Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation
Summary
Some pathogenic bacteria form thick capsules that both block immune responses through inhibition of complement deposition and phagocytosis and stimulate a weak response resulting from a lack of T-cell involvement. Contrary to this model, capsular polysaccharides from 23 serotypes of Streptococcus pneumoniae have been successfully used in a multivalent vaccine in the absence of a carrier protein. Furthermore, type I pneumococcal polysaccharide (Sp1) has been shown to activate T cells in vivo and in vitro via an uncharacterized mechanism. In the present report, we demonstrate that Sp1 utilizes the major histocompatibility complex (MHC) class II pathway in antigen-presenting cells (APCs) for processing and presentation. APCs internalize and process Sp1 through a nitric oxide-dependent mechanism and, once inside the cell, it associates with MHC II proteins in an H-2M-dependent manner that leads to in vivo T-cell activation. These results establish that Sp1 activates T cells which can lead to abscess formation in mice through an H-2M-dependent polysaccharide antigen presentation pathway in APCs, potentially contributing to pneumococcal polysaccharide vaccine efficacy through the recruitment of T-cell help.
Introduction
Bacterial carbohydrates are generally thought to impede immune recognition and response through a variety of innate mechanisms, while the adaptive immune response to carbohydrate-encapsulated bacterial pathogens is poorly understood. Deposition of complement proteins on the bacterial surface and antibody-mediated opsonization are usually reduced by capsular polysaccharides because they block exposure of surface proteins.1 Specific immune activation may also be thwarted through molecular mimicry and immunogenic tolerance. For example, the capsular carbohydrate structure of group B Neisseria meningitidis (α2→8 sialic acid) is remarkably similar to that of glycans on the neural cell adhesion molecule (N-CAM).2 Perhaps most importantly, carbohydrates alone do not typically activate T cells via major histocompatibility complex (MHC) presentation like protein antigens.1 It is thought that these molecules initiate an innate response primarily through pathogen-associated molecular pattern (PAMP) receptors3,4 and weak humoral responses via B-cell receptor crosslinking.5–7 As a result, responses to carbohydrate antigens often do not lead to full immunological protection against the pathogen nor immunoglobulin isotype switching, as a result in part of a lack of T-cell help.8
During the late 1970s and early 1980s, many attempts at using capsular polysaccharides alone as vaccine candidates largely failed because of a lack of lasting protective responses, especially in children. These experiments ultimately led to the idea of protein conjugation in which the polysaccharide of interest was covalently attached to an immunogenic carrier protein to increase the immunogenic properties of the carbohydrate. Conjugation typically results in the activation of T cells and initiation of T-cell help and memory,9,10 although several polysaccharide-only vaccines do exist. Perhaps the best example is PneumoVax®,11,12 a vaccine against Streptococcus pneumoniae, a Gram-positive bacterium known as a causative agent in pneumonia, bacteremia, otitis media, meningitis and peritonitis, with millions of cases per year in the USA alone. According to the Centers for Disease Control and Prevention, S. pneumoniae carries a mortality rate of 14% in patients with invasive disease (http://www.cdc.gov/ncidod/aip/research/spn.html) and has increasingly developed antibiotic resistance. Essentially all of the 90 serotypes described to date carry a thick polysaccharide capsule. PneumoVax® includes a mixture of 23 unconjugated S. pneumoniae capsular polysaccharides, representing 88% of the invasive serotypes, to provide good broad-spectrum protection in adults.13
The relative success of this vaccine in adults suggests that pneumococcal carbohydrates are stronger immunogens than many other bacterial polysaccharides and points to their central role in immune recognition during infection. Interestingly, type I S. pneumoniae produces a capsular polysaccharide (Sp1) that shows the ability to activate T cells in the absence of protein conjugation.14–16 Sp1 is comprised of a non-branched trisaccharide repeating unit [→3)-α-2,4-dideoxy-4-amino-D-FucNAc-(1→4)-α-D-GalAp-(1→3)-α-D-GalAp-(1→] that carries an alternating single positive charge and two negative charges, making the polymer zwitterionic (Fig. 1a).17 Although carbohydrates are traditionally considered T cell-independent antigens as mentioned above, Sp1 and other zwitterionic polysaccharides (ZPSs) induce abscess formation in rodent models of peritoneal infection16 in a T cell-dependent fashion.18 Studies have confirmed that Sp1 activates CD4+ T cells in vitro,19 and can be used to generate rat T-cell hybridomas that show polysaccharide specificity.15 These hybridomas failed to recognize conventional peptide antigens and no specific Vβ requirements within the αβ T-cell receptors (αβTCRs) were apparent, suggesting that the recognition may be similar to that of peptide antigens.15 Consistent with traditional antigen-presenting cell (APC)-mediated T-cell activation mechanisms, exposure of APCs to Sp1 induces B7-2 and CD40L up-regulation,14 while antibodies against the MHC II allele human leucocyte antigen (HLA)-DR blocks Sp1-mediated in vitro T-cell activation.19 These data collectively point to the newly defined MHC II-αβTCR driven mechanism20 for T-cell stimulation by Sp1.
