Janus kinase 3-deficient T lymphocytes have an intrinsic defect in CCR7-mediated homing to peripheral lymphoid organs
Summary
Chemokine-mediated signalling involves the activation of a Janus kinase (Jak) pathway. We have previously shown that Jak3 mediates CCR9 and CXCR4 signalling in response to CCL25 and CXCL12 in BM progenitors and thymocytes. The lack of peripheral lymph nodes and Peyer's patches observed in Jak3–/– mice suggested a possible role of Jak3 in CCR7-mediated homing to these organs. Here, we demonstrate phosphorylation of Jak3 in peripheral lymphocytes in response CCL19 and CCL21. In addition, Jak3–/– naïve T cells and pharmacologically inhibited Jak3+/+ T lymphocytes have impaired chemotactic responses towards these ligands. Interestingly, CCR7 expression was higher in Jak3–/– thymocytes compared to their Jak3+/– littermates, indicating that the impaired migration must be caused by impaired CCR7-mediated signalling, in the absence of Jak3. In addition, adoptive transfer experiments showed that Jak3+/+ mice reconstituted with Jak3–/– green fluorescent protein (GFP)+ bone marrow progenitors had reduced T-lymphocyte homing to peripheral and mesenteric lymph nodes, compared to reconstitution with Jak3+/+ GFP+ progenitors. Furthermore, reciprocal transfer experiments indicated that Jak3–/– stromal cells were not responsible for the deficient T-cell homing. Finally, we performed direct competitive homing assays and demonstrated that Jak3–/– T lymphocytes have a clear defect in homing to peripheral and mesenteric lymph nodes, while migration to spleen was moderately impaired. Our data demonstrates that Jak3–/– T lymphocytes have an intrinsic defect in CCR7-mediated homing to peripheral lymphoid organs.
Introduction
Chemokines are a superfamily of chemotactic cytokines acting primarily through G protein-coupled, seven transmembrane domain receptors,1 which are involved in a variety of immune responses including lymphoid organogenesis and leukocyte trafficking during homeostasis and inflammation reviewed in.2,3
In homeostasis, chemokines have been implicated in homing of bone marrow (BM) progenitors to the thymus4, in migration of thymocytes through the thymic stroma5 and in homing of mature lymphocytes to secondary lymphoid organs.6 In addition, they have been implicated in T-cell development in the thymus7–9 and in lymphoid organogenesis.10–12
In addition, chemokines control both entry and exit of lymphocytes from the secondary lymph nodes (LN). During migration of lymphocytes to lymphoid organs, two receptors, CCR7 and CXCR5, have been shown to regulate lymphocyte traffic, directing and positioning lymphocytes inside the lymphoid organ to allow different cell–cell interactions.13 These receptors are expressed both by lymphocytes and the high endothelial venules (HEV).14
Most T and B cells enter LNs by passing through HEV. CCR7 receptor expressed in these cells makes them responsive to gradients of homeostatic chemokines CCL19/ELC and CCL21/SLC expressed in the HEV15 (reviewed in 16). Although CCL19 and CCL21 share the same receptor, CCR7, they are functionally different in relocating T lymphocytes from the primary lymphoid organ to secondary lymphoid organs. CCL21 is highly expressed in the venules17 (reviewed in 2) and participates in the homing of lymphocytes into the organ through the HEV; while CCL19 is expressed in the lymphatic endothelium and in interstitial cells in the LN, but can be transcytosed to the luminal surface of the HEV.15 This chemokine may also be involved in promoting encounters between T cells and antigen-bearing dendritic cells (DC) cells, allowing T-cell activation.18 In addition, CCL19, in association with CXCL13, participates in the migration of activated B cells into the T-cell zone.19 In contrast, lymphocyte entry to the spleen does not occur through HEV and it has been shown to be less dependent on chemokine-mediated signals, as demonstrated by the lack of sensitivity to pertussis toxin.16,20,21
Naïve T cells also express CCR9 and CXCR4 receptors, and respond to CCL25 and CXCL12 ligands, respectively. It is not clear whether these receptors may have a key role in the entry of T lymphocytes into the secondary lymph organs. Instead, CCR9 has an important role in T-cell homing to intestinal mucosa.22
The role of CCR7 and its ligands in homing to lymphoid organs has been demonstrated by the use of knock out mice. CCR7-deficient mice show defects in the migration of naïve T cells into the T-cell zones of lymphoid follicles, and have affected their cellular distribution.23 In these mice, DC migration to the T-cell zones is also impaired24 resulting in a reduction of primary T-cell responses, as demonstrated by analysis of delayed-type hypersensitivity responses.23,25
On the other hand, a mouse naturally deficient in CCL19 and CCL21-Ser (plt/plt mice (‘paucity’ of LN T cells)), has severe defects in homing naive T cells to LNs and Peyer's patches (PP).26,27 In addition, it displays a defect in the organization of T cells within the T-cell zone of lymphoid follicles and impaired migration of activated DC into T-cell zones. Nevertheless, these mice are capable of mounting T-cell immune responses that, presumably, indicates that these ligands may be redundant for these functions (reviewed in 28).
CCR7 also has a role in defining T-cell subsets with different homing capabilities. Currently, two cell types have been described displaying different features: short-lived effector T cells and memory T cells. It has been suggested that T cells leaving the LN are either central memory T cells (TCM) or effector memory T cells (TEM).29 TCM cells show preferential homing to lymphoid organs and BM and express CCR7, CXCR4, as well as l-selectin and interleukin (IL)-7Rα. In contrast, TEM do not express CCR7 and have low levels of l-selectin. These cells do not recirculate to LN but remain in periphery or migrate to non-lymphoid organs.18 Recently, it was proposed that CCR7, like the sphingosine-1-phosphate receptor (S1P1), participates in the mechanism controlling the egress and re-entry of T cells from the periphery into afferent lymphatics and LN.30–32
We and others have shown that the Janus kinase (Jak) pathway is involved in chemokine receptor mediated signalling both in cell lines and primary cells33–35 (reviewed in 36). In particular, Jak3, unlike the rest of the kinases from the Janus family, is mainly expressed by haematopoietic cells. Furthermore, Jak3 is associated to the common γ chain (γc), shared by the cytokine receptors to IL-2, 5, 7, 9, and 15. Upon ligand binding, Jak3 phosphorylates the Stat proteins, which then become dimerized and enter into the nucleus, where they act as activators of transcription of specific sequences (reviewed in 37).
