercellular adhesion molecule 1 (ICAM-1), CD1a and CD86] and might be mediated by modulations in extracellular signal-regulated kinase 1 (ERK1) and ERK2 phosphorylation.
Materials and methods
Media
All media constituents were purchased from Gibco-Invitrogen (Paisley, UK) unless otherwise stated. Human CD14+ monocytes were cultured in RPMI-1640 with l-glutamine supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich, Dorset, UK), Glutamax I, penicillin/streptomycin, 2-mercaptoethanol, non-essential amino acids, sodium pyruvate, IL-4 at approximately 1000 U/ml, and granulocyte–macrophage colony-stimulating factor (GM-CSF) at 50 ng/ml (Leucomax, Sandoz, the Netherlands). The MLRs were cultured in the above media without IL-4 and GM-CSF. The NDP-MSH (Sigma-Aldrich) was dissolved in sterile phosphate-buffered saline (PBS) and added in vitro at a final concentration of 10−8 m.
Antibodies, reagents and chemicals
All antibodies for flow cytometry were purchased from BD Biosciences (Oxford, UK) unless otherwise stated. Anti-mitogen-activated protein kinase (MAPK) -activated (diphosphorylated ERK1 and ERK2) antibody (M8159; Sigma-Aldrich, St Louis, MO) was used to probe Western blots.
Monocyte isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated from human buffy coats by Ficoll–Hypaque gradient centrifugation. The cells were washed in RPMI-1640 medium with 1% FCS, counted by trypan blue exclusion and cryopreserved in liquid nitrogen. Monocytes were purified from the PBMCs using a magnetic antibody cell sorting CD14+ isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). They were then seeded at a cell density of 3 × 105/ml in six-well plates (Costar-Corning, Cambridge, UK) in medium supplemented with GM-CSF and IL-4 for 4 days, after which they were stimulated with lipopolysaccharide (LPS) from Salmonella enterica (Sigma-Aldrich) at 1 μg/ml for a further 40 hr to produce mature DCs. Differentiation of DCs was checked after 6 days by staining with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies CD14, MHC class I [human leucocyte antigen (HLA) -A, -B, -C], CD83 (Immunotech, Marseilles, France) and phycoerythrin (PE)-conjugated antibodies against CD1a, MHC class II (HLA-DR) and CD86. Biotinylated antibodies such as ICAM-1 (CD54) were detected by incubation with PE-conjugated streptavidin. Unstained cells served as a negative control. Samples were analysed on a Becton Dickinson FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
Reverse transcription–polymerase chain reaction
Total RNA from DCs was prepared using Trizol (Gibco). Linear polyacrylamide was used as a carrier in the precipitation step. The resulting RNA was treated with DNAse to remove any DNA contamination. First-strand complementary DNA (cDNA) was synthesized using Superscript ΙΙ reverse transcriptase (Invitrogen) and for each sample a reaction without enzyme was included to control for the amplification of contaminating genomic DNA. The cDNA was used as a template in a polymerase chain reaction (PCR) with gene-specific primers (Table 1). The cDNA from the melanoma cell line WM266.4 was used as a positive control for MC-1R, MC-3R and MC-4R because these receptors were found to be over-expressed in this cell line.10 The cDNA from A375-SM, a human cutaneous melanoma cell line, was used as a positive control for MC-2R and MC-5R. The PCR products were run on 1% agarose gels by electrophoresis and visualized with ultraviolet illumination.
| Primer (5′ to 3′) | Sequence | Expected PCR product size (bp) |
|---|---|---|
| MC-1R upper | GCCACCATCGCCAAGAACCG | 416 |
| MC-1R lower | ATAGCCAGGAAGAAGACCAC | |
| MC-2R upper | GAAGCACATTATCAACTCGTATG | 539 |
| MC-2R lower | GCGACGTGAAGGTGATCAGTCTG | |
| MC-3R upper | GAGCAGCAGCGCCTTCTGTG | 462 |
| MC-3R lower | CGAGTAGACGATGAACACCAC | |
| MC-4R upper | CAATAGCCAAGAACAAGAATC | 564 |
| MC-4R lower | GACAACAAAGACGCCAATCAG (see ref. 13) | |
| MC-5R upper | CATTGCTGTGGAGGTGTTTCT | 425 |
| MC-5R lower | GTAGGTGGATTCTGAGTACAG (see ref. 13) |
- bp, base pairs; PCR, polymerase chain reaction.