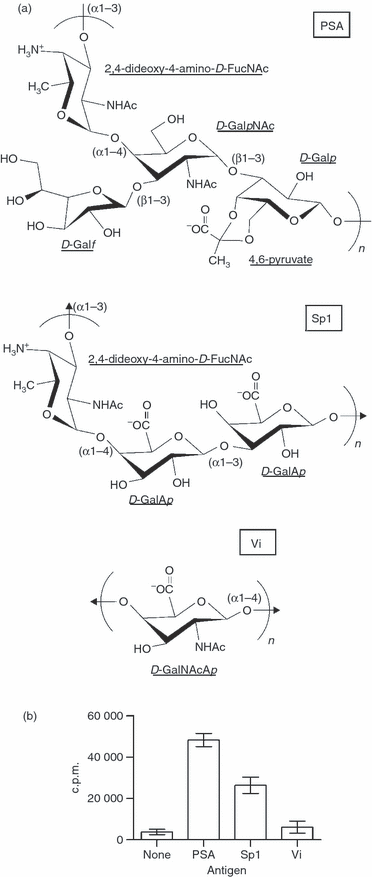
Repeating unit structures for polysaccharide A (PSA), type I pneumococcal polysaccharide (Sp1), and the Vi antigen from the capsule of Salmonella typhi. (a) Sp1 (middle) contains a trisaccharide repeating unit, with a positively charged free amine and negatively charged carboxylates shown to illustrate the zwitterionic nature of the polysaccharide. PSA (top) also contains a zwitterionic motif, although the Vi antigen (bottom) does not. (b) T-cell proliferation at day 6 of stimulation with 50 μg/ml PSA, Sp1 or Vi. Both zwitterionic polysaccharides PSA and Sp1 induced proliferation over medium background (10–15-fold), yet the Vi antigen did not. c.p.m., counts per minute.
T-cell activation mediated by the capsular polysaccharide A (PSA; Fig. 1a)20 from the commensal Gram-negative anaerobe Bacteroides fragilis is an important aspect of immune system development in the gut of gnotobiotic mice colonized with commensal organisms, suggesting that bacterial polysaccharide processing and presentation may represent a critical symbiotic host mechanism by which gut homeostasis is maintained.21 In contrast, Gram-positive pathogens such as S. pneumoniae and Staphylococcus aureus types 5 and 822 also express capsular ZPSs that are known to induce CD4+ T-cell activation during infection.
In the present study, the mechanism of pneumococcal polysaccharide Sp1 processing and presentation was determined. We found that Sp1 is processed by APCs into a low-molecular-weight form, and requires nitric oxide (NO) production and H-2M activity for MHC II-dependent surface presentation. Furthermore, Sp1 directly associates with MHC II proteins in vitro, and in vivo T-cell activation leading to abscess formation is dependent upon H-2M activity for MHC II loading. Sp1 binding to MHC II can be competed with soluble peptides, suggesting that the carbohydrate and peptide antigen binding domains overlap and supporting our findings on the H-2M dependence for loading and in vivo T-cell activation. These data conclusively demonstrate that NO-dependent carbohydrate processing and binding by MHC II proteins within the traditional endosomal pathway of APCs are critical mechanisms by which capsular polysaccharides from pathogenic bacteria are presented by the adaptive immune system. Moreover, the ability to utilize the traditional MHC II pathway suggests that ZPS antigens such as Sp1 from polysaccharide-only vaccines such as Pneumovax® facilitate efficacy through the induction of T-cell help.
Materials and methods
Sp1 purification and labelling
The Sp1 component of PneumoVax®, prepared by Merck & Co. (Whitehouse Station, NJ), was purchased from the American Type Culture Collection (ATCC; Manassas, VA). The pneumococcal C substance was removed from this preparation as previously described.19 Purified Sp1 was pooled and stored dry at −80°. Polysaccharide labelling was accomplished using carbodiimide [N-cyclohexyl-N′-(2-morpholinoethyl) carbodiimide metho-p-toluenesulphonate] reduction to generate reactive centres from 10% of the carboxylate groups.23 This site was then either biotinylated using biotin hydrazide (Pierce, Rockford, IL), tritiated with tritiated sodium borohydride (GE Healthcare, Piscataway, NJ), or fluorescently tagged with AlexaFluor594® hydrazide (Molecular Probes, Eugene, OR). Reactions were quenched with sodium borohydride and the products stored in dH2O at −20°.
Cells and animals
Raji cells, MHC II−/− Raji cells (RJ2.2.5), and splenocytes were cultured in RPMI-1640 medium (ATCC) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY). RAW 264.7 macrophages were cultured in Dulbecco’s modified Eagle’s medium (ATCC) supplemented with 10% fetal bovine serum (FBS). Murine splenocytes were isolated from wild-type (C57Bl/6), inducible nitric oxide synthase (iNOS)−/− (B6·129P2-Nos2tm1Lau), or H-2M−/− (murine homologue of HLA-DM; B6.129S4-H-2DMatm1Dim/J) mice. All animals were purchased from Jackson Laboratories (Bar Harbor, ME).