Jak3–/– mice38–40 showed a defect in very early T-cell development41 that results in a severely hypoplastic thymus, although almost nearly normal numbers of T cells are generated and can reach the periphery. However, Jak3 seems to be crucial for T-cell homeostasis, because T lymphocytes have increased susceptibility to apoptosis and show an activated/anergic phenotype.42 Interestingly, despite the accumulation of T and B lymphocytes in the spleen of Jak3–/–, these mice show absence of peripheral LN and PP, suggesting a role of Jak3 in homing to these peripheral lymphoid organs. Interestingly, the phenotype of these mice resembles to that shown by CCR7-deficient mice and the plt/plt mice.23,27
We have previously shown that Jak3 is involved in the signalling pathway of CCR9 and CXCR4 in murine thymocytes. By analysing the phenotype of the Jak3–/– mouse, we also identified an impaired migration of BM progenitors and thymocytes towards thymus-expressed chemokines CCL25 and CXCL12,34 indicating that Jak3–/– cells may have intrinsic defects in chemokine-mediated homing to lymphoid organs. Now, we have further analysed the role of the Jak3 signalling pathway in CCR7-mediated responses in peripheral lymphoid organs. Here, we demonstrate that Jak3 is phosphorylated in response to CCL19 and CCL21 in LN cells, and that both pharmacological inhibition of Jak3 or the use of Jak3–/– naïve T lymphocytes, results in impaired chemotactic responses towards these ligands, indicating that Jak3 is required for CCR7-mediated migration. Furthermore, adaptive transfer experiments and direct competitive homing assays demonstrate that Jak3-deficient T lymphocytes have intrinsic defects in homing to peripheral lymphoid organs.
Materials and methods
Cytokines
Mouse recombinant IL-7 (rIL-7) and chemokines SLC/CCL21 ELC/CCL19 were obtained from Peprotech (Piscataway, NJ).
Antibodies
Antiphosphotyrosine monoclonal antibody (4G10) was obtained from Upstate Biotechnology (Lake Placid, NY), Polyclonal goat anti-Jak3 antibody (sc-1078, Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase-labelled anti-mouse and anti-rabbit immunoglobulin antibodies (Amersham Pharmacia Biotech, Inc, NJ) were used as secondary reagents. ELC-Fc fusion protein was kindly provided by Dr U. von Andrian (Harvard Medical School, Boston, MA). Fluoroscein isothiocyanate (FITC)-labelled polyclonal Anti-human immunoglobulin G (IgG) was obtained from Zymed (Invitrogen, Carlsbad, CA).
Mice
Jak3-deficient mice (Jak3–/–) (C57BL/6-JAK3tm11jb) and C57BL/6 mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and kept in pathogen free conditions in our animal facility. Six to 10-week-old homozygous Jak3–/–mice or Jak3 heterozygous littermates (Jak3+/–) were used in our experiments. In some cases C57BL/6 (Jak3+/+) age-matched mice, were also used. Actin-green fluorescent protein (GFP) transgenic mice on the C57BL/6 background were obtained from Dr M. Okawa43 (Genome Information Research Center, Osaka University, Japan). These mice were crossed with Jak3–/– mice for a few generations in order to obtain actin-GFP transgenic mice on the Jak3–/– background.
Lymphocyte preparation
Total LN cells were obtained from peripheral LNs (maxillary, axillary, inguinal), mesenteric LNs, spleen and thymi, were prepared after mechanical disaggregation, using a 50 µm nylon mesh. They were washed twice with Hank's balanced salt solution buffer (HBSS) before use for chemotaxis and phosphorylation analysis. For spleen and BM cells, cell suspensions were initially incubated with red cell lysis solution followed by two washes.
Chemotaxis assays
Cell migration assays were performed using a 48-well modified Boyden microchemotaxis chamber, as described before.34 Briefly, increasing concentrations (10–1000 ng/ml) of the chemokines were tested in the lower wells of the chamber. 5 × 106 cells from Jak3–/–, Jak3+/– or Jak3+/+ mice were resuspended in chemotaxis medium (HBSS, bovine serum albumin 0·05%). First, cells were fluorescently labelled with calcein-AM (1 µg/ml, Molecular Probes, Eugene OR) for 15 min at 37°, washed twice with HBSS, and resuspended at 5 × 106 cells/ml in chemotaxis medium and allowed to migrate through fibronectin-coated pore polycarbonate membranes (Neuroprobe, Gaithersburg, MD). After 2 hr, migrated cells were quantified using a Molecular Imager (Bio-Rad, Hercules, CA). Viability of cells was assessed, before the chemotaxis assay by trypan blue exclusion staining.
For inhibition experiments, LN cells from Jak3+/+ C57BL/6 mice were resuspended in medium (8 × 106 cells/ml in RPMI-1640, 10% fetal calf serum) containing specific Jak3 inhibitor, WH1-P131 (80 µg/ml, Calbiochem, San Diego, CA), Pertussis toxin (PTX, 200 ng/ml, Sigma Chemicals, St Louis, MO), or control buffer (dimethyl sulphoxide (DMSO), Sigma Chemicals). Cells were incubated for 2 hr at 37°. Under these conditions, neither Jak3 inhibitor nor DMSO treatment affected cell viability. Cells were then washed twice with HBSS and tested in chemotaxis assays, as before.
