Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases
Summary
Human neutrophil migratory responses to Toll-like receptor (TLR) agonists were studied using videomicroscopy. When challenged with lipopolysaccharide (LPS, TLR4 agonist) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4, TLR2 agonist), neutrophils displayed enhanced motility, which was found to reflect increased random migration but not directed migration (chemotaxis). Enhanced neutrophil motility was detected within 10 min after stimulation with LPS or P3CSK4, and was sustained for more than 80 min. Stimulation of neutrophils with LPS or P3CSK4 resulted in the activation of extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK), which preceded neutrophil migration. TLR-mediated neutrophil migration was strongly suppressed by pretreatment of cells with U0126 (MAPK/ERK kinase inhibitor) but not with U0124 (an inactive analogue of U0126) or SB203580 (a p38 MAPK inhibitor), and was almost completely abolished by pretreatment of cells with U0126 and SB203580 in combination. Randomly migrating neutrophils in response to LPS or P3CSK4 displayed directed migration when further challenged with gradient concentrations of N-formyl-methionyl-leucyl-phenylalanine (FMLP) or platelet-activating factor (PAF). These findings indicate that TLR agonists stimulate human neutrophil migration via the activation of ERK and p38 MAPK, and FMLP- or PAF-induced neutrophil chemotaxis is not affected by the pre-exposure of cells to TLR agonists.
Abbreviations:
-
- ECL
-
- enhanced chemiluminescence
-
- ERK
-
- extracellular signal-regulated kinase
-
- FCS
-
- fetal calf serum
-
- FMLP
-
- N-formyl-methionyl-leucyl-phenylalanine
-
- G-CSF
-
- granulocyte colony-stimulating factor
-
- GM-CSF
-
- granulocyte–macrophage CSF
-
- GRO
-
- growth-related oncogene
-
- HBSS
-
- Hanks' balanced salt solution
-
- IL
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- MAPK
-
- mitogen-activated protein kinase
-
- MCP-1
-
- monocyte chemotactic protein-1
-
- MEK
-
- MAPK/ERK kinase
-
- MIP-2
-
- macrophage inflammatory protein-2
-
- NF-κB
-
- nuclear factor-κB
-
- PAF
-
- platelet-activating factor
-
- PBS
-
- phosphate buffered saline
-
- P3CSK4
-
- N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine
-
- SDS
-
- sodium dodecyl sulfate
-
- TCA
-
- trichloroacetic acid
-
- TLR
-
- Toll-like receptor
-
- TNF-α
-
- tumour necrosis factor α.
Introduction
Neutrophils play an important role in the innate immune system. Neutrophils arrive quickly at sites of infection and form the first line of defence against invading micro-organisms. They also play a major role in inflammation and tissue injury, contributing to the pathogenesis of a variety of inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease and acute respiratory distress syndrome.1 Neutrophils are known to be activated by various inflammatory mediators, including granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage CSF (GM-CSF), tumour necrosis factor-α (TNF-α) and Toll-like receptor (TLR) agonists.2–4 Human neutrophils express most of the TLRs described to date, including TLR2 and TLR4.5 TLR4 is a receptor for Gram-negative bacteria, lipopolysaccharide (LPS) and some viruses. TLR2 is a receptor for Gram-positive bacteria, lipopeptides, lipoteichoic acid and peptidoglycans, and TLR2 agonists signal via TLR2/TLR1 or TLR2/TLR6 heterodimers. TLR agonists exert pleiotropic effects on human neutrophils. For example, stimulation of human neutrophils with TLR agonists results in interleukin (IL)-8 production, up-regulation of CD11b, down-regulation of L-selectin, delayed apoptosis, enhanced phagocytosis and enhanced N-formyl-methionyl-leucyl-phenylalanine (FMLP)-induced superoxide release.6–11 In regard to neutrophil migration, it has been reported that TLR agonists inhibit IL-8-induced chemotaxis, which may be partly ascribed to down-regulation of the IL-8 receptors, CXCR1 and CXCR2.5,9,12 On the other hand, it has been reported that LPS pretreatment of human neutrophils decreases the IL-8- or growth-related oncogene (GRO)-α-induced expression of G-protein-coupled receptor kinases and CXCR2 internalization, and consequently enhances neutrophil migration to chemokines.13 Thus, the effect of TLR agonists on neutrophil chemotaxis appears to be complex. Although it has been demonstrated that TLR agonists modulate neutrophil chemotaxis induced by chemokines, the direct effect of TLR agonists on neutrophil migration has not been investigated. Here, we show that human neutrophils display enhanced random migration in response to LPS (a TLR4 agonist) and N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4, a TLR2/1 agonist). The results suggest that TLR-mediated neutrophil migration is regulated by the co-ordinated action of extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK). The results also show that neutrophil chemotaxis induced by FMLP or platelet-activating factor (PAF) is not affected by the pre-exposure of cells to TLR agonists.
