Chronic exposure in vivo to thyrotropin receptor stimulating monoclonal antibodies sustains high thyroxine levels and thyroid hyperplasia in thyroid autoimmunity-prone HLA-DRB1*0301 transgenic mice
J. C. Flynn and J. A. Gilbert contributed equally to this work.
Summary
We have examined the induction of autoimmunity and the maintenance of sustained hyperthyroidism in autoimmunity-prone human leucocyte antigen (HLA) DR3 transgenic non-obese diabetic (NOD) mice following chronic stimulation of the thyrotropin receptor (TSHR) by monoclonal thyroid-stimulating autoantibodies (TSAbs). Animals received weekly injections over the course of 9 weeks of monoclonal antibodies (mAbs) with strong thyroid-stimulating properties. Administration of the mAbs KSAb1 (IgG2b) or KSAb2 (IgG2a), which have similar stimulating properties but different TSH-binding blocking activity, resulted in significantly elevated serum thyroxine (T4) levels and thyroid hyperplasia. After the first injection, an initial surge then fall in serum T4 levels was followed by sustained elevated levels with subsequent injections for at least 63 days. Examination of KSAb1 and KSAb2 serum bioactivity showed that the accumulation of the TSAbs in serum was related to their subclass half-lives. The thyroid glands were enlarged and histological examination showed hyperplastic follicles, with minimal accompanying thyroid inflammation. Our results show that chronic in vivo administration of mAbs with strong thyroid-stimulating activity resulted in elevated T4 levels, suggesting persistent stimulation without receptor desensitization, giving a potential explanation for the sustained hyperthyroid status in patients with Graves' disease. Moreover, despite the presence of HLA disease susceptibility alleles and the autoimmune prone NOD background genes, chronic stimulation of the thyroid gland did not lead to immune cell-mediated follicular destruction, suggesting the persistence of immunoregulatory influences to suppress autoimmunity.
Abbreviations:
-
- CTLA-4
-
- cytotoxic T-lymphocyte associated antigen-4
-
- DR3
-
- HLA-DRB1*0301
-
- HLA
-
- human leucocyte antigen
-
- MHC
-
- major histocompatibility complex
-
- mAbs
-
- monoclonal antibodies
-
- mTg
-
- mouse thyroglobulin
-
- NOD
-
- non-obese diabetic
-
- OD
-
- optical density
-
- Tg
-
- thyroglobulin
-
- TPO
-
- thyroid peroxidase
-
- TSAbs
-
- thyroid-stimulating autoantibodies
-
- TSH
-
- thyrotropin
-
- TSHR
-
- thyrotropin receptor
-
- T4
-
- thyroxine
-
- TT4
-
- total thyroxine.
Introduction
Some of the most common autoimmune conditions affecting humans belong to thyroid autoimmune disorders, which include Graves' disease and Hashimoto's destructive thyroiditis.1 In Graves' disease, the hyperthyroidism is directly caused by thyroid-stimulating autoantibodies (TSAbs) to the thyrotropin receptor (TSHR) that mimic the effects of the hormone TSH to hyperstimulate thyroid function.2 The thyroid glands in patients with Graves' disease are enlarged as a result of thyroid hyperplasia and some show foci of intrathyroidal inflammatory infiltrates, including well-maintained germinal centres with abundant plasma B cells.3 Another cardinal feature of patients with Graves' disease is the presence of autoantibodies to other thyroid antigens, such as thyroid peroxidase (TPO) and thyroglobulin (Tg), which are commonly associated with Hashimoto's thyroiditis.1 The reasons for the recruitment of TPO and Tg as autoantigens in patients with Graves' disease are not completely understood, but it is believed that the intense and continuous stimulation of the gland may result in leakage of these potential antigens into the periphery.1 For example, Tg is a by-product of thyroxine (T4) release and we have shown a three- to five-fold increase of circulatory Tg after 3 days of TSH infusion via an osmotic pump, coincident with T4 release that peaked within 24 hr.4 There is also strong genetic susceptibility to disease development, contributed predominantly by major histocompatibility complex (MHC) antigens, cytotoxic T-lymphocyte-associated antigen-4 and the protein tyrosine phosphatase 22 (PTPN22) gene.5 Amongst the MHC genes, the HLA-DRB1*0301 (DR3) class II genes have been recognized as important susceptibility elements for disease in patients with thyroid autoimmune disease.6