Analysis of Jak3 tyrosine phosphorylation. LN cells were isolated as described and washed once with HBSS. 3 × 107 cells were resuspended in 300 µl of the same medium containing varying concentration of chemokines (50–100 ng/ml) and incubated at 37° for up to 15 min. As a positive control, cells were incubated with rIL-7 (50 ng/ml) for 5 min at 37°. Stimulations were terminated and total lysates were prepared in 1% Triton-X-100 lysis buffer containing phosphatase and protease inhibitors, as previously described.34 Supernatants were first incubated with goat anti-Jak3 antibody and then immunoprecipitated with recombinant protein-G sepharose (Invitrogen, Carlsbad, CA). Immunoprecipitated and total lysates, were resolved on a 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to Immobilon-P membranes (Millipore, Bedford, MA). For phosphotyrosine44 analysis, Western blots were performed using antiphosphotyrosine antibody (4G10) followed by horseradish peroxidase-labelled anti-mouse immunoglobulin (Amersham Pharmacia, UK). Membranes were developed by chemiluminiscence (ECL, Amersham Pharmacia). As a control for protein loading, blots were stripped and reprobed with a rabbit anti-Jak3 antibody. Densitometric analysis was performed on the blots using an FX Imager (Bio-Rad, Hercules, CA) and phosphorylation was calculated as the OD ratio: PY/Jak3.
Analysis of CCR7 expression
Flow cytometry. To analyse CCR7 expression on T lymphocytes a CCL19-Fc recombinant fusion protein was used, as previously described.45 Briefly, total thymocytes or lymphocytes were stained with anti-CD4 antigen-presenting cell (APC) and anti-CD8 cychrome, both from Pharmingen (Becton Dickinson (BD), Mountain View, CA), and then incubated with CCL19-Fc supernatant for 30 min at 37°, followed by FITC-labelled anti-human IgG polyclonal antibody (Zymed, San Francisco, CA). Cells were acquired in a FACScalibur® flow cytometer (BD) and data were analysed using Cell Quest Pro® software (BD). For competitive transfer assays, splenocytes were also analysed for CCR7 expression, using anti-CD4 APC and anti-CD8 biotin followed by streptavidin APC cy7 (Pharmingen, BD). In this case, cells were acquired in a fluorescence-activated cell sorting (FACS) Aria (BD) and data was analysed using the FACS Diva® software. For statistical analysis of the differences, FlowJo® software (BD) was used and Chi-squared and Kolmogorow–Smirnoff indexes were calculated. Histograms were considered significantly different when Tx > 4.
Quantitative reverse transcription–polymerase chain reaction (RT–PCR). Total RNA was isolated from C57BL/6 or Jak3–/– thymocytes using RNA STAT-60 (Iso-Tex, Friendswood, TX) as described34 and according to the manufacturer's protocol. cDNA was synthesized from DNAse I-treated total RNA using Superscript II reverse transcriptase (Invitrogen) and oligo dT. Real-time PCR was performed by amplifying one-tenth of total cDNA yield, with SYBR Green PCR Core Kit (Applied Biosystems, Foster City, CA) on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems), as previously described.34 Primers used in the PCR amplification were the following:
CCR7 sense: 5′-ACAGCGGCCTCCAGAAGAACAGCGG-3′; CCR7 antisense: 5′-TGACGTCATAGGCAATGTTGAGCTG-3′, CXCR4 sense: 5′-AGAAGCTAAGGAGCATGACGGA-3′, CXCR4 antisense: 5′-ACTGCCTTTTCAGCCAGCAGTT-3′, CCR9 sense: 5′-CAGGCAGCTGCAGTGGTCCTCTCCC-3, CCR9 antisense: 5-′TGTGCAAGGCTGGGCTGTCTTTGC-3′, hypoxanthine-guanine phosphoribosyltransferase (HPRT) sense: 5′-CCTGCTGGATTACATTAAGGCACTG-3′, HPRT antisense: 5′-GTCAGGGGCATATCCAACAACAAAC-3′.
Statistical analysis. For analysis of RT–PCR data, the program Qgene (available at http://gene-quantification.com/download.htmlqgene) was used.44 Analysis of the data was performed as previously described.34 An unpaired two-tailed Student's t-test was performed to determine statistical significance. P-values <0·05 were considered as significant.
In vivo homing assays. BM cells (5 × 106) from Jak3–/– GFP+ or Jak3+/+ GFP+ BM cells were injected intravenously into irradiated (900 rad) C57BL/6 recipient mice. Reconstituted mice were analysed 6–8 weeks after transfer. Peripheral and mesenteric LN, spleen, BM and thymi were harvested from reconstituted mice, cell suspensions prepared and cells stained with anti-CD4, anti-CD8 and anti-CD19 antibodies to assess the cellular composition of these lymphoid organs. Four-colour staining was analysed using the FACScalibur flow Cytometer (BD).
Competitive lymphocyte homing assay. Jak3+/– and Jak3–/– splenocytes were differentially labelled using orange (4·5 µm) and green (0·3 µm) cell trackers, respectively (Molecular Probes). Briefly, cells were incubated with cell tracker in PBS for 30 min at 37° in the dark. Then, they were washed twice and incubated with PBS in the dark for an extra 30 min at 37°. After a final wash cells were resuspended at 5 × 107 cell/ml in PBS then mixed 1 : 1 and injected intravenously (a total of 10 million) into C57BL/6 mice. Eighteen hr later, peripheral/mesenteric LN and spleens were harvested and percentages of green and orange cells were determined. Cell suspensions were also stained with CD4-APC or CD19-APC and CD8-biotin antibodies, followed by streptavidin APCcy7 (as described above). Phenotypic analysis of the migrated cells was performed after ‘gating’ the green and orange subpopulations, respectively. Five-colour staining was analysed using the FACS Aria flow Cytometer (BD).