FITC-dextran uptake
Fluorescein isothiocyanate-dextran (1 mg/ml; Mr = 40500; Sigma-Aldrich) was added to immature and mature DCs cultured in the presence and the absence of NDP-MSH for 5, 20 or 60 min at 37° in PBS containing 1% FCS. A 0-min control was kept on ice throughout the duration of the experiment for each DC culture condition to assess the non-specific FITC signal. The cells were washed four times with PBS and cell fluorescence was analysed on a Becton Dickinson FACSCalibur flow cytometer.
Confocal microscopy
For immunofluoresence confocal microscopy, immature and mature DCs cultured in the presence or absence of NDP-MSH (10−8 m) were allowed to adhere to polylysine glass slides for 30 min at 37°. Cells were fixed for 20 min in 4% paraformaldehyde in PBS pH 7·4 and stained for 40 min at room temperature with MHC I (HLA-A, -B, -C monoclonal antibodies) diluted 1 : 10 in PBS + 1% bovine serum albumin + 2% FCS. The slides were washed in PBS for 5 min, fixed again in 4% paraformaldehyde and washed in PBS once more. Slides were counterstained three times with ToPro3 to label cell nuclei and mounted in VectaShield mounting medium (Vector Laboratories, Inc., Burlingame, CA). Samples were examined using a Leica TCS SP2 confocal microscope.
Allogeneic MLRs
Mature and immature DCs were plated into 96-well flat bottom micro-plates (Costar Corning, High Wycombe, UK) in titrated amounts (1·2 × 104 to 3·75 × 102) per well. CD3+ T cells were purified using a human T-cell enrichment column kit (HTCC-10; R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. These T cells were used as allogeneic responders to DCs and were added at a concentration of 2 × 105 per well. Wells containing DCs alone were set up as a measure of potential background. The MLRs were pulsed with [3H]thymidine (Amersham Life Sciences, Buckinghamshire, UK) on day 5 for 16 hr and were then harvested and counted in a scintillation counter. The results are presented as the mean counts per minute of triplicate cultures.
Western blots
Immature and mature DCs were generated from CD14+ monocytes under the aegis of IL-4 and GM-CSF and in the presence or absence of NDP-MSH (10−8 m) for 6 days. Dendritic cells were washed in PBS and were re-suspended in 1% nonidet P-40 lysis buffer. Forty micrograms of protein from each sample was loaded per lane onto an 8% sodium dodecyl sulphate polyacrylamide gel and was transferred onto a polyvinylidene fluoride membrane (Schleicher & Schuell BioScience, Dassel, Germany). Membranes were blocked in Tris-buffered saline (TBS) containing 1·5% bovine serum albumin and 0·1% Tween-20 at room temperature for an hour. Blots were probed with activated Anti-MAPK antibody (ERK1/2) (Sigma-Aldrich). After they were immunostained overnight at 4° and washed, blots were incubated with secondary antibody (horseradish peroxidase-conjugated goat anti-mouse antibody). Immunoreactive bands were visualized using a chemiluminescence ECL kit (Amersham Life Sciences).
Statistics
The results of the MLRs are expressed as arithmetic means of triplicates together with standard deviations and were statistically analysed using the Student’s t-test. A P value of < 0·05 was considered significant.