T-cell activation assays
Antigen-stimulated T-cell proliferation was measured by [3H]thymidine incorporation. Briefly, primary mononuclear cells were isolated from peripheral blood on a Lymphoprep gradient (Sigma-Aldrich, St Louis, MO). A portion of the sample to be used as the APC population was treated with 25 μg/ml mitomycin C for 30 min at 37° to prevent proliferation. Untreated mononuclear cells were used to isolate T cells on a nylon wool column. Cells were incubated in the column for 1 hr at 37° and then eluted with pre-warmed media. T cells and mitomycin C-treated APCs were co-cultured in equal numbers (105 each) with and without 50 μg/ml antigen for 6 days. On day 6, the cells were pulsed with 1 μCi [3H]thymidine for 6 hr to allow DNA incorporation into dividing cells and then the cells were harvested and analysed by scintillation counting.
Endosomal processing assays
Intracellular processing was assessed by incubating 107 Raji B cells or RAW 264.7 macrophages with 0·5 mg [3H]Sp1 overnight. Endosome isolation was performed as previously described.20 Radioactive Sp1 was analysed for molecular mass on a Superose 12 molecular sieve column fitted to a fast protein liquid chromatography (FPLC) system (BioRad, Hercules, CA) to measure cleavage compared with the input Sp1.
Co-immunoprecipitation assays
Wild-type, iNOS−/− and H-2M−/− splenocytes were incubated with 0·5 mg [3H]Sp1 overnight. Surface MHC II was removed by papain digestion [0·1 mg/ml papain; 10 mm Tris-HCl, pH 7·5; 1 mm ethylenediaminetetraacetic acid (EDTA); 1%β-mercaptoethanol] and immunoprecipitated with anti-HLA-DR mAb (clone L243) or an isotype control and protein A-agarose resin (Sigma, St Louis, MO). For total Sp1 present, the resin was quantified by scintillation counting. For analysis of Sp1 size bound to MHC II, the resin was boiled for 20 min and the released Sp1 was passed through a Superose 12 molecular sieve column as above and fractions quantified by scintillation counting.
Confocal microscopy
Human Raji or RJ2.2.5 B cells (106) were incubated with AlexaFluor594®-conjugated Sp1 (50 μg/ml) for 3 hr, then fixed with 4% paraformaldehyde for 30 min and stained with AlexaFluor488®-conjugated anti-HLA-DR mAb (clone L243). The cells were analysed by confocal microscopy using a Zeiss Pascal confocal microscope (×63 objective lens; Carl Zeiss, Thornwood, NY). For blocking endocytosis, all conditions were the same except that 20 μm colchicine or 20 μm cytochalasin D was added to cells 1 hr prior to Sp1 to inhibit endocytosis.
In vitro processing
[3H]Sp1 (1 mg/ml) in dual acetate and phosphate-buffered saline (A/PBS) at pH 5·0 or 7·3 was treated with ozone gas using an ozone generator (AquaZone; Red Sea Ltd, Houston, TX) at constant flow rate (100 mg/hr) for varying lengths of time and analysed for size on a Superose 12 molecular sieve column equilibrated in PBS and fitted to an FPLC system (BioRad) to measure cleavage. Fractions were quantified by scintillation counting and plotted against elution volume.
Binding studies
MHC II binding was performed with ozone-cleaved (for 15 min, except as indicated) and biotinylated Sp1. In vitro binding studies were performed essentially as previously described,20 except for the use of HLA-DR1 (DRA*0101, DRB1*0101; gift from Dr Stephen DeWall, Harvard Medical School) as the model MHC II protein24 and 10 μm Sp1 (except where indicated) at pH 5·0. Binding conditions were altered in some cases with the addition of 0·5 m NaCl or different pH values. The amount of Sp1 bound was quantified by time-resolved fluorometry using europium-conjugated streptavidin and lanthanide chelation as previously described.20 Data reflect the mean ± standard error of mean with at least n = 3 for all data points. Binding affinity (Kd) was determined through non-linear regression of the binding curve with varied Sp1 concentration using a single site–single ligand model (Prism v3.03; GraphPad Software, San Diego, CA). Competition assays with tetanus toxin peptide (TTp) were performed with static Sp1 concentration (10 μm) as above, but with varied TTp concentrations. TTp and Sp1 were added to wells at the same time. Bound Sp1 was detected by time-resolved fluorescence as above.
Abscess studies
Wild-type and H-2M−/− mice were injected with 50 μg of Sp1 and sterilized cecal contents as previously described.16 Animals with at least one peritoneal abscess 7 days following challenge were counted as positive.
Statistical analyses
All statistical analyses were performed using Instat v.3.06 (graphpad Software). Each analysis is indicated in the corresponding figure legend for clarity.