Results
Jak3 is required for CCL19 and CCL21-induced migration
Response to CCL19 and CCL21 has been shown to be crucial for homing of lymphocytes to peripheral lymphoid organs. Jak3 has been involved in chemokine mediated signalling. We hypothesized that the absence of peripheral LN and PP in Jak3–/– mice might be caused by the inability of naive Jak3–/– lymphocytes to respond to chemokines expressed in these lymphoid organs, in the absence of Jak3. In an attempt to investigate the role of Jak3 signalling in CCR7 dependent migration, we used first a Jak3 specific inhibitor using conventional chemotaxis assays. Figure 1(a) shows the chemotaxis response of total wild type LN cells towards CCL21, that were pretreated with the specific Jak3 inhibitor (WHI-P131, 80 µg/ml) or with pertussis toxin (PTX, 200 ng/ml). Chemotactic response of untreated LN cells showed a peak around 100 ng/ml, while cells treated with specific Jak3 inhibitor or PTX (which inhibits Gαi proteins), completely abrogated the migration response to CCL21 (Fig. 1a). To further confirm a migratory defect of naïve T lymphocytes in the absence of Jak3, we also performed chemotaxis assays using total thymocytes obtained from 4–6-week-old Jak3+/+ or Jak3–/– mice towards CCR7 ligands. As shown, in Fig. 1(b), Jak3–/– thymocytes, were unable to migrate towards CCL19 or CCL21, similarly to thymocytes pretreated with PTX. In contrast, thymocytes from Jak3+/– control littermates, showed a typical chemotactic response towards these chemokines showing a peak of response at 500 ng/ml and 100 ng/ml, respectively. As previously reported, Jak3 deficiency does not affect chemotactic responses towards other inflammatory chemokines, such as CCL4, CCL5 and CCL11.34
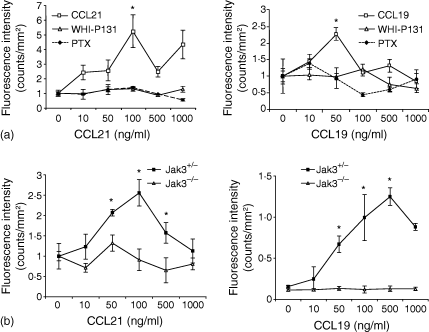
Chemotactic responses towards CCL19 and CCL21 are impaired in the absence of Jak3. (a) Pharmacological inhibition of Jak3 inhibits chemotactic responses of lymph node cells towards CCL19 and CCL21. Cells were prestained with calcein-AM and allowed to migrate, in a chemotaxis assay, in the presence 0–1000 ng/ml chemokines, as described in Materials and Methods. Data shows a representative experiment (n = 4) and migration index is calculated as fluorescence intensity and is expressed as counts/mm2. (b) Chemotaxis assay of total thymocytes from C57BL/6 mice or Jak3–/– mice. Jak3–/– thymocytes show impaired chemotactic responses towards CCL19 and CCL21. Data are from a representative experiment (n = 4) showing the mean of triplicate wells ± SE (standard error). Statistical analysis was performed using the unpaired two-tailed Student's t-test. Asterisks indicate a P-value of <0·05.
Jak3–/– thymocytes express increased levels of CCR7
Expression of CCR7 is regulated during thymocyte development (reviewed in 9). Positively selected thymocytes, increase CCR7 expression7 allowing mature CD4 and CD8 single positive (SP) thymocytes to migrate towards the medulla, where CCL19 and CCL21 are highly expressed. Mature thymocytes can then exit the thymus through the venules present in the medullary region and home to secondary lymphoid organs6 using a mechanism that involves both CCR7 and sphingosine 1 phosphate receptor (S1P1) signalling.32 Because Jak3 thymocytes failed to migrate towards CCL19 and CCL21, we needed to assess the levels of CCR7 expression on Jak3–/– thymocytes to exclude the possibility that the lack of migration was caused by reduced levels of this receptor. Real time PCR analysis was performed from Jak3+/+ or Jak3–/– adult thymi. As shown in Fig. 2(a), we detected increased levels of CCR7 in Jak3–/– mice when compared with wild type mice. In contrast, CCR9 and CXCR4 levels in Jak3–/– thymocytes appeared to be not significantly different from those in wild type mice, confirming data previously reported.34 Although unexpected, this result might indicate a feed-back mechanism to compensate for an impaired CCR7 mediated signalling, which may be in agreement with a previous report demonstrating that Jak3 deficient cells, show increased levels of γc.46

Expression of CCR7 in Jak3–/– thymocytes. (a) Real time RT–PCR was performed on cDNA obtained from total RNA isolated from 6-week-old Jak3–/– and C57BL/6 mice (pools of six mice). Murine CCR7, CCR9, CXCR4 and hypoxanthine-guanine phosphoribosyltransferase (HPRT)-specific primers were used. Data shows a representative experiment (n = 6) performed in triplicate. Data (mean ± standard deviation) are plotted as relative chemokine receptor (CKR) mRNA expression, calculated by normalizing the values obtained with the CCRs primers with their respective HPRT controls, as described in Materials and Methods. Analysis of significance was performed using the program Qgene, as previously described.44 Asterisks indicate P < 0·01. (b) Surface expression of CCR7. Wild type or Jak3–/– thymocytes from 6–8-week-old mice were stained with anti-CD4-APC and anti-CD8-cychrome monoclonal antibodies, in combination with soluble CCL19-Fc fusion protein followed by FITC anti-human immunoglobulin. Analysis of the four major thymocyte subpopulations (DN, DP, CD4 and CD8) shows an increase in MFI in all subpopulations obtained from Jak3–/– mice compared to wild type mice. Data shows a representative experiment (n = 8). Green line: secondary reagent alone; blue line: Jak3+/+ red line: Jak3–/–.