Materials and methods
Reagents
Re-extracted LPS from Escherichia coli was purchased from List Biological Laboratories (Campbell, CA). Synthetic bacteria-like lipopeptide P3CSK4, SB203580 (p38 MAPK inhibitor), U0126 [MAPK/ERK kinase (MEK) inhibitor] and U0124 (an inactive analogue of U0126) were purchased from Calbiochem (San Diego, CA). FMLP and PAF (C16: 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) were purchased from Sigma (St Louis, MO). Alexa Fluor 546-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR). Conray was purchased from Mallinckrodt (St Louis, MO). Ficoll and the enhanced chemiluminescence (ECL) Western blotting system were purchased from Amersham Pharmacia Biotech (Bucks., UK). Rabbit polyclonal antibodies against ERK1/2, Thr202/Tyr204-phosphorylated ERK1/2, p38 MAPK and Thr180/Tyr182-phosphorylated p38 MAPK were purchased from Cell Signaling Technology (Beverley, MA).
Preparation of cells
Human neutrophils were prepared from healthy adult donors, as described previously,3 using dextran sedimentation, centrifugation with Conray-Ficoll and hypotonic lysis of contaminating erythrocytes. Neutrophil fractions contained more than 98% neutrophils. Cells were suspended in Hanks' balanced salt solution (HBSS) containing 10 mmN-2-hydroxyethyl-piperazine-N′-2-ethane-sulfonic acid (pH 7·4). Informed consent was obtained from all subjects.
Time-lapse recording and analysis of cell migration
Neutrophils (3 × 105/ml), suspended in HBSS containing 1% human serum, were placed in a glass-bottom dish (MatTek Co., Ashland, MA), the surface of which was precoated with fetal calf serum (FCS) to prevent spontaneous neutrophil adherence to the glass surface. After pre-incubation for 30 min at 37°, LPS or P3CSK4 (10 µl) was added to the cell suspension (1 ml) in the dish to obtain the desired final concentration. A uniform concentration of each agonist was obtained by gentle mixing. The cell suspensions were kept at 37°, a warmer being placed under the dish. Cells were monitored under a microscope (Olympus, Tokyo, Japan) with a ×40 phase-contrast objective lens, and the videotape recording was performed as described previously.14 The videotape recording was converted to digital imaging for analysis. The migration distance and speed were determined using the image analysis software move-tr/2d (Library Co., Tokyo, Japan). The centre of the cell body was traced every 2 seconds with the software to determine the migration distance during the period required. The migration speed was determined by measuring the distances that cells travelled during each 5-min period. When required, cells were pretreated with various inhibitors (U0126, U0124 and SB203580) for 30 min at 37° before stimulation with LPS or P3CSK4. Cell viability was determined by the Trypan Blue exclusion test, and the inhibitors used were not toxic to neutrophils.
Migration assay with the micropipette method
Neutrophils were placed in a glass-bottom dish, as described above. LPS (500 µg/ml), P3CSK4 (10 µg/ml), FMLP (1 µm) or PAF (1 µm) was loaded into a sterile injection capillary, Femtotip II (Eppendorf, Hamburg, Germany). A gradient was formed by slow release of the stimuli from the tip into the medium by using an Eppendorf microinjector, CellTram vario. Cells were monitored under a microscope, and the videotape recording was performed as described above.
Migration assay with the Boyden chamber method
LPS (1 µg/ml) or P3CSK4 (1 µg/ml) was put into the upper and/or lower wells of Boyden chambers (Neuroprobe, Cabin John, MD). The lower well was separated from the upper well by a polycarbonate filter with a 5-µm pore diameter. Neutrophils (1 × 106/ml, 50 µl) suspended in HBSS were put into the upper well, and the chamber was placed in a humidified incubator under 5% CO2 for 2 hr at 37°. After incubation, the filters were washed, fixed and stained with Mayer's Hematoxylin, and were then mounted on the glass slides. The cells migrating through the filter were counted under a light microscope in a high-power field (×400).14
Determination of actin re-organization
Actin re-organization was analyzed using confocal laser-scanning microscopy, as described previously.2 Neutrophils (1 × 107/ml, 50 µl), suspended in HBSS containing 1% human serum, were stimulated with LPS (1 µg/ml) or P3CSK4 (1 µg/ml) on FCS-coated glass coverslips for 20 min at 37°. After incubation, the cells were fixed with 3·7% paraformaldehyde and permeabilized with 0·2% Triton X-100 in phosphate-buffered saline (PBS). Then, the cells were incubated with Alexa Fluor 546-conjugated phalloidin (0·2 U/ml) in the dark for 30 min at room temperature. Fluorescence images were photographed with a confocal laser-scanning microscope (Zeiss LSM510; Zeiss, Welwyn Garden City, UK).