One seldom recognized feature of Graves' disease is that there are no animal species other than humans that develop thyroid-stimulating antibodies. Various protocols to induce experimental Graves' disease have been reported over the years and these have led to improved models in terms of potency, disease incidence and severity of hyperthyroidism.7 We recently reported that humanized, transgenic mice expressing human leucocyte antigen (HLA) DR3 in the absence of endogenous class II molecules on a non-obese diabetic (NOD) background were more prone to human Tg-induced autoimmune thyroiditis than the wild-type NOD mouse.8 Moreover, the DR3 transgenic animals were also susceptible to experimental Graves' hyperthyroidism, which was accompanied by lymphocytic infiltration and antibodies to Tg in some animals.9 Interestingly, in other studies using HLA-DRB1*0301 transgenic mice on a C57BL/10 background, no hyperthyroidism or thyroid inflammation developed,10 confirming the importance of the MHC class II locus together with other autoimmune susceptibility genes for disease.
We recently generated thyroid-stimulating monoclonal antibodies (mAbs) from a mouse model of Graves' disease, which powerfully stimulate the TSHR and act as full agonists, akin to the ligand TSH.11 The two mAbs KSAb1 and KSAb2 are highly potent and act as full agonists to the TSHR at low nanomolar concentrations, but also exhibit important differences in their EC50 values at lower doses.11 In addition, different studies in an acute setting have demonstrated that single injections of low microgram quantities of anti-TSHR mAbs in mice induce rapid elevation of serum T4 levels, which peak around 24 hr before returning to normal levels by 48–72 hr.11–13 In the present study, using two stimulatory mAbs, we investigated the effect of chronic stimulation of the TSHR on thyroid function. In addition, we investigated whether chronic stimulation of the thyroid gland over a prolonged period of 9 weeks would provoke autoimmune responses, similar to those observed in patients with Graves' disease. We performed the chronic exposure study in the DR3 transgenic Ab0/NOD mice to include the necessary genetic susceptibility elements to provoke potential autoimmunity.
Materials and methods
HLA-DRB1*0301 transgenic NOD mice
NOD mice expressing HLA-DR3 in the absence of endogenous (IAg7) class II molecules were generated, raised and typed in the pathogen-free Immunogenetics Mouse Core facility at the Mayo Clinic before shipment as previously described.8 Briefly, the HLA-DRA/DRB1*0301 (DR3) transgene was introduced into class II-negative Ab0 mice14 backcrossed to C57BL/10 mice,15 which were then backcrossed to NOD mice for several generations (N8). IAg7 and DR3 expressions were determined by polymerase chain reaction and flow cytometric analysis of peripheral blood leucocytes, respectively.8 DR3+ Ab0/NOD mice of both sexes were used at 12–14 weeks of age and maintained in a specific pathogen-free facility on acidified water. A veterinarian supervised animal care and all procedures were performed in accordance with accredited institutional guidelines.
Chronic stimulation by passive transfer of anti-TSHR mAbs
KSAb1 and KSAb2 immunoglobulin G (IgG) were purified from culture supernatants of the hybridomas by protein A–Sepharose chromatography.11 The purity of the IgG was > 95% as judged by sodium dodecyl sulphate–polyacrylamide gel electrophoresis analysis. Groups of three male (for KSAb1) or three female (for KSAb2) DR3+ Ab0/NOD mice were injected intravenously into the tail vein with 10 μg KSAb1 or KSAb2 mAb, respectively, in saline buffer every week for 8 weeks (totalling nine injections). For injections at weeks 0, 1, 3, 5 and 7, sera were collected from the animals 24 hr before and after each injection. For preimmune serum samples, the mice injected with KSAb1 and KSAb2 were bled 4 days and 1 day, respectively, before the first injection.
