A ceramide-1-phosphate analogue, PCERA-1, simultaneously suppresses tumour necrosis factor-α and induces interleukin-10 production in activated macrophages
Summary
Tight regulation of the production of the key pro-inflammatory cytokine tumour necrosis factor-α (TNF-α) is essential for the prevention of chronic inflammatory diseases. In vivo administration of a synthetic phospholipid, named hereafter phospho-ceramide analogue-1 (PCERA-1), was previously found to suppress lipopolysaccharide (LPS)-induced TNF-α blood levels. We therefore investigated the in vitro anti-inflammatory effects of PCERA-1. Here, we show that extracellular PCERA-1 potently suppresses production of the pro-inflammatory cytokine TNF-α in RAW264.7 macrophages, and in addition, independently and reciprocally regulates the production of the anti-inflammatory cytokine interleukin-10 (IL-10). Specificity is demonstrated by the inability of the phospholipids ceramide-1-phosphate (C1P), sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) to perform these activities. Similar TNF-α suppression and IL-10 induction by PCERA-1 were observed in macrophages when activated by Toll-like receptor 4 (TLR4), TLR2 and TLR7 agonists. Regulation of cytokine production is demonstrated at the mRNA and protein levels. Finally, we show that, while PCERA-1 does not block activation of nuclear factor (NF)-κB and mitogen-activated protein kinases by LPS, it elevates the intracellular cAMP level. In conclusion, the anti-inflammatory activity of PCERA-1 seems to be mediated by a cell membrane receptor, upstream of cAMP production, and eventually TNF-α suppression and IL-10 induction. Thus, identification of the PCERA-1 receptor may provide new pharmacological means to block inflammation.
Abbreviations:
-
- ACTH
-
- adrenocorticotropic hormone
-
- ATCC
-
- American Type Culture Collection
-
- BSA
-
- bovine serum albumin
-
- C8-C1P
-
- C8-ceramide-1-phosphate
-
- cAMP
-
- cyclic AMP
-
- CERA-1
-
- ceramide analogue-1
-
- CRH
-
- corticotropin-releasing hormone
-
- DMEM
-
- Dulbecco’s modified Eagle’s minimal essential medium
-
- DMSO
-
- dimethyl sulphoxide
-
- DTT
-
- dithiothreitol
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- EGTA
-
- ethyleneglycoltetraacetic acid
-
- EIA
-
- enzyme immunoassay
-
- EP2
-
- prostaglandin E2 receptor
-
- ERK
-
- extracellular signal-regulated kinase
-
- FACS
-
- fluorescence-activated cell sorting
-
- FBS
-
- fetal bovine serum
-
- GPCR
-
- G-protein coupled receptor
-
- IBMX
-
- isobutylmethylxanthine
-
- IL-10
-
- interleukin-10
-
- JNK
-
- c-jun N-terminal kinase
-
- LC-MS
-
- liquid chromatography mass spectrometry
-
- LDL
-
- low-density lipoprotein
-
- LPA
-
- lysophosphatidic acid
-
- LPS
-
- lipopolysaccharide
-
- MAP
-
- mitogen-activated protein
-
- MSH
-
- melanocyte-stimulating hormone
-
- NF-κB
-
- nuclear factor κB
-
- NP
-
- nonyl phenoxylpolyethoxylethanol
-
- PACAP
-
- pituitary adenylate cyclase activating peptide
-
- PBS
-
- phosphate-buffered saline
-
- PCERA-1
-
- phospho-ceramide analogue-1
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PGE2
-
- prostaglandin E2
-
- PKA
-
- protein kinase A
-
- PLA
-
- phospholipase A
-
- PMSF
-
- phenylmethylsulphonyl fluoride
-
- PVDF
-
- polyvinylidene fluoride
-
- RI
-
- relative intensity
-
- rTNF-α
-
- recombinant TNF-α
-
- RT-PCR
-
- reverse transcription–polymerase chain reaction
-
- S1P
-
- sphingosine-1-phosphate
-
- SDS–PAGE
-
- sodium dodecyl sulphate–polyacrylamide gel electrophoresis
-
- SMA
-
- difluoromethylene analogue of sphingomyelin
-
- TLR
-
- Toll-like receptor
-
- TNF-α
-
- tumour necrosis factor α
-
- VIP
-
- vasoactive intestinal peptide
Introduction
Activation of Toll-like receptors (TLRs) by specific pathogens leads to the production of pro-inflammatory mediators that initiate an inflammatory process. This is followed by a counter anti-inflammatory response that prevents excessive prolonged damage to the host. Loss of inflammatory balance can lead to pathological events.1,2 Cytokines play a major role in mediating the signals that regulate the inflammatory and anti-inflammatory responses. Tumour necrosis factor (TNF)-α, the first cytokine to be released after activation of essentially all TLRs, is mainly produced by monocytes and tissue macrophages, and is regarded as the key pro-inflammatory cytokine.3,4 TNF-α receptors are ubiquitously expressed, and therefore TNF-α exerts multiple effects on a broad range of cell types in the immune system, and outside it.5 TNF-α as a primary cytokine amplifies and prolongs the inflammatory response by activating immune cells to release other pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, and IL-8 as well as other inflammatory mediators.6 Interestingly, TNF-α also enhances the TLR-induced production of the key anti-inflammatory cytokine IL-10,7 which in turn suppresses TNF-α to complete the negative regulatory feedback cycle.8
While timed secretion of TNF-α is crucial for overcoming infections, systemic exposure to high levels of TNF-α causes septic shock that is often fatal.3 Moreover, local unregulated release of TNF-α may lead to chronic inflammation and auto-immune diseases, such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis.9 These conditions are currently treated with TNF-α blockers, the monoclonal antibodies infliximab and adalimumab, and the soluble TNF-α receptor fusion protein etanercept.10 In addition, anti-TNF-α therapy is being considered for the treatment of a variety of diseases in which TNF-α contributes to the pathology, such as in asthma,11 cardiac failure,12 diabetes,13 and cancer.14,15 Therapeutic application of the approved anti-TNF-α drugs is, however, hampered by the general disadvantages of protein drugs, such as the lack of oral availability, limited distribution, possible immunogenic reactions, and high cost of therapy.16 In light of these limitations, there is a continuous search for novel, efficient and safe anti-inflammatory drugs, in particular low-molecular-weight inhibitors of TNF-α production. Other approaches for blocking pro-inflammatory cytokines such as IL-1217 or inducing the anti-inflammatory cytokine IL-10 are currently under clinical evaluation.18
A synthetic phospholipid molecule, 1-methyl-2-(3-methoxyphenyl)-2-(octanoylamino)ethyl-disodium-phosphate, named hereafter phospho-ceramide analogue-1 (PCERA-1; Fig. 1), was recently described as a potent in vivo suppressor of LPS-induced TNF-α secretion.19,20 Evaluation of the structure–activity relationships has demonstrated that both the phosphate20,21 and the lipidic19,22 portions of PCERA-1 are required for activity. The chemical and pharmacological features of PCERA-1 led us to postulate the existence of a novel phospholipid-binding receptor, which can modulate TNF-α production. The field of phospholipid-binding G-protein coupled receptors (GPCRs) is a rapidly expanding area of research, amenable to modulation using reverse pharmacological approaches, as was demonstrated by the discovery of the mechanism of action of the clinical immunosuppressant FTY720, which in its phosphate-ester form is a highly potent non-selective agonist of sphingosine-1-phosphate (S1P) receptors.23 FTY720 and selective S1P1 agonists enabled the dissection of the roles of phospholipid-binding receptors in lymphocyte recirculation.24–27 Following these findings, it became clear that other endogenous phospholipids might also have receptor-dependent signalling roles. As the endogenous phospholipid mediators S1P and lysophosphatidic acid (LPA) are not known to suppress in vivo TNF-α production, it is conceivable that PCERA-1 has unique signalling properties.
