Eosinophils infiltrate thyroids, but have no apparent role in induction or resolution of experimental autoimmune thyroiditis in interferon-γ−/− mice
Summary
Granulomatous experimental autoimmune thyroiditis (G-EAT) is induced by mouse thyroglobulin (MTg)-sensitized splenocytes activated with MTg and interleukin (IL)-12. Our previous studies showed that, when used as donors and recipients, interferon (IFN)-γ−/− and wild-type (WT) DBA/1 mice both develop severe G-EAT. Thyroid lesions in IFN-γ−/− mice have many eosinophils and few neutrophils, while those in WT mice have extensive neutrophil infiltration and few eosinophils. Thyroid lesions in IFN-γ−/− mice consistently resolve by day 40–50, whereas those in WT mice have ongoing inflammation and fibrosis persisting for more than 60 days. To determine if the extensive infiltration of eosinophils in thyroids of IFN-γ−/− mice contributes to thyroid damage and/or early resolution of G-EAT, anti-IL-5 was used to inhibit migration of eosinophils to thyroids. G-EAT severity was compared at day 20 and day 40–50 in IFN-γ−/− recipients given anti-IL-5 or control immunoglobulin G (IgG). Thyroids of anti-IL-5-treated IFN-γ−/− mice had few eosinophils and more neutrophils at day 20, but G-EAT severity scores were comparable to those of control IgG-treated mice at both day 20 and day 40–50. Expression of chemokine (C-X-C motif) ligand 1 (CXCL1) mRNA was higher and that of chemokine (C-C motif) ligand 11 (CCL11) mRNA was lower in thyroids of anti-IL-5-treated IFN-γ−/− mice. IL-5 neutralization did not influence mRNA expression of most cytokines in IFN-γ−/− mice. Thus, inhibiting eosinophil migration to thyroids did not affect G-EAT severity or resolution in IFN-γ−/− mice, suggesting that eosinophil infiltration of thyroids occurs as a consequence of IFN-γ deficiency, but these cells have no apparent pathogenic role in G-EAT.
Abbreviations:
-
- G-EAT
-
- granulomatous experimental autoimmune thyroiditis
-
- mTG
-
- mouse thyroglobulin
-
- WT
-
- wild type
Introduction
Granulomatous experimental autoimmune thyroiditis (G-EAT) is induced by adoptive transfer of mouse thyroglobulin (MTg)-primed splenocytes activated in vitro with MTg and interleukin (IL)-12.1–4 The adoptive transfer model of G-EAT is an excellent experimental model with which to study the contributions of various cells and inflammatory mediators in induction and resolution of autoimmune inflammation.1–6 Our previous studies showed that G-EAT lesions in recipients of activated splenocytes reach maximal severity 20 days after cell transfer and evolve over time to two distinct outcomes: either inflammation resolves, or there is continuing inflammation with development of fibrosis.6–8 When interferon (IFN)-γ−/− or wild-type (WT) DBA/1 mice are used as donors and recipients, both develop G-EAT with similar severity scores 20 days after cell transfer.6–8 However, thyroid lesions in IFN-γ−/− mice have many eosinophils and almost no neutrophils, while those in WT mice have many neutrophils and very few eosinophils, with fibrosis and necrosis.6–8 Thyroid lesions in IFN-γ−/− mice consistently resolve by day 40–50, whereas those in WT mice have ongoing inflammation and fibrosis that persists for more than 60 days.6–8 These results suggest that differential infiltration of neutrophils versus eosinophils could contribute to the different outcomes of G-EAT in WT versus IFN-γ−/− mice.
Eosinophils are multifunctional leucocytes that play important roles in asthma and several other inflammatory processes.9 Eosinophils are frequently associated with tissue remodelling and fibrosis in allergy as well as other diseases, including Riedel’s thyroiditis and pulmonary fibrosis.10–14 IL-5 regulates the activation, differentiation, recruitment and survival of eosinophils.9 A humanized monoclonal anti-IL-5 has been evaluated in clinical trials for treatment of allergies, asthma and other hypereosinophilic syndromes.9,15–18 Further studies are needed to increase our understanding of the roles of eosinophils and IL-5 in inflammatory responses and other diseases in which hypereosinophilia occurs.