Measurement of residual KSAb1 and KSAb2 IgG in serum
Residual KSAb1 and KSAb2 IgG levels present in the sera of the animals following intravenous injections were measured as TSHR-stimulating activity by bioassay using human TSHR-transfected JP09 cells. The assay was performed essentially as described elsewhere11 using 3 μl heat-inactivated serum in triplicate wells using isotonic Hanks' balanced salt solution buffer containing sucrose and HEPES supplemented with the phosphodiesterase inhibitor, isobutyl-1-methylxanthine (0·5 mm) (IBMX, Sigma-Aldrich, Dorset, UK). The cAMP released into the medium was measured by radioimmunoassay (R & D Systems, Oxford, UK) and the results were expressed as pmol/ml.
Assessment of total serum T4 hormone levels
Total thyroid hormone, TT4, was determined with 25 μl serum using a commercial kit (DS Laboratories, Oxford, UK). Due to the volume of serum required, these measurements were performed in single samples.
Determination of antibodies to mouse Tg
Serum titres to mouse Tg (mTg) were determined by enzyme-linked immunosorbent assay (ELISA) generally as described elsewhere.16 Serum dilutions were added to mTg-coated plates (1 μg/well) (Immulon II; Dynex Technologies, Shantilly, VA) and incubated at 4° overnight. Alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL) was added for 60 min (37°) followed by substrate p-nitrophenyl phosphate (Sigma Diagnostics, St Louis, MO) for 30 min at room temperature. Optical densities (OD) were determined at 405 nm. Anti-mTg standard sera and normal mouse sera were used as positive and negative controls, respectively. Data were expressed as mean OD after subtracting background.
Thyroid pathology
Thyroids were examined 65 days after the initial anti-TSHR mAb injection. Thyroid glands were examined grossly and histological sections were obtained. Mononuclear cell infiltration was determined by evaluating 60–70 vertical sections (hameatoxylin & eosin stain, 10–15 step levels) throughout both lobes. Individual pathology scores, based on a scale of 0–4, were expressed as per cent of thyroid gland infiltration: 0, no infiltration; 0·5, > 0–10% infiltration, with multiple foci of infiltration without follicular destruction; and 1·0, > 10–20% infiltration with follicular destruction.15
Results
Chronic stimulation of TSHR in vivo and thyroid function
In in vitro studies, KSAb1 and KSAb2 IgG stimulate cAMP production in human TSHR-transfected Chinese hamster ovary cells (JP09 cells) by showing full agonist activity, with EC50 values of 9·4 and 93 ng/ml, respectively.11 For passive transfer studies, we selected a dose of 10 μg for both KSAb1 and KSAb2 mAbs (greater than an EC50 dose of 1000-fold and 100-fold, respectively). In this manner, we ensured an adequate dose of the agonist mAbs in the animals, which exceeded by three and two log folds for KSAb1 and KSAb2, respectively. To maintain the chronicity of TSHR stimulation of the gland, the injections were repeated every week for 9 weeks and serial blood samples were collected 1 day before and 1 day after alternate injections with the mAbs. Serum T4 levels were measured in all the serial blood samples as an indicator of the hyperthyroid status. After the first intravenous injection of mAb, a robust surge in serum T4 was observed which peaked on day 1. Serum T4 levels were elevated by approximately 2·5- and 4-fold in KSAb1- and KSAb2-challenged mice, respectively (shown as means in Fig. 1a,b, respectively). Following the first injection, serum T4 levels subsequently declined (day 6, Fig. 1a,b), but remained elevated compared with the basal levels for both the mAb-treated animal groups (Fig. 1a,b). Analysis of serum T4 levels 1 day after the second injection on day 8 revealed a marginal increase in the mean values for both KSAb1- and KSAb2-injected mice (Fig. 1a,b). Subsequent injections of KSAb1 or KSAb2, particularly on day 21 and day 35, when blood samples were also collected before and after the injections, confirmed the continued elevated levels of serum T4 levels. Interestingly, the overall trend for sustained hyperthyroidism was maintained (Fig. 1a,b). Continued weekly injections of the mAbs, for example, on day 49, showed no dramatic differences in response of the glands to TSHR stimulation where serum T4 levels stabilized and plateaued in both KSAb1-treated and KSAb2-treated animals (Fig. 1a,b). Finally, analysis of serum samples on day 63 following 9 weeks of sustained stimulation indicated that hyperthyroidism was maintained, compared with the basal serum T4 levels before the start of the injection schedule (Fig. 1a,b). For the duration of the experiment, the animals did not show any sign of discomfort or weight loss. To summarize, chronic in vivo exposure of mice to continued weekly injections of KSAb1 or KSAb2 led to rapid onset of hyperthyroidism, which was maintained throughout the course of antibody-induced stimulation of the thyroid gland.