Next, to assess CCR7 expression at the protein level, we performed cell surface staining on the four major thymocyte subpopulations: CD4– CD8– (DN) CD4+ CD8+ (DP), CD4+ (CD4 SP) and CD8+ (CD8 SP), using a CCL19-Fc fusion protein45 in combination with anti-CD4 and anti-CD8 monoclonal antibodies. It has been previously reported that, similar to other chemokine receptors expressed by thymocytes, expression of CCR7 is developmentally regulated.8 CCR7 is transiently expressed by a small subpopulation of immature thymocytes (DN1–DN2), decreases in DP thymocytes and is re-expressed after thymocytes have been positively selected and differentiate into mature CD4 and CD8 SPs47,48 (reviewed in 49). As shown in Fig. 2(b), thymocytes from Jak 3–/– mice expressed increased levels of CCR7, as detected by increased mean fluorescent intensity (MFI), confirming the results obtained with real time PCR analysis. Subpopulation analysis indicated that all thymocyte subpopulations showed increased levels of CCR7, although DNs usually showed the highest increase. To further investigate the origin of the cells expressing those high levels of CCR7 within the DN subpopulation, we performed staining with anti-CD44 and anti-CD25 to identify DN1 (CD44+ CD25–), DN2 (CD44+ CD25+), DN3 (CD44– CD25+) and DN4 (CD44– CD25–) subpopulations. Interestingly, CCR7 expression appeared to be restricted to the DN3 subpopulation (not shown). Therefore, the absence of Jak3 appears to modify the normal expression pattern of CCR7 in thymocyte subpopulations at different stages of T-cell development.
Jak3 becomes tyrosine phosphorylated following CCL19 and CCL21 stimulation. Confirmation that Jak3 is involved in the signalling pathway of CCR7 in peripheral naive T cells resulted from phosphorylation experiments using peripheral LN cells from 6 to 8 weeks old C57BL/6 mice. We stimulated the cells with CCL19 and CCL21 (100–200 ng/ml) for 15 s and up to 15 min or with IL-7 (50 ng/ml) for 5 min, as a positive control. In Fig. 3 a representative experiment shows that Jak3 becomes tyrosine phosphorylated as early as 15 s after stimulation with 100 or 200 ng/ml of CCL21 and CCL19. Time course experiments showed that the peak of phosphorylation was between 15 and 45 s and it decreased after 1 min of stimulation (not shown). To assess the levels of Jak3 in LN cells, gels were stripped and reprobed with antibody specific for Jak3. The level of tyrosine phosphorylation was assessed by calculating the OD ratio phosphotyrosine/Jak3, after densitometric analysis of the blots, showing a moderate but significant phosphorylation of Jak3 in response to CCL19 and CCL21 (Fig. 3, bottom panel).
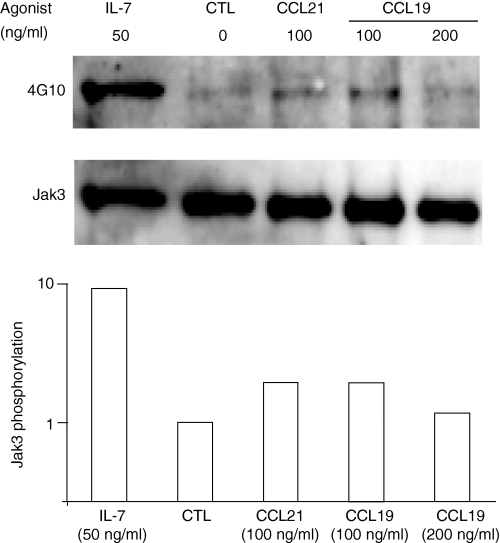
Jak3 becomes tyrosine phosphorylated in murine T lymphocytes in after stimulation with CCL19 and CCL21. Lymph node cells (40 × 106) from 6–8-week-old C57BL/6 mice were stimulated for 15 s, with 100–200 ng/ml of CCL19 and CCL21. Jak3 phosphorylation was analysed after immunoprecipitation and blotting with antiphosphotyrosine antibody, 4G10 (top panel). Cell lysates were separated by SDS–PAGE. As a positive control, T lymphocytes were stimulated with recombinant IL-7 (50 ng/ml) for 5 min. After stripping, the membrane was reprobed with an anti-Jak3 polyclonal antibody (bottom panel). Densitometric analysis was performed on phosphotyrosine and Jak3 blots, and the phosphorylation level of Jak3 was calculated as the ratio of the densitometric intensities of the antiphosphotyrosine signal to the anti-Jak3 signal of the respective bands. The data were normalized to the values obtained with lymph node cells treated in media alone. A representative experiment is shown (n = 3). CTL: Control media alone.
In vivo homing of Jak3-deficient lymphocytes is impaired
CCR7 mediated signalling is required for homing of T lymphocytes to peripheral lymph nodes and Peyer's patches (reviewed in 50). To investigate whether that the lack of homing to these organs in Jak3 knock out mice may be caused by an intrinsic migration defect of Jak3–/– lymphocytes, we crossed Jak3–/– mice with mice expressing GFP under the actin promoter (actin-GFP transgenic mice). We performed adoptive transfer experiments in which we reconstituted irradiated C57BL/6 mice with either Jak3–/– GFP+ or Jak3+/+ GFP+ BM progenitors. Thus, in these chimeric mice, lymphocyte development and homing would occur in the presence of a normal stroma, while the Jak3 deficiency would be restricted to the haematopoietic cell compartment. Mice were analysed 6–8 weeks after transfer and determination of total cell numbers and the percentage of GFP+ cells in each lymphoid organ was assessed by fluorescence-activated cell sorting (FACS) analysis. As shown in Fig. 4(a), reconstitution of BM was achieved to the same extent when either Jak3–/– GFP+ or Jak3+/+ GFP+ BM progenitors were used in the transfer (∼40–60%). As expected, the percentage of GFP+ thymocytes was always lower in Jak3–/– GFP+ BM transfers, indicating that Jak3–/– T-cell precursors have an intrinsic deficiency when competing with residual Jak3+/+ progenitors once they reach the thymus, as has been previously described. However, analysis of peripheral lymphoid organs showed that while Jak3+/+ BM transfers led to efficient homing of Jak3+/+ GFP+ to peripheral and mesenteric LN (approximately 60–80% of lymphocytes were GFP+), only a small percentage of Jak–/– GFP+ cells reached the peripheral and mesenteric LN (∼10–15%; Fig. 4a), and a similar decrease was seen in PP (not shown). A summary of BM transfer experiments is presented in Fig. 4(b) (n = 5). Subpopulation analysis of the cells that reached the peripheral (Fig. 4c) and mesenteric LNs (not shown) indicated that both CD4+ and CD8+ Jak3–/– T lymphocytes have similar impaired migration toward these lymphoid organs. In contrast, homing to the spleen was less severely impaired (1/2–1/3 of that obtained with Jak3+/+ BM progenitors), indicating that entry of lymphocytes to the spleen is less dependent on CCR7-mediated signals reviewed in.21 The differential reconstitution pattern observed in the chimeric mice resembles the homing pattern observed in Jak3–/– mice, in which lymphocyte homing deficiency affects preferentially peripheral LN and PP, while homing to the spleen appears to be less affected.40 These results suggested that the deficiency in T-cell homing was caused by defects in the haematopoietic cell compartment.