Results
DCs express mRNA for MC-Rs other than MC-1R
We first analysed the pattern of expression of the five known MC-Rs in DCs and their monocyte precursors. The reverse transcription (RT-) PCR was performed using primers specific for MC-1R, MC-2R, MC-3R, MC-4R and MC-5R. Total RNA was obtained from CD14+ magnetic bead-purified monocytes before and after they had been differentiated in vitro to DCs in the presence of GM-CSF and IL-4. LPS was used to induce DC maturation.11 A PCR product for MC-1R of the expected size of 416 base pairs was detected in reverse-transcribed mRNA from monocytes and immature and mature DCs (Fig. 1a,b). The MC-Rs MC-2R to MC-5R were also detected by RT-PCR in DCs but not in monocytes.
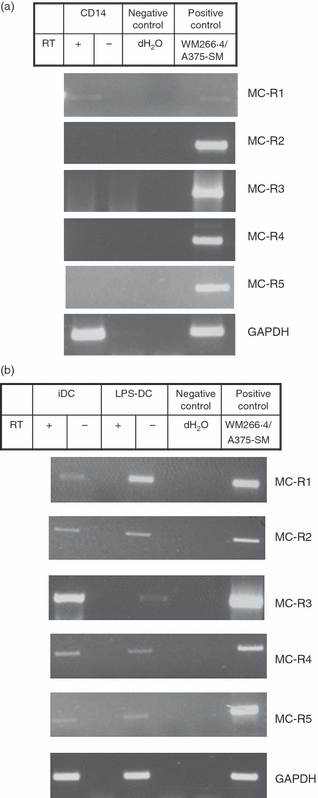
Expression of melanocortin receptor (MC-R) -specific messenger RNA by human monocytes and dendritic cells (DCs). Total RNA was obtained from CD14+ monocytes before and after they had been differentiated in vitro to DCs. The DCs were treated with lipopolysaccharide (LPS; 1 μg/ml) for 40 hrs to generate mature DCs. Transcript levels for the five known MC-Rs were determined by reverse transcription–polymerase chain reaction using oligonucleotides specific for each receptor. The reverse transcriptase-negative samples are included to control for DNA contamination. (a) CD14+ monocytes express messenger RNA for MC-1R whereas (b) DCs express MC-1R as well as the other four known MC-Rs. GAPDH (bottom) was used as a loading control. Abbreviations used: iDC, immature DCs; LPS-DC; mature DCs; negative control: dH2O; positive control: complementary DNA from cell lines WM266.4 or A375-SM.
Antigen uptake (FITC-dextran) remains unchanged but surface expression of CD86, ICAM-1 and CD1a is down-regulated in DCs treated with NDP-MSH
Once we had established that DCs expressed the mRNA for MC-1R, we analysed the effect that NDP-MSH had on DC phenotypic maturation and function. CD14+ monocyte precursors were cultured in media supplemented with GM-CSF and IL-4 for 4 days to generate DCs and were stimulated with LPS at 1 μg/ml for a further 40 hr to produce mature DCs. The NDP-MSH (10−8 m) was either present or absent in the media throughout the 6 days. This synthetic analogue is more stable under in vitro culture conditions and has a higher binding affinity than native α-MSH.1,12 Treatment with NDP-MSH did not appear to affect the ability of DCs to display cell surface markers associated with DC maturation and differentiation such as the maturation marker CD83 and MHC class Ι and ΙΙ (Fig. 2). However, surface expression levels of the co-stimulatory molecule CD86 (B7.2), the adhesion molecule CD54 (ICAM-1) and the antigen-presenting molecule CD1a were reduced on NDP-MSH-treated DCs in comparison with untreated controls (2, 3).