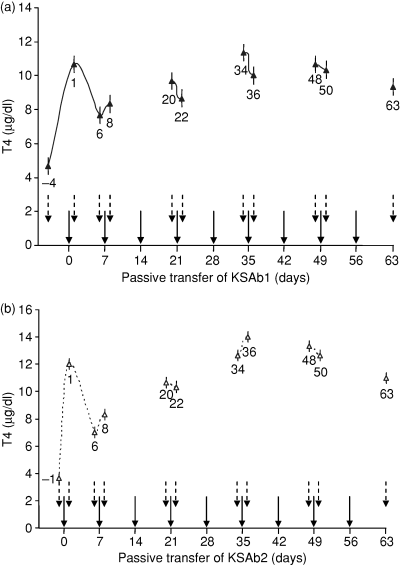
Chronic thyroid stimulation in vivo with thyroid-stimulating mAbs results in elevated serum T4 levels, with no signs of refractoriness. Animals were injected with 10 μg KSAb1 (a) and KSAb2 (b) every week as indicated (solid arrows). Pre-immune samples were obtained 4 days (KSAb1) or 1 day (KSAb2) before the first injection [indicated as − 4 and − 1 day in (a) and (b), respectively]. Animals were bled after the first week of injection, and then 1 day before and 1 day after the injections on alternate weeks (dotted arrows), and serum T4 levels measured. The data shown represent the means of duplicate measurements. The mean baseline T4 levels in the normal group of HLA-DR3 transgenic Ab0/NOD mice range from 3 to 4 μg/dl. With the limitations in obtaining regular serial bleeds from the animals for a prolonged time, the animals were not bled on days 14, 28, 42 and 56 and hence were not evaluated at those particular time-points. The numbers next to the data points refer to days after the first injection. The bars at each time-point indicate standard mean error.
Clearance of KSAb1 and KSAb2 IgG following passive transfer
Following the weekly passive transfer injections of KSAb1 and KSAb2, we investigated the residual levels of the mAbs in the animals by assessing the biopotency of the serum in terms of cAMP stimulatory activity in JP09 cells. The results are shown in Fig. 2. After the injection on day 0, a surge in the levels of serum KSAb1 and KSAb2 was apparent on day 1. A small reduction in the potency of the mAbs following the first injection, for example on day 6, indicated clearance (or metabolism) of the mAbs from the circulation (Fig. 2). Subsequent injections for KSAb1 and KSAb2 showed different rates of accumulation and clearance from the circulation. Thus, the rate of clearance of KSAb1 was more pronounced than that observed with KSAb2, such that by day 63 the levels of residual KSAb1 activity appeared substantially different from those for activity of KSAb2. Interestingly, the slow clearance rates of KSAb2 (IgG2a) compared to KSAb1 (IgG2b) correlated with the known half-lives of their subclasses.17 Nevertheless, despite the accumulation of KSAb2 in the serum of the mice (Fig. 1), there were no apparent differences in the hyperthyroid status of the animals when compared to KSAb1-challenged animals.