Results
Sp1 activates T cells in vitro
The zwitterionic polysaccharides PSA and Sp1 were compared with a T cell-independent carbohydrate molecule called the Vi antigen from the capsule of Salmonella typhi in T-cell stimulation assays to demonstrate immunological activity. The chemical structures of all three polysaccharide repeating units are shown in Fig. 1(a). Peripheral blood mononuclear cells were isolated and used as a source of APCs and T cells. Mononuclear cells were used as APCs and treated with mitomycin C to prevent proliferation, while T cells were isolated from an untreated mononuclear cell sample using a nylon wool column. Using [3H]thymidine incorporation into dividing cells, we found that both PSA and Sp1 induced nearly 10- to 15-fold proliferation of the T cells over medium-only background (Fig. 1b). In contrast, the T cell-independent Vi antigen failed to induce proliferation. These data confirm previously published findings15 showing that Sp1 activates T cells in vitro.
Sp1 co-localizes with MHC II
Our first step in determining the mechanism by which Sp1 activates CD4+ T cells employed confocal microscopy to measure the intracellular and cell surface localization of both MHC II protein and Sp1. Human Raji B cells were cultured with AlexaFluor594®-conjugated Sp1 (red) for 3 hr, fixed with paraformaldehyde and stained with AlexaFluor488®-conjugated anti-MHC II (green) monoclonal antibody (mAb) to assess co-localization (yellow). We found significant co-localization of MHC II and Sp1 fluorescence inside vesicular compartments as well as co-localization and increased local concentrations of MHC II on the surface of the APCs (Fig. 2a–b), demonstrating that Sp1 enters vesicles containing MHC II, commonly called MIIC, following endocytosis. Surface co-localization of MHC II and Sp1 further suggests that surface-bound Sp1 could be the result of a direct interaction with MHC II proteins.
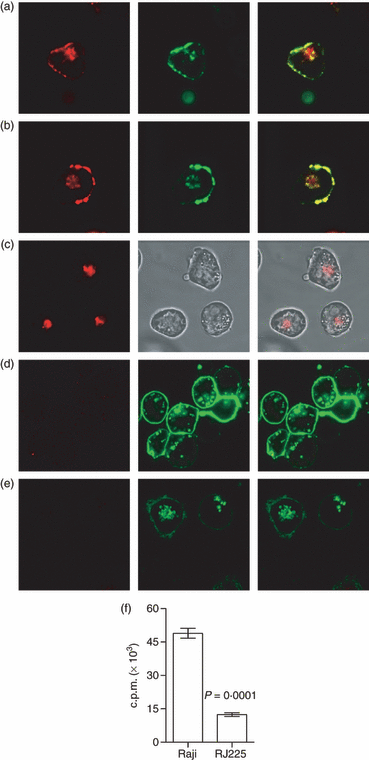
Type I pneumococcal polysaccharide (Sp1) is presented by major histocompatibility complex class II (MHC II) on antigen-presenting cells (APCs) following endocytosis. (a, b) Human Raji B cells were incubated with AlexaFluor594®-conjugated Sp1 (red) and then stained with monoclonal antibody (mAb) against MHC II (green). Sp1 and MHC II co-localize (yellow; merged image on right) both intracellularly and on the APC surface, suggesting but not confirming a direct molecular interaction. (c) MHC II−/− RJ2.2.5 B cells show internal localization of Sp1 (red; left) but not on the cell surface (see brightfield image; centre), demonstrating that surface localization of Sp1 is dependent upon MHC II expression. Raji cells pretreated with the cytoskeletal disruptor colchicine (d) or cytochalasin D (e) showed a complete blockade of Sp1 internalization and a failure to localize Sp1 on the surface, indicating that MHC II presentation is dependent upon Sp1 binding within intracellular compartments. (f) Raji cells were incubated with [3H]Sp1 overnight. Surface-localized MHC II was then removed via papain digestion and immunoprecipitated. Radioactive Sp1 co-immunoprecipitated with MHC II from wild-type Raji B cells, while B cells lacking MHC II (RJ2.2.5) showed significantly less Sp1 immunoprecipitation (mean ± standard error of the mean; P = 0·0001; unpaired t-test), confirming direct binding and presentation of Sp1 by MHC II on the surface of APCs. c.p.m., counts per minute.
In order to establish direct MHC II contacts with carbohydrate antigen during surface localization of Sp1, Raji B cells lacking MHC II expression, called RJ2.2.5, were incubated with AlexaFluor594®-Sp1 as above and viewed by confocal microscopy. In these cells, Sp1 was efficiently internalized, yet was not detectable on the cell surface (Fig. 2c). Wild-type Raji and RJ2.2.5 cells were also incubated with [3H]Sp1 overnight and the MHC II was removed by papain digestion and immunoprecipitated with α-HLA-DR monoclonal antibody (mAb). Immunoprecipitates from Raji B cells, but not RJ2.2.5 B cells, contained significant amounts of radiolabelled Sp1 (Fig. 2f). The amount of radioactivity in the RJ2.2.5 sample was equivalent to results obtained using an isotype control antibody (not shown). Together, these data show that surface localization of Sp1 is the result of MHC II-mediated binding and presentation.