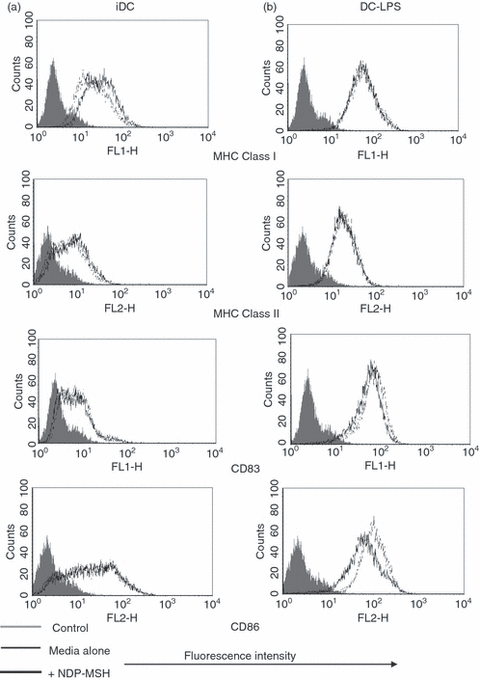
Flow cytometric analysis of [Nle4, DPhe7]-α-melanocyte-stimulating hormone (NDP-MSH) treated and untreated mature and immature dendritic cells (DCs). The DCs were generated in vitro as described in the Materials and methods section in the presence or absence of NDP-MSH. DCs were treated with lipopolysaccharide (LPS; 1 μg/ml) for 40 hr to generate mature DCs. DCs were then incubated with major histocompatibility complex (MHC) Class I (HLA-A,B,C), MHC Class II (HLA-DR), CD86 and CD83 antibodies and were analysed by flow cytometry. NDP-MSH does not influence markers associated with DC maturation, with the exception of CD86 which is down-regulated in NDP-MSH-treated mature DCs. Filled histograms (control), thin lines (DCs in media) and thick lines (DCs treated with NDP-MSH). Data are representative of three separate experiments.
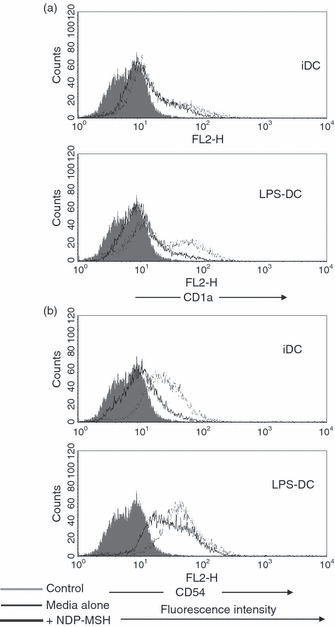
The effect of [Nle4, DPhe7]-α-melanocyte-stimulating hormone (NDP-MSH) on CD1a and CD54 expression on dendritic cells (DCs). The DCs were generated in culture in the presence or absence of NDP-MSH (10−8 m) and stimulated with lipopolysaccharide (LPS; 1 μg/ml) for 40 hr to produce mature DCs. Cells were then incubated with antibodies against (a) CD1a and (b) CD54 (intercellular adhesion molecule 1) and analysed by flow cytometry. NDP-MSH causes down-regulation of CD1a and CD54 on DCs. Filled histograms (control), thin lines (DCs in media) and thick lines (DCs treated with NDP-MSH).
As DC immaturity is characterized by a high level of endocytosis, the effect of NDP-MSH on immature DC antigen uptake was assayed using FITC-dextran (Fig. 4). The NDP-MSH did not appear to affect the endocytic capabilities of immature DCs because treatment with the hormone did not disrupt FITC-dextran uptake.