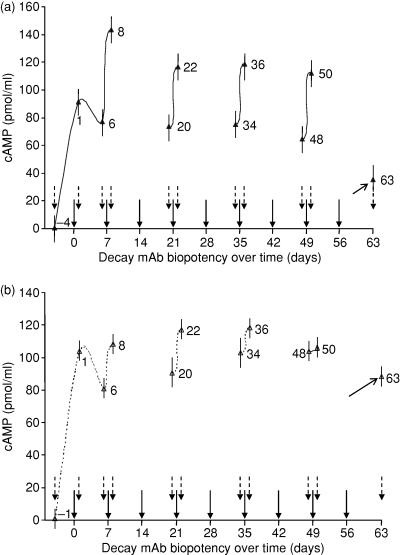
Chronic injections of (a) KSAb1 (IgG2b) and (b) KSAb2 (IgG2a) results in accumulation of the mAbs in serum according to their subclass half-lives. The residual activity of the stimulatory IgG present in the serum of the animals treated by passive transfer of the mAbs was evaluated by bioassay in JP09 cells and shown as cAMP generated in pmol/ml. Animals were injected with 10 μg KSAb1 or KSAb2 every week as indicated (solid arrows). Pre-immune samples were obtained 4 days (KSAb1) or 1 day (KSAb2) before the first injection (indicated as − 4 and − 1 day in (a) and (b), respectively). Animals were bled after the first week of injection, and then 1 day before and 1 day after the injections on alternate weeks (dotted arrows), and their TSHR stimulatory activity was assessed by cAMP production. The stimulatory activity remaining in the serum on day 63 is indicated (marked arrow on day 63). As mentioned in the legend to Fig. 1, the animals were not bled on days 14, 28, 42 and 56. The bars at each time-point indicate standard mean error.
Induction of autoimmune response and thyroid gland histology
Sustained stimulation of the thyroid gland by TSHR-stimulating antibodies may lead to the induction of an autoimmune response to thyroid antigens such as Tg and TPO, a cardinal feature of patients with active Graves' disease. We tested the day 65 serum samples for antibodies to mouse Tg by ELISA. As shown in Table 1, compared to sera from control, age-matched DR3+ Ab0/NOD mice, only one mouse (KSAb2 #3) was positive for mTg antibodies. Examination of the thyroid glands from KSAb1-challenged and KSAb2-challenged animals revealed grossly enlarged glands with vascular congestion compared to control animals (Fig. 3a,b). Histological analysis of all the glands from mAb-treated mice revealed enlarged follicles with hyperplastic papillary infolding of the epithelium (Fig. 3c,d). However, none of the thyroid sections demonstrated any significant inflammatory cell infiltrate above background.
| Anti-TSHR mAb | Mouse no. | Anti-mTg titres | Thyroiditis % thyroid infiltration | ||
|---|---|---|---|---|---|
| Serum dilution (O.D.) | |||||
| 1 : 5 | 1 : 10 | 1 : 20 | |||
| KSAb1 | 1 | 0·26 | 0·22 | 0·17 | >0–10 |
| 2 | 0·23 | 0·18 | 0·13 | >0–10 | |
| 3 | 0·19 | 0·16 | 0·15 | 0 | |
| KSAb2 | 1 | 0·28 | 0·22 | 0·17 | 0 |
| 2 | 0·26 | 0·22 | 0·17 | 0 | |
| 3 | 0·70 | 0·47 | 0·34 | 0 | |
| Control | 1 | 0·21 | 0·17 | 0·13 | 0 |
| 2 | 0·26 | 0·24 | 0·20 | >0–10 | |
| 3 | 0·15 | 0·12 | 0·09 | 0 | |
| 4 | 0·14 | 0·11 | 0·08 | >0–10 | |
| 5 | 0·16 | 0·13 | 0·10 | 0 | |
| 6 | 0·23 | 0·18 | 0·13 | 0 | |
- Male (KSAb1) and female (KSAb2) HLA-DR3 transgenic Ab0/NOD mice were injected intravenously with 10 μg anti-TSHR mAb every week for 8 weeks. On day 65, mice were killed, and serum antibody titres to mTg and thyroid pathology were determined. Control mice were unmanipulated male and female DR3+ Ab0/NOD mice.