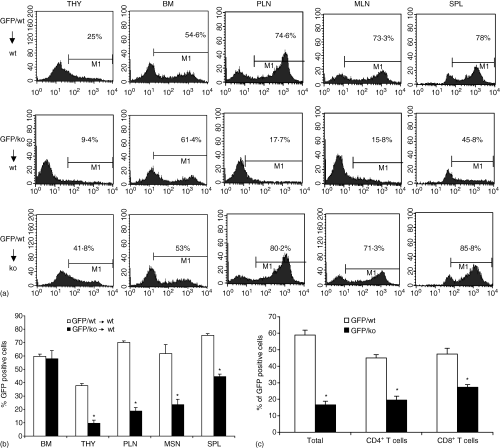
BM adoptive transfers show impaired T-cell homing to peripheral lymph nodes. BM cells (5 × 106) from Jak3+/+ GFP+ or Jak3–/– GFP+ mice were transferred intravenously into irradiated C57BL/6 mice. Six to 8 weeks after transfer, peripheral lymphoid organs were harvested and analysed for the presence of GFP+ cells. (a) Percentages of GFP+ cells from bone marrow, thymus, peripheral, mesenteric lymph nodes and spleen are indicated. Data shows a representative experiment (n = 5). Bottom panel, reciprocal BM transfer experiments using GFP+ Jak3+/+ BM progenitors into irradiated Jak3–/– mice. (b) Summary of BM transfer experiments. Bars represent percentages of GFP+ cells in each organ and are expressed as mean values ± standard error (n = 10). (c) Subpopulation analysis of the T lymphocytes that migrated to the lymph nodes of recipient C57BL/6 mice showed that both CD4 and CD8 T lymphocytes had a significant reduction in homing to peripheral lymph nodes. Representative experiment of a total of three (n = 5 mice, *P < 0·05).
IL-7 receptor signalling has been shown to play a crucial role in lymphoid organogenesis (reviewed in 12). In order to investigate whether the Jak3 deficient stroma was also responsible for the deficient T cell homing, we performed reciprocal experiments, transferring Jak3+/+ GFP+ BM progenitors into irradiated Jak3–/– mice. As shown in Fig. 4(a) (lower panels), Jak 3+/+ GFP+ lymphocytes were able to home efficiently to peripheral, mesenteric LNs and spleen of Jak3–/– recipient mice. The percentages of GFP+ cells were above 70% in peripheral and mesenteric LN and over 80% in spleen. In contrast, PP were not detectable in the Jak3 reconstituted mice (data not shown), indicating that this lymphoid tissue may have more specific requirements.12,51,52 Interestingly, reconstitution of thymus was higher than in experiments using wild type BM progenitors transferred into wild type mice (Fig. 4a). This indicates that Jak3+/+ progenitors have an advantage when competing with residual Jak3–/– haematopoietic cells. Therefore, the defect in lymphocyte homing to peripheral LN observed in Jak3–/– mice cannot be explained by a defect in the stromal cell compartment but rather by an intrinsic defect in the haematopoietic cell compartment.
Direct competitive homing assays demonstrate a defect in Jak3–/– lymphocyte homing
To further prove that Jak3–/– T lymphocytes have a homing defect, we performed direct competitive transfer experiments. For these assays, equal amounts of wild type and Jak3–/– splenocytes were differentially labelled (Jak3+/+ with orange and Jak3–/– with green cell trackers, respectively) and injected intravenously in 1 : 1 ratio (a total of 10 million) into non-irradiated C57BL/6 mice. Eighteen hr later, mice were killed and peripheral lymphoid organs removed and analysed by FACS for the presence of green and orange-labelled cells. Figure 5(a) shows the results of a representative experiment demonstrating that homing of Jak3–/– lymphocytes to peripheral and mesenteric LNs was greatly impaired (50–70-fold decrease). On the other hand, homing to the spleen was less severely impaired, showing around six- to eightfold decrease when compared to that of Jak3+/+ lymphocytes. A summary of all competitive homing experiments is shown in Fig. 5(b) (n = 13). In some experiments, dyes were swapped obtaining a similar decrease in homing when comparing percentages of Jak 3–/– (in orange) versus Jak 3+/+ (green) splenocytes (not shown). The decrease in lymphocyte homing was not caused by reduced levels of CCR7 in Jak3–/– splenocytes. As shown in Fig. 5(c), analysis of splenic T cells previous to injection, showed that both CD4+ and CD8+ T lymphocytes showed normal levels of CCR7 on their surface, indicating that the defect in homing to the LN must be the result of defective CCR7-mediated signalling in the absence of Jak3.

In vivo competitive homing assays. Jak3 deficient T lymphocytes show impaired homing to peripheral lymphoid organs. Splenocytes from C57BL/6 or Jak3–/– mice were differentially labelled with orange (Jak3+/–) or green (Jak3–/–) cell trackers. A mixture of Jak3+/+ and Jak3–/– cells (1 : 1 ratio) was injected intravenously and 18 hr later, the ratio of orange/green cells was calculated after analysis of lymphocytes obtained from peripheral, mesenteric lymph nodes and spleen. (a) A representative experiment, indicating the percentages of orange and green that reached the lymphoid organs. Homing of Jak3–/– lymphocytes to peripheral and mesenteric lymph nodes showed a 50–70-fold decrease when compared with Jak3+/+ lymphocytes, while homing of Jak3–/– splenocytes to spleen was reduced six- to eightfold, when compared with Jak3+/– splenocytes. (b) Summary of competitive homing experiments shows that there are significant differences in homing of Jak3+/– versus Jak3–/– splenocytes, towards all secondary lymphoid organs (n = 13, P < 0·001). (c) Analysis of CCR7 expression on Jak3+/– or Jak3–/– splenocytes previously to the competitive transfer. CCL19-Fc staining shows normal levels of CCR7 on all Jak3–/– splenic subpopulations. Data shows a representative experiment (n = 6). Green line: secondary reagent alone; purple line: Jak3+/–; red line: Jak3–/–.