The differential migration of eosinophils versus neutrophils to thyroids of IFN-γ−/− and WT mice during the development of G-EAT offers a unique opportunity to examine the role of eosinophil trafficking to sites of inflammation and to investigate the potential role of these cells in the induction and resolution of inflammation. Neutralization of IL-5 markedly inhibited migration of eosinophils to thyroids of IFN-γ−/− mice during development of G-EAT. However, IL-5 neutralization had no effect on the severity or rate of resolution of inflammation in G-EAT, suggesting that eosinophil migration has no apparent pathogenic role in G-EAT.
Materials and methods
Mice
WT and IFN-γ−/− DBA/1 mice were produced in our animal facilities at the University of Missouri as previously described.6–8 Both male and female mice (6–10 weeks old) were used.
Induction of G-EAT
G-EAT was induced as previously described.1,5 Briefly, mice were injected intravenously (i.v.) twice at 10-day intervals with 150 μg of MTg3 and 15 μg of lipopolysaccharide (LPS) (Escherichia coli 011:B4; Sigma Chemical Co., St Louis, MO). Seven days later, donor spleen cells were re-stimulated in vitro with 25 μg/ml MTg and 5 ng/ml IL-12.1 Cells were harvested after 72 hr and washed twice, and 3·5 × 107 cells were transferred i.v. to 500-Rad irradiated syngeneic recipients.
Anti-IL-5 treatment
Anti-IL-5 was purified from culture supernatants of the anti-IL-5-producing hybridoma TRFK-5 (provided by Dr Robert Coffman, DNAX Research Institute, Palo Alto, CA, USA) using protein G. IFN-γ−/− recipients of IFN-γ−/− donor cells were given 300 μg of anti-IL-5 intraperitoneally (i.p.) or rat immunoglobulin G (control IgG) every 4 days beginning on the day of cell transfer until euthanasia. WT recipients of WT donor cells were used for comparison.
Evaluation of G-EAT histopathology and fibrosis
Thyroids were removed from groups of five or six recipient mice 20 days (peak of disease) or 40–50 days (fibrosis versus resolution) after cell transfer.1–6 Thyroids were fixed in formalin, sectioned and stained with haematoxylin and eosin (H&E), and scored quantitatively for G-EAT severity (the extent of inflammatory cell infiltration and thyroid follicle destruction) using a scale of 1+ to 5+, as described previously.6 1+ thyroiditis is defined as an infiltrate of at least 125 cells in one or several foci; 2+ is 10–20 foci of cellular infiltration involving up to 25% of the gland; 3+ indicates that 25–50% of the gland is infiltrated; 4+ indicates that > 50% of the gland is destroyed by infiltrating inflammatory cells; and 5+ indicates virtually complete destruction of the thyroid with few or no remaining follicles. Thyroid lesions were also evaluated qualitatively. Thyroid lesions in WT mice had enlargement and proliferation of thyroid follicular cells, with numerous histiocytes, multinucleated giant cells, and increased numbers of neutrophils in addition to mononuclear cell infiltration. The more severely inflamed thyroids (4–5+ severity scores) also had microabscess formation, necrosis, and focal fibrosis, and inflammation generally extended beyond the thyroid to involve adjacent muscle and connective tissue.1–5,19 G-EAT lesions in IFN-γ−/− mice with 4–5+ severity scores differed from those in WT mice in that they generally had less fibrosis and minimal necrosis and lesions generally did not extend outside the thyroid. G-EAT lesions in IFN-γ−/− mice had very few neutrophils, but many eosinophils.6–8
Immunohistochemistry
Neutrophils were detected in frozen thyroid sections using a rat anti-neutrophil monoclonal antibody (mAb) (RB6-8C5; American Type Culture Collection, Rockville, MD).6,20 Frozen thyroid sections were fixed in acetone for 10 min at 4°. Goat anti-rat antibody (1 : 500; Caltag Laboratories, Burlingame, CA) was used as the secondary antibody, with 3-diaminobenzidine tetrahydrochloride (DAB; Sigma) as the chromogen. Slides were counterstained with haematoxylin. Rat IgG was used as a negative control and staining was always negative. The same method was used for IL-5 staining except that the primary antibody was rabbit anti-IL-5 polyclonal Ab (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-rabbit IgG (Santa Cruz) was used as the secondary antibody. NovaRED (Vector Laboratories, Burlingame, CA) was used as the chromogen.