Superantigens are distinct from conventional protein antigens in several ways, but two defining characteristics are that superantigens associate with MHC II directly on the cell surface without passage through the APC and they do not require proteolytic processing. Raji B cells were therefore treated either with the microtubule elongation inhibitor colchicine or the actin inhibitor cytochalasin D during exposure to antigen to confirm Sp1 entry via endocytosis and to test whether internalization was required for MHC II-mediated presentation. Internal and surface-localized Sp1 was eliminated in both cases (Fig. 2d–e). These data demonstrate that Sp1 binding to MHC II must occur within intracellular compartments following traditional antigen endocytosis, much like conventional protein antigens.
Sp1 processing and the endosome
Given the requirement for intracellular loading of Sp1 onto MHC II, we next sought to determine if Sp1 undergoes processing to small fragments in much the same way as conventional protein antigens are cleaved to peptides. Both murine RAW 264.7 macrophages and human Raji B cells were incubated with native high-molecular-weight [3H]Sp1 overnight. Vesicular compartments were isolated by differential centrifugation and lysed, and the contents were analysed by size exclusion chromatography. The elution pattern from the Superose 12 column showed that intact Sp1 was large and eluted near the column void volume (approx. 10 ml; Fig. 3), yet vesicular Sp1 isolated from macrophages and B cells also contained low-molecular-weight Sp1 (Fig. 3a and b, respectively). These data show that both APCs process Sp1 to smaller oligosaccharides within vesicular compartments following endocytosis.
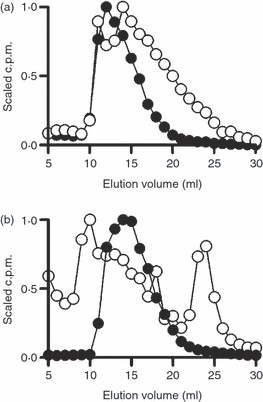
Antigen-presenting cells (APCs) process type I pneumococcal polysaccharide (Sp1) in vesicular compartments. Murine RAW 264.7 macrophages (a) and human Raji B cells (b) were incubated with [3H]Sp1 overnight and mechanically lysed, and the vesicular compartments were isolated by differential centrifugation. Vesicular contents were analysed on a Superose 12 molecular sieve column to determine the size of Sp1. Endosomal Sp1 (open circles) showed a marked reduction in size compared with control Sp1 (closed circles), indicating intracellular processing of Sp1 to lower molecular weight species in both APC types. c.p.m., counts per minute.
Sp1 processing and oxidation
The presence of low-molecular-weight Sp1 within APCs raises two significant questions: what is the mechanism of cleavage, and is it required for presentation? First, chemical cleavage of polysaccharides by ozone, or ozonolysis, has been shown to be an efficient method of depolymerizing carbohydrates.25,26 In order to determine if oxidation could be responsible for Sp1 cleavage within APCs, [3H]Sp1 was treated with ozone gas in near-neutral pH PBS for 0, 15 or 30 min and then analysed for size on a Superose 12 column as before. Ozone led to a significant reduction in molecular mass of Sp1 in a time-dependent manner (Fig. 4a), suggesting that the oxidative burst in APCs that occurs early following endocytosis could account for intracellular Sp1 processing. Interestingly, when this experiment was repeated at pH 5·0 to mimic the acidic environment of late endosomes and lysosomes, no oxidative cleavage was seen (Fig. 4b). These data suggest that, if oxidation plays a key role in intracellular processing of Sp1, it must occur early in the vesicular pathway, prior to acidification.

Oxidative cleavage of type I pneumococcal polysaccharide (Sp1). Sp1 was resuspended in dual acetate and phosphate-buffered saline (A/PBS) at pH 7·3 (a) or at pH 5·0 (b) and treated with ozone for varying times. At pH 7·3, the size of Sp1 decreased with increased time (closed circle 0 min, open square 15 min, and open triangle 30 min), indicating that Sp1 is readily cleaved through chemical oxidation at the near-neutral pH found in early endosomes. However, no cleavage occurred under the acidic conditions typical of late endosomes and lysosomes. (c) Wild-type (WT) C57/Bl6 mouse splenocytes were isolated and incubated with full-length [3H]Sp1 overnight. Surface major histocompatibility complex class II (MHC II) was removed by papain digest and immunoprecipitated. Bound Sp1 was removed from MHC II by Pronase digestion and then analysed on a Superose 12 molecular sieve column. The presented form of Sp1 was much smaller than intact Sp1 (compare to panel a, filled circles), indicating that only processed Sp1 can be presented by MHC II. (d) In similar experiments, splenocytes from wild-type and inducible nitric oxide synthase (iNOS)−/− animals were used and the total co-immunoprecipitated Sp1 was quantified (mean ± standard error of the mean). The wild-type cells co-precipitated Sp1 with MHC II, but splenocytes from iNOS−/− animals showed significantly reduced Sp1 (P = 0·0033; unpaired t-test) corresponding to only background radioactivity when compared with the monoclonal antibody (mAb) isotype control (cont.; P = 0·8328; unpaired t-test) results for wild-type animals, indicating that processing is dependent upon nitric oxide (NO) production. c.p.m., counts per minute.