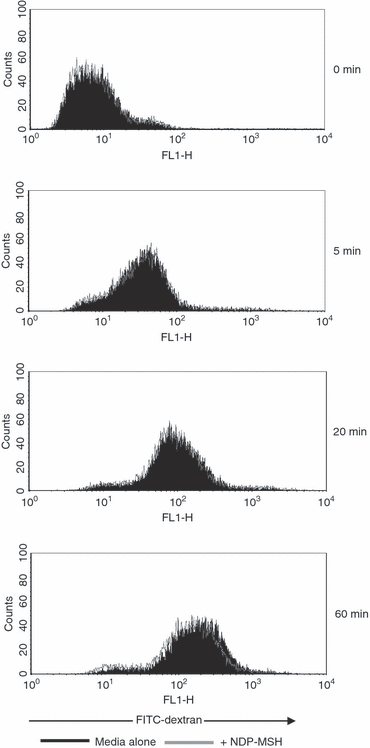
Flow cytometric analysis of endocytic activity of immature dendritic cells (DCs) pretreated with [Nle4, DPhe7]-α-melanocyte-stimulating hormone (NDP-MSH). Filled histograms show the uptake of fluorescein isothiocyanate (FITC) -dextran at 37° by immature DCs cultured in medium alone. Overlay represents the uptake of immature DCs treated with NDP-MSH in parallel experiments. Endocytic activity was also measured at 4° (0 min sample) to control for non-specific fluorescence. NDP-MSH does not affect DC endocytosis. Data are representative of three independent experiments.
NDP-MSH does not affect dendritic cell morphology
Immature DCs generated in the absence or presence of NDP-MSH were stained for MHC class I and expressed this antigen-presenting molecule at low levels (Fig. 5a,b). Upon stimulation with LPS, dendritic membrane processes developed and MHC class I expression was up-regulated, as revealed by bright green surface-staining (Fig. 5c,d). No difference was observed in the dendrite formation in NDP-MSH-treated or untreated DCs, indicating that this hormone does not affect the cytological differentiation of monocytes into DCs.
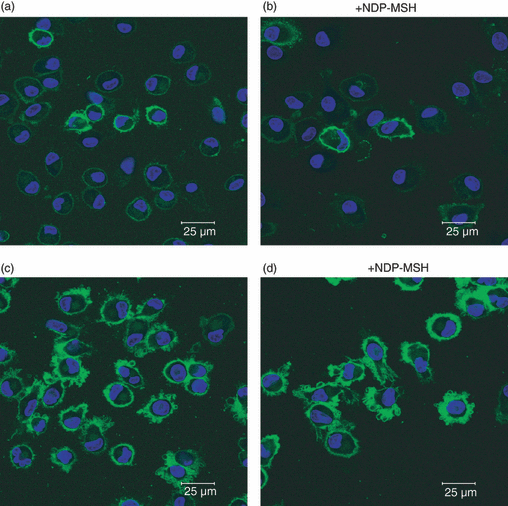
Confocal microscopy of [Nle4, DPhe7]-α-melanocyte-stimulating hormone (NDP-MSH)-treated and untreated mature and immature dendritic cells (DCs). The DCs generated from monocytes after 6 days in culture in RPMI-1640 containing granulocyte–macrophage colony-stimulating factor and interleukin-4 were stained with fluorescein isothiocyanate-conjugated major histocompatibility complex (MHC) class I (HLA-A,B,C). Low numbers of immature DCs stained positive, with green surface-staining of cell membranes (a and b). After treatment with lipopolysaccharide, MHC class I was up-regulated and dendritic processes were exhibited on cell surfaces (c and d). No significant difference in the cytological appearance was observed between NDP-MSH-treated (b and d) or untreated (a and c) cells. The horizontal bar indicates 25 μm.
NDP-MSH-treated mature DCs are markedly less efficient at stimulating allogeneic T cells
The down-regulation of surface molecules on DCs by NDP-MSH prompted us to investigate the effect of this hormone on DC function as stimulators for allogeneic CD3+ T cells in MLRs. Although DCs are known not to proliferate once they have differentiated from their precursors, values of mean counts per minute from DCs alone were subtracted as background from the results. The DCs generated and matured in the presence of NDP-MSH were significantly less potent at stimulating allogeneic T cells than their untreated counterparts, to an approximately three-fold degree (Fig. 6a). Surprisingly, immature DCs, which are characterized by a low capacity for T-cell activation because of low expression of costimulatory molecules, were more effective at stimulating T-cell proliferation than NDP-MSH-treated mature DCs. Parallel experiments repeated with NDP-MSH present in the MLRs generated results very similar to MLRs conducted in the absence of NDP-MSH (Fig. 6b). This indicates that when mature DCs are forced to develop in the presence of NDP-MSH, such DCs are impaired in their ability to prime T cells even when NDP-MSH is eventually removed from their environment (Fig. 6a).