Serum thyroxine (T4) assay
Serum T4 levels were determined using a T4 enzyme immunoassay kit (Biotecx Labs, Houston, TX) according to the manufacturer’s instructions. Results are expressed as μg T4/dl of serum. Using this assay, T4 values for normal mouse serum ranged from 4 to 10 μg/dl; values < 3 μg/dl are considered low.20
Quantitative real-time PCR
RNA was isolated from individual thyroid lobes of recipient mice using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed as previously described.20–22 Levels of IL-10, IL-17, chemokine (C-X-C motif) ligand 1 (CXCL1) and chemokine (C-C motif) ligand 11 (CCL11) were quantified by real-time PCR using the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Amplification was performed in a total volume of 25 μl for 40 cycles, and product was detected using SYBR Green (ABgene, Rochester, NY). Samples were run in triplicate and relative expression levels were determined by normalizing expression of each target to hypoxanthine-guanine phosphoribosyl transferase. Expression levels of normalized samples are shown as relative expression units. Real-time PCR primers for IL-10 and IL-17 were previously described.22,23 Primers for CXCL1 were: sense, 5′-TGCACCCAAACCGAAGTCAT-3′; antisense, 5′-TTGTCAGAAGCCAGCGTTGAC-3′; and for CCL11, they were: sense, 5′-CTGCTTGATTCCTTCTCTTTCCTAA-3′; antisense, 5′-GGAACTACATGAAGCCAAGTCCTT-3′.
Statistical analysis
Experiments were repeated at least three times. Statistical analysis was performed using an unpaired two-tailed Student’s t-test. Values with a P-value < 0·05 were considered significant and are designated by an asterisk or diamond in the figures.
Results
Effect of anti-IL-5 on eosinophil and neutrophil infiltration into thyroids of IFN-γ−/− mice
Eosinophils are predominant in thyroids of IFN-γ−/− recipients of splenocytes from IFN-γ−/− donors 20 days after cell transfer, whereas thyroids of WT recipients of WT donor cells have extensive infiltration by neutrophils.6–8 IL-5 regulates eosinophil production,9 and neutralization or gene knockout of IL-5 decreases eosinophil infiltration in models of allergy and other inflammatory diseases.9,24–28 To determine if the presence of eosinophils in IFN-γ−/− thyroids plays a role in determining the severity or outcome of G-EAT, anti-IL-5 was used to inhibit migration of eosinophils to thyroids of IFN-γ−/− mice. Very few eosinophils with typical pink granule staining were present in thyroids of WT mice (Fig. 1a), whereas many eosinophils were present in thyroids of IFN-γ−/− mice with G-EAT (Fig. 1b). Thyroids of IFN-γ−/− recipients given anti-IL-5 had many fewer eosinophils (Fig. 1c), indicating that the amount of anti-IL-5 was sufficient to inhibit infiltration of most eosinophils into thyroids of IFN-γ−/− mice. Thyroids of IFN-γ−/− mice given anti-IL-5 (Fig. 1f,i) had more neutrophils than thyroids of IFN-γ−/− mice given IgG (Fig. 1e,h), but the extent of neutrophil infiltration was always much less than in thyroids of WT mice (Fig. 1d,g). Numbers of eosinophils (Fig. 1j, pink column) and neutrophils (Fig. 1j, brown column) in five or six randomly selected high-power fields for three individual mice per group (magnification: ×1000) were manually counted and results are summarized (Fig. 1j).
