Glycogen synthase kinase 3 activity during development of bone marrow-derived dendritic cells (DCs) essential for the DC function to induce T helper 2 polarization
Summary
Dendritic cells (DCs) polarize naive CD4+ T cells to either T helper 1 (Th1) or Th2 cells. We examined the role of glycogen synthase kinase 3 (GSK3) activity during DC development from murine bone marrow (BM) cells. DCs were generated by culturing lineage-marker-negative BM cells with granulocyte–macrophage colony-stimulating factor in the presence or absence of a specific inhibitor of GSK3 (Gi), SB415286, for 6 days. DCs generated in the presence (GiDC) or absence (control DC) of SB415286 similarly exhibited a conventional DC phenotype (CD11b+ B220– CD8–). These DCs were mixed with allogeneic CD4+ T cells and the ability to polarize Th1 or Th2 cells was evaluated. The GiDCs exhibited markedly impaired function to induce Th2 polarization compared to control DCs. In contrast, the ability of GiDCs to generate Th1 cells was slightly higher than that of control DCs. CD86 expression and CD40-mediated interleukin-6 production were completely diminished in GiDCs, which might be associated with the impaired ability of the GiDCs to induce Th2 differentiation. These results suggest that the GSK3 activity during DC development is essential for the establishment of the DC function to induce Th2, but not Th1, differentiation.
Abbreviations:
-
- allo-MLR
-
- allogeneic mixed leucocyte reaction
-
- BM
-
- bone marrow
-
- BMDC
-
- BM-derived DC
-
- CD40L
-
- CD40 ligand
-
- CREB
-
- CAMP response element binding protein
-
- DCs
-
- dendritic cells
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- FCS
-
- fetal calf serum
-
- FITC
-
- fluorescein isothiocyanate
-
- GM-CSF
-
- granulocyte–macrophage colony-stimulating factor
-
- GSK3
-
- glycogen synthase kinase 3
-
- IFN
-
- interferon
-
- IgG
-
- immunoglobulin G
-
- IL
-
- interleukin
-
- mAb
-
- monoclonal antibody
-
- LN
-
- lymph nodes
-
- LPS
-
- lipopolysaccharide
-
- MACS
-
- magnetic-activated cell sorting
-
- 2-ME
-
- 2-mercaptoethanol
-
- MHC
-
- major histocompatibility complex
-
- NF-κB
-
- nuclear factor κB
-
- PBS
-
- phosphate-buffered saline
-
- PE
-
- phycoerythrin
-
- TCR
-
- T-cell receptor
-
- Th cell
-
- T helper cell
-
- TLR
-
- Toll-like receptor
-
- TNF-α
-
- tumour necrosis factor-α.
Introduction
Dendritic cells (DCs), the most potent antigen-presenting cells, play a critical role in both innate and adaptive immunity by producing chemokines and cytokines as well as presenting antigens to the antigen-specific T cells. DCs polarize naive CD4+ T cells to T helper 1 (Th1) or Th2 cells.1,2 Th1 cells, producing interferon-γ (IFN-γ), are crucial in promoting the cellular immunity that clears intracellular bacteria and viruses, whereas Th2 cells, releasing interleukin-4 (IL-4), IL-5, IL-10 and IL-13, induce humoral immune responses against extracellular parasites.3 Th1/Th2 polarization is regulated by various factors including antigen dose, the nature and amount of costimulations, and the cytokine environment around the differentiating cells.4,5 The level and type of costimulations during the T-cell priming also influence the Th1/Th2 polarization. In contrast to Th1 induction, Th2 differentiation strictly requires the costimulation to be mediated via CD28 interactions with its ligands, CD80 and CD86, expressed on DCs.6–8 During antigen presentation, DCs produce various cytokines, such as IL-6, IL-12, IL-23 and IL-27, that regulate Th1 or Th2 polarization. The DC-produced IL-12, IL-23 and IL-27 induce Th1 polarization,9,10 while the IL-6 is involved in Th2 promotion and Th1 inhibition.11–13
Glycogen synthase kinase 3 (GSK3), a serine/threonine kinase, is involved in the Wnt signalling pathway.14 Two closely related isoforms of GSK3 have been identified, GSK3-α and GSK3-β, which share 97% sequence similarity within their kinase catalytic domains.15 In the murine system, deletion of the GSK3-β gene leads to embryonic lethality characterized by extensive liver degeneration,16 while the phenotype of mice lacking GSK3-α has not yet been reported. GSK3 regulates many cellular functions including glycogen metabolism, cell-cycle control and proliferation.17 GSK3 can both positively and negatively affect a variety of transcription factors that are critical in regulating pro- and anti-inflammatory cytokine synthesis.18–20 Martin et al.21 reported that GSK3 inhibition resulted in substantial increases in IL-10 production and suppressed the release of proinflammatory cytokines [IL-12p40, IL-6 and tumour necrosis factor-α (TNF-α)] in human monocytes or peripheral blood mononuclear cells upon Toll-like receptor (TLR) stimulation. Moreover, GSK3 inhibition conferred significant protection against lipopolysaccharide- (LPS) induced lethality in mice.22
Recently, it has been reported that GSK3 is a crucial enzyme for the differentiation and maintenance of an immature phenotype of human monocyte-derived DCs.21 However, a role of GSK3 in the DC function for Th1 or Th2 differentiation remains unclear. We demonstrate herein that GSK3 activity during DC development from bone marrow (BM) cells is essential for the subsequent DC function to induce Th2, but not Th1, polarization.