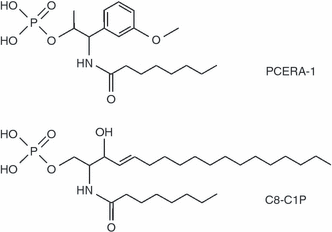
Structure of phospho-ceramide analogue-1 (PCERA-1) and C8-ceramide-1-phosphate (C8-C1P).
While an extensive structure–function relationship was previously demonstrated in vivo,19–22,28,29 the mechanism of PCERA-1 activity remained poorly understood. In particular, no data was made available regarding the identity of the TNF-α producing cells which are affected by PCERA-1, regarding the biochemical step by which PCERA-1 regulates TNF-α production, or regarding its effect on production of other cytokines. Thus the purpose of our studies has been to elucidate key aspects of regulation of TNF-α production by this novel phospholipid-like molecule. As the majority of in vivo LPS-induced TNF-α is produced by tissue macrophages, we decided to use RAW264.7, a mouse macrophages cell line, for these studies. We show here that PCERA-1 can act in a specific extracellular manner on activated macrophages, and that its effect is TLR-general, rather than specific for a particular TLR. Additionally, we show that PCERA-1 suppresses production of the pro-inflammatory cytokine TNF-α while it independently induces production of the anti-inflammatory cytokine IL-10. These effects were observed at the protein as well as the mRNA level, and are suggested to occur via the second messenger cyclic AMP (cAMP).
Materials and methods
Reagents and cell culture
LPS (Escherichia coli serotype 055:B5), imiquimod, peptidoglycan, isobutylmethylxanthine (IBMX), LPA, polymyxin-B, phenylmethylsulphonyl fluoride (PMSF) and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO). Trypsin, l-glutamine, penicillin and streptomycin were purchased from Biological Industries (Beit Haemek, Israel). Dulbecco’s modified Eagle’s minimal essential medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA). Bovine serum albumin (BSA) was purchased from Amresco (Solon, OH). C8-ceramide-1-phosphate (C8-C1P) and sphingosine-1-phosphate (S1P) were purchased from Avanti Polar lipids (Alabaster, AL), dissolved in ethanol and then diluted in culture medium containing 4% fatty acid-free BSA. A neutralizing monoclonal anti-mouse IL-10 antibody, rat immunoglobulin G (IgG) isotype control, recombinant mouse TNF-α (rTNF-α) and enzyme-linked immunosorbent assay (ELISA) reagent sets for TNF-α and IL-10 were purchased from R&D Systems (Minneapolis, MN). The cAMP enzyme immunoassay (EIA) kit was purchased from Cayman Chemicals (Ann Arbor, MI). Antibodies against doubly phosphorylated mitogen-activated protein (MAP) kinases, general MAP kinases, α-tubulin and proliferating cell nuclear antigen (PCNA) were obtained from Sigma, while the antibody against the p65 subunit of NF-κB was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Infrared dye-labelled secondary antibodies and blocking buffer were obtained from Li-Cor Biosciences (Lincoln, NE). Nitrocellulose membranes were purchased from Sartorius (Gollingen, Germany) and Immobilon-FL polyvinylidene fluoride (PVDF) membranes were purchased from Millipore (Billerica, MA). Complete protease inhibitor mixture was purchased from Roche (Mannheim, Germany). NAP-5 columns were purchased from GE Healthcare (Little Chalfont, UK). PCERA-1 and its non-phosphorylated analogue, CERA-1, were synthesized according to published procedures.21,29 PCERA-1 was dissolved in phosphate-buffered saline (PBS) and freshly diluted in culture medium, while CERA-1 was initially dissolved in ethanol and then diluted in culture medium containing 4% fatty acid-free BSA. Mouse RAW264.7 macrophage cells, obtained from the American Type Culture Collection (ATCC, Rockville, MD), were grown to 80–90% confluence in DMEM medium supplemented with 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (hereafter culture medium) and 10% FBS, at 37° in a humidified incubator with 5% CO2.
Lysis buffers
Buffer A contained 1% Triton X-100, 50 mm Tris (pH 8·0), 100 mm NaCl, 50 mmβ-glycerophosphate, 40 mm NaF, 1 mm sodium orthovanadate, 1 mm ethylenediaminetetraacetic acid (EDTA), 1 mm ethyleneglycoltetraacetic acid (EGTA) and 30% glycerol. Buffer B contained 10 mm HEPES, pH 7·9, 10 mm KCl, 0·25 μm dithiothreitol (DTT) and 0·1 mm EDTA. Buffer C contained 1% NP-40, 0·025% sodium dodecyl sulphate (SDS), 1% deoxycholic acid, 50 mm Tris, pH 8·0, 0·4 m NaCl and 0·1 mm EDTA. In addition, all lysis buffers contained ‘complete’ protease inhibitor mixture, diluted according to the manufacturer’s instructions, and supplemented with 1 mm PMSF.