In vitro oxidation and cleavage of Sp1 establish that chemical processing could occur in APCs and the co-immunoprecipitation experiments establish a direct MHC II-mediated presentation mechanism for Sp1. In order to determine if cleavage is required for presentation and if cellular oxidation plays a role in Sp1 processing, splenocytes from wild-type C57Bl/6 were isolated and incubated with [3H]Sp1 to allow processing and presentation to occur. MHC II was then removed from the cell membrane with papain digestion and immunoprecipitated with a-MHC II mAbs and protein A resin. Co-precipitated Sp1 was then released with a brief Pronase digestion to remove the protein and then analysed by Superose 12 chromatography. We found that only low-molecular-weight Sp1 was bound to the immunoprecipitated MHC II protein (Fig. 4c), demonstrating that only small Sp1 products associate with MHC II and therefore carbohydrate processing is required for MHC II-mediated Sp1 presentation. Furthermore, splenocytes from mice lacking a functional iNOS enzyme were used in similar co-immunoprecipitation experiments to determine the role of cellular oxidation within this pathway. The data show that only wild-type APCs presented Sp1, while the amount of radioactivity in iNOS−/− pellets was indistinguishable from that detected in the isotype control antibody (P = 0·8328) assays from wild-type cells (Fig. 4d). Collectively, these data indicate not only that processing is required for presentation, but that production of NO is a key step in the processing mechanism.
Binding of Sp1 and MHC II
Based on the co-immunoprecipitation data, binding of Sp1 is dependent upon processing. To better understand the role of carbohydrate size in MHC II binding, purified HLA-DR1 ectodomain (DR1) was immobilized onto Immulon 2HB ELISA plates and then incubated with biotinylated Sp1 treated with ozone for varying lengths of time to generate varying sizes. Bound Sp1 was detected by europium-conjugated streptavidin and time-resolved lanthanide fluorescence (TRF). In strong support of the immunoprecipitation findings, we observed that maximal binding of Sp1 to DR1 (Fig. 5a) was seen after 15 and 30 min of ozone cleavage (5- to 30-kDa size range; see Fig. 4a), demonstrating strong size dependence on binding in that high and very low molecular mass Sp1 do not readily associate with MHC II. These data mirror the MHC II association of conventional peptide antigens, which tend to be between 8 and 20 residues.
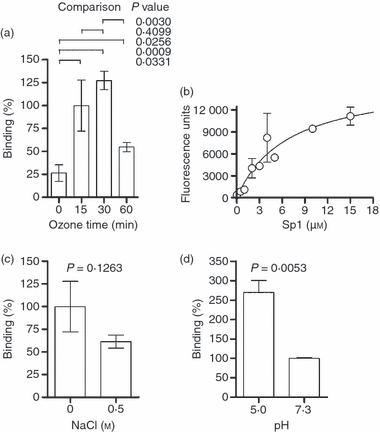
Type I pneumococcal polysaccharide (Sp1) binds directly to human leucocyte antigen (HLA)-DR1 with high affinity. Purified soluble ectodomains of HLA-DR1 were coated on Immulon 2HB 384-well plates and then used to assess binding [24 hr at 37° in dual acetate and phosphate-buffered saline (A/PBS) buffer at pH 5·0] of biotinylated Sp1 by europium-conjugated streptavidin and time-resolved fluorescence detection. All data shown are the mean ± standard error of the mean. (a) Sp1 was cleaved for varying lengths of time with ozone and then used in binding assays to measure the effect of Sp1 size on binding. Maximal binding was seen with 15–30 min of ozone treatment (5–30-kDa size range), but there was reduced binding with intact (0 min) or very small Sp1 (60 min) samples, indicating that cleavage but not total degradation to monosaccharides is required for MHC II binding. Data were scaled to the 15-min data point and P-values from unpaired t-tests are shown for various comparisons. (b) Using 15-min ozone-treated Sp1 at varying concentrations, a binding curve was collected and fitted using non-linear regression. The affinity (Kd) of Sp1 for HLA-DR1 (6·8 ± 2·9 μm) was determined by the ligand concentration at half maximal binding. The effects of NaCl (c; normalized to 0 m NaCl) and pH (d; normalized to pH 7·3) on binding were assessed in similar binding experiments. Ionic strength reduces Sp1 binding only modestly (P = 0·1263; unpaired t-test), suggesting a minor role for electrostatic interactions, while acidic pH showed optimal binding over neutral pH (P = 0·0053; unpaired t-test), suggesting that MHC II binding probably occurs in the acidified MHC II compartment in antigen-presenting cells.
Using this quantitative TRF binding assay, we next measured the binding affinity using a static concentration of DR1 coated onto the wells and varied ‘pre-processed’ Sp1 concentrations in pH 5·0 buffer to mimic intracellular compartments. We found that Sp1 associates with DR1 in a saturable manner with a binding affinity (Kd) of 6·8 ± 2·9 μm (Fig. 5b), which falls within the broad range of known antigen affinities for MHC II proteins.1 Interestingly, increasing the ionic strength of the binding conditions with NaCl decreased association only marginally, suggesting that electrostatic bonds are a minor component of the association (Fig. 5c). Finally, we found that binding occurs preferentially at acidic pH (Fig. 5d), which further supports the observation that Sp1 cannot load onto MHC II proteins at the cell surface as a result not only of a lack of processing, but also of a lack of binding ability at near-neutral pH.