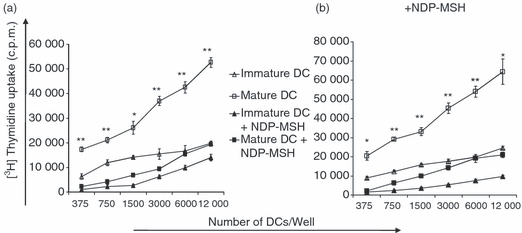
[Nle4, DPhe7]-α-melanocyte-stimulating hormone (NDP-MSH) impairs the ability of dendritic cells (DCs) to stimulate allogeneic T-cell proliferation even upon removal of NDP-MSH from the medium. CD14+-enriched cells were cultured in granulocyte–macrophage colony-stimulating factor and interleukin-4 for 6 days to generate DCs either in the absence or presence of NDP-MSH. Mature DCs were produced by stimulating DCs with lipopolysaccharide (1 μg/ml) for 40 hr. Allogeneic T cells were then stimulated for 5 days with titrated numbers of DCs in 96-well plates in (a) the absence or (b) the presence of NDP-MSH according to the Materials and methods section. Cell proliferation was evaluated after pulsing with [3H]thymidine at 1 μCi/well and is expressed as the mean counts per minute (c.p.m.) of triplicate wells. Error bars represent standard deviations. Thymidine uptake by DCs and T cells alone was subtracted as background. Analysed by Student’s t test: *P < 0·005, **P < 0·0005 indicated for mature DCs versus mature DCs treated with NDP-MSH. Results are representative of six separate experiments.
NDP-MSH inhibits ERK1/2 phosphorylation in LPS-activated DCs
Cell lysates were made from immature and mature DCs that had been generated from CD14+ monocytes in the presence or absence of NDP-MSH (10−8 m). Cell lysates were then used to detect the phosphorylated forms of ERK1/2. Total ERK protein was used as a loading control.
Western blotting with anti-phospho-ERK revealed a greater degree of activation (phosphorylation) of ERK in LPS-treated mature DCs than in immature DCs (Fig. 7). However, phosphorylated ERK levels in mature DCs generated with NDP-MSH in the media were lower than in mature DCs generated in the absence of the hormone (Fig. 7).
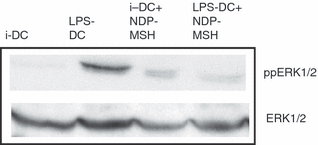
The effect of [Nle4, DPhe7]-α-melanocyte-stimulating hormone (NDP-MSH) on extracellular signal-regulated kinase (ERK) phosphorylation immature and mature dendritic cells (DCs). The phosphorylation of ERK in immature and mature DCs cultured with or without NDP-MSH in the medium was determined by Western blot. Forty micrograms of protein was loaded per well track. Blots were stripped and reblotted with anti-ERK1/2 to measure total ERK as a loading control. An immunoblot representative of two different experiments is shown. iDC, immature DCs; LPS-DC, mature DCs.
Discussion
Although it has been long established that α-MSH regulates the immune response by down-regulating adhesion molecules on antigen-presenting cells such as macrophages and monocytes, little is known about the effect this hormone has upon DCs, key mediators of the immune response.4,13 We therefore decided to investigate the effect of NDP-MSH (an α-MSH analogue) on DC function and development. We report that NDP-MSH decreases the ability of DCs to generate allogeneic T-cell reactions in vitro and this may be the result of the down-regulation of stimulatory molecules on DCs.
