Increased interleukin-10 production and Th2 skewing in the absence of 5-lipoxygenase
Summary
Eicosanoids (prostaglandins and leukotrienes) are important mediators of inflammatory responses. These lipid mediators may also regulate the production of peptide mediators of the immune system. In this study, we investigated the effect of the absence of 5-lipoxygenase (5-LO)-derived leukotrienes on interleukin (IL)-10 production. IL-10 is a key regulator of immune and inflammatory responses, and previous studies have suggested that prostaglandins effect their immunosuppressive functions in part by stimulation of IL-10 production. We therefore investigated whether leukotriene production would have a similar role in regulation of IL-10 production. We have made the striking observation that absence of 5-LO-derived leukotrienes results in increased IL-10 production with a concomitant decrease in the production of pro-inflammatory cytokines, including tumour necrosis factor (TNF)-α and IL-12. Moreover, T-cell cytokine production in the absence of 5-LO-derived leukotrienes results in increased IL-4 production and decreased interferon (IFN)-γ production. This may be in part secondary to increased IL-10 production and its effects on dendritic cell function resulting in altered T-cell differentiation. These findings indicate that, in addition to the central role leukotrienes play in the acute inflammatory response, endogenous leukotrienes are also important regulators of inflammatory cytokine production, via regulation of IL-10 production and in vivo differentiation of T cells.
Introduction
Leukotrienes are a class of potent biological mediators called eicosanoids which are derived from arachidonic acid. The eicosanoids include both cyclooxygenase products (prostaglandins and thromboxanes) and 5-lipoxygenase (5-LO) products, known as leukotrienes. Leukotrienes have been implicated in the pathophysiology of multiple diseases including asthma, atopic dermatitis, psoriasis, arthritis and inflammatory bowel disease.1,2
The synthesis of leukotrienes is tightly regulated. Arachidonic acid released from endogenous phospholipids by phospholipase A2 is subsequently sequestered at the nuclear envelope and brought into contact with the 5-LO enzyme by an accessory protein termed 5-lipoxygenase activating protein (FLAP). The enzyme 5-LO catalyses the formation of 5-hydroperoxy-6,8,11,14-eicosotetraenoic acid (5-HPETE) and its subsequent conversion to leukotriene A4 (LTA4). The conversion of arachidonic acid to LTA4 by 5-LO is the critical initial enzymatic step in the formation of all leukotrienes.3 5-LO is expressed primarily in immune cells including granulocytes, macrophages, dendritic cells and mast cells.4,5 Low levels of 5-LO expression have also been detected in non-immune cells such as keratinocytes6 and colon epithelial cells.7–9
LTA4, the initial product of 5-LO, is subsequently metabolized to one of two different classes of leukotrienes: the pro-inflammatory leukotriene LTB4, or LTC4 and its metabolites LTD4 and LTE4, also known as the slow-reacting substances of anaphylaxis. The enzyme LTA4 hydrolase catalyses the conversion of LTA4 to LTB4. LTB4 has multiple pro-inflammatory biological activities. LTB4 is a very potent chemoattractant for neutrophils, eosinophils, monocytes and dendritic cells.10,11 LTB4 can also increase adhesion of leucocytes to vascular endothelium and induce neutrophil degranulation.12,13 Recently, a role for LTB4 in the initiation of the adaptive immune responses has also been demonstrated. T lymphocytes express B leukotriene receptor 1 (BLT1), a high-affinity receptor for LTB4.14,15 LTB4 signalling through BLT1 mediates CD4+ and CD8+ T-cell chemotaxis into inflamed tissue.14,15 Both LTB4 and LTC4 have been reported to regulate the development of T helper 2 (Th2)-type immune responses.16,17 Taken together, these studies suggest that LTB4 has an important role in innate immune responses and may also participate in the development of adaptive immune responses. The second metabolic pathway for LTA4 involves the conjugation of LTA4 to glutathione by the enzyme leukotriene C4 synthase. LTC4 is the prototype leukotriene for the class of cysteinyl leukotrienes originally described as the slow-reacting substances of anaphylaxis. LTC4 is also known as an important mediator of pulmonary allergic responses. LTC4 is a potent bronchoconstrictor18 and promotes bronchial mucous secretion.19 Unlike LTB4, the cysteinyl leukotrienes do not induce leucocyte migration into inflamed tissue. However, cysteinyl leukotrienes may contribute to inflammation via increased vascular permeability and subsequent oedema formation.12
The production of multiple cytokines has been reported to be altered in vitro by 5-LO-derived leukotrienes.20,21 The functional significance of these in vitro observations has not been established in vivo. Interleukin (IL)-10, a cytokine with potent anti-inflammatory and immunoregulatory activity, can be regulated by lipid (prostaglandin) mediators and is a candidate cytokine for regulation by leukotrienes. IL-10 has been shown to be a potent macrophage deactivator, blocking the induced synthesis of tumour necrosis factor (TNF)-α, IL-1, IL-6, IL-8 and granulocyte–macrophage colony-stimulating factor (GM-CSF) by human monocytes22 and mouse peritoneal macrophages.23 IL-10 also indirectly suppresses the synthesis of interferon (IFN)-γ by helper T cells24 and natural killer (NK) cells.25 IL-10 is also a central regulator of the production of IL-12,26 a cytokine key for the induction of Th1-type adaptive immune responses.27,28 Moreover, absence of IL-10 in vivo results in the promotion of Th1-type immune responses.29 Several studies have demonstrated that prostaglandins can induce IL-10 production.30,31 In a study of murine dendritic cells it was convincingly demonstrated that one of the mediators of prostaglandin E2 (PGE2)-induced immunosuppression was IL-10.31 However, the relationship between IL-10 and the regulation of lipid mediators is complex. We have found that IL-10 is a negative regulator of the production of cyclooxygenase-2 (COX-2) derived prostaglandins, and absence of IL-10 resulted in a marked increase in lipopolysaccharide (LPS)-induced COX-2 expression and PGE2 production.32 The role of leukotrienes in the production of IL-10 has not been completely elucidated. Although Zaitsu et al. have reported that IL-10 does not regulate leukotriene production in vitro,33 others have demonstrated that IL-10 in vitro can inhibit leukotriene production by inhibiting the induction of FLAP.34 In an in vitro study of murine dendritic cells, it was reported that pharmacological blockade of BLT1, the high-affinity receptor for LTB4, resulted in increased IL-10 production.21 This latter study suggested that 5-LO-derived leukotrienes may negatively regulate IL-10 production. The functional significance of these in vitro observations has not been established in vivo.