Determination of free and protein-bound (PCERA-1)
PCERA-1 (10 μm) was incubated for 2 hr at 37° in culture medium, with or without serum (5% FBS). Free PCERA-1 and protein-bound PCERA-1 were completely separated on a NAP-5 exclusion chromatography column. A collection efficiency of 56% for the low-molecular-weight fraction was determined by mass spectrometry (MS), using PCERA-1 (in the absence of serum proteins) as a probe. A collection efficiency of 88% for the high-molecular-weight fraction was determined by a modified Bradford protein assay (details below), with serum proteins as a probe. For release and quantification of protein-bound PCERA-1, the high-molecular-weight fraction was diluted 1 : 4 with methanol at −20°, vortexed for 1 min, and concentrated back to the original volume using a speed-vac.
The concentration of PCERA-1 in the two fractions was measured by liquid chromatography mass spectrometry (LC-MS). Briefly, the PCERA-1 sample (5 μl) was applied to a SB-C18 column (Agilent Zorbax, 2·1 mm × 75 mm; Agilent Technologies Inc., Santa Clara, CA) on an Agilent 1100 LC system. Solvents A and B were 0·1% formic acid in H2O or in acetonitrile, respectively, and the flow rate was 0·25 ml/min. Isocratic elution with 90% solvent A for 3 min was followed by a linear gradient ramping to 100% solvent B at 15 min, with the column effluent being sent to an Agilent 6410 triple quadrupole mass spectrometer. PCERA-1 was quantified by monitoring the signature m/z transition state 388 → 290 and using 388 → 164 as a qualifying transition. Analysis was carried out in positive ion mode with a capillary voltage of 4000 V, a fragmentor voltage of 115 V and a dwell time of 250 milliseconds, and a collision energy of 15 V. A calibration curve of PCERA-1 standards (2·5–50 pmol) was obtained before and after all samples, to correct for signal drift. Duplicate analysis of each sample was used to determine an average and standard deviation, and the whole experiment was repeated twice.
Macrophage activation assay
RAW264.7 macrophages were maintained for 48 hr prior to the experiment in 96-well plates, at 2 × 105 cells/well, in culture medium supplemented with 5% FBS, at 37° in a humidified incubator with 5% CO2. The cells were stimulated with LPS (10–100 ng/ml) at 37° for 2 hr in the presence or absence of PCERA-1 (10 μm). TNF-α and IL-10 secretion to the medium was measured by ELISA.
Cytokine and cAMP measurements
Measurements of TNF-α and IL-10 levels in supernatants of RAW264.7 cells were performed with commercially available ELISA reagent sets, according to the manufacturer’s instructions, using a microplate reader (Bio-Tek, Winooski, VT). The cytokines were undetectable (< 20 pg/ml) in the absence of LPS. The samples were stored at −80° until use. All experiments were repeated as least three times. Measurements of intracellular cAMP levels in RAW264.7 cells were performed with a commercially available EIA kit, according to the manufacturer’s instructions.
Viability assay
RAW264.7 macrophages were seeded in a six-well plate at 8 × 105 cells/well and cultured for 48 hr as above for the activation assay. The cells were treated with LPS (100 ng/ml) and/or PCERA-1 (10 μm) for 2 hr at 37°, harvested with cold PBS, centrifuged (1000 g for 3 min), and re-suspended in PBS at 1·6 × 106 cells/ml. Viability was measured by FACS (Easy Cyte Mini System; Guava, Hayward, CA) using a Guava Viacount reagent kit, according to the manufacturer’s instructions. Analysis was performed using the Guava cyto soft software.
MAP kinase phosphorylation assay
RAW264.7 macrophages were maintained for 24 hr prior to the experiment in 12-well plates, at 8 × 105 cells/well, in culture medium supplemented with 0·1% FBS. The cells were stimulated with LPS (100 ng/ml) at 37° for 30 min in the presence or absence of PCERA-1 (10 μm). The cells were washed twice with cold PBS and lysed for 1 hr at 4° with buffer A. Cell extracts were centrifuged (14 000 g, 15 min at 4°) and the supernatants were stored at −80°.
NF-κB activation assay
RAW264.7 macrophages (3 × 106 cells per T-25 flask) were grown in culture medium supplemented with 10% FBS for 48 hr, up to a confluence of 90%. The cells were stimulated with LPS (1 μg/ml) at 37° for 1 hr in the presence or absence of PCERA-1 (0·1–10 μm). The cells were harvested with trypsin, centrifuged (1000 g for 3 min), and washed twice with cold PBS. Separation of the cytosolic and nuclear fractions was accomplished by a modification of a procedure of Zilberman et al.30 Briefly, cell pellets were re-suspended in lysis buffer B, and allowed to swell on ice for 15 min. Then, 2·5% NP-40 was added, and the samples were centrifuged at 900 g for 5 min. The cytosolic fraction was collected and stored at −80° until use, while the nuclear pellet was washed again with buffer B, re-suspended in buffer C and homogenized using a Dounce Wheaton homogenizer (Wheaton Science, Millville, NJ) and tight pestle. The nuclear extracts were kept on ice for 1 hr with intermittent vortexing prior to centrifugation at 20 000 g for 10 min. The supernatant (nuclear fraction) was stored at −80° until use.
Western blotting
Cell extracts (30 μg protein) were boiled for 5 min in sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) buffer and subjected to 10% SDS-PAGE, and proteins were transferred to a nitrocellulose (NF-κB assay) or Immobilon-FL PVDF (MAP kinase assay) membrane. Two-colour imaging and quantitative analysis of western blots were performed using the Odyssey infrared imaging system (Li-Cor Biosciences), according to the manufacturer’s instructions.
Protein determination
Protein was determined by a modification of the Bradford procedure, which yields linear results, increased sensitivity, and reduced detergent interference, as previously described by Zor and Selinger.31 BSA served as a standard.