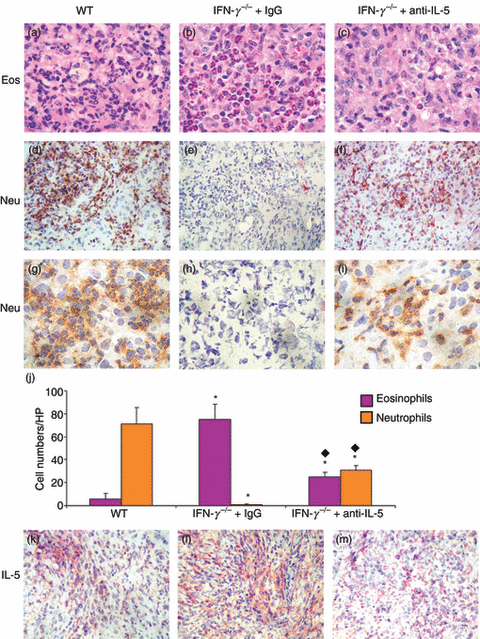
Interleukin (IL)-5 neutralization decreases eosinophil (Eos) and increases neutrophil (Neu) infiltration into thyroids of interferon (IFN)-γ−/− mice. (a–c) Haematoxylin and eosin (H&E) staining 20 days after cell transfer. Eosinophils are identified by dark pink granules. (d–i) Immunohistochemical staining for neutrophils (dark brown). Numbers of eosinophils (j, black column) and neutrophils (j, white column) in five or six randomly selected high-power (HP) fields of three individual mice in each group (magnification: ×1000) were manually counted and results are summarized (j). A significant difference between thyroids of rat immunoglobulin G (IgG)-treated or anti-IL-5-treated IFN-γ−/− versus wild-type (WT) mice is indicated by an asterisk (P < 0·05) and a significant difference between thyroids of rat-IgG-treated versus anti-IL-5-treated IFN-γ−/− mice is indicated by a diamond (P < 0·05). (k–m) IL-5 staining (red). Thyroids from each group had 5+ severity scores (a–i and k–m). Magnification: (a–c and g–i) ×1000; (d–f and k–m) ×400. Photos are representative of three experiments.
Consistent with the extensive infiltration by neutrophils, thyroids of WT recipients had extensive necrosis at day 20, whereas there was little necrosis in thyroids of IFN-γ−/− mice given anti-IL-5 (Table 1). Eosinophils and neutrophils had largely disappeared in all thyroids by day 40–50 (Table 1). These results indicate that administration of anti-IL-5 leads to less eosinophil infiltration and more neutrophil infiltration into thyroids of IFN-γ−/− mice.
| WT | IFN-γ−/− + IgG | IFN-γ−/− + anti-IL-5 | ||||
|---|---|---|---|---|---|---|
| Day 20 | Day 40–50 | Day 20 | Day 40–50 | Day 20 | Day 40–50 | |
| G-EAT severity score | 4·8 ± 0·2 | 4·9 ± 0·1 | 4·1 ± 0·5 | 1·5 ± 0·4 | 4·6 ± 0·2 | 2·4 ± 0·5 |
| Eosinophils1 | −/+ | − | ++++ | + | + | + |
| Neutrophils | ++++ | + | − | − | +/++ | − |
| Necrosis2 | ++/+++ | − | − | − | −/+ | − |
| Fibrosis2 | + | +++ | −/+ | − | −/+ | − |
| CXCL1 | ++++ | ND | ++ | ND | ++++ | ND |
| CCL11 | ++ | ND | ++++ | ND | ++ | ND |
| Serum T43 | 2·8 ± 0·4 | 1·2 ± 0·2 | 4·1 ± 0·8 | 5·1 ± 0·4 | 3·3 ± 0·4 | 7·5 ± 1·2 |
- CCL11, chemokine (C-C motif) ligand 11; CXCL1, chemokine (C-X-C motif) ligand 1; ND, not determined.
- 1Four to six thyroids from at least three separate experiments were examined from each group. Infiltration of neutrophils and eosinophils and necrosis were evaluated by H&E and immunostaining at days 20 and 40–50. The intensity was graded as follows: −, not detectable; +, weak; ++, moderate; +++, strong; ++++, very strong. The range of intensity for each group is indicated (e.g. −/+). Thyroids from normal DBA/1 mice were also stained and were all negative.
- 2Intensity of fibrosis as determined by Trichrome staining or necrosis as determined on H&E stained slides was graded as follows: −, not detectable; +, weak; ++, moderate; +++, strong; and ++++, very strong.
- 3Low serum T4 is defined as < 3·0 μg/dl.
Effect of anti-IL-5 on protein expression of IL-5 in thyroids
Protein expression of IL-5 at day 20 (Fig. 1k–m) was increased in thyroids of IFN-γ−/− mice given control IgG compared with that in thyroids of WT (Fig. 1l,k) or IFN-γ−/− mice given anti-IL-5 (Fig. 1l,m). As IL-5 neutralization correlates with reduced eosinophil infiltration and increased neutrophil infiltration into thyroids of IFN-γ−/− mice, this provides an excellent opportunity to address the role of eosinophils versus neutrophils in G-EAT resolution.