Despite multiple in vitro studies demonstrating a potential role of 5-LO products in the regulation of multiple cytokines, the functional significance of these changes in vivo is not clear. The generation of mice with a targeted disruption of the 5-LO gene (5LO–/–)35,36 has allowed further definition of the actions of 5-LO-derived products in complex physiological systems. We have compared 5LO–/– and wild-type mice to determine the importance of endogenous 5-LO-derived leukotrienes in the regulation of IL-10 production and the development of adaptive immune responses.
Materials and methods
Animals
Wild-type 129/SvEv mice were obtained from Taconic Farms (Germantown, NY). 5-LO-deficient mice (5LO–/–) on a mixed 129/B6 background were obtained from Jackson Laboratories (Bar Harbor, ME) and backcrossed five generations to the 129/SvEv strain; subsequently heterozygous mice were mated to generate homozygous founder mice for the breeding colonies used to generate mice for the studies. Mice were maintained in microisolator cages under specific-pathogen-free, Helicobacter-free conditions at the animal care facility at the University of Iowa.
Reagents
LTB4, LTC4, U-75302 and 14,15-dehydro-LTB4 were obtained from Cayman Chemical (Ann Arbor, MI). Nuclease-resistant phosphorothioate-modified oligodeoxynucleotides (ODNs) were obtained from the Coley Pharmaceutical Group (Wellesley, MA). LPS from Escherichia coli (serotype 0111:B4) was obtained from Sigma (St Louis, MO).
Spleen cell cultures
Spleen cells from wild-type or 5LO–/– mice were cultured at 5 × 106 cells/ml in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol, penicillin (100 U/ml) and streptomycin (100 U/ml) in 12-well tissue culture plates (Costar, Corning, NY). Cells were incubated in medium alone or medium supplemented with E. coli LPS at 1·0 µg/ml or CpG oligonucleotides (1·0 µg/ml). The immunostimulatory ODN 1826 (TCCATGACGTTCCTGACGTT) and non-stimulatory control ODN 1982 (TCCAGGACTTCTCTCAGGTT) were used for this study. As the trends in cytokine production were similar at 18, 24 and 48 hr poststimulation, subsequent studies used the 24-hr time-point. Supernatants from triplicate cultures were harvested after 24 hr and stored at −80° before analysis for cytokine concentration.
Leukotrienes in vitro
To evaluate the role of leukotrienes in the regulation of cytokine production, in some cultures LTB4 (100 nm to 1 µm) or LTC4 (1 µm) was added at the initiation of the culture. To further test the role of LTB4, in some cultures an LTB4 antagonist, 14-15-dehydro-LTB4 (1 µm) or U-75302, was added to in vitro cultures. The 1B13A cell line, which produces a blocking antibody to the IL-10 receptor (IL-10R), was a kind gift from DNAX (Palo Alto, CA).
Enzyme-linked immunosorbent assay (ELISA) for murine cytokines
Sandwich ELISAs were used to measure cytokine concentrations in supernatants from cell cultures. The concentrations of TNF-α, IL-6, IL-12p70, IFN-γ, IL-4, IL-5, IL-10 and IL-13 in cell culture supernatants were determined using BD OptEIA™ Mouse ELISA Sets (BD Pharmingen, San Jose, CA).
Quantification of PGE2
PGE2 levels in tissue culture supernatants were determined using a PGE2 enzyme immunoassay (EIA) kit from Cayman Chemical Company, as per the manufacturer's instructions.
PGE2 production from bone-marrow-derived macrophages
Single-cell suspensions of bone marrow cells were isolated from the femur/pelvis of wild-type or 5LO–/– mice and cultured at 2·5 × 105 cells/ml for 7 days in RPMI-1640 supplemented with 10% FCS, 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol, penicillin (100 U/ml) and streptomycin (100 U/ml) in 12-well tissue culture plates (Costar) and GM-CSF (200 U/ml). Cells were incubated in medium alone or in medium supplemented with E. coli LPS at 1·0 µg/ml or CpG oligonucleotides (1·0 µg/ml).
Flow cytometry
Spleens were mechanically disrupted and a single-cell suspension was acquired after red blood cell lysis using AKC buffer (0·15 m NH4Cl, 10 mm KHCO3 and 0·1 mm Na2EDTA, pH 7·2). Cells at a concentration of 1 × 106 cells/ml were incubated with 10% rat serum and then labelled with 10 µg/ml of phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated antibody. Cells were stained for B220 (RA3-6B2), Mac-1 (M1/70), CD4 (HM40-3), CD8α (53-6·7), CD11c (HL3), major histocompatibility complex (MHC) class II I-Ab (M5/114·15·2), CD25 (PC61), CD40 (HM40-3), CD69 (H1·2F3), CD45Rb (C353·16A), CD80 (16-10 A1) and CD86 (GL1) (all from eBiosciences, San Diego, CA). Flow cytometry was performed on a Becton Dickinson (Frankin Lakes, NJ) FACScan and analysis performed using the FlowJo software program (Tree Star, Ashland, OR).
Isolation of splenic dendritic cells
Spleens were injected with a solution containing liberase (0·1 units/ml; Roche Diagnostics Corp., Indianapolis, IN) and DNase (40 units/ml; Roche Diagnostics Corp.) in phosphate-buffered saline (PBS) and incubated for 30 min at 37°. Spleens were then mechanically disrupted and single-cell suspensions were made. The cells were washed with balanced salt solution (BSS) and centrifuged through Ficoll (Sigma, St. Louis, MO). Viable mononuclear cells were collected from the interface and washed in BSS. For flow cytometric analysis, the cells were resuspended in staining buffer consisting of 5% bovine calf serum (HyClone Laboratories, Inc., Logan, UT) and 0·1% sodium azide in BSS supplemented with 10 µg/ml anti-CD16/32 (FcgRIII/II) (2·4G2; eBiosciences) to decrease non-specific staining.
Dendritic cell culture protocol
For isolation of dendritic cells for culture, spleen cell suspensions were prepared as described above. Dendritic cells were enriched by positive selection using anti-mouse CD11c-coated magnetic beads in a magnetic field (autoMACS; Miltenyi Biotec, Auburn, CA). Populations were > 95% pure upon flow cytometric analysis. Purified dendritic cells were incubated in 96-well plates at a concentration of 2 × 106 cells/ml. Cells were incubated for 24 hr in control medium [McCoy's medium enriched with 10% FCS, essential and non-essential amino acids, sodium pyruvate, penicillin (100 U/ml) streptomycin (100 U/ml), 2 mm l-glutamine and 0·05 mm 2-mercaptoethanol] or medium supplemented with LPS (1 µg/ml) or CpG oligonucleotides (1 µg/ml). After 24 hr of incubation at 37°, the supernatants were harvested for cytokine determination by ELISA.