Binding competition and antigen exchange
A hallmark of the conventional peptide antigen presentation mechanism is the involvement of H-2M, a protein that catalyses the exchange of resident self peptide with antigenic peptides within the MIIC.27 In contrast, superantigens associate with MHC II at the cell surface and binding is typically enhanced in the presence of peptides. Binding analysis of pre-processed Sp1 titrated with increasing concentrations of tetanus toxin peptide (TTp; residues 830–844) shows that Sp1 binding competes with peptide binding on DR1 (Fig. 6a), suggesting that the binding domains for these two antigens overlap.
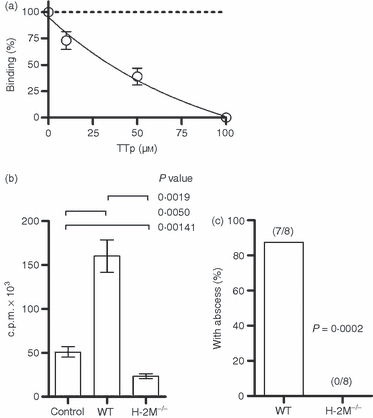
Type I pneumococcal polysaccharide (Sp1) competes for major histocompatibility complex class II (MHC II) binding via H-2M activity. All data shown are mean ± standard error of the mean. (a) In vitro competition binding experiments were performed by adding tetanus toxin peptide (TTp) at varying concentrations to assays containing Sp1 and DR1. Binding of Sp1 was competitive with peptide in a concentration-dependent fashion, demonstrating that peptide binding and carbohydrate binding are mutually exclusive. (b) Co-immunoprecipitation of MHC II from surface extracts of mouse splenocytes incubated with [3H]Sp1 revealed that Sp1 is not presented by MHC II from H-2M−/− cells [P = 0·0019; unpaired t-test; control is an isotype monoclonal antibody (mAb) with wild-type (WT) cells], showing that antigenic exchange catalysis by H-2M is required for Sp1 presentation. (c) For in vivo T-cell activation studies, wild-type and H-2M−/− mice were injected with 50 μg of Sp1 and sterile cecal content adjuvant to induce abscess formation. Wild-type mice developed abscesses, whereas the H-2M−/− mice failed to do so (P = 0·0002; unpaired t-test), confirming that, like in vitro cellular presentation (b), in vivo T-cell activation requires MHC II-mediated Sp1 presentation catalysed by H-2M because of mutual exclusivity of binding (a). c.p.m., counts per minute.
If peptide and polysaccharide MHC II binding is mutually exclusive in that only one type of antigen can bind at once, it would be expected that H-2M would play a significant role in Sp1 presentation. To test this hypothesis, splenocytes from wild-type or H-2M−/− animals were incubated with intact [3H]Sp1 overnight. The surface MHC II was removed with papain and immunoprecipitated as above. Wild-type animals presented Sp1 effectively, yet animals lacking H-2M failed to present Sp1 (Fig. 6b). To confirm that this reduction in presentation results in decreased in vivo T-cell activation, wild-type and H-2M−/− animals were challenged with Sp1 and sterilized cecal contents as previously described to induce abscesses. As expected, the H-2M−/− animals failed to form abscesses (Fig. 6c), indicating a lack of in vivo T-cell activation mediated by Sp1 because of a lack of MHC II binding and presentation.
Discussion
A conventional protein antigen usually activates T helper cells in the context of MHC II proteins through the endocytic pathway in APCs.28,29 Exogenous proteins can enter this MHC II pathway where they are processed into peptides, loaded onto MHC II with the help of H-2M, and presented on the surface in a processed form bound to MHC II. Here, we show that the zwitterionic polysaccharide Sp1 from S. pneumoniae, a component of the PneumoVax® vaccine, bears all of the hallmarks of this conventional protein pathway which requires cell entry, processing, and MHC II expression for presentation on APCs, while in vivo T-cell activation is dependent upon H-2M-assisted MHC II presentation.
Confocal microscopy confirmed that Sp1 is internalized by B cells and co-localizes with MHC II proteins, demonstrating that Sp1-containing endosomes deliver their content to the canonical MHC II compartment (MIIC). When this mechanism proceeds uninhibited, Sp1 is presented on the cell surface through direct interactions with MHC II protein; however, inhibition of endocytosis leads to not only a loss of Sp1 internalization, but also loss of Sp1 surface localization. These data indicate that MHC II-dependent presentation of Sp1 occurs only if the polysaccharide is allowed to associate with MHC II within the cell.