We corroborate that human CD14+ monocytes only express MC-1R and none of the other MC-Rs at mRNA level13 and that peripheral blood-derived DCs express MC-1R (Fig. 1).14,15 However, unlike other researchers, we detected transcripts specific for MC-2R to MC-5R in DCs by RT-PCR.15 This might indicate that expression of MC-Rs is related to a maturation or activation process in an anti-inflammatory response. While expression levels of MC-1R increased in DCs post-maturation with LPS, the opposite was observed with MC-3R, a receptor that couples to inositol triphosphate as well as cAMP.16 It has been reported that MC-1R-deficient mice, which express MC-3R on their macrophages, lack obvious immunological defects, suggesting that MC-3R and not just MC-1R alone mediates the anti-inflammatory effects of melanocortins.17 Evidence for MC-1R, MC-3R and MC-5R expression has been detected in human macrophages18 and we detected MC-1R, MC-2R and MC-4R mRNA in human T and B lymphocytes by RT-PCR (data not shown). This indicates that it is possible that DCs express all of the known MC-Rs as other immunocompetent cells appear to express MC-Rs other than MC-1R. As such, several MC-Rs may have a function in immune regulation, possibly at different peptide concentrations or by activating different signalling pathways.19 As all of the MC-Rs, except MC-2R, bind α-MSH to some degree, it would be interesting to determine which specific MC-Rs are involved in our study. This is dependent on the development of neutralizing antibodies specific for each different receptor.20
We observed that human DCs generated in the presence of NDP-MSH displayed cell surface markers associated with a mature DC phenotype, such as CD80, CD83, MHC class Ι and class ΙΙ (Fig. 2). Furthermore, in corroboration of previous reports, we observed down-regulation of CD86 expression, a co-receptor in T-cell activation, in mature DCs after NDP-MSH was administered (Fig. 2).15,21 However, in contrast with other reported findings, we observed that cell-surface expression of CD1a was down-regulated on DCs treated with NDP-MSH (Fig. 3a).15 CD1a presents lipid and glycolipid antigens from cancer and micro-organisms to T cells.22–25 It is noteworthy that CD1a+ CD14− DCs (Langerhans’cells) cause greater stimulation of allo-T cells in MLRs than either CD1a− dermal DCs or CD1a− Langerhans’cells precursors.26 The down-regulation of CD1a− on NDP-MSH-treated DCs may therefore contribute to their impaired ability to prime T cells (Fig. 6). Although other researchers differentiated DCs in the absence of α-MSH and then incubated them with the hormone (10−12 m) for 48 hr,15 we exposed DCs to NDP-MSH (10−8 m) throughout their development from monocytes for 6 days, which may explain discrepant findings. Levels of α-MSH within the range of the concentration used in our experiment (10−10 to 10−8 m) have been reported in physiological and pathological conditions.27,28
α-Melanocyte-stimulating hormone profoundly reduces the expression levels of ICAM-1 (CD54) in human dermal microvascular endothelial cells, melanoma cells, T cells and DCs.21,29,30 Our findings corroborate that NDP-MSH demonstrates a suppressive effect on ICAM-1 in DCs (Fig. 3b).21 The ICAM-1 is up-regulated on DCs once they have been exposed to infectious agents and binds to lymphocyte function-associated antigen-1, an integrin on T cells. It may therefore enhance T-cell activation by strengthening physical contact between DCs and T cells. 31,32 When expression levels of ICAM-1 are reduced with UV-B light, stimulatory function of mature DCs in MLRs is abrogated. 33 We also observed that ICAM-1 surface expression was down-regulated on NDP-MSH-treated DCs and they were impaired in their allostimulatory function (3, 6). This impairment is irreversible because mature NDP-MSH-treated DCs could not prime T cells effectively even when NDP-MSH was subsequently removed from their microenvironment (Fig. 6a). Conversely, mature DCs that developed from monocytes in the absence of NDP-MSH were not impeded in their ability to stimulate T cells when this hormone was introduced into their environment (Fig. 6b). As such, in order for NDP-MSH to mediate a disruptive effect on mature DC function, DCs have to be exposed to α-MSH throughout their development from monocyte precursors.