Antigen-specific antibody production
To induce a Th1-type immune response, mice were immunized intraperitoneally with 50 µg of trinitrophenyl-keyhole limpet hemocyanin (TNP-KLH) (Biosource, Camarillo, CA) plus 10 µg of the immunostimulatory CpG oligonucleotide 1826 (sequence: TCC ATG ACG TTC CTG ACG TT). To induce a Th2-type immune response, mice were immunized intraperitoneally with 50 µg of TNP-KLH (Biosource) in alum. Serum was collected 28 days later for measurement of TNP-specific antibody production. Isotype-specific immunoglobulin M (IgM), IgG1, IgG2A and IgE anti-TNP levels were determined as follows: 96-well ELISA plates were coated with isotype-specific capture antibodies at a concentration of 1–5 µg/ml in buffer consisting of 0·05 m Tris [tris(hydroxymethyl)aminomethane, pH 9·5] and 0·02% NaN3. The capture antibodies utilized were goat anti-mouse IgM, anti-mouse IgG1 and IgG2a (Southern Biotechnology Associates, Birmingham, AL). The anti-IgE coating antibody was EM95 (a kind gift of Thomas Waldschmidt, University of Iowa). Coated plates were blocked with 5% dry milk–PBS. Control monoclonal antibodies (for standard curves) and serum samples appropriately diluted in 5% dry milk–PBS were added and similarly incubated. After washing, 0·5 µg of biotin-conjugated TNP–human gammaglobulin (JacksonImmunoResearch, West Grove, PA) diluted in 5% dry milk–PBS was added to each well, and the plates were further incubated. Alkaline phosphatase strepavidin (Sigma, St Louis) diluted in 5% dry milk–PBS was added after washing. Substrate (pNPP) was obtained from Sigma and used exactly as per the manufacturer's directions. The reaction was stopped by addition of 3 N NaOH, and absorbance was measured at a dual wavelength of 405 and 540 nm with a microplate Autoreader EL311 (Bio-Tek Instruments, Winooski, VT). All washes between steps were carried out with 0·05% Tween 20–PBS. Antibody concentrations were determined from standard curves. The control monoclonal antibodies used for standard curves were for 4G2F8 (mouse IgM anti-TNP monoclonal antibody), 1B7 (mouse IgG1 anti-TNP monoclonal antibody), B4D4 (mouse IgG2a anti-TNP monoclonal antibody), and A3B1 (mouse IgE anti-TNP monoclonal antibody). The TNP-specific monoclonal antibodies were affinity-purified by passage of hybridoma culture supernatants over TNP–bovine gammaglobulin–Sepharose 6 followed by elution with TNP–glycine, and were kind gifts from Dr Thomas Waldschmidt (University of Iowa).
Schistosoma immunization protocol
Wild-type 129/SvEv mice and 5LO–/– mice were immunized with Schistosoma using the following protocol. Schistosome eggs were aseptically collected from the livers of schistosome-infected hamsters as previously described.37 Washed Schistosoma mansoni ova were suspended in PBS and then frozen and stored in liquid nitrogen. Wild-type 129/SvEv mice and 5LO–/– mice were exposed to 20 µg of freeze/thaw-killed Schistosoma egg antigen (SEA) subcutaneously in incomplete Freund's adjuvant and simultaneously 5000 freeze/thaw-killed schistosome eggs were administered by intraperitoneal injection on day 0. Seven days later, the animals were re-exposed to eggs by a second intraperitoneal injection of 5000 freeze/thaw-killed schistosome eggs. Five days after the second exposure (day 12), mice were killed and spleen cells were harvested for in vitro cell culture.
Cell culture
Spleen cells were cultured in RPMI-1640 (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 10 mm HEPES buffer, 2 mm l-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma). Cells were cultured for 5 days at 5 × 106 cells/well in six-well plates in medium alone or with 5 or 1 µg/ml SEA. Supernatants were subsequently harvested for cytokine measurement.
T-cell culture
T cells were enriched by positive selection using rat anti-mouse CD4-coated magnetic beads in a magnetic field (autoMACS; Miltenyi Biotec). Populations were > 95% pure upon flow cytometric analysis. Purified CD4+ T cells were incubated at a concentration of 2 × 106 cells/ml in 96-well plates coated with anti-CD3. Culture supernatants were collected after 24 hr of stimulation and frozen at −80° until analysis of cytokine concentration by ELISA. In some experiments the purified CD4+ T cells were incubated with plate-bound anti-CD3 and soluble anti-CD28 (10 µg/ml). In order to assess the effect of exogenous leukotrienes on CD4+ T-cell cytokine production, in some experiments LTB4 (1 µm) or LTC4 (1 µm) was added at the initiation of culture.
Statistical analysis
Significant differences between experimental groups were evaluated by the non-parametric Mann–Whitney U-test. A P-value of < 0·05 was considered statistically significant.
Results
Characterization of wild-type and 5-LO-deficient mice
5LO–/– mice on a 129/SvEv background were evaluated and compared with wild-type controls. The 5LO–/– mice appeared healthy. Evaluation of the internal organs at necropsy revealed no evidence of disease. The percentages of B cells, T cells, and dendritic cells in the spleens of wild-type and 5LO–/– mice were evaluated. The number of spleen cells was essentially the same for each cell type in both wild-type and 5LO–/– mice (data not shown). Thus the absence of 5-LO did not result in obvious disease or alter the development of the immune system.
Inflammatory cytokine production is decreased and IL-10 production is increased in LPS- or CpG DNA-stimulated spleen cells from 5LO–/– mice
To further assess the effect of absence of leukotrienes on the immune response, we stimulated spleen cell cultures with either LPS or CpG DNA to stimulate Toll-like receptor (TLR)4- or TLR9-mediated IL-10 and inflammatory cytokine production. Consistently, LPS-stimulated spleen cells from 5-LO-deficient mice produced approximately 25% less inflammatory cytokines, including IL-12p70, IL-6, TNF-α and IFN-γ(Figs 1a–d). In contrast, production of IL-10 from LPS-stimulated 5-LO-deficient spleen cells was significantly increased as compared with wild-type spleen cells (Fig. 2). Similar results were seen with immunostimulatory CpG oligonucleotide (data not shown). As we noted increased IL-10 production from 5-LO-deficient spleen cells, we tested whether addition of anti-IL-10R antibody (1B13A, which blocks IL-10 action via blockade of the receptor) would increase LPS-induced cytokine production from the 5-LO-deficient cells. When spleen cell cultures were stimulated in the presence of blocking antibody to the IL-10R, both wild-type and 5LO–/– spleen cells produced increased levels of inflammatory cytokines. However, the level of cytokine production by 5LO–/– spleen cells stimulated in the presence of anti-IL-10R antibody remained lower than that of similarly stimulated wild-type spleen cells (Figs 1a–d).