Furthermore, in some experiments, subpopulation analysis of the migrated cells was performed to assess whether the homing deficiency was preferentially affecting a specific T-cell subset. As shown in Fig. 6(a), previous to injection, staining of splenocytes from donor Jak3–/– or Jak3+/+ mice, showed a reduction in the percentage of CD8+ T lymphocytes in spleen of Jak3–/– compared to Jak3+/– spleens, as had been previously reported. Eighteen hr after transfer, when we analysed the subpopulations within the orange (Jak3+/–) and green cells (Jak3–/–) that reached the peripheral LN (Fig. 6b), mesenteric LN (Fig. 6c) and spleen (Fig. 6d), we detected an increased percentage (double) of CD4 positive cells in the Jak3–/– lymphocytes that home to the peripheral and mesenteric LN. However, this increase did not reflect a preferential homing of CD4+ Jak3–/– T lymphocytes, because a similar increase was observed in the homing of splenocytes obtained from Jak3+/+ mice. Rather, this increase may suggest a preferential entry of CD4+ T lymphocytes into lymphoid organs at early time points. In contrast, percentages of CD4+ and CD8+ T cells that reached the spleen, were identical to the percentages detected in Jak3+/+ splenocytes previously to injection (compare with Fig. 6a), while Jak3–/– lymphocytes homing to spleen also showed an enrichment of CD4+ T cells and a decrease in the percentage of B cells (Fig. 6d). Figure 6(e) shows the summarized data of these competitive homing assays indicating the percentages of CD4+ and CD8+ splenocytes, previous to injection and after homing to peripheral lymph nodes and spleen (n = 7, P < 0·005).

Subpopulation analysis from in vivo competitive homing assays. (a) Characterization of splenocytes from Jak3+/– and Jak3–/– mice, previous to the competitive transfer. A decrease in CD8+ T lymphocytes is detected in spleen of Jak3–/– mice (b–d). FACS analysis of migrated cells indicating the percentage of orange (wt) and green (ko) cells recovered from peripheral, mesenteric LNs and spleen, 18 hr after transfer. A representative experiment is shown. Subpopulation analysis of peripheral lymphoid organs showed increased percentages of CD4+ T cells both in Jak3+/– and Jak3–/– lymphocytes that reached the peripheral (b) and mesenteric (c) lymph nodes, while a reduction of B cells was observed. (d) Subpopulation analysis in the spleen. (e) Summarized data of competitive homing assays showing the percentages of CD4+ and CD8+ splenocytes, previous to injection and after homing to peripheral lymph nodes and spleen (n = 7, P < 0·005).
Discussion
Homing of naïve T lymphocytes to peripheral LN was shown to depend on CCR7-mediated responses towards CCL21 and CCL19. CCL21 is highly expressed on HEV, while CCL19 is expressed by stromal cells surrounding HEV, but can be transcytosed to the luminal surface of the HEV, enabling efficient T-cell homing to peripheral LN and PP. Entry of lymphocytes through HEV has been shown to involve chemokine-mediated integrin activation, allowing circulating leucocytes to undergo firm adhesion to the endothelium and cell arrest. Initial experiments showed that a PTX-sensitive signalling pathway was involved in this process.53 However, the requirement of other signalling proteins has been less studied. The involvement of a tyrosine kinase-dependent pathway was proposed by Stein et al. using a Jak inhibitor (AG490) to investigate the adhesion of lymphocytes to HEVs, using intravital microscopy.54 The authors demonstrated that CCR7-dependent integrin activation and firm adhesion to the endothelium was dependent of a Jak pathway. However, using this approach they were unable to investigate the role of these kinases using in vivo homing assays.
We have used Jak3-deficient mice, which lack peripheral LN and PP, and investigated the role of Jak3 in CCR7-mediated homing to peripheral lymphoid organs. Because no peripheral LN are present in these mice, we used thymocytes to evaluate the response of naïve T cells towards CCL21 and CCL29 and showed that Jak3–/– thymocytes were unable to migrate towards these chemokines. Presumably, deficient migration of Jak3–/– thymocytes towards these chemokines, which are highly expressed in the thymic medulla47 (reviewed in 9), might result in differences in cell localization, as has been described in CCR7-47,48 and CCR9-deficient22,55 mice. In this context, architectural abnormalities have previously been shown in thymi of Jak3–/– mice.38 Recent data has shown that a DN1–DN2 subpopulation also expresses CCR7 transiently in the thymus. The functional implication of this expression was assessed in CCR7–/– mice, showing an accumulation of immature DN thymocytes in the cortical medullary junction.48 This reflected the inability of DN thymocytes to exit the medullary region and migrate towards the subcapsular region, where most DN thymocytes are located in wild type mice. Interestingly, analysis of CCR7 in Jak3–/– thymi demonstrated that all thymocyte subpopulations expressed higher levels of CCR7, indicating that the impaired chemotactic responses observed cannot be explained by reduced levels of chemokine receptor on the surface of Jak3–/– lymphocytes, but rather by impaired CCR7-mediated signalling. In this context, our results confirmed that Jak3 becomes tyrosine phosphorylated, as early as 15 s, in peripheral lymphocytes in response to CCL19 and CCL21.