Consistent with these findings, internalized Sp1 is cleaved to a low-molecular-weight species that is found associated with MHC II at the cell surface. This mechanism of processing is dependent upon the production of NO and demonstrates that Sp1 processing inside APCs is a required mechanism that facilitates the binding of small Sp1-derived oligosaccharides to MHC II. In vitro binding assays confirm that association occurs at an affinity comparable to that for other MHC II-specific antigens, although the binding affinity for mouse MHC II has yet to be determined, and association is remarkably sensitive to Sp1 molecular mass. Finally, Sp1 binding competes with peptides in an H-2M-dependent fashion, leading to the conclusion that the binding domain for Sp1 significantly overlaps with the traditional peptide binding groove found in all MHC proteins. In total, these data show that, despite being a polysaccharide, Sp1 appears to behave like a conventional MHC II-dependent antigen.
In the traditional MHC II pathway, acidification of the MIIC is crucial for a number of reasons.28,29 First, the proteases that are responsible for protein antigen processing and invariant chain (Ii) cleavage are preferentially active in an acidic environment. The second function of acidification is the optimal activity of H-2M, which catalyses the exchange of the class II-associated invariant chain (CLIP) peptide for an antigenic peptide. In the polysaccharide antigen pathway, pH is critical for antigen binding but not processing. The lack of oxidative cleavage at near-neutral pH shows that NO-mediated processing must occur early in the endocytic pathway, before acidification. However, the competition of Sp1 and peptide together with the reliance on H-2M activity shows that the low pH is required to allow Ii cleavage by Cathepsin S to free the binding site on MHC II and to allow normal levels of H-2M activity. In fact, another recent study has demonstrated that the acidification inhibitor Bafilomycin A1 blocks Sp1-mediated T-cell activation in vitro.14 Together with the observed acidic pH optimum for in vitro binding even in the absence of H-2M, these data demonstrate that APC internalization of Sp1 is required to allow processing and to employ the traditional proteases within an acidic environment to facilitate antigen binding and ultimately surface presentation by MHC II (Fig. 7).
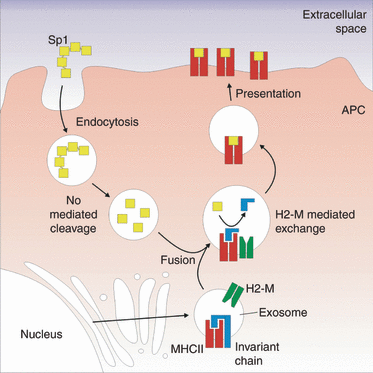
Model of the type I pneumococcal polysaccharide (Sp1) processing and presentation mechanism. Our data support a pathway in which Sp1 is internalized by antigen-presenting cells (APCs) via endocytosis and then cleaved to low-molecular-weight fragments in a nitric oxide-dependent fashion. These vesicles fuse with exosomes carrying the major histocompatibility complex class II (MHC II) machinery, forming the MHC II compartment. Cathepsin S cleaves the invariant chain, enabling the exchange of the class II-associated invariant chain (CLIP) peptide for Sp1 catalysed by H-2M. The loaded MHC II–Sp1 complex is then trafficked to the cell surface where it can be recognized by T cells.
The implications of these findings are many fold. We have previously shown that the ZPS antigen PSA from commensal B. fragilis follows an MHC II-dependent pathway to activate T cells now known to help stabilize the immune system through exposure in the gut during bacterial colonization and symbiosis. The discovery that Sp1 follows a similar pathway in APCs is of great importance because of the fact that S. pneumoniae is a pathogen that can cause invasive disease. This distinction from B. fragilis suggests that the downstream T-cell response is probably dissimilar for these two polysaccharide antigens. Indeed, there is no evidence showing that pneumococcal vaccination with PneumoVax® significantly alters B. fragilis colonization, as would be expected if the T-cell recognition and tolerance of PSA was not specific and resulted in a down-regulation of the Sp1 response. Nonetheless, the similarity of processing and presentation mechanisms for PSA and Sp1 (Fig. 7) shows that the adaptive immune response can effectively utilize carbohydrates as ‘conventional-like’ antigens that activate CD4+ T cells following processing, loading onto MHC II, and ultimately recognition by T cells.
These findings solidify the carbohydrate antigen processing and presentation pathway recently discovered using B. fragilis polysaccharide20 through demonstrating that other unrelated bacteria carry carbohydrates that activate T cells and utilize MHC II and an NO-dependent cleavage mechanism to achieve presentation. Furthermore, we report the binding affinity between a carbohydrate antigen and human MHC II, while showing for the first time that H-2M is required for T-cell activation mediated by a carbohydrate. It is interesting to note that PneumoVax® is a somewhat rare example of a polysaccharide-only vaccine that has shown strong efficacy. Sp1 is one of 23 pneumococcal polysaccharides that comprise this vaccine and is one of the 14 polysaccharides in the originally licensed product, and thus the data presented in this article lead to renewed questions about how Sp1 might contribute to PneumoVax® efficacy through specific T helper cell recruitment during immunization.
Acknowledgements
We would like to thank Dr Stephen DeWall for helpful advice and for providing purified HLA-DR1 and Dr Qun Wang for Sp1 purification and 1H-NMR analysis of Sp1 samples. This work was supported by a grant to DLK through the National Institutes of Health, National Institute of Allergy and Infectious Disease (AI039576) and a grant to BAC through the National Institutes of Health, National Institute of Allergy and Infectious Disease (AI062707).