Western blotting
Western blotting was performed as described previously.15 Neutrophils (4 × 106/ml) suspended in HBSS containing 1% human serum were placed in a FCS-coated dish. After pre-incubation for 30 min at 37°, the cells were stimulated with LPS or P3CSK4 at 37°. When required, the cells were pretreated with U0126 (10 µm) and/or SB203510 (10 µm) for 30 min at 37°. The reactions were terminated by the addition of trichloroacetic acid (TCA). The final TCA concentration was 10%. The cells were collected with a cell scraper, washed with acetone containing 10 mm dithiothreitol and then lysed with ×2 sample buffer [4% sodium dodecyl sulfate (SDS), 20% glycerol, 10% mercaptoethanol, and a trace amount of Bromophenol Blue dye in 125 mm Tris-HCl, pH 6·8], heated at 100° for 5 min, and then frozen at −80° until use. Samples were subjected to 10% SDS gel electrophoresis. After electrophoresis, proteins were electrophoretically transferred from the gel onto a nitrocellulose membrane in a buffer containing 25 mm Tris, 192 mm glycine and 20% methanol at 2 mA/cm2 for 1·5 hr at 25°. Residual binding sites on the membrane were blocked by incubating the membrane in Tris-buffered saline (pH 7·6) containing 0·1% Tween 20 and 5% nonfat dry milk for 2 hr at 25°. The blots were incubated with appropriate primary antibody overnight at 4°. After washing, the membrane was incubated with appropriate secondary antibody conjugated with horseradish peroxidase, and the antibody complexes were visualized by the ECL detection system, as directed by the manufacturer. Immunoreactive bands were quantified by nih image on a Macintosh computer.
Statistical analysis
An analysis of variance (ANOVA) followed by a multiple comparison test or the Student's t-test was used to determine statistical significance.
Results
Stimulation of human neutrophil migration by TLR agonists
Neutrophils were stimulated with LPS (1 µg/ml) or P3CSK4 (1 µg/ml) and the neutrophil migratory responses were monitored under a videomicroscope. The time-lapse recording showed that neutrophils displayed morphological change (polarization with adherence) within 10 min after stimulation with LPS or P3CSK4, and then started migration (Fig. 1). The migration speed reached a maximum within 40 min, which was sustained for more than 80 min (1, 2, upper panel). The centre of the cell body was traced every 2 seconds with the software to determine the migration distance. The centre of the cell body was slightly changed from time to time, despite no active migration, and the software recognized this change as migration. This explains why apparent migration distance (migration speed) was detected at 0–5 min after stimulation with LPS or P3CSK4. To exclude this false migration, we measured the direct distance between the starting point and the arrival point that neutrophils reached 5 min thereafter (Fig. 2a). The direct distance reached a maximum level at 20–30 min after stimulation with LPS or P3CSK4 (Fig. 2b, lower panel). The effect of LPS or P3CSK4 on neutrophil migration was dependent on the concentrations of agonist used (Fig. 2c). An almost identical effect was obtained at 0·1 µg/ml LPS and 1 µg/ml P3CSK4. Stimulation of neutrophils with LPS or P3CSK4 resulted in F-actin redistribution, a finding consistent with the induction of cell migration by these agonists (Fig. 2d).
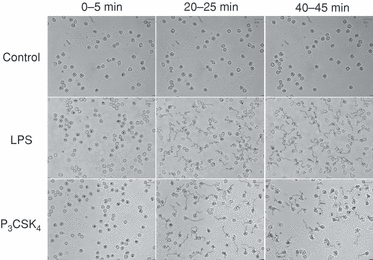
Stimulation of neutrophil migration by Toll-like receptor (TLR) agonists. Each image represents the tracks that neutrophils moved along at 0–5 min, 20–25 min or 40–45 min after stimulation with lipopolysaccharide (LPS) (1 µg/ml) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4) (1 µg/ml). The images shown are representative of five independent experiments.