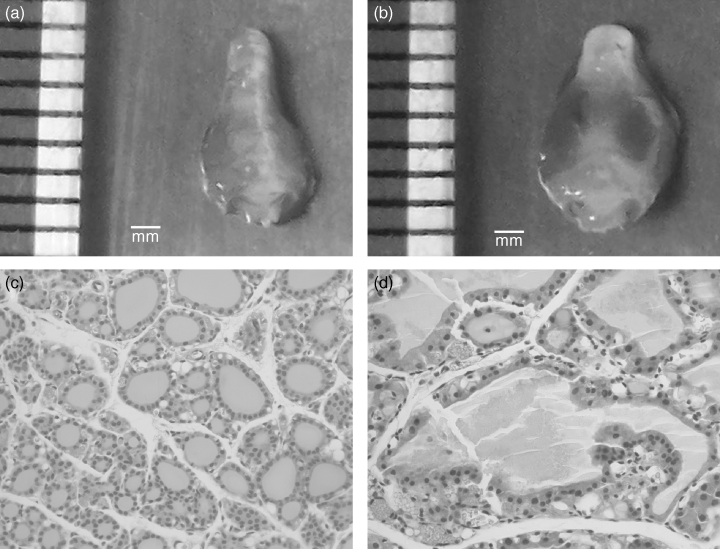
Chronic anti-TSHR mAb injections result in enlarged thyroids and hyperplastic follicles. DR3+ Ab0/NOD mice were injected intravenously with 10 μg KSAb2 mAb weekly for 8 weeks and killed 9 days later. Compared to gross (a) and microscopic (c) analysis of thyroid pathology from a normal, age-matched mouse, the KSAb2 mAb-treated mouse showed a grossly enlarged thyroid gland with increased erythema from vascular congestion (b) and dilated thyroid follicles with hyperplastic papillary infoldings of the epithelium (d). Original magnifications: (a) and (b), × 3; (c) and (d), ×400.
Discussion
The development of mAbs with strong thyroid-stimulating properties has led to studies evaluating their pathogenic mechanisms in vivo by passive transfer to naive mice.11–13 Using two mAbs with powerful agonistic properties to the TSHR, our data in animals subject to chronic stimulation of the TSHR show that the thyroid gland continues to respond by secreting thyroid hormone, similar to the clinical situation in Graves' disease patients. Measurement of the residual bioactivity of the mAbs in the serum of the injected animals showed the presence of the stimulating antibodies throughout the period of chronic exposure, confirming sustained stimulation of the gland during the course of the study.
The isotypes of KSAb1 (IgG2b) and KSAb2 (IgG2a) are different.11 Analysis of the decay of biopotency of KSAb1 and KSAb2 shows that the residual levels of KSAb2 were generally maintained at higher levels than KSAb1. This was particularly pronounced after several weeks of repeated injections, where the levels of KSAb2 appeared to plateau compared to KSAb1, where the levels continued to fluctuate. These findings are in accordance with the known half-lives of mouse IgG subclasses in serum, which have been determined to be 4–6 days (IgG2b) and 6–8 days (IgG2a).17 Thus, one explanation for the observed heterogeneity of the induced experimental Graves' disease models in inbred strains of mice7 may be related to the specific subclasses of induced antibodies with TSAb activity, where their half-lives determine the degree of hyperthyroidism present in the different animals. In patients with Graves' disease, such a scenario may not be relevant because almost all TSAb activity is known to reside in the IgG1 subclass.18 On the other hand, in Graves' patients, the clearance rate would be offset by continuing production of TSAb to maintain a persistent level of stimulation. It is not inconceivable that continuous stimulation of the TSHR could help promote the autoimmune process leading to thyroid infiltration as seen in some patients, although our attempt to provide chronic stimulation did not result in autoimmune thyroiditis in these mice.