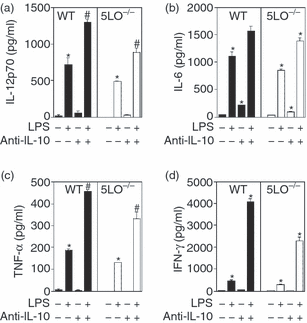
Inflammatory cytokine production from wild-type and 5-lipoxygenase (LO)-deficient spleen cells. Spleen cells from wild-type and 5LO–/– mice (5 × 106 cells/ml) were cultured in the presence or absence of lipopolysaccharide (LPS) (1 µg/ml). Supernatants were harvested after 24 hr of culture and analysed for cytokine levels by enzyme-linked immunosorbent assay (ELISA): (a) interleukin (IL)-12p70; (b) IL-6; (c) tumour necrosis factor (TNF)-α; (d) interferon (IFN)-γ. In some experiments, blocking antibody to the IL-10 receptor (1B13A; 10 µg/ml) was added at the initiation of the cultures. Data are representative of three independent experiments. *P < 0·05, for 5LO–/– mice versus wild-type mice.
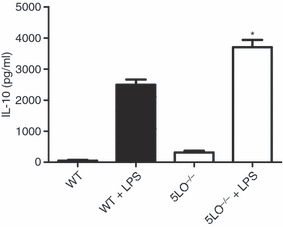
Interleukin (IL)-10 production from lipopolysaccharide (LPS)-stimulated wild-type and 5-lipoxygenase (LO)-deficient spleen cells. Spleen cells from wild-type and 5LO–/– mice (5 × 106 cells/ml) were cultured in the presence or absence of LPS (1 µg/ml). Supernatants were harvested after 24 hr of culture and analysed for IL-10 levels by enzyme-linked immunosorbent assay (ELISA). Data are representative of three independent experiments. *P < 0·05, for 5LO–/– mice versus wild-type mice.
IL-6 production from spleen cells is induced by exogenous LTB4
In order to further assess the regulation of cytokine production by leukotrienes, we determined the extent to which exogenous LTB4 or LTC4 altered cytokine production from wild-type or 5LO–/– cells. The addition of exogenous LTB4 led to increased IL-6 production from spleen cells (Fig. 3a). Similar results were obtained with bone-marrow-derived macrophages (Fig. 3b) and splenic dendritic cells (Fig. 3c) from wild-type and 5LO–/– mice. Although LPS induced IL-6 production, the combination of LTB4 and LPS did not lead to further increases in IL-6 production from spleen cells or bone-marrow-derived macrophages (Figs 3a and b), although a small increase in production was noted from splenic dendritic cells from 5LO–/– mice (Fig. 3c). The effect of exogenous LTB4 on IL-10 production from LPS-stimulated spleen cells from wild-type or 5-LO-deficient mice was variable, with decreased IL-10 production seen in some experiments and no change in other experiments. The addition of LTB4 did not induce de novo production of IFN-γ or IL-12, nor did exogenous LTB4 increase LPS-induced IFN-γ or IL-12 production from wild-type or 5LO–/– spleen cells (data not shown). The addition of exogenous LTC4 did not alter IL-6, IL-10, IL-12p70, IFN-γ or TNF-α production from unstimulated cells or from LPS- or CpG-stimulated wild-type or 5-LO-deficient spleen cells (data not shown).
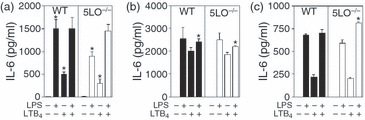
Effect of the leukotriene LTB4 on interleukin (IL)-6 production from wild-type and 5-lipoxygenase deficient cells. (a) Spleen cells (5 × 106 cells/ml), (b) bone-marrow-derived macrophages (2 × 106 cells/ml) or (c) splenic dendritic cells (2 × 106 cells/ml) from wild-type and 5LO–/– mice were cultured in the presence or absence of lipopolysaccharide (LPS) (1 µg/ml). In some cell cultures, LTB4 (1 µm) was added at the initiation of culture. Supernatants were harvested after 24 hr of culture and analysed for IL-6 levels by enzyme-linked immunosorbent assay (ELISA). Data are representative of three independent experiments. *P < 0·05, for 5LO–/– mice versus wild-type mice.
Prostaglandin production did not differ between wild-type and 5LO–/– mice. When bone marrow macrophages from wild-type and 5LO–/– mice were stimulated with the calcium ionophore A23187, the amount of PGE2 produced was essentially the same in both strains (wild type, mean ± SD; 300 ± 10 pg/ml; 5LO–/–, 290 ± 15 pg/ml). The amounts of PGE2 produced from LPS- or CpG-stimulated peritoneal macrophages or splenic dendritic cells were not significantly different in cells from 5LO–/– mice as compared with those from wild-type mice (data not shown).
Cytokine production by stimulated CD4+ T cells from 5LO–/– mice is skewed to a Th2-type profile
Given the increased IL-10 production by LPS- or CpG-stimulated 5LO–/– spleen cells, we evaluated cytokine production from globally stimulated CD4+ T cells from wild type and 5LO–/– mice. Purified splenic T cells isolated from 5-week-old mice were cultured for 24 hr in the presence of anti-CD3 and cytokine production was assessed at 24 hr. Globally stimulated splenic CD4+ T cells from 5-LO-deficient mice produced significantly more IL-4 and IL-13 than wild-type CD4+ T cells (Figs 4a and b). Moreover, the production of IL-10 from 5LO–/– spleen cells was significantly greater than that from wild-type spleen cells (Fig. 4c). Conversely, production of IFN-γ from stimulated 5-LO-deficient CD4+ T cells was significantly lower than that from stimulated wild-type T cells (Fig. 4d). Similar results were obtained when purified splenic CD4+ T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 (data not shown). Addition of exogenous LTB4 (1 µm) significantly decreased IL-10 production (mean ± SD; 1579·3 ± 106·8 versus 1011·3 ± 83·3; P < 0·001) from 5-LO-deficient CD4+ T cells. Exogenous LTB4 did not alter IL-10 production from wild-type CD4+ T cells. Exogenous LTB4 did not significantly alter IL-4, IL-13 or IFN-γ production from stimulated wild-type or 5-LO-deficient CD4+ T cells and addition of exogenous LTC4 (1 µm) did not alter production of these cytokines from wild-type or 5LO–/– CD4+ T cells (data not shown).
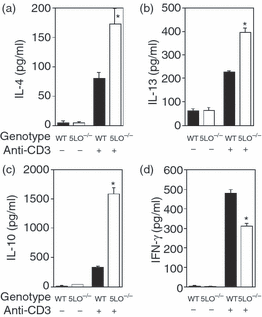
T-cell cytokine production from wild-type and 5-lipoxygenase deficient CD4+ T cells. (a) Interleukin (IL)-4; (b) IL-13; (c) IL-10; (d) interferon (IFN)-γ. Purified CD4+ T cells (2 × 106 cells/ml) were cultured in the presence or absence of plate-bound anti-CD3. Supernatants were harvested after 24 hr of culture and analysed for cytokine levels by enzyme-linked immunosorbent assay (ELISA). Data are representative of three independent experiments. *P < 0·05, for 5LO–/– mice versus wild-type mice.