Quantitative reverse transcription–polymerase chain reaction (RT-PCR)
The mRNA levels of TNF-α and IL-10 in RAW264.7 cells were quantified by real-time PCR. The cells were seeded in a six-well culture plate at 1 × 106 cells/well and cultured for 48 hr in culture medium supplemented with 5% FBS. The cells were then treated with LPS (100 ng/ml) with or without PCERA-1 (10 μm) for 1 hr at 37°. Total RNA was isolated using the MasterPure RNA purification kit (Epicentre Biotechnologies, Madison, WI), and 1 μg of RNA from each sample was reverse transcribed into cDNA using the Verso cDNA synthesis kit (Thermo Scientific, Waltham, MA). Quantification of cDNA (50 ng total) was preformed on the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), using TaqMan probes, purchased from Qiagen (Valencia, CA) for TNF-α measurement, and from primerDesign (Southampton, UK) for IL-10 and actin measurements.
Statistical analysis
All the data were analysed using Student’s t-test wherever applicable. In all cases, differences of P < 0·05 were considered to be significant. Effective concentration (EC50) values were calculated by non-linear regression curve fitting, using the sigmaplot software (Systat Software Inc., San Jose, CA).
Results
In vitro activity of PCERA-1 in RAW264.7 macrophage cells
To assess the in vitro effect of PCERA-1 on LPS-stimulated TNF-α secretion, mouse RAW264.7 macrophages were incubated with various concentrations of PCERA-1 and stimulated with LPS for 2 hr. Figure 2(a) shows that PCERA-1 suppressed production of the pro-inflammatory cytokine TNF-α with an EC50 of 100 ± 20 nm (mean ± SD of 3 independent experiments), and that maximum inhibition was observed at a concentration of approximately 1·0 μm. Interestingly, PCERA-1 increased in vitro production of the anti-inflammatory cytokine IL-10, with an identical EC50 of 100 ± 20 nm (Fig. 2a). As PCERA-1 is a phospholipid-like molecule, it is expected to bind to serum albumin present in the culture medium. Such binding decreases the actual concentration of PCERA-1 which is free and available for interaction with the receptor. We thus measured the concentration of free PCERA-1 in the serum-supplemented culture medium, using size exclusion chromatography (separating protein-bound and protein-free PCERA-1) followed by quantification using LC-MS analysis. The distribution of PCERA-1 (10 μm total) between the protein-bound and protein-free fractions was 3888 ± 605 and 628 ± 37 pmol, respectively, while total PCERA-1 (in the absence of serum) was 5000 ± 953 pmol (mean ± SD). Thus, roughly 87 ± 3% of PCERA-1 (at 10 μm) is bound to serum proteins, indicating that the actual EC50 for free PCERA-1, available for interaction with its receptor, is 13 ± 2 nm (calculated as 13% of 100 nm). This calculation represents a maximal estimation because at the total concentration of 100 nm, which yields a 50% effect, a lower fraction of PCERA-1 may be protein-free than at 10 μm. Yet, we cannot rule out the possibility that protein-bound PCERA-1 is also active.
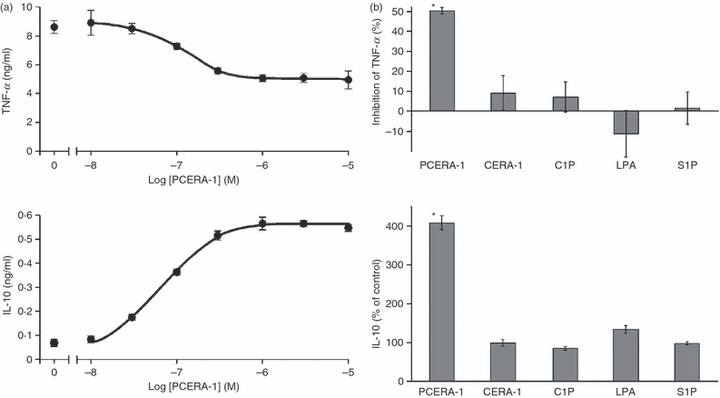
Phospho-ceramide analogue-1 (PCERA-1) specifically suppresses tumour necrosis factor (TNF)-α and enhances interleukin (IL)-10 production in RAW264.7 macrophages. (a) Mouse macrophage RAW264.7 cells were incubated at 37° for 2 hr with the indicated concentrations of PCERA-1 and with lipopolysaccharide (LPS; 100 ng/ml). TNF-α and IL-10 release to the medium was measured by enzyme-linked immunosorbent assay (ELISA). Each data point represents the mean ± standard deviation (SD) (n = 6). (b) The cells were incubated with LPS (100 ng/ml) in the presence or absence of PCERA-1, its dephosphorylated derivative CERA-1, C8-ceramide-1-phosphate (C8-C1P), lysophosphatidic acid (LPA) or sphingosine-1-phosphate (S1P) (each 10 μm, except for S1P which was 5 μm). The vehicle, which included 0·5% ethanol, had no significant effect on cytokine release. The error bars represent the sum of standard deviations (SDs) (n = 6) obtained for the measurements of sample and control (LPS only). The results are representative of at least three independent experiments. *P < 0·004, for cells incubated with LPS and PCERA-1, compared with LPS only.
Attesting to specificity, the dephosphorylated form of PCERA-1 (CERA-1) had no significant effect on the production of TNF-α or IL-10 (Fig. 2b). Furthermore, no significant effect on cytokine production was observed for C8-ceramide-1-phosphate (C8-C1P), a phospholipid that has close structural similarity to PCERA-1 (1, 2), or for the native extracellular phospholipid mediators S1P and LPA, which are also related in structure to PCERA-1 (Fig. 2b). Finally, neither PCERA-1 nor LPS affected macrophage viability (75 ± 5% for untreated or treated cells).
We examined whether the continuous presence of PCERA-1 was necessary for the suppression of LPS-induced TNF-α production. Preincubation of the macrophages with PCERA-1 followed by its removal prior to LPS addition resulted in a loss of PCERA-1 activity (Fig. 3a). Similarly, prostaglandin E2 (PGE2), an agonist of a cell-surface receptor, suppressed TNF-α production only when co-incubated with LPS. In contrast, dexamethasone, an agonist of a nuclear receptor, efficiently suppressed TNF-α production even when the LPS stimulus was applied after its preincubation and removal from the cell culture medium (Fig. 3a).