Effect of anti-IL-5 on induction and resolution of G-EAT in IFN-γ−/−mice
Because anti-IL-5 markedly reduced eosinophil infiltration and resulted in increased neutrophil infiltration in thyroids of IFN-γ−/− mice (Fig. 1 and Table 1), we hypothesized that inhibiting infiltration of eosinophils into thyroids using anti-IL-5 might influence the severity and/or rate of resolution of G-EAT. To determine if inhibiting eosinophil infiltration into IFN-γ−/− thyroids would influence the severity or rate of resolution of G-EAT, WT and IFN-γ−/− recipients were given control IgG or anti-IL-5 as described in the Materials and methods. Eosinophil infiltration of thyroids and G-EAT severity together with resolution were all evaluated in each individual experiment. WT mice developed very severe G-EAT 20 days after cell transfer (2, 3). Anti-IL-5 had no effect on G-EAT severity in WT recipients (data not shown). Consistent with our previous studies,6–8 IFN-γ−/− mice given control IgG or anti-IL-5 also developed severe G-EAT at day 20 (2, 3; P > 0·05). However, eosinophils were predominant in thyroids of control IgG-treated IFN-γ−/− mice, while eosinophils were greatly reduced and neutrophils were increased in thyroids of anti-IL-5-treated IFN-γ−/− mice (Fig. 1 and Table 1). Thyroids of most WT recipients still had very severe (5+) G-EAT (average severity score: 4·8) at day 40–50 (2, 3), while thyroid lesions in most IFN-γ−/− mice given control IgG or anti-IL-5 had either resolved or were beginning to resolve with G-EAT severity scores of 1–3+ (average severity score: 1·5–2·4) at day 40–50 (2, 3). Although G-EAT resolution occurs earlier in mice lacking IFN-γ, inhibition of the migration of eosinophils into thyroids of IFN-γ−/− mice has no apparent effect on the severity or resolution of G-EAT.
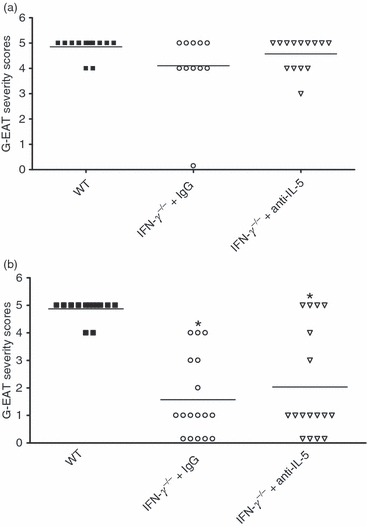
Interleukin (IL)-5 neutralization reduces eosinophil infiltration but has no effect on the severity or resolution of granulomatous experimental autoimmune thyroiditis (G-EAT) in interferon (IFN)-γ−/−mice. G-EAT severity scores were evaluated 20 days (a) and 40–50 days (b) after cell transfer on a scale from 0+ to 5+. Each symbol represents the G-EAT severity score of an individual mouse. Average G-EAT severity scores at day 20 were comparable for all three groups of mice (P > 0·05). Average G-EAT severity scores at day 40–50 for wild-type (WT) mice (4·8 ± 0·2) differed from those of IFN-γ−/− mice treated with control immunoglobulin G (IgG) (1·5 ± 0·4) or anti-IL-5 (2·4 ± 0·5). IFN-γ−/− mice treated with control immunoglobulin G (IgG) or anti-IL-5 had similar severity scores (P > 0·05). A significant difference in the severity scores of anti-IL-5-treated or control IgG-treated IFN-γ−/− mice compared with WT mice at day 40–50 is indicated by an asterisk (P < 0·05). Results are pooled from three separate experiments.