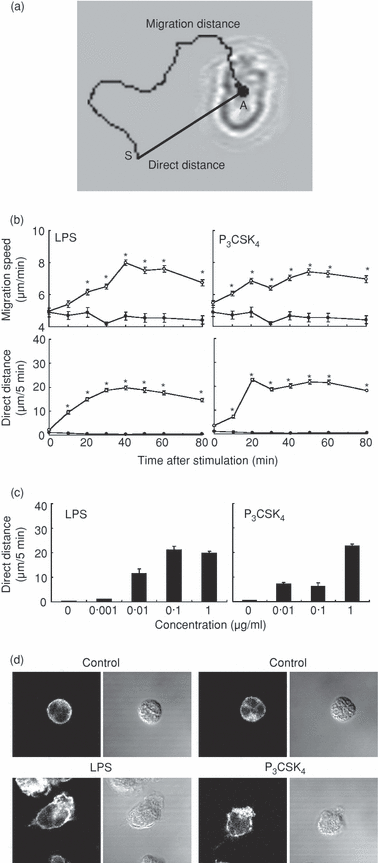
Stimulation of neutrophil migration by Toll-like receptor (TLR) agonists. (a) The migration distance represents the distance that cells actually travelled during 5-min period. The direct distance represents the distance between the starting point (S) and the arrival point (A) that cells reached 5 min thereafter. (b) Neutrophils were stimulated with lipopolysaccharide (LPS) (1 µg/ml) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4) (1 µg/ml). The migration speed represents the mean speed calculated from the migration distance that neutrophils traveled during each 5-min period. The direct distance that neutrophils traveled during each 5-min period was determined according to the image shown in (a). The data are expressed as the mean ± standard error of the mean (SEM) of three independent experiments. At least 50 cells were analyzed for each point. Closed circle, control cells. *Significantly greater compared with control cells and cells at minute 0 (P < 0·01). (c) Neutrophils were stimulated with the indicated concentrations of LPS or P3CSK4. The direct distance was determined 40–45 min after LPS stimulation and 20–25 min after P3CSK4 stimulation, respectively. Essentially identical results were obtained when the other time-points were used. The data are expressed as the mean ± SEM of three independent experiments. (d) Neutrophils were stimulated with LPS (1 µg/ml) for 40 min or with P3CSK4 (1 µg/ml) for 20 min at 37°. Left panel: F-actin distribution was analyzed with confocal microscopy. Right panel: Nomarski images. The images shown are representative of three independent experiments.
TLR agonists induce random migration, but not chemotaxis
The study with videomicroscopy revealed that TLR agonists directly stimulated human neutrophil migration. To determine whether TLR agonists induce random migration or directed migration (chemotaxis), the Boyden chamber method was used. As shown in Fig. 3(a), LPS induced neutrophil migration when added to the upper well or to both wells, but not when added to the lower well alone. Similar results were obtained when P3CSK4 was used instead of LPS. P3CSK4 induced neutrophil migration when added to the upper well or to both wells, but not when added to the lower well alone. Under the same conditions, FMLP induced remarkable neutrophil chemotaxis (Fig. 3a).
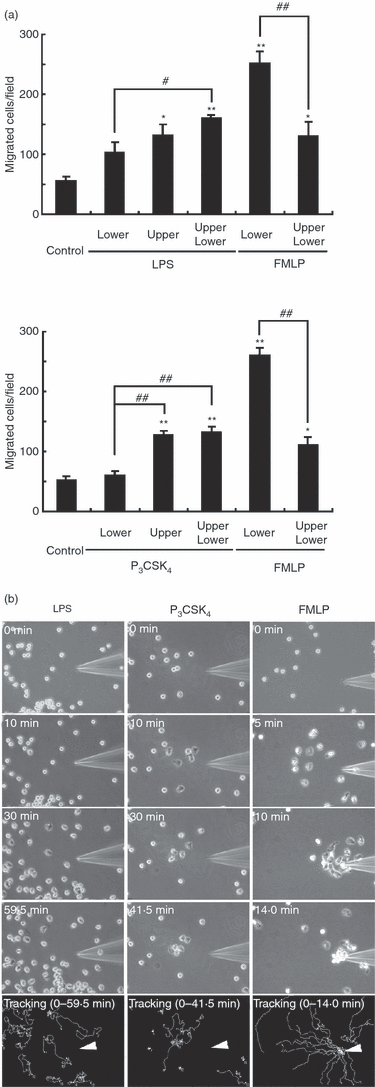
Analysis of neutrophil migration with the Boyden chamber method and the micropipette method. (a) Migration assay with the Boyden chamber method. Lipopolysaccharide (LPS) (1 µg/ml) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4) (1 µg/ml) was added to the upper and/or lower wells. As a positive control, N-formyl-methionyl-leucyl-phenylalanine (FMLP) (0·1 µm) was used. The neutrophil suspension was added to the upper well. After incubation for 2 hr, the number of cells migrating to the lower well was determined. The data are expressed as the mean ± SEM of three independent experiments. *P < 0·05, **P < 0·01 (significantly greater as compared with control cells). #P < 0·05, ##P < 0·01 (significant difference). (b) Migration assay with the micropipette method. LPS (500 µg/ml), P3CSK4 (10 µg/ml) or FMLP (1 µm) was loaded into a sterile injection capillary, Femtotip II. A gradient was formed by slow release of the stimuli from the tip into the medium by using an Eppendorf microinjector. The images at the indicated time-points after the addition of LPS, P3CSK4 or FMLP are shown. The tracks that neutrophils moved along during the indicated time-periods are shown at the bottom. An arrowhead indicates the micropipette tip. The images shown are representative of three independent experiments.