There are a few studies that have examined the in vivo effects of chronic stimulation of TSHR with TSH or antibodies with contradictory results. Our earlier study4 and those of others19,20 have examined the chronic stimulation of mice (and rats) with osmotic pumps or regular injections of TSH, respectively. Thus, a rapid decline in serum T4 levels in response to further stimulation with TSH, suggesting desensitization of the gland, was noted in some studies4,19 but not in others.20 Similarly, stimulation of the thyroid gland in vivo with antibodies in both acute and chronic situations has also given mixed results.11–13,21 In a recent chronic exposure study13 immunocompromised mice treated with pristane to grow a hamster-derived hybridoma as ascites secreting TSAb over a 2-week period resulted in refractoriness of the gland, with serum T4 levels in the treated mice comparable to those in the preimmune controls. This in vivo protocol is significantly different from the procedure described in this report, which used a measured bolus of purified mAb for the stimulating protocol. Other studies using thyroid-stimulating mAbs in passive transfer by single injections to mimic acute stimulatory conditions have also given contradictory results.11–13 In two different studies, including our study with KSAb1 and KSAb2 mAbs, passive transfer of microgram quantities of the stimulatory mAbs into BALB/c mice led to rapid onset of hyperthyroidism.11,12 The elevated serum T4 levels were maintained following stimulation of the receptor by the TSAbs for a number of days in our study at a dose that was two to three log fold greater than the EC50 value of the stimulatory concentration of the mAbs.11,12 It can be argued that single serum T4 measurements may not accurately reflect the degree of hyperthyroidism in vivo as a variety of factors, such as T4 half-life and frequent blood drawings may affect the readings. It is for this reason that the animals were bled every alternate week following administration of the stimulating mAbs. Other studies have examined the effect of stimulating antibodies at an acute level. For example, the acute passive transfer study of the hamster TSAb mAb injected into CBA/J mice showed that serum T4 levels were elevated within 24 hr, followed subsequently by significant decrease in T4 levels.13 The results from this study indicated antibody-mediated receptor desensitization as a potential mechanism, which was also supported in part by in vitro studies. Nevertheless, the possibility remains that the differences in these acute study models may be the result of the clearance rates of the murine-derived and hamster-derived mAbs in mice or perhaps the two log difference in the Kd of the thyroid-stimulating mAbs.11–13
Acute stimulation studies by passive transfer in vivo of thyroid-stimulating mAbs have also given contrasting results on thyroid inflammation. In our recent study with single injections of KSAb1 and KSAb2 into BALB/c mice, we failed to trigger an inflammatory response to the thyroid gland11 but contrasting results were reported in another study.12 In this report, sustained stimulation of the TSHR in autoimmunity-prone, DR3 transgenic animals did not provoke an inflammatory response because the thyroid glands did not show any signs of mononuclear cell infiltration over the background level that we had observed in DR3+ Ab0/NOD mice.9 Nor did we observe antibodies to Tg except in one of the six mice. It is clear, however, that regulatory influences to suppress autoimmune responses persist despite prolonged stimulation of the thyroid. The possibility remains that more aggressive and sustained exposure to the stimulating mAbs may trigger higher levels of anti-Tg antibodies and perhaps thyroid inflammation, as is present in patients with Graves' disease.
In summary, we provide evidence for sustained in vivo stimulation of the TSHR by passive transfer of potent mAbs with thyroid-stimulating activity. The possibility exists that there may be desensitization of the gland following repeated stimulation of the receptor with the TSAbs, although we observed stimulation. We did not examine in vivo desensitization of the thyroid gland, but the overall results show that, if desensitization was present, the phenotype observed was dominated by stimulation to maintain the serum T4 levels. Since only stimulating antibodies were given, there were no blocking antibodies to provide competition, as would be present in experimentally induced models or patients with Graves' disease. Thus, thyrotoxicosis as maintained in the animals during the period of chronic exposure could help explain continuing hyperthyroidism in patients. Understanding the mechanisms of the dominance of stimulation by chronic exposure in vivo with these TSAbs can provide a greater insight into the maintenance of thyrotoxicosis in patients with Graves' disease.
Acknowledgments
We are grateful to Julie Hanson and her staff for breeding the mice and to Ann Mazurco for excellent histological preparations. This work was supported in part by DK45960 from NIDDK and St John Hospital (to Y.M.K.) and by a Training grant (to J.A.G.).