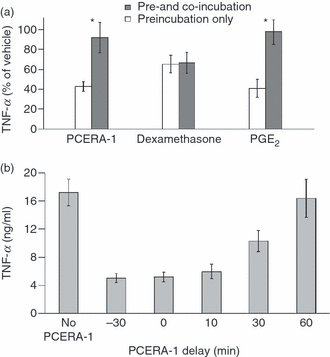
Co-incubation with lipopolysaccharide (LPS), rather than preincubation, is required for tumour necrosis factor (TNF)-α suppression by phospho-ceramide analogue-1 (PCERA-1). (a) Mouse macrophage RAW264.7 cells were preincubated at 37° for 40 min with one of the indicated TNF-α suppressors [PCERA-1 (10 μm), prostaglandin E2 (PGE2) (0·1 μm) or dexamethasone (2 μm)] or with vehicle. The cells were washed and incubated at 37° for 2 hr with LPS (100 ng/ml) and with a fresh solution of one of the above TNF-α suppressors (open bars) or with vehicle (solid bars). TNF-α release to the medium was measured by enzyme-linked immunosorbent assay (ELISA). Each point represents the mean of six replicates, relative to TNF-α level measured for LPS only. The error bars represent the sum of standard deviations (SDs) (as a percentage) obtained for measurements in the presence and absence of the TNF-α suppressor. The results are representative of at least three independent experiments. *P < 0·001 for cells incubated with PCERA-1 or PGE2 together with LPS, compared with cells preincubated with PCERA-1 or PGE2 only before LPS addition. (b) Mouse macrophage RAW264.7 cells were incubated with LPS (100 ng/ml) at 37° for 3 hr. PCERA-1 (10 μm) was added at the indicated time-points relative to LPS addition. TNF-α release to the medium was measured by ELISA. Each point represents the mean ± SD (n = 6).
Significantly, preincubation of the macrophages with PCERA-1, prior to LPS addition, was not required for its activity, as evident from the identical activities of PCERA-1 when added 30 min before LPS (without removal) or co-added with LPS (Fig. 3b). However, the ability of PCERA-1 to suppress prior LPS-induced TNF-α production was strictly time-dependent. Full inhibitory activity was demonstrated when PCERA-1 was added no later then 10 min after LPS, while a delay of 1 hr resulted in a major loss of its activity, although LPS incubation time was increased from 2 to 3 hr (Fig. 3b). The ability of PCERA-1 to amplify LPS-induced IL-10 production was similarly time-dependent (data not shown). Taken together, our results demonstrate that PCERA-1 can specifically exert a robust anti-inflammatory effect on LPS-stimulated macrophages in culture, in an extracellular time-dependent manner.
TNF-α suppression and IL-10 induction are independent
As the modulations of TNF-α and IL-10 production by PCERA-1 had identical concentration dependences (Fig. 2a) and were found to be on similar time scales (data not shown), we hypothesized that PCERA-1 binds a single receptor that reciprocally regulates production of TNF-α and IL-10. In order to experimentally determine whether indeed both TNF-α suppression and IL-10 induction are primary effects of PCERA-1 or whether one of them is a secondary effect of the other, we measured cytokine production by LPS-stimulated RAW264.7 cells treated with or without PCERA-1 in the presence or absence of a neutralizing anti-IL-10 antibody or a recombinant TNF-α protein (rTNF-α). The anti-IL-10 antibody was added to eliminate the possible TNF-α suppression mediated by PCERA-1-induced IL-10, while rTNF-α was added to eliminate the possible effect of the PCERA-1-suppressed TNF-α level on IL-10 production. We found that PCERA-1-mediated suppression of TNF-α production was not affected by the anti-IL-10 antibody (Fig. 4a), although the antibody totally sequestered the secreted IL-10 (data not shown). An IgG isotype-matched control antibody also did not significantly affect the level of either TNF-α or IL-10. This result indicated that TNF-α suppression is not mediated by PCERA-1-induced IL-10 in these cells. Similarly, addition of exogenous rTNF-α to PCERA-1-treated cells, up to the level of LPS-induced TNF-α obtained in the absence of PCERA-1, did not change IL-10 secretion (Fig. 4b). We therefore conclude that IL-10 induction and TNF-α suppression are independent effects of PCERA-1 in activated macrophages.
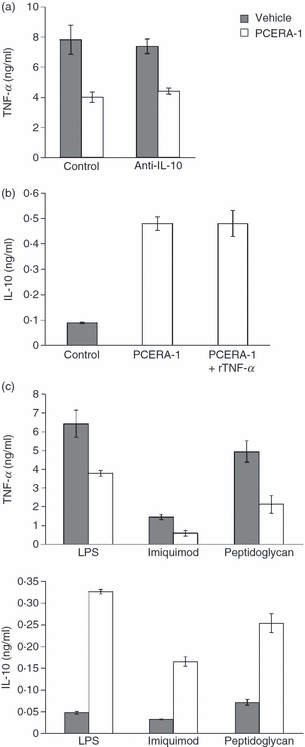
The anti-inflammatory effects of phospho-ceramide analogue-1 (PCERA-1) are independent of each other and do not depend on a specific inflammatory stimulus. Mouse macrophage RAW264.7 cells were incubated at 37° for 2 hr with lipopolysaccharide (LPS) [Toll-like receptor 4 (TLR4) agonist, 10 ng/ml, panels a–c], imiquimod (TLR7 agonist, 100 μm, panel c) or peptidoglycan (TLR2 agonist, 30 μg/ml, panel c) in the presence (open bars) or absence (solid bars) of PCERA-1 (10 μm). Interleukin (IL)-10 and tumour necrosis factor (TNF)-α levels in the culture medium were measured by enzyme-linked immunosorbent assay (ELISA). Each data point represents the mean ± standard deviation (SD) (n = 6). P < 0·001 for cells incubated with PCERA-1, compared with cells incubated with vehicle. (a) A neutralizing monoclonal anti-IL-10 antibody (3 μg/ml) was added to observe the effect of PCERA-1-induced IL-10 on TNF-α production. IL-10 neutralization was verified by ELISA. (b) Recombinant TNF-α (rTNF-α, 6 ng/ml) was exogenously added to the PCERA-1-treated cells to complement the TNF-α level to that of the control samples.