The neutrophil migratory response to TLR agonists was also studied by using the micropipette method. As shown in Fig. 3(b), neutrophils migrated towards the micropipette tip containing FMLP. By contrast, neutrophils never migrated towards the micropipette tip containing LPS or P3CSK4, but displayed random migration. These findings indicate that both LPS and P3CSK4 induce random migration, but not chemotaxis.
Involvement of ERK and p38 MAPK in TLR-mediated neutrophil migration
Stimulation of neutrophils with LPS or P3CSK4 resulted in the phosphorylation of ERK and p38 MAPK. Significant phosphorylation of ERK and p38 MAPK was detected within 10 min after stimulation with LPS or P3CSK4(Fig. 4a). Maximum levels of phosphorylation of ERK and p38 MAPK were detected 20 min after LPS stimulation and 10 min after P3CSK4 stimulation, respectively. The effect of LPS or P3CSK4 on the phosphorylation of ERK and p38 MAPK was dependent on the concentrations of agonist used (Fig. 4b). The effective concentrations of LPS or P3CSK4 required to induce phosphorylation of ERK and p38 MAPK were almost identical to those used to induce neutrophil migration, and phosphorylation of ERK and p38 MAPK preceded neutrophil migration (2, 4).
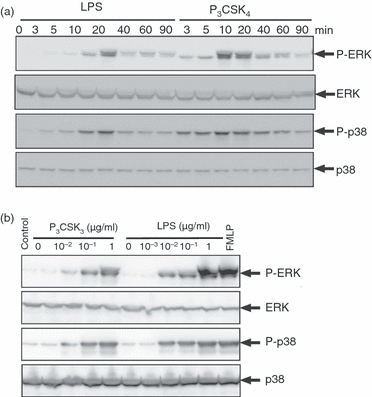
Phosphorylation of extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) in neutrophils stimulated by lipopolysaccharide (LPS) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4). (a) Neutrophils were stimulated with LPS (1 µg/ml) or P3CSK4 (1 µg/ml) for the indicated time-periods at 37°. (b) Neutrophils were stimulated with the indicated concentrations of LPS for 20 min or with P3CSK4 for 10 min at 37°. Immunoblotting was performed using antibodies against ERK1/2, p38 MAPK and the phosphorylated forms of ERK1/2 and p38 MAPK. As a positive control, neutrophils were stimulated with N-formyl-methionyl-leucyl-phenylalanine (FMLP) (0·1 µm) for 1 min at 37°. The cell lysates equivalent to 4 × 105 cells were loaded onto each lane. The results shown are representative of three independent experiments.
Our recent studies showed that ERK and p38 MAPK are involved in actin reorganization in human neutrophils stimulated by pro-inflammatory cytokines (G-CSF, GM-CSF and TNF-α), and G-CSF induces human neutrophil random migration via the activation of MEK/ERK.2,14 These findings raise the possibility that TLR-mediated neutrophil migration might be regulated by ERK and p38 MAPK. This possibility was explored by using specific inhibitors against MEK (an upstream kinase of ERK) and p38 MAPK. As shown in Fig. 5, LPS-induced neutrophil migration was strongly suppressed by the pretreatment of cells with U0126 (a MEK inhibitor), but not with U0124 (an inactive analogue of U0126) or SB203580 (a p38 MAPK inhibitor). LPS-induced neutrophil migration was almost completely abolished when cells were pretreated with U0126 + SB203580 in combination (Fig. 5).
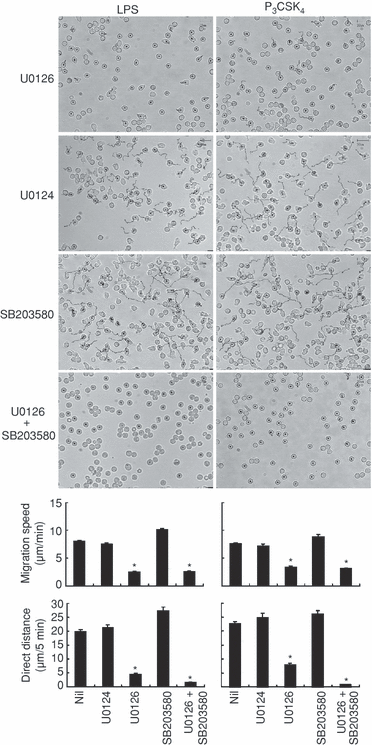
Effects of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase (MEK) and p38 MAPK inhibitors on Toll-like receptor (TLR)-mediated neutrophil migration. Neutrophils were pretreated with U0126 (10 µm), U0124 (10 µm), SB203580 (10 µm), or U0126 (10 µm) plus SB203580 (10 µm) for 30 min at 37°, and thereafter stimulated with lipopolysaccharide (LPS) (1 µg/ml) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4) (1 µg/ml). Upper panel: the tracks that neutrophils moved along 40–45 min after LPS stimulation or 20–25 min after P3CSK4 stimulation. Lower panel: the effects of MEK and p38 MAPK inhibitors on the migration speed and the direct distance were assessed 40–45 min after LPS stimulation or 20–25 min after P3CSK4 stimulation. Essentially identical results were obtained at other time-points. The data are expressed as the mean ± standard error of the mean (SEM) of three independent experiments. At least 50 cells were analyzed for each experiment. *Significantly suppressed by U0126 or U0126 plus SB203580 (P < 0·01).