Given the differences in CD4+ T-cell cytokine production we assessed the level of activation of CD4+ T cells from wild-type and 5LO–/– mice; however, no differences were noted. The level of expression of CD69 was similar in wild-type and 5-LO-deficient CD4+ T cells. Moreover, the ratio of CD45RBhigh to CD45RBlow was the same in wild-type and 5-LO-deficient mice (data not shown). In addition, the percentage of splenic CD4+ CD25+ T cells was essentially the same in 5LO–/– and wild-type mice (10% and 8%, respectively).
Immunization of 5-LO-deficient mice results in the skewing towards a Th2 immune response
The induction of antibody responses to T-dependent antigens is known to require CD4+ helper T cells. As global stimulation of splenic CD4+ T cells from 5LO–/– mice resulted in increased Th2-type cytokine production, we assessed whether absence of 5-LO-derived leukotrienes would alter the T-dependent (TD) antibody response. To determine the antibody response to a T-dependent antigen, wild-type and 5-LO-deficient mice were immunized with TNP-KLH using either CpG oligonucleotide 1826 as an adjuvant, which is known to induce a Th1-type immune response, or alum, which is known to induce a Th2-type immune response. When immunized with alum, 5-LO-deficient mice generated increased levels of TNP-specific IgG1 and IgE, whereas the levels of TNP-specific IgM and IgG2a were the same in the two strains (Fig. 5). In contrast, when wild-type and 5LO–/– mice were immunized with CpG oligonucleotide as an adjuvant, the levels of anti-TNP IgM, IgG1, IgG2a and IgE were not statistically different between the two animal strains (Fig. 5).
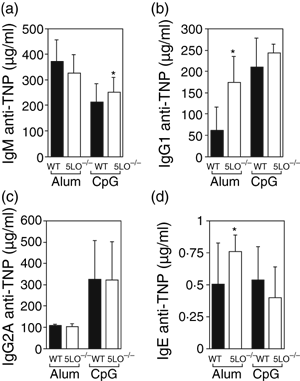
Comparison of antigen-specific immunoglobulin responses in wild-type and 5-lipoxygenase deficient (5LO–/–) mice. Mice were immunized with TNP-KLH with either alum or CpG 1826 oligonucleotide as the adjuvant. Serum from 10 animals per group was collected 28 days post immunization and antigen-specific serum immunoglobulin M (IgM), IgG1, IgG2A and IgE responses were evaluated by enzyme-linked immunosorbent assay (ELISA). (a) Day 28 IgM anti-TNP response. (b) Day 28 IgG1 anti-TNP response. (c) Day 28 IgG2a anti-TNP response. (d) Day 28 IgE anti-TNP response. *P < 0·05, for 5LO–/– mice versus wild-type mice.
To further assess the development of the Th2 immune response induced in the absence of 5-LO-derived leukotrienes, wild-type or 5LO–/– mice were immunized with SEA and non-viable Schistosoma eggs. In wild-type mice, this protocol generates a strong Th2 immune response.37 After immunization of wild-type and 5LO–/– mice, SEA-induced cytokine production from spleen cells was determined. SEA-stimulated cytokine production from 5LO–/– CD4+ T cells was skewed towards a Th2 response, with significantly increased IL-4 production as compared with wild-type mice (Fig. 6). Moreover, IFN-γ production was significantly greater in wild-type than in 5-LO-deficient mice, both in unstimulated cell cultures and with SEA stimulation. While production of IL-5 from unstimulated spleen cells was significantly greater in 5LO–/– mice, SEA-induced production of IL-5 and IL-13 was equivalent in the two strains. SEA-stimulated IL-10 and IL-6 production was similar in wild-type and 5LO–/– mice (Fig. 6).
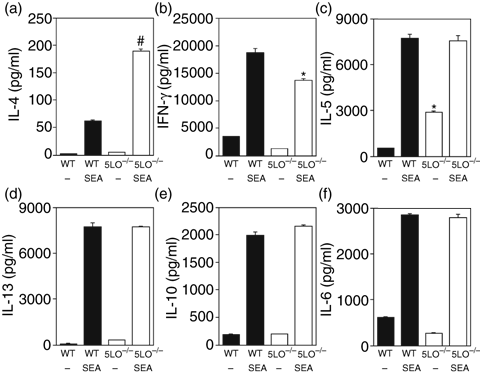
Spleen cell cultures from Schistosoma egg antigen (SEA)-immunized wild-type and 5-lipoxygenase deficient (5LO–/–) mice. Mice were immunized with SEA and non-viable Schistosoma eggs on day 0 and boosted with Schistosoma eggs on day 7. Spleens were harvested on day 12 and spleen cells (5 × 106 cells/ml) placed in control medium or medium supplemented with SEA antigen (5 µg/ml). Supernatants were harvested after 5 days of culture and analysed for cytokine levels by enzyme-linked immunosorbent assay (ELISA): (a) interleukin (IL)-4; (b) interferon (IFN)-γ; (c) IL-5; (d) IL-13; (e) IL-10; (f) IL-6. Data are representative of two experiments. *P < 0·05 and #P < 0·001, for 5LO–/– mice versus wild-type mice.
Splenic dendritic cells from 5LO–/– mice have altered cytokine production
As dendritic cells are the key antigen-presenting cells in the development of CD4+ T-cell responses, we determined the extent to which absence of 5-LO altered the dendritic cell compartment. Although the numbers of splenic dendritic cells were not significantly different between 5-LO-deficient and wild-type mice (data not shown), we found that absence of endogenous 5-LO resulted in altered cytokine production. Splenic dendritic cells from 5LO–/– mice produced less IL-12p70 and TNF-α than wild-type dendritic cells (Figs 7a and b). In contrast, splenic dendritic cells from 5LO–/– mice produced large amounts of IL-10 in response to LPS stimulation (Fig. 7c). Similar results were found when dendritic cells were stimulated with the stimulatory CpG oligonucleotide 1826 (data not shown). The addition of LTB4 (1 µm) or LTC4 (1 µm) to dendritic cell cultures did not induce cytokine production, nor did the addition of exogenous leukotrienes alter LPS-induced cytokine production from wild-type or 5LO–/– dendritic cells (data not shown).