PCERA-1-mediated modulation of cytokine production by agonists of TLR2, TLR4 and TLR7
To further establish the general anti-inflammatory character of PCERA-1 activity, we incubated RAW264.7 cells with or without PCERA-1 in the presence of either LPS (a TLR4 agonist), peptidoglycan (a TLR2 agonist) or imiquimod (a TLR7 agonist). As shown in Fig. 4(c), TNF-α suppression by PCERA-1 was general and independent of the specific TLR activated. Additionally, IL-10 production was markedly up-regulated by PCERA-1, in the presence of any of the TLR agonists (Fig. 4c). Induction of IL-10 by PCERA-1 in the absence of a TLR agonist was as low as induction by the TLR agonist alone, indicating a synergistic IL-10 production (data not shown). To rule out a possible contamination of LPS in the peptidoglycan and imiquimod samples, we added polymyxin-B, a specific LPS blocker, and observed diminished cytokine production in the presence of LPS but not in the presence of the TLR2 or TLR7 agonist (data not shown).
Intracellular signalling of PCERA-1
To provide further insight into the molecular mechanism underlying the PCERA-1-dependent reciprocal regulation of TNF-α and IL-10 release, the mRNA levels of these cytokines in RAW264.7 cells were quantified by real-time RT-PCR. Figure 5 shows that PCERA-1 reduced the LPS-induced TNF-α mRNA level by 33%, while it elevated the IL-10 mRNA level 2·7-fold over the LPS-induced level. Actin served as a control for the quantity of total mRNA analysed. These results suggest that PCERA-1 is involved in regulation of TNF-α and IL-10 gene expression in RAW264.7 cells.
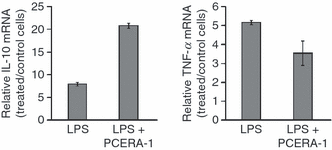
Phospho-ceramide analogue-1 (PCERA-1) modulates mRNA levels of tumour necrosis factor (TNF)-α and interleukin (IL)-10. Mouse macrophage RAW264.7 cells were incubated in duplicates with or without PCERA-1 (10 μm) and stimulated with lipopolysaccharide (LPS; 100 ng/ml) for 1 hr at 37°. Total RNA was isolated from the cells and cytokine mRNA levels were measured by quantitative reverse transcription–polymerase chain reaction (RT-PCR). TNF-α and IL-10 mRNA levels were normalized using internal actin mRNA levels and presented as fold-increase over control [Dulbecco’s modified Eagle’s minimal essential medium (DMEM)] cells. P < 0·001 for cells incubated with LPS + PCERA-1, compared with cells incubated with LPS alone. Cells incubated with PCERA-1 in the absence of LPS showed a non-significant difference from control cells. The results are representative of two independent experiments.
LPS-induced production of TNF-α depends primarily on the transcription factor NF-κB,32 and on the mitogen-activated protein (MAP) kinases p38, extracellular signal-regulated kinases (ERK) 1/2 and c-jun N-terminal kinase (JNK).33 We therefore sought to examine the effect of PCERA-1 on LPS-induced activation of these key mediators. Figure 6(a) shows that PCERA-1 was unable to inhibit the LPS-induced nuclear translocation, and hence activation, of the p65 subunit of NF-κB. Figure 6(b) shows that PCERA-1 was unable to significantly affect the LPS-induced phosphorylation, and hence activation, of any of the three examined MAP kinases. It should be noted that PCERA-1 did not block activation of these signalling pathways, when measured either at the peak of activation (Fig. 6; 1 hr for NF-κB and 0·5 hr for MAP kinases) or at earlier time-points (data not shown). The relatively low basal activation of NF-κB, and even lower basal activation of MAP kinases, may be attributable to serum factors or growth conditions in general.
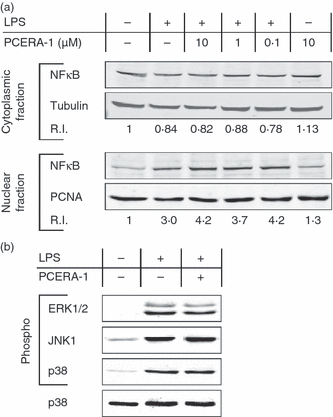
Phospho-ceramide analogue-1 (PCERA-1) does not block activation of nuclear factor (NF)-κB and mitogen-activated protein (MAP) kinases. (a) NF-κB activation. Mouse macrophage RAW264.7 cells were stimulated with lipopolysaccharide (LPS; 1 μg/ml) for 1 hr at 37°, in the absence or presence of the indicated concentration of PCERA-1. Dulbecco’s modified Eagle’s minimal essential medium (DMEM) served as vehicle. Cytosolic and nuclear fractions (30 μg of protein) were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and, after transfer to nitrocellulose, the membranes were probed with an anti-NF-κB-p65 antibody, together with an antibody against either tubulin (cytosolic reference) or proliferating cell nuclear antigen (PCNA, nuclear reference) for normalization. The normalized relative intensity (RI) of NF-κB-p65 in the unstimulated cytosol or nucleus was set to 1. The results are representative of three independent experiments. (b) MAP kinase activation. Mouse macrophage RAW264.7 cells were stimulated with LPS (100 ng/ml) for 30 min at 37°, in the presence or absence of PCERA-1 (10 μm). DMEM served as vehicle. Cell lysates (30 μg of protein) were subjected to SDS-PAGE and, after transfer to Immobilon-FL polyvinylidene fluoride (PVDF), the membranes were probed with antibodies against the doubly phosphorylated form of each MAP kinase, and with a general antibody against p38 for normalization. The treatments had no effect on the total level of p38 (shown here), extracellular signal-regulated kinase 1/2 (ERK1/2) or c-jun N-terminal kinase (JNK) (data not shown). The results are representative of four independent experiments.
Finally, as cAMP is known to mediate TNF-α suppression34 and IL-10 induction7 through a multitude of endogenous extracellular mediators (i.e. PGE2), we sought to determine whether PCERA-1 affects the cAMP level. Figure 7 shows that PCERA-1 indeed elevated the intracellular cAMP level fivefold relative to the vehicle. The observed cAMP increase was LPS-independent and comparable to that induced by PGE2 (data not shown). The structurally related phospholipid C8-C1P (Fig. 1) had no significant effect on cAMP level (Fig. 7). Taken together, our results suggest that PCERA-1 specifically increases intracellular cAMP to a functionally significant level.
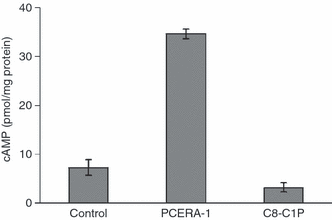
Phospho-ceramide analogue-1 (PCERA-1) elevates the intracellular cyclic AMP (cAMP) level. Mouse macrophage RAW264.7 cells were preincubated with the cAMP-phosphodiesterase inhibitor isobutylmethylxanthine (IBMX; 1 mm) for 10 min at 37° prior to the addition of PCERA-1 (100 μm), C8-ceramide-1-phosphate (C8-C1P; 100 μm) or vehicle, for an additional period of 10 min. Intracellular cAMP was then measured by enzyme immunoassay (EIA). Each data point represents the mean ± standard deviation (n = 6).