Similar findings were obtained when P3CSK4 was used instead of LPS (Fig. 5). P3CSK4-induced neutrophil migration was strongly suppressed by the pretreatment of cells with U0126, but not by pretreatment with U0124 or SB203580. P3CSK4-induced neutrophil migration was almost completely abolished when cells were pretreated with U0126 + SB203580 in combination.
Effects of U0126 and SB203580 on TLR agonist-induced phosphorylation of ERK and p38 MAPK
As shown in Fig. 6, LPS- or P3CSK4-induced phosphorylation of ERK was markedly inhibited by the pretreatment of cells with 10 µm U0126, the concentration used in the present experiment. On the other hand, LPS- or P3CSK4-induced phosphorylation of p38 MAPK was unaffected by the pretreatment of cells with U0126 or SB203580 as expected, because SB203580 is an inhibitor of p38 MAPK, but not of MKK3/6, an upstream kinase of p38 MAPK.2,16 LPS- or P3CSK4-induced phosphorylation of ERK was increased by the pretreatment of cells with SB203580. This effect of SB203580 is unlikely to be nonspecific because we found that ERK phosphorylation induced by cytokines (G-CSF, GM-CSF and TNF-α) was not increased by the pretreatment of cells with SB203580.2,14 Increased ERK phosphorylation by the pretreatment of cells with SB203580 may be ascribed to increased MEK activity because the enhancing effect of SB203580 on ERK phosphorylation was inhibited by U0126 (Fig. 6).
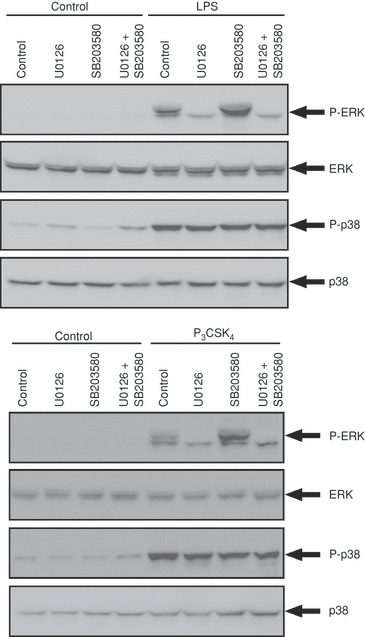
Effects of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase (MEK) and p38 MAPK inhibitors on Toll-like receptor (TLR) agonist-induced phosphorylation of ERK and p38 MAPK. Neutrophils were pretreated with U0126 (10 µm), SB203580 (10 µm), or U0126 (10 µm) plus SB203580 (10 µm) for 30 min at 37°, and thereafter stimulated with lipopolysaccharide (LPS) (1 µg/ml) for 20 min or with N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4) (1 µg/ml) for 10 min. Immunoblotting was performed using antibodies against ERK1/2, p38 MAPK, and the phosphorylated form of ERK1/2 and p38 MAPK. The results shown are representative of three independent experiments.
FMLP- or PAF-induced neutrophil chemotaxis is not affected by pre-exposure of cells to TLR agonists
Controversial results have been reported for the effect of TLR agonists on human neutrophil chemotaxis (i.e. TLR agonists inhibit or enhance chemokine-induced neutrophil migration).5,9,12,13 Therefore, we re-examined the effect of TLR agonists on FMLP-induced neutrophil migration using the micropipette method. Neutrophils were pre-exposed to uniform concentrations (1 µg/ml) of LPS or P3CSK4 for 30 min and thereafter challenged with gradient concentrations of FMLP. As shown in Fig. 7(a), randomly migrating neutrophils in response to LPS or P3CSK4 displayed directed migration when further challenged with gradient concentrations of FMLP. In addition, the migration speed of cells towards the micropipette tip was not affected by the pre-exposure of cells to LPS or P3CSK4 (Fig. 7b). The pre-incubation of cells with LPS or P3CSK4 for 1 hr gave similar results (data not shown). These findings are consistent with a previous report5 demonstrating that FMLP-induced Ca2+ flux in human neutrophils is not affected by the pretreatment of cells with LPS. Similar findings were obtained when PAF was used instead of FMLP. Randomly migrating neutrophils in response to LPS or P3CSK4 displayed directed migration when further challenged with gradient concentrations of PAF (images not shown). In addition, the migration speed of cells towards the micropipette tip was not affected by the pre-exposure of cells to LPS or P3CSK4. The migration speed of PAF-stimulated neutrophils was slower than that of FMLP-stimulated cells (Fig. 7c). These findings indicate that FMLP- or PAF-induced neutrophil chemotaxis is not affected by the pre-exposure of cells to TLR agonists, at least under the conditions employed.