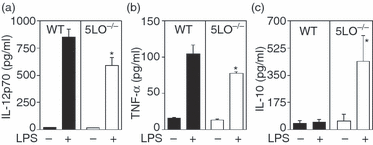
Cytokine production from wild-type and 5-lipoxygenase (LO)-deficient splenic dendritic cells. (a) Interleukin (IL)-12p70; (b) tumour necrosis factor (TNF)-α; (c) IL-10. Purified splenic dendritic cells (2 × 106 cells/ml) were cultured in the presence or absence of lipopolysaccharide (LPS) (1 µg/ml). Supernatants were harvested after 24 hr of culture and analysed for cytokine levels by enzyme-linked immunosorbent assay (ELISA). Data are representative of three independent experiments. *P < 0·05 and #P < 0·01, for 5LO–/– mice versus wild-type mice.
To further evaluate the splenic dendritic cells, flow cytometry was performed to assess the dendritic cell subtype distribution and level of dendritic cell activation. No differences between wild-type and 5LO–/– mice were noted in the percentage of splenic dendritic cells that were positive for CD11b, CD8α or B220. However, 5LO–/– dendritic cells expressed lower levels of surface activation markers than wild-type dendritic cells. The levels of expression of MHC class II and CD86 were decreased on splenic dendritic cells from 5LO–/– mice as compared with wild-type splenic dendritic cells (Figs 8b and d), whereas the expression of CD80 and CD40 was similar (Figs 8a and c). After 48 hr of culture, the expression of CD80 on dendritic cells from 5LO–/– mice was decreased as compared with wild-type dendritic cells (Fig. 9a). When stimulated with LPS, the induction of CD80, CD86 and CD40 was similar in splenic dendritic cells from 5-LO-deficient and wild-type mice (Fig. 9). The addition of LTB4 (1 µm) or LTC4 (1 µm) to dendritic cell cultures did not increase the expression of cell surface markers of activation (MHC class II, CD80 and CD86) (data not shown). Flow cytometric assays were also performed to assess the phagocytic activity of 5-LO-deficient and wild-type splenic dendritic cells. No differences were noted in the ability of splenic dendritic cells to phagocytosis FITC-dextran or FITC-albumin, nor did the addition of LTB4 (1 µm) alter phagocytosis from either wild-type or 5-LO-deficient splenic dendritic cells (data not shown).
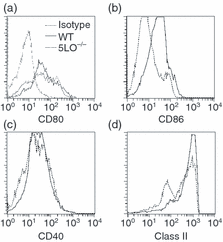
Phenotype of purified splenic dendritic cells. Splenic dendritic cells were purified from wild-type and 5-lipoxygenase (LO)-deficient (5LO–/–) mice and examined by flow cytometry as described in the ‘Materials and methods’. Profiles are representative of three independent experiments. (a) CD80; (b) CD86; (c) CD40; (d) major histocompatibility complex (MHC) class II.
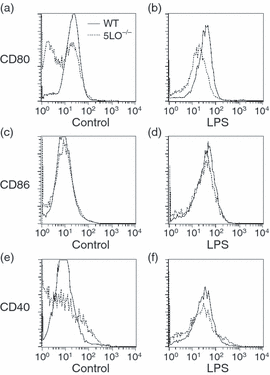
Phenotype of lipopolysaccharide (LPS)-stimulated splenic dendritic cells. Dendritic cells from wild-type and 5-lipoxygenase (LO)-deficient (5LO–/–) mice were purified and placed in control medium or medium containing LPS (1 µg/ml). Flow cytometry was performed after 48 hr in culture. Profiles are representative of three independent experiments. (a) CD80, control; (b) CD80, LPS; (c) CD86, control; (d) CD86, LPS; (e) CD40, control; (f) CD40, LPS.
Discussion
We have used 5LO–/– and wild-type mice to further define the regulatory role of endogenous leukotrienes in inflammatory responses elicited by LPS and in the development of T-cell immune responses. Our studies show that LPS stimulation of spleen cells from 5LO–/– mice consistently resulted in decreased production of inflammatory cytokines. In contrast, LPS stimulation of 5LO–/– spleen cells resulted in enhanced IL-10 production. When we assessed dendritic cells, we found that LPS stimulation of splenic dendritic cells from 5LO–/– mice resulted in increased IL-10 production and decreased production of IL-12 and pro-inflammatory cytokines. Given these alterations in cytokine production, we assessed T-cell immune responses in wild-type and 5LO–/– mice. Global stimulation of T cells from 5LO–/– mice resulted in increased IL-10 production and decreased IFN-γ production as compared with wild-type mice. Immunization of 5LO–/– mice with a Th2 immunization protocol resulted in enhanced production of IL-4 and decreased production of IFN-γ in 5LO–/– mice as compared with wild-type mice. Taken together, these data suggest that endogenous leukotriene production is an important regulator of cytokine production and T-cell differentiation.
Leukotrienes are potent lipid mediators that have pleiotropic effects on numerous types of cells of the immune system. The first step in the generation of leukotrienes is catalysed by 5-LO. Lipoxygenases are a class of non-haem iron-containing dioxygenases that catalyse incorporation of molecular oxygen into polyunsaturated fatty acid substrates. The initial product of 5-LO is 5-hydroperoxy-6,8,11,14-eicosatetraenoic acid. This is further metabolized to LTA4. LTA4 is subsequently metabolized either to LTB4 by LTA4 hydrolase or to the initial cysteinyl-containing leukotrienes, LTC4. Mice with targeted deletions of the 5-LO gene have been generated in order to evaluate the role of 5-LO-derived metabolites in immune and inflammatory responses.35,36 These mice are not capable of generating leukotrienes. The initial studies of 5LO–/– mice demonstrated that absence of 5-LO does not alter the normal development of the mouse, nor were there alterations in the number or phenotype of lymphoid or myeloid cells.35,36 Similar results were obtained in our studies, in which 5LO–/– mice were used on a 129/SvEv background. Using 5LO–/– mice, it has been demonstrated that leukotrienes have activity in both acute and chronic inflammatory responses. For example, topical application of arachidonic acid results in acute inflammation that has both a vascular permeability component (with increased oedema) and a cellular component (neutrophil infiltration). The acute inflammatory response is decreased in 5LO–/– mice.35 A similar phenomenon was noted in FLAP-deficient mice.38
A key finding in our study was that LPS stimulation of 5-LO-deficient spleen cells consistently resulted in decreased pro-inflammatory cytokine production. This cytokine production is probably from splenic macrophages, and previous studies have indicated that macrophage functions can be modulated by 5-LO metabolites. Macrophages are known to possess leukotriene receptors,39 including the high-affinity LTB4 receptor (BLT1).40 Receptor expression is regulated by cellular activation and cytokine production. In a study of human monocytes/macrophages, it was found that LPS activation and cytokine production induced alterations in the expression of BLT1.40 IFN-γ and TNF-α were found to down-regulate BLT1 expression, whereas IL-10 increased BLT1 mRNA and protein. In fact, pretreatment of the cells with IFN-γ decreased BLT1 expression and the cells were no longer responsive to LTB4in vitro.40 Furthermore, LTB4 has been shown to stimulate murine macrophages for bacteriocidal activity both in vivo and in vitro.41 Leukotrienes may also modulate cytokine production. In a study of human monocytes, LTB4 elicited TNF-α production.42 Using 5-LO and FLAP inhibitors, it was observed that blockade of leukotriene production decreased LPS-stimulated TNF-α production from murine macrophages.20 These results are similar to those obtained in our studies, in that we found decreased production of multiple cytokines, including TNF-α and IL-12, in 5-LO-deficient mice. Thus, macrophages express receptors for LTB4, these receptors are regulated by the state of cellular activation and cytokine production, and in our hands the absence of 5-LO-derived metabolites resulted in altered (decreased) pro-inflammatory cytokine production. Similar to our findings, the addition of 5-LO metabolites such as LTB4 or LTC4 did not restore cytokine production.20 There may be several reasons for this observation. For example, not all 5-LO metabolites (e.g. LTD4, LTE4 and 5-HETE) were tested. It is also possible that the 5-LO metabolites placed in culture were not effective because of oxidation of the lipid. Moreover, the role that leukotrienes may play could be restricted to a particular phase of activation, and it is possible that the leukotriene added at the beginning of culture was no longer present at the time when it could have activated the cultured cells.