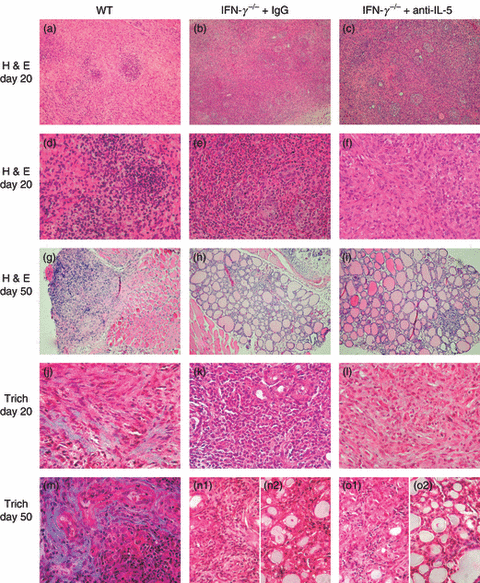
The decrease in eosinophil migration to thyroids produced by anti-interleukin (IL)-5 has no effect on fibrosis at day 40–50. (a–i) Haematoxylin and eosin (H&E) staining of thyroids from wild-type (WT), anti-IL-5-treated or control immunoglobulin G (IgG)-treated interferon (IFN)-γ−/− mice at day 20 (a–f) or day 50 (g–i). Granulomatous experimental autoimmune thyroiditis (G-EAT) reached maximal severity (5+) in WT mice (a, d) and control IgG-treated (b, e) or anti-IL-5-treated IFN-γ−/− mice (c, f) at day 20. Inflammation was sustained in thyroids of WT mice with fibrosis and atrophy (5+ severity) at day 50 (g), whereas inflammation resolved (1+ severity) in control IgG-treated (h) or anti-IL-5-treated IFN-γ−/− mice (i). (j–o) Trichrome (Trich) staining for collagen in thyroids of WT (j, m), control IgG-treated (k, n) and anti-IL-5-treated (l, o) IFN-γ−/− mice at day 20 (j–l) or day 50 (m–o). (n1, o1) Control IgG-treated (n1) or anti-IL-5-treated (o1) IFN-γ−/− mice with 4–5+ severity score at day 50. (n2, o2) Control IgG-treated (n2) or anti-IL-5-treated (o2) IFN-γ−/− mice with 1–2+ severity score at day 50. Magnification: (a–c and g–i) ×100; (d–f and j–o) ×400. Photos are representative of three experiments.
Effect of anti-IL-5 on thyroid fibrosis and serum T4 levels
WT mice with severe G-EAT develop thyroid fibrosis which is very severe 40–50 days after cell transfer, and mice with severe thyroid fibrosis also have low serum T4.1–8,20–23 In contrast, thyroids of IFN-γ−/− mice have minimal fibrosis at day 20, and even less fibrosis at day 40–50 when inflammation is resolving6–8,29 and serum T4 levels are usually normal.6 To determine if the severity of fibrosis was influenced by inhibiting eosinophil migration into thyroids of IFN-γ−/− mice, Masson’s Trichrome staining was used to assess collagen deposition in thyroids 20 and 40–50 days after cell transfer. In general, thyroids with very severe (5+) G-EAT at day 20 had some fibrosis, and there was less fibrosis in thyroids of isotype IgG-treated (Fig. 3k) or anti-IL-5-treated IFN-γ−/− mice (Fig. 3l) than in thyroids of WT mice 20 days after cell transfer (Fig. 3j). By day 40–50, fibrosis was more extensive in the thyroids of WT mice (Fig. 3m,j), but there was considerably less fibrosis in the thyroids of IFN-γ−/− mice given control IgG (Fig. 3n2) or anti-IL-5 (Fig. 3o2). This was true even when G-EAT severity scores at day 40–50 were comparable (4–5+) (Fig. 3n1,o1) to those in WT recipients. These results suggest that the decreasing infiltration of eosinophils into thyroids of IFN-γ−/− mice given anti-IL-5 had little effect on the severity of thyroid fibrosis (Table 1).
WT mice with severe thyroid fibrosis have been shown to have low serum T4, whereas mice with minimal fibrosis usually have normal serum T4 levels.1,5–8 In this study, serum T4 levels were low in some mice with 5+ severity scores in all three groups at day 20. By day 40–50, all WT mice had low serum T4, whereas IFN-γ−/− recipients (both anti-IL5 and control IgG groups) all had T4 levels within the normal range (Table 1; data for individual mice not shown).