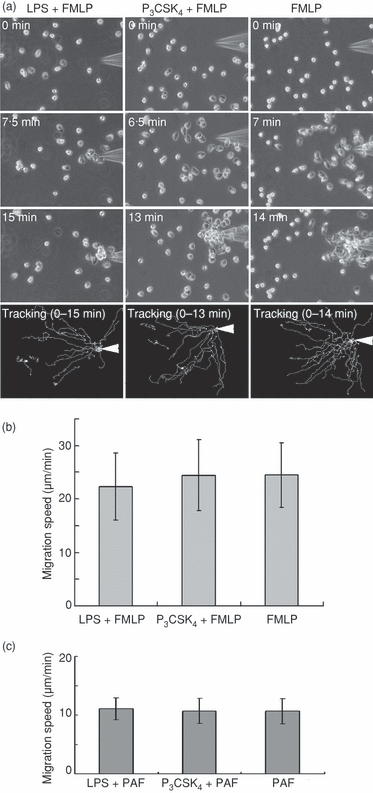
Effect of pre-exposure of neutrophils to Toll-like receptor (TLR) agonists on N-formyl-methionyl-leucyl-phenylalanine (FMLP)- or platelet-activating factor (PAF)-induced chemotaxis. Neutrophils were pre-exposed to uniform concentrations (1 µg/ml) of lipopolysaccharide (LPS) or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteinyl-seryl-(lysyl)(3)-lysine (P3CSK4) for 30 min at 37°, and thereafter challenged with gradient concentrations of FMLP or PAF using a micropipette. FMLP (1 µm) or PAF (1 µm) was loaded into a sterile injection capillary. (a) The images at the indicated time-points after the addition of FMLP are shown. The tracks that neutrophils moved along during the indicated periods are shown at the bottom. An arrowhead indicates the micropipette tip. The images shown are representative of three independent experiments. (b) The migration speed of neutrophils towards the micropipette tip containing FMLP. The data are expressed as the mean ± standard deviation (SD) of 20–30 cells analyzed in one experiment. An additional two experiments gave similar results. (c) The migration speed of neutrophils towards the micropipette tip containing PAF. The data are expressed as the mean ± SD of 20–30 cells analyzed in one experiment. An additional two experiments gave similar results.
Discussion
Previous studies focused the modulatory effects of TLR agonists on neutrophil chemotaxis induced by chemokines5,9,12,13 and the direct effect of TLR agonists on neutrophil migration was not studied. In the present experiments, we showed that LPS (a TLR4 agonist) and P3CSK4 (a TLR2 agonist) by themselves are potent stimuli for inducing random migration in human neutrophils, and MEK/ERK plays a major role in neutrophil migration induced by these agonists. These findings are consonant with our recent observation that G-CSF induces random migration in human neutrophils and that MEK/ERK plays a major role in G-CSF-induced neutrophil migration.14 The present experiments also show that p38 MAPK plays a regulatory role in neutrophil migration induced by TLR agonists, and FMLP- or PAF-induced neutrophil chemotaxis is not affected by the pre-exposure of cells to TLR agonists.
We used three different methods to demonstrate the direct effect of TLR agonists on neutrophil migration. Under the condition of uniform concentrations of LPS or P3CSK4, neutrophils displayed morphological change within 10 min after stimulation and then started migration. The studies with the Boyden chamber method and the micropipette method revealed that neutrophils displayed random migration, but not chemotaxis, in response to stimulation with LPS or P3CSK4.