Dendrites increase the surface area to volume ratio of mature DCs, allowing them to interact with large numbers of T cells in the surrounding area and capture antigen.34,35 No difference was observed in the amount of dendrites on NDP-MSH-treated DCs compared with untreated cells, indicating that the impairment observed in DC function in MLRs is not the result of alterations in DC morphology (Fig. 5).
Upon binding to its receptor, α-MSH exerts its signal via the adenylate-cyclase-mediated conversion of ATP to cAMP and different signalling cascades can then be acted upon, including the phosphatidyl inositol 3-kinases, protein kinase A (PKA) pathway or mammalian MAPKs (ERK1 and ERK2).4,36,37 The best-characterized MAPK cascade is that activated by Ras and Raf proteins.38,39
Phosphorylated ERK was expressed at very low levels in immature DCs but was up-regulated in LPS-treated mature DCs, corroborating other findings (Fig. 7).11 Phosphorylation was also observed in both immature and mature DCs generated in the presence of NDP-MSH but phospho-ERK levels were lower in mature DCs in comparison with their untreated counterparts (Fig. 7). Researchers demonstrate that ERK phosphorylation impairs the phenotypic maturation of immature DCs.40 Although phospho-ERK was up-regulated above baseline levels in NDP-MSH-treated immature DCs, no change in their endocytic activity was observed (4, 7). Immunofluoresence confocal microscopy also revealed that LPS-matured DCs generated in the presence of NDP-MSH exhibited typical DC morphology, including membrane blebbing and dendrite formation (Fig. 5). Hence, despite detecting similar phospho-ERK levels in NDP-MSH treated immature and mature DCs, the latter had differentiated into a mature DC lineage and were not CD14+ monocytes or immature DCs. The activation of ERK by cAMP is linked to V-raf murine sarcoma viral oncogene homolog B1 (B-RAF) whereas through PKA, cAMP inhibits C-RAF and downstream ERK phosphorylation. Previous treatment of DCs with NDP-MSH may therefore suppress the ability of LPS to activate ERK signalling (Fig. 7).11,38,41
The transcription factor nuclear factor-κB (NF-κB) transcriptionally activates several genes including MHC Ι and ΙΙ, CD86 and ICAM-1.11,42,43 Other researchers report that melanocortins exert their immunomodulating effects on immune cells and human dermal microvascular endothelial cells by suppressing NF-κB and therefore ICAM.30,42,44 It has been reported that ERK1 and ERK2 are on an independent signal transduction pathway from NF-κB but that agents such as LPS can activate both signal transduction pathways.11 The NDP-MSH may act similarly or suppress ICAM-1 and CD86 expression through cAMP elevation and PKA activation rather than ERK, as cAMP can couple to NF-κB and cAMP can cross-talk with RAS/RAF/MEK/ERK.43,45 However, it has also been reported that elevated cAMP levels do not inhibit ICAM-1 (CD54) expression.46 As such, although the precise cell signalling mechanisms by which α-MSH mediates its anti-inflammatory effect on DCs remains unclear and complex, our observation that the phosphorylation levels of ERK1/2 in DCs were altered by NDP-MSH treatment provides an insight into the mechanism that may be used by NDP-MSH to modulate DC function (Fig. 7).
We report novel findings that DCs express mRNA for all the known MC-Rs and that NDP-MSH-treated DCs have poor allostimulatory properties. Down-regulation of adhesion and antigen-presenting molecules may be responsible for this impaired DC function. Although our data do not explain the relative contribution of these molecules to the anti-inflammatory effects observed, our findings do further support the notion that native α-MSH regulates immune responses through direct action on host cells and could be a powerful means of manipulating the immune response.
Acknowledgements
We acknowledge financial support from the Royal Marsden Hospital. We also wish to thank Richard Marais for helpful comments, Eva Fragkoudi for expert assistance with the critical reading of this manuscript and David Robertson for technical assistance with the confocal microscopy. The final manuscript was penned by the corresponding author.
Disclosure
The author has no conflicts of interest to disclose.