Discussion
Recently published data showed that a synthetic phospholipid-like molecule, named PCERA-1, potently suppresses TNF-α production in LPS-challenged mice, by an unknown mechanism.28 As TNF-α plays a distinctive role in the initiation and progression of autoimmune diseases,6,9 we sought to investigate the mechanism of action by which PCERA-1 inhibits TNF-α production.
The following experimental evidence indicates that PCERA-1 specifically binds a receptor expressed on the cell membrane of activated macrophages. (i) The α-methyl group (Fig. 1) protects PCERA-1 from dephosphorylation which might enable membrane permeability but would be detrimental for its activity, in vivo20,21 as well as in vitro (Fig. 2b). Thus, the inactivity of CERA-1 suggests that PCERA-1 is unable to cross the cell membrane and therefore acts in an extracellular manner. (ii) The lipid moiety of PCERA-1, which is also required for its activity,19,22 may enable its insertion into the cell membrane. We used C8-C1P, a structurally related phospholipid, as a negative control (Fig. 2b) to rule out a non-specific effect on membrane structure, leading to an aberrant response to LPS. (iii) In order to suppress TNF-α production, PCERA-1 must be present during incubation of the macrophages with LPS (Fig. 3a). This mode of TNF-α suppression is similar to that of PGE2, which acts extracellularly via membrane receptors, and at the same time opposite to that of dexamethasone, which is cell-permeable and suppresses TNF-α production via a nuclear receptor (Fig. 3a). The finding that preincubation of the cells with PCERA-1 before LPS addition is not required for its activity (Fig. 3b) indicates that a signalling cascade initiated by PCERA-1 occurs in parallel to LPS signalling. This is in contrast to the modulation of TNF-α production in macrophages by omega-3 polyunsaturated fatty acids, which requires a long preincubation but no co-incubation with LPS, and thus is suggested to occur following incorporation of the fatty acids into the cell membrane and possibly their metabolism.35 (iv) Identical concentration-dependence curves were obtained for the independent effects of PCERA-1 on production of two different cytokines, TNF-α and IL-10, suggesting that a single receptor is responsible for both effects. (v) In support of a protein receptor for PCERA-1, the activity of PCERA-1 is highly dependent on the stereochemistry, being over two orders of magnitude more potent for the [1S,2R] stereoisomer relative to the [1S,2S] stereoisomer.20 Taken together, the above findings argue in favour of a specific membrane phospholipid-binding receptor, modulating production of key cytokines in activated macrophages, in an anti-inflammatory direction.
To date two subfamilies of phospholipid-binding receptors have been characterized, for which the endogenous extracellular mediators S1P and LPA serve as agonists. While these phospholipids affect inflammatory processes36–38 via GPCRs,39,40 they are not known to suppress in vivo TNF-α production. Moreover, neither S1P nor LPA had a significant effect on TNF-α or IL-10 release from LPS-stimulated RAW264.7 macrophages (Fig. 2b), implying a distinct receptor for PCERA-1. Interestingly, low-density lipoprotein (LDL)-derived oxidized phospholipids have been previously implicated in LPS signalling inhibition,41 mediated by cAMP,42 partially via the PGE2 receptor EP2.43 This receptor is, however, unlikely to be the PCERA-1 receptor as PCERA-1 contains a fully saturated and reduced fatty acid rather than an oxidized fatty acid moiety which is considered to be the common binding motif of EP2 agonists. Additionally, it should be noted that another LDL-derived oxidized phospholipid suppresses TNF-α, but does not activate that receptor,43 indicating that its receptor is yet to be discovered.
To date, no cell surface receptor has been identified for ceramide-1-phosphate (C1P). The drug studied here was named phospho-ceramide analogue-1 (PCERA-1) because of its molecular similarity to C1P (Fig. 1). However, a synthetic C8-C1P was unable to mimic the effects of PCERA-1 on cytokine production (Fig. 2b). This may represent a real functional difference, or may be attributable either to the different stereochemistries of PCERA-1 and C1P, or to the short chain of synthetic C1P, compared with natural C1P. In fact, the generic ‘ceramide’ includes over 50 distinct molecular species.44 Functionally, C1P is mainly considered a second messenger, which regulates survival and apoptosis.45 While several reports claimed that exogenous C1P may regulate inflammation by direct activation of phospholipase A2 (PLA2) and subsequent PGE2 production,46,47 Tauzin et al. have shown that this observation may be an artifact resulting from the use of dodecane as a C1P solvent.48 In this regard, it should be noted that PCERA-1 is water-soluble, and that dodecane was not used in our study for any purpose.
In contrast to C1P, which is not known to affect cytokine production, ceramide is implicated in LPS signalling, as it was shown to be endogenously produced upon LPS stimulation of RAW264.7 macrophages,49 and to mimic some of the effects of LPS upon exogenous addition.49,50 Conflicting results have been obtained for the effect of exogenous ceramide on TNF-α production, with some reports showing induction in unstimulated macrophages,51,52 and others showing no such effect in either unstimulated or LPS-stimulated macrophages.49,50 Interestingly, a recent report using intestinal epithelial cells showed that difluoromethylene analogue of sphingomyelin (SMA)-7, a C1P derivative with stereochemistry similar to that of PCERA-1, suppressed LPS-induced NF-κB activation and subsequent production of the pro-inflammatory cytokine IL-8, by direct inhibition of a ceramide-producing enzyme, sphingomyelinase.53 The relevance of endogenous ceramide signalling to the activity of PCERA-1 remains to be explored.