TLR-mediated neutrophil migration was strongly suppressed in the presence of the MEK inhibitor, suggesting that ERK plays a major role in neutrophil migration. These findings are consistent with our recent studies showing that ERK is selectively activated in human neutrophils stimulated by G-CSF, and ERK is involved in G-CSF-induced neutrophil migration.3,14 The role of ERK in cell migration has also been demonstrated in various cell lines, and ERK-mediated activation of myosin light chain kinase and calpain 2 (m-calpain) is proposed to be involved in the stimulation of cell migration.17,18 In human neutrophils, it has been recently reported that calpain 1 (µ-calpain) functions as a negative regulator in cell migration by inhibiting the activation of Cdc42 and Rac,19 and calpain 2 is a component of the frontness signal that promotes polarization during chemotaxis.20 Our recent study showed that MEK/ERK is involved in redistribution of the phosphorylated myosin light chain and F-actin, rather than myosin light chain phosphorylation, in G-CSF-stimulated human neutrophils.14 The role of ERK in calpain 2 recruitment to the leading edge of polarized neutrophils remains to be determined.20
TLR-mediated neutrophil migration was not suppressed in the presence of the p38 MAPK inhibitor, whereas it was almost completely abolished in the presence of the MEK and p38 MAPK inhibitors. These findings suggest that p38 MAPK plays a regulatory role in neutrophil migration induced by TLR agonists. It has been reported that the stimulation of human neutrophils with LPS results in p38 MAPK-mediated activation of Cdc42,21 suggesting that the p38 MAPK/Cdc42 pathway might be partly involved in the regulation of LPS-induced neutrophil migration. A recent study showed that TNF-α induces a stop signal through a p38 MAPK pathway, promoting firm neutrophil adhesion and inhibiting IL-8- or C5a-induced neutrophil polarization and chemotaxis. TNF-α-induced human neutrophil polarization and migration are enhanced in the presence of the p38 MAPK inhibitor.22 Our previous studies showed that p38 MAPK is involved in GM-CSF- or TNF-α-induced neutrophil adherence.23 These findings, taken together, suggest that p38 MAPK may contribute not only to migration but also to firm adhesion in neutrophils stimulated by TLR agonists or pro-inflammatory cytokines. Using murine neutrophils lacking Cdc42 GTPase-activating protein, Szczur et al. have recently reported that high ERK activity with increased Cdc42 activity is associated with enhanced chemokinesis in response to FMLP stimulation and integrin ligation.24
It has been reported that FMLP-induced human neutrophil chemotaxis is dependent on the activation of p38 MAPK, but not of ERK, and that IL-8-induced human neutrophil chemotaxis is independent of the activation of p38 MAPK and ERK.25–28 G-CSF-induced human neutrophil random migration is dependent on the activation of ERK.14 The results presented here show that TLR-mediated human neutrophil random migration is dependent on the activation of ERK and p38 MAPK. These findings suggest that the roles of ERK and p38 MAPK in human neutrophil migration or chemotaxis are dependent on the agonists used. Independent regulation of neutrophil migration induced by FMLP and TLR agonists may also be supported by the findings that FMLP-induced neutrophil chemotaxis is not affected by pre-exposure of cells to TLR agonists. The agonist-dependent role of ERK and p38 MAPK in neutrophil migration may be closely associated with the signaling molecules concomitantly activated by stimulation with each agonist, as neutrophil migration may be regulated by multiple molecules according to the agonists used. It is also plausible that the balance of ERK and p38 MAPK activity may be important for the decision of the neutrophil migratory response.
The signaling pathways activated in human neutrophils stimulated by TLR agonists include ERK, p38 MAPK, c-Jun N-terminal kinase, nuclear factor-κB (NF-κB) and phosphatidylinositol 3-kinase.9,11,29 The role of each signaling pathway in the TLR-mediated activation of human neutrophils is not fully understood. NF-κB is involved in human neutrophil survival and the production of pro-inflammatory cytokines, such as IL-1β, TNF-α, IL-8, macrophage inflammatory protein-2 (MIP-2) and monocyte chemotactic protein-1 (MCP-1).5,7,10,30–32 LPS-induced NF-κB activation and TNF-α production in human neutrophils is regulated by p38 MAPK.32 LPS-induced c-Jun N-terminal kinase activation in adherent human neutrophils is mediated by phosphatidylinositol 3-kinase, and is involved in MCP-1, but not TNF-α and IL-8, expression.30 TLR2-mediated phosphatidylinositol 3-kinase activation causes activation of ERK and p38 MAPK in murine neutrophils, and is involved in TNF-α and MIP-2 production.33 TLR4-mediated human neutrophil survival is dependent on the activation of NF-κB, ERK and p38 MAPK.10 These findings suggest that cross-talk occurs between each signaling pathway in neutrophils stimulated by TLR agonists. Although TLR2 and TLR4 agonists exert similar effects on neutrophils, there are some differences in the biological effects of these agonists on human neutrophils. For example, human neutrophil apoptosis is suppressed by TLR4 agonist, but the anti-apoptotic effect of TLR2 agonist is minimal.10
In this study, we showed that LPS (TLR4 agonist) and P3CSK4 (TLR2 agonist) by themselves are potent stimuli for inducing random migration in human neutrophils, and that ERK and p38 MAPK are involved in the regulation of TLR-mediated neutrophil migration. The direct stimulation of neutrophil migration by TLR agonists, an early event induced by these agonists, may be physiologically relevant and may contribute to the host defence against invading micro-organisms, possibly, in part, by accelerating the clearance of micro-organisms through increased chance of contact between migrating neutrophils and micro-organisms.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research, Japan, and Osaka City University Research Foundation.