Immune and inflammatory responses are mediated by cytokines produced by lymphocytes and other cell types but also by arachidonic acid metabolites (prostaglandins and leukotrienes). These cytokines and lipid mediators form a network with one another and can regulate each other's production. As an example of this type of regulation, we previously demonstrated that endogenous IL-10, a central regulator of the immune and inflammatory responses, controls cyclooxygenase-2 expression and prostaglandin production.43 Several lines of evidence suggest that the production of IL-10 may be regulated by leukotrienes. In an in vivo study of children with asthma treated with montelukast, a cysteinyl leukotriene receptor (LTR)-1 antagonist, it was found that blockade of the CysLTR1 resulted in an increase in the serum level of IL-10.44 Moreover, in an in vitro study, antigen stimulation of peripheral blood mononuclear cells from patients with asthma in the presence of the leukotriene receptor antagonist montelukast resulted in increased IL-10 production.45 In an in vitro study of murine dendritic cells, it was reported that pharmacological blockade of BLT1, the high-affinity receptor for LTB4, resulted in increased IL-10 production.21 These data suggest that 5-LO-derived metabolites (both LTB4 and LTC4) can negatively regulate IL-10 production. Consistent with this, in our study we found that LPS stimulation of mononuclear cells from 5LO–/– mice resulted in increased IL-10 production. In addition, similar to the study of antigen activation of human T cells in the presence of montelukast,45 activation of T cells from 5LO–/– mice with anti-CD3 also resulted in increased IL-10 production. The increased production of IL-10 from 5-LO-deficient mononuclear cells had physiological consequences, as incubation of mononuclear cells from 5LO–/– mice with anti-IL-10 resulted in increased LPS-stimulated cytokine production. These data suggest that one mechanism for the decrease in cytokine production from spleen of mononuclear cells from 5LO–/– mice is the increased production of IL-10.
Although we found that anti-IL-10 treatment increased cytokine production from 5-LO-deficient mononuclear cells, indicating that the IL-10 produced functionally altered LPS-stimulated cytokine production, the levels of inflammatory cytokines produced were not as high as those seen with wild-type cells. Although not directly assessed in this study, potentially this may be a result of inhibition of the LTB4 receptor signalling by IL-10, as the cells from 5LO–/– mice may be exposed in vivo to increased levels of IL-10. IL-10 may also alter the responsiveness of cells to leukotrienes by altering receptor signalling pathways. Preincubation of mononuclear cells with IL-10 has been previously demonstrated to inhibit LPS-induced cytokine production,23 and it has also been demonstrated that IL-10 can cause uncoupling of cell surface chemokine receptors from intracellular signalling pathways.46 In a study of human neutrophils (which have a strong response to LTB4), IL-10 significantly inhibited LPS-induced priming for fMLP-induced production of LTB4. Receptor desensitization may also explain the inability of exogenous LTB4 to normalize LPS-induced cytokine production from 5-LO-deficient spleen cells (a phenomenon previously noted by others20).
The role of IL-10 in regulation of the production of LTB4 is less clear. In an in vitro study of human neutrophils, it was reported that IL-4 and IL-13 could up-regulate LTB4 production from A23187-stimulated neutrophils, whereas IL-10 had no effect.33 Similarly, we found that absence of IL-10 had no effect on calcium ionophore-stimulated production of LTB4. Moreover, we found no difference between wild-type and IL-10-deficient mice in the production of the cysteinyl leukotriene LTC4 (data not shown). In contrast, others have demonstrated that IL-10 in vitro can inhibit leukotriene production by inhibiting the induction of FLAP.34 Differences between these studies may reflect genetic differences in the strain of mouse used as well as differences in the type and duration of the stimulus used.