Inflammation is a complex process involving the simultaneous production of many cytokines.1 We demonstrate here that PCERA-1 was able to down-regulate TNF-α and up-regulate IL-10 production in LPS-induced RAW264.7 macrophages. Similar results were obtained in primary peritoneal macrophages (data not shown), attesting that the activity of PCERA-1 is not limited to a tumour cell line, and highlighting the importance of these findings for elucidation of the mechanism of action of PCERA-1. The viability data rule out a general toxic effect and the up-regulation of IL-10 release by PCERA-1 is inconsistent with either a general toxic/suppressive effect or a simplified mechanism of LPS-TLR4 signalling blockade. Furthermore, to assess the generality of the anti-inflammatory activity of PCERA-1, we replaced the TLR4 agonist LPS with either imiquimod or peptidoglycan, which are specific agonists for TLR754 and TLR2,55 respectively. We found that PCERA-1 can suppress TNF-α production and enhance the production of IL-10, regardless of the type of inflammatory stimulus dictating activation of a specific TLR. Moreover, we observed consistent inhibition of LPS-induced TNF-α secretion from RAW264.7 macrophages for at least 24 hr (data not shown), illustrating that PCERA-1 inhibits the release of TNF-α rather than postponing the onset of its release. We can therefore conclude that the anti-inflammatory activity of PCERA-1 is genuine and universal rather than limited to modulation of the TLR4 pathway.
Interleukin-10 and TNF-α have a reciprocal relationship where TNF-α promotes the production of IL-10 while IL-10 inhibits the expression of TNF-α.8 We therefore decided to determine whether TNF-α inhibition by PCERA-1 is dependent on the prior elevation of IL-10 in the RAW264.7 cell line. Using a neutralizing anti-IL-10 antibody, we showed that the effect of PCERA-1 on TNF-α release was independent of IL-10 activity. In addition, by adding exogenous TNF-α to the PCERA-1-treated RAW264.7 cells, we showed that IL-10 levels were elevated regardless of the TNF-α level. Moreover, IL-10 was modestly induced by PCERA-1 even in the absence of LPS and concomitant TNF-α release (data not shown). These results indicate that reduced TNF-α secretion and increased IL-10 production are independent consequences of PCERA-1 treatment. The identical concentration dependences of these effects imply that a single PCERA-1-binding receptor regulates production of both cytokines.
LPS-induced expression of TNF-α depends primarily on the transcription factor NF-κB,32 and on the MAP kinase family member p38, at the levels of transcription,56 mRNA stability,57 and translation.58 In addition to p38, other MAP kinase family members, ERK1/2 and JNK, are also involved in TNF-α induction.33 As PCERA-1 did not block the activation of these key signalling pathways by LPS, we reasoned that PCERA-1 rather activates a distinct pathway that blocks TNF-α production.
The second messenger cAMP initiates a signalling cascade that culminates in suppression of transcription at the TNF-α promoter34 and enhancement of transcription at the IL-10 promoter.7 The cAMP pathway is activated by a variety of external stimuli, which are occasionally complex negative feedback loops initiated by TNF-α.59 Among the mediators that utilize this mechanism are α-melanocyte-stimulating hormone (MSH),60 adrenocorticotropic hormone (ACTH) and corticotropin-releasing hormone (CRH),61 adrenaline,62 adenosine,63 vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP),64 and prostaglandin I2 (PGI2) and PGE2.65 As PCERA-1 elevates cAMP in RAW264.7 macrophages to a level comparable to that of PGE2, it is reasonable to assume that cAMP mediates TNF-α suppression and IL-10 induction by PCERA-1. Consistently, H-89, a specific inhibitor of the cAMP-dependent protein kinase A (PKA), blocked the effects of PCERA-1 on TNF-α and IL-10 production (data not shown).
To further delineate the mechanism of activity of PCERA-1 we determined the mRNA levels of TNF-α and IL-10 by quantitative RT-PCR. Our results clearly demonstrate that PCERA-1 reduces the accumulation of TNF-α mRNA, while it increases that of IL-10. Thus, the effect of PCERA-1 on TNF-α and IL-10 release from macrophages, following induction by LPS, involves at least in part modulation of transcription or mRNA stability. The finding that PCERA-1 must be co-added with LPS in order to effectively suppress TNF-α and induce IL-10 production indicates that PCERA-1 affects an early event in LPS signalling, and is consistent with regulation of transcription by PCERA-1.
Transcription at the TNF-α promoter is activated co-operatively by several LPS-induced transcription factors,33,66 with NF-κB as the primary regulator, binding at several distinct enhancer sites.67,68 We have shown here that, while PCERA-1 modulates the TNF-α mRNA level, it does not inhibit NF-κB activation. Consistently, PCERA-1 is able to partially, rather than fully, suppress TNF-α production. We suggest that cAMP response element-binding protein (CREB), activated by PCERA-1-elevated cAMP, acts as a repressor at the TNF-α promoter. Alternatively, PCERA-1 may inhibit a transcription factor, distinct from NF-κB, which induces TNF-α transcription in response to LPS.
To conclude, in this study we have demonstrated that PCERA-1, a phospholipid-like molecule, has the capability to affect production of key pro- and anti-inflammatory cytokines in activated macrophages. Our findings are consistent with a mechanism of activity in which PCERA-1 acts as an extracellular modulator, to influence in parallel the mRNA and consequently also protein levels of TNF-α and IL-10. These effects are likely to be mediated, at least in part, by the second messenger cAMP, which is elevated by PCERA-1. The endogenous phospholipid mediators S1P and LPA, for which cell membrane G-protein coupled receptors have been identified, do not suppress TNF-α or induce IL-10 production in LPS-stimulated RAW264.7 cells. We therefore propose that PCERA-1 binds a distinct phospholipid-binding receptor, upstream of an anti-inflammatory signalling pathway.
Acknowledgements
The research was supported by grants from the European Commission (IRG #021862), from Teva Pharmaceutical Industries Ltd, from the public committee for allocation of Estate funds at Israel’s Ministry of Justice (#3223), and from the Israel Science Foundation (#907/07). GL-R received post-doctoral fellowships from the Wise and the Pikovsky-Valachi foundations. TZ was financially supported by Israel’s Ministry of Absorption. We are grateful to Nava Silberstein for superb technical assistance. Amir Philosoph, Dana Feldman, Dana Shaham, and Matan Kalman are also acknowledged for technical assistance. Antibodies against MAP kinases were a generous gift from Dr Rony Seger. We thank Peter Ding and Dr Mark Parnell for chemical synthesis of PCERA-1 and Dr Germana Sanna and Dr Anthony H. Futerman for helpful discussions. We are grateful to Dr Uriel Zor, Dr Meir Shinitzky, Dr Joel Hirsch, and Tamir Avni for critical reading of the manuscript.