To investigate the relevance of this impaired migration we performed adoptive transfer experiments in vivo. In these experiments we used Jak3–/– GFP + or Jak3+/+ GFP+ BM progenitors to reconstitute irradiated wild type mice. These chimeric mice allowed Jak3–/– or Jak3+/– haematopoietic cells to develop and migrate in the presence of a normal stromal cell compartment. Our results showed that Jak3–/– T lymphocytes have an intrinsic defect in homing to peripheral, mesenteric LN and PP, while homing to the spleen was less impaired. These results confirm previous data indicating that entry to the spleen is less dependent on chemokine-mediated signals.20
Although we have previously demonstrated that Jak3 is also involved in CCR9- and CXCR4-mediated signalling in naïve T cells34 it has been shown that CCR7 plays the main role in T lymphocyte homing to the peripheral LN.16 On the other hand, it has been shown that CXCL12 is able to mediate CCR7 independent homing to peripheral LN only of memory but not in naïve T lymphocytes.56 Finally, analysis of CCR9-deficient mice has shown an impaired homing of intraepithelial CD8 αβ T lymphocytes in the small intestine but normal T-cell homing to peripheral LN.57 Our results demonstrate that Jak3 participates in the signalling pathway of CCR7 in naïve T lymphocytes and that this kinase is required for the chemotactic response of T lymphocytes towards CCL19 and CCL21. In addition, the absence of peripheral LN in Jak 3–/– mice is also reminiscent of the phenotype of CCR7–/– mice and the plt/plt mice, arguing in favour that the homing deficiencies observed in Jak3–/– mice must be primarily the result of impaired CCR7-mediated signalling, in the absence of Jak3. Similarly, homing to the peripheral LN was not completely abrogated, suggesting that other receptors may be involved in the entry of T lymphocytes into these organs.
Jak3 is constitutively associated to the γc, which is an important component of IL-7R-mediated signalling, recently shown to be critical during lymphoid organogenesis.12 More specifically, it has been shown that lymphoid tissue development requires the initial interaction of CD4+ CD3– CD45+ IL-7Rα+ haematopoietic cells (‘inducer cells’) with resident vascular cell adhesion molecule-1 (VCAM-1)+ stromal cells (‘organizer cells’). Delivery of lymphotoxin (LT)α1β2 to LTβ receptor expressing ‘organizer’ cells, triggered by IL-7R signalling in ‘inducer’ cells, leads to expression of chemokines and further up-regulation of VCAM-1 promoting the recruitment of lymphocytes and finally allowing lymphoid organ development. Although IL-7Rα is critical for PP development, the requirement of this receptor for LN development has not yet been demonstrated.11 However, because Jak3 is constitutively associated to IL-7 receptor γc, the lack of peripheral LN and PP observed in Jak3–/– mice might also be caused by the lack of IL-7Rα signalling in the ‘inducer’ cells. Therefore, we investigated the contribution of a Jak3 deficiency in the stromal cells and its possible contribution to the in vivo homing defect. For this purpose, we performed reciprocal adoptive transfer experiments using Jak3+/+ GFP+ BM progenitors to reconstitute irradiated Jak3–/– mice. Although numbers of lymphocytes were always lower, Jak3+/+ lymphocytes were able to reach all peripheral organs, except for PP. Interestingly, a higher percentage of GFP+ lymphocytes were frequently detected in these mice in most lymphoid organs (BM, thymus, lymph nodes and spleen) compared to transfers in which Jak3+/+ GFP+ progenitors were used to reconstitute Jak3+/+ mice. This data further indicates that defects in lymphoid organogenesis cannot completely explain the lack of peripheral lymph nodes observed in Jak3–/– mice. Our data is in agreement with a previous report suggesting that the lymph node anlagen may develop independently of the IL-7R signalling pathway.58 In contrast, IL-7R-mediated signalling seems to be more strictly required for development of PP.12
We have also demonstrated that the impaired chemotactic responses as well as the deficient in vivo homing cannot be explained by reduced levels of CCR7. Similar to our previous observation in the thymus, CCR7 levels in Jak3–/– naïve T lymphocytes were not lower than in wild type T lymphocytes; rather, in some mice increased levels of this receptor were observed in splenic T lymphocytes (not shown). Therefore, this indicates that the impaired homing observed in Jak3–/– T lymphocytes is caused by a deficient CCR7-mediated signalling.
Although the molecular mechanisms underlying this deficient migration are still unknown, one possible explanation might be impaired chemokine-mediated integrin activation. Thus, we could postulate, that the lack of signalling through CCR7 in the absence of Jak3, might impair leucocyte function-associated-1 activation and/or clustering, which is an event required for extravasation and entry into LN through HEV.59 Another possibility would be that Jak3 participates in signalling pathways involved in cytoskeleton reorganization in response to chemokines. In this context, it was previously reported that Jak2 is required for tyrosine phosphorylation of PI3 kinase, focal adhesion kinase, and paxillin, in haematopoietic progenitors in response to CXCL12.60 Furthermore, recent data has demonstrated the association of Janus kinases with proteins that interact with the microtubule network, such as the Jamip1, suggesting a potential role of Janus kinases in processes involved in chemokine induced migration, such as in cell polarization.61 Altogether, this evidence suggests that Jak3 might also be involved in T lymphocyte cytoskeleton reorganization in response to CCL21 and CCL19 chemokines. However, to further investigate specific chemokine-induced signalling pathways that may be affected in the absence of Jak3, more detailed studies are still needed. In summary, our data demonstrates that Jak3 has a crucial role in CCR7-mediated T lymphocyte homing to peripheral lymphoid organs.
Acknowledgements
We want to thank Dr U. von Andrian (Harvard University, MA) for providing the CCL19-Fc fusion protein, and Dr M. Okawa (Osaka University, Japan), for providing the actin-GFP transgenic mice. We also thank MVZ Gerardo Arrellín for animal care assistance, M.C. Carlos Castellanos and B. Ramsés Chávez for technical assistance. This work was supported by DGAPA Grant #IN234002, Universidad Nacional Autónoma de México, México. IL was supported by a doctoral fellowship from CONACYT # 203753 and from DGEP, UNAM.