Effect of IL-5 neutralization on expression of cytokines and chemokines in thyroids of IFN-γ−/− mice
The balance between pro- and anti-inflammatory cytokines or chemokines produced by thyroid-infiltrating inflammatory cells could contribute to the differential infiltration of eosinophils versus neutrophils in thyroids. To determine if anti-IL-5 modulated cytokine gene expression in recipient thyroids, mRNA was isolated from thyroids of WT and IFN-γ−/− mice given control IgG or anti-IL-5. Expression of pro- and anti-inflammatory cytokines and chemokines known to be important for trafficking of neutrophils versus eosinophils30 was determined by RT-PCR or real-time PCR on RNA isolated 20 days after cell transfer, when differences in neutrophils and eosinophils in WT versus IFN-γ−/− mice were maximal. No cytokine or chemokine mRNA was detected in normal thyroids (Fig. 4). IL-17 is a pro-inflammatory cytokine known to be regulated by IFN-γ.31–33 However, mRNA expression of IL-17 was lower in thyroids of IFN-γ−/− mice given control IgG or anti-IL-5 compared with its expression in WT thyroids (Fig. 4a), as previously shown in this model.6 Consistent with the mRNA expression level, protein expression of IL-17 was also reduced in thyroids of IFN-γ−/− mice with or without anti-IL-5 treatment compared with WT (data not shown). However, mRNA expression of IL-10, an important anti-inflammatory cytokine, was increased in thyroids of IFN-γ−/− mice with or without anti-IL-5 treatment compared with WT (Fig. 4b). The increased IL-10 in thyroids of IFN-γ−/− mice may contribute to the earlier resolution of G-EAT which is controlled, at least in part, by IL-10.22
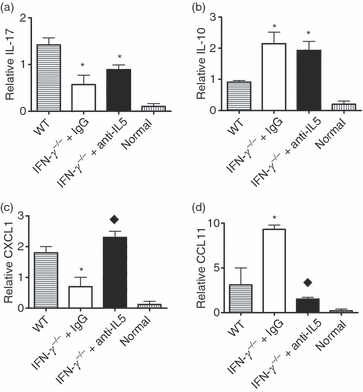
Real-time polymerase chain reaction (PCR) analysis of interleukin (IL)-17, IL-10, chemokine (C-X-C motif) ligand 1 (CXCL1) and chemokine (C-C motif) ligand 11 (CCL11) mRNA expression in thyroids. (a–d) mRNA expression levels of IL-17, IL-10, CXCL1 and CCL11 in normal thyroids and thyroids of wild-type (WT) and control immunoglobulin G (IgG)-treated or anti-IL-5-treated interferon (IFN)-γ−/− mice 20 days after cell transfer are shown. Bars are mean results for thyroids of four or five individual mice ± standard error of the mean (SEM). Results are expressed as the mean relative ratio to hypoxanthine-guanine phosphoribosyl transferase (HPRT) and are representative of three independent experiments. A significant difference between control IgG-treated or anti-IL-5-treated IFN-γ−/− mice versus WT mice is indicated by an asterisk (P < 0·05). A significant difference between anti-IL-5-treated versus IgG-treated IFN-γ−/− mice is indicated by a diamond (P < 0·05).
Expression of CXCL1 and CCL11 in thyroids was associated with the relative infiltration of thyroids by neutrophils versus eosinophils. Expression of the neutrophil-attracting chemokine CXCL1 was lower in thyroids of IFN-γ−/− mice given control IgG compared with WT mice or IFN-γ−/− mice given anti-IL-5 (Fig. 4c). In contrast, expression of the eosinophil-attracting chemokine CCL11 was higher in thyroids of control IgG-treated IFN-γ−/− mice compared with WT or IFN-γ−/− mice given anti-IL-5 (Fig. 4d). Thus, expression of CXCL1 was associated with the extent of neutrophil infiltration, while expression of CCL11 was associated with the extent of eosinophil infiltration into thyroids. Expression of other pro- and anti-inflammatory cytokines, such as TNF-α, inducible nitric oxide synthase (iNOS), IL-5, IL-13 and transforming growth factor (TGF)-β, was also examined in these studies. Although there were differences in expression between thyroids of WT and IFN-γ−/− mice, as previously shown,6,8 there were no differences in expression of any of these molecules when comparing thyroids of control IgG-treated and anti-IL-5-treated IFN-γ−/− mice (data not shown).
Discussion
An unexpected and interesting finding in this study is that manipulating eosinophil numbers in thyroids during development of G-EAT had no effect on the severity and/or earlier resolution of inflammation in IFN-γ−/− compared with WT mice. This is consistent with the fact that anti-IL-5 had no effect on expression of major pro- and anti-inflammatory cytokines in thyroids of IFN-γ−/− mice. To our knowledge, this is the first report using a murine thyroiditis model to address the role of IL-5 and eosinophils in autoimmune inflammation.