A consistent finding in our studies was a shift in T-cell cytokine production to a Th2-type profile. Global stimulation of T cells from 5LO–/– mice resulted in increased IL-10, IL-4 and IL-13 production and decreased production of IFN-γ. Consistent with this profile, when 5LO–/– mice were immunized with TNP and alum, increased titres of IgG1 and IgE were obtained as compared with wild-type mice. In contrast to global stimulation of T cells with anti-CD3, slightly different results were noted in an antigen-specific T-cell response. Immunization with SEA using a protocol known to promote Th2 T-cell differentiation37 resulted in a marked increase in antigen-stimulated T-cell production of IL-4 and decreased IFN-γ production in 5LO–/– mice as compared with wild-type mice. However, in our antigen-specific immunization protocol in 5LO–/– mice, skewing to a Th2 profile was incomplete as we did not find increased IL-13 production. It is interesting to note that the 5-LO inhibitor Zileuton has clinical benefit in asthma, a disease characterized by T-cell skewing to a Th2 phenotype.47 Rather than immune modulation, the beneficial effect of 5-LO inhibition in asthma could, however, be secondary to increased IL-10 production dampening the chronic inflammation present in asthma. Alternatively, the major benefit of 5-LO inhibition in asthma may be a result of inhibition of the production of the cysteinyl leukotrienes, which are potent bronchoconstrictors18 and promote bronchial mucous secretion.19
The altered T-cell cytokine profile in 5-LO-deficient mice may be secondary to increased IL-10 production from dendritic cells, which are the key antigen-presenting cells for naïve T cells. IL-10 is an important regulator of dendritic cell function. IL-10 is known to be a potent inhibitor of IL-12 production, which is central for the cellular immune responses as it induces differentiation of naïve T cells to a Th1-type phenotype.48 Studies of IL-10 and dendritic cells of various of subsets demonstrated that exogenous IL-10 could inhibit production of IL-12 and the expression of costimulatory molecules by the dendritic cells.49,50 This inhibition correlated with the ability of IL-10 in vitro to inhibit primary alloantigen responses.51 Moreover, further work has demonstrated that IL-10 treatment of dendritic cells can induce or contribute to a state of anergy in alloantigen or peptide-antigen activated T cells.52,53
Different dendritic cell populations producing IL-10 have been identified from the Peyer's patch and the lung. In a study examining dendritic cells from the intestine,54 it was demonstrated that stimulation of Peyer's patch dendritic cells by CD40 resulted in high levels of IL-10 production, whereas no IL-10 was produced from CD40-stimulated spleen dendritic cells. Moreover, the Peyer's patch dendritic cells ex vivo were found to prime in vitro for IL-4 and IL-10 production from T cells. Peyer's patch dendritic cells also primed the T cells for much lower production of IFN-γ as compared with spleen dendritic cells. Thus, small intestinal dendritic cells from the Peyer's patch made significant amounts of IL-10. Moreover, IL-10 production by these dendritic cells had an important functional effect, skewing T-cell differentiation to a Th2 phenotype. In a study of dendritic cells from the lung,55 it was found that IL-10-producing T cells altered antigen responsive, in this study inducing anergy to pulmonary antigens. In our studies, the increased dendritic cell production of IL-10 may have resulted in both decreased IL-12 production and the skewing of T cells to a Th2 phenotype. Moreover, we found that splenic dendritic cells from 5LO–/– mice had decreased expression of activation markers, suggesting a more immature phenotype. This phenotype may be the result of increased in vivo exposure to IL-10 in the microenvironment and may also have altered the differentiation of the T cells.56
Altered T-cell cytokine production in 5LO–/– mice may be attributable to the absence of direct T-cell modulation by leukotrienes. Although leukotrienes are generally thought of as mediators of acute inflammatory responses, recent studies have suggested an important role for leukotrienes in the regulation of T lymphocytes.14 T lymphocytes are known to express receptors for both LTB4 and cysteinyl leukotrienes.57–59 LTB4 is a potent chemotactic factor for CD4+ and CD8+ T lymphocytes. This effect is mediated through the BLT1 receptor, which in mice is expressed on effector CD4+ and CD8+ T lymphocytes,14,60 demonstrating that leukotrienes can interact directly with T cells. In our in vitro studies, we found that exogenous LTB4 decreased IL-10 production from anti-CD3-stimulated T cells from 5LO–/– mice. Thus, the T-cell cytokine phenotype noted in our studies could be in part attributable to absence of endogenous LTB4 with subsequent alteration in T-cell migration.
Altered prostaglandin production could potentially alter cytokine production in 5-LO-deficient mice. The effect of 5-LO deficiency on prostaglandin production has not been consistent in published reports and it remains controversial whether absence of the leukotriene biosynthetic pathway results in shunting of arachidonic acid into the prostaglandin pathway. This is important, as prostaglandins are potent mediators of inflammatory and immune responses and alterations in their levels may affect these responses. PGE2in vitro can inhibit cytokine production, including IL-12 production and function61–63 and TNF-α production.64,65 Prostaglandins may also regulate T-cell differentiation and function,63,66 favouring the production of Th2 T cells over pro-inflammatory Th1 T cells. Moreover, previous studies have demonstrated that PGE2 can induce IL-10 production.31,66,67 Studies of the 5-LO-deficient mouse strain36 as well as FLAP-deficient mice38 reported increased prostaglandin production from calcium ionophore-stimulated macrophages. Similar to our results, in a study by Chen et al.35 increased production of prostaglandins was not detected in calcium ionophore-stimulated macrophages from a different strain of 5-LO-deficient mice. It is possible that alternative stimulation of the immune cells (for example with zymosan) may lead to different results. Moreover, other factors in vivo (which may be strain dependent) may be altering the production of prostaglandins in leukotriene-deficient mice. However, in our models, the phenotype of increased IL-10 production, decreased inflammatory cytokine production and increased Th2 T-cell responses was not correlated with increased PGE2 production.
Our study demonstrated that 5LO–/– mice had increased IL-10 production, decreased pro-inflammatory cytokine production and elevated Th2-type cytokine production. Thus, endogenous 5-LO-derived lipid mediators clearly modify inflammatory and immune responses. In contrast to the results of our study, other investigators have reported increased production of Th2-type immune responses in the absence of 5-LO-derived products.68 Differences between our studies and other reports are probably the result of genetic differences, differences in the models tested, and differences in the tissue compartment studied. A classic example in immunology of the role of genetic influences in the development of immune responses is the finding that Leishmania infection in C57Bl/6 mice results in the development of a protective Th1 T-cell immune response, whereas Leishmania-infected BALB/c mice develop a Th2 T-cell response.69 Alterations in the immune and inflammatory responses in various leukotriene-dependent models have been noted to be dependent upon strain differences. Strain differences have been noted in arachidonic acid-induced inflammation38 as well as in collagen-induced arthritis in FLAP-deficient mice.70 The role of genetic modifiers in inflammatory responses mediated by leukotrienes was illustrated by a study by Goulet.71 Using three different strains that were deficient in 5-LO (129/SvEv, C57Bl/6 and DBA), it was clearly demonstrated that genetic factors determined the contribution of leukotrienes in several models of acute inflammatory responses. Moreover, it was also shown that the role of leukotrienes in a model of arachidonic acid-induced inflammation depended upon the specific tissue being examined.71 For example, absence of 5-LO had no effect on arachidonic acid-induced peritoneal inflammation in DBA mice, whereas 5-LO-derived leukotrienes played a major role in arachidonic acid-induced cutaneous inflammation in this strain.71 Differences in results between our study and others may reflect differences in the relative roles of LTB4 and cysteinyl leukotrienes in the models tested (for example, models dependent upon neutrophil infiltration and activation versus models of asthma), genetic differences between mouse strains, and differences in the tissue compartment that was assessed.
In summary, our studies have demonstrated that absence of 5-LO-derived leukotrienes in our mouse model led to increased IL-10 production with concomitant decrease in inflammatory cytokines from stimulated myeloid cells (macrophages and dendritic cells). Moreover, absence of 5-LO-derived leukotrienes resulted in a skew in CD4+ T-cell differentiation in vivo to a Th2 phenotype. These data support the concept that 5-LO-derived leukotrienes in vivo regulate both innate and adaptive immune responses.
Acknowledgements
This research was supported financially by the Crohn's and Colitis Foundation of America (Research Award to DJB) and NIH (RO1 DK 060718-01A2).