Eosinophilia is a classic feature of several human diseases such as parasitic infections, inflammatory bowel disease, asthma, Churg–Strauss syndrome, eosinophilic esophagitis and eosinophilic gastroenteritis.9,34–38 Eosinophils have many functions, including antigen presentation and exacerbation of inflammatory responses through their ability to secrete various cytokines and lipid mediators.9,35 Eosinophils are important inflammatory cells, for example in sites of allergic inflammation, and they have been shown to affect both tissue injury and remodelling,9,37,39 and they have been implicated in promoting fibrosis in several diseases.10–14 IL-5 regulates the activation, differentiation, recruitment and survival of eosinophils,9 and neutralizing IL-5 can block infiltration of eosinophils into synovial tissues34 and sites of allergic inflammation.40
Although the role of IL-5 in the differentiation, proliferation and migration of eosinophils has been well established,9 it remains unclear how important IL-5 and eosinophils are to the development and/or progression of clinical diseases including autoimmune diseases. In fact, several clinical trials using anti-IL-5 mAb in patients with asthma have failed to improve symptoms, although IL-5 seems to be responsible for the accumulation of eosinophils in blood and tissues.41–43 In this study, we took advantage of the differential migration of eosinophils versus neutrophils to thyroids of IFN-γ−/− and WT mice during development of G-EAT to examine the potential role of eosinophil trafficking to sites of autoimmune inflammation in G-EAT induction and resolution. In this model, eosinophils contribute substantially to thyroid inflammation in IFN-γ−/− mice with G-EAT, as they are one of the major cell types infiltrating IFN-γ−/− thyroids from day 10–21 after cell transfer.8 However, inhibition of the migration of most eosinophils to the thyroid by administration of anti-IL-5 had little effect on G-EAT severity scores. Although anti-IL-5 markedly reduced the contribution by eosinophils to thyroid inflammation, other cells such as neutrophils increased in number and the end result was a similar severity score (defined as the percentage of the thyroid replaced by infiltrating inflammatory cells) in thyroids of IFN-γ−/− mice given control IgG or anti-IL-5. Therefore, a similar degree of inflammation of the thyroid (severity score) can result from the activity of different inflammatory cells and cytokines or chemokines. This is consistent with results reported in other animal models of autoimmune disease such as experimental autoimmune encephalomyelitis, experimental autoimmune uveitis and gastritis, where different subsets of T cells can induce similar damage of a particular organ, but the manifestations of the inflammatory response can differ.44–46
Eosinophils can play an important role in repair of inflammation and fibrosis.9–14,47–50 However, inhibiting migration of eosinophils into thyroids of IFN-γ−/− mice had no apparent effect on resolution of inflammation or development of fibrosis in thyroids of IFN-γ−/− mice. By day 40–50, thyroid lesions in IFN-γ−/− mice still resolved without fibrosis after reduction of eosinophil infiltration. These results are in agreement with results reported by others for mouse models of bleomycin-induced pulmonary fibrosis, bronchial asthma and colitis and reports on the failure of anti-IL-5 therapy in humans.16,17,27,51,52
The balance between pro- and anti-inflammatory cytokines produced by thyroid-infiltrating inflammatory cells contributes to the outcome of G-EAT.6–8,20–23,29 Thyroids of anti-IL-5-treated IFN-γ−/− mice expressed less CCL11 mRNA and higher CXCL1 mRNA compared with IgG-treated IFN-γ−/− mice. This correlated with the reduced eosinophils and increased neutrophils in thyroids of anti-IL-5-treated IFN-γ−/− mice. However, IL-5 neutralization did not lead to changes in expression of other pro- or anti-inflammatory cytokines in thyroids of IFN-γ−/− mice. Thyroid lesions in IFN-γ−/− mice with G-EAT resolve without fibrosis, while those in WT mice have extensive fibrosis and do not resolve (Table 1). The primary difference between WT and IFN-γ−/− mice that apparently controls development of fibrosis and resolution of inflammation is the presence or absence of IFN-γ.6,29 IFN-γ−/− mice also have increased production of IL-10 (Fig. 4) which plays an important role in G-EAT resolution.22 Inhibition of eosinophil infiltration into thyroids has no effect on these disease parameters, suggesting that IFN-γ and IL-10, but not IL-5 or eosinophils, play a critical role in G-EAT resolution and development of fibrosis.
Acknowledgements
We thank Patti Mierzwa and Alicia Duren for technical assistance. This work was supported by National Institutes of Health Grant DK35527 (to HB-M) and a fellowship from the Arthritis Foundation Eastern Missouri Chapter (to YF).
Disclosures
None.