Materials and methods
Mice
Female BALB/c and C57BL/6 (B6) mice were obtained from Japan SLC (Hamamatsu, Japan) and were maintained in the specific pathogen-free conditions of our animal facility at Hokkaido University. All animals were used at 6–10 weeks of age. The animal experimentation was approved by the Hokkaido University Animal Care and Use Committee (Sapporo, Japan) and followed its regulations.
Reagents and antibodies
Murine recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) was purchased from PeproTech (London, UK). LPS from Escherichia coli (055:B5) and SB415286, a specific inhibitor of GSK3, were obtained from Sigma Chemical Co (St Louis, MO). Purified anti-mouse CD3 monoclonal antibody (mAb) (145-2C22), phycoerythrin- (PE) conjugated anti-mouse CD40 mAb (3/23), fluorescein isothiocyanate- (FITC) conjugated anti-mouse CD80 mAb (16-10A1), FITC-conjugated anti-mouse CD86 mAb (GL1), biotin-conjugated anti-mouse I-Ab mAb (AF6-120.1), PE-conjugated anti-mouse H-2Kb mAb (AF6-88.5), biotin-conjugated anti-mouse H-2Db mAb (KH95), FITC-conjugated anti-mouse CD11b mAb (M1/70), PE-conjugated anti-mouse CD11c mAb (HL3), PE-conjugated anti-mouse CD4 mAb (RM4-5), PE-conjugated anti-mouse TLR-4 mAb (MTS510), and streptavidin–peridinin chlorophyll protein (PerCP) were obtained from BD Biosciences Pharmingen (San Diego, CA). Anti-phosphoglycogen synthase (Ser641) antibody, anti-β-actin antibody, and horseradish-peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody were obtained from Cell Signaling (Beverly, MA).
DC generation from BM cells
BM-derived DCs (BMDCs) were generated as previously described.23 BM cells were prepared from femur and tibia BM of 6- to 10-week-old B6 mice. After lysis of erythrocytes, major histocompatibility complex (MHC) class II, CD45R- (B220), CD4- and CD8-positive cells were removed using magnetic-activated cell sorting (MACS) system (Miltenyi Biotec, Bergisch Gladbach, Germany). DC generation was conducted in RPMI-1640 (Sigma Chemical Co.) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 5% heat inactivated fetal calf serum (FCS), 20 ng/ml GM-CSF, and 50 µm 2-mercaptoethanol (2-ME). The lineage-marker-negative BM cells were cultured at a density of 1 × 106 cells/ml/well (24-well plate) in the medium containing vehicle (0·1% dimethyl sulphoxide) or SB415286. On day 2, the medium was gently exchanged for fresh medium containing vehicle or SB415286. On day 4, non-adherent granulocytes were removed without dislodging clusters of developing DCs and fresh medium containing vehicle or SB415286 was added. On day 6, free-floating and loosely adherent cells were collected and DCs were positively selected using anti-CD11c (N418) MicroBeads and a MACS column (Miltenyi Biotec). The purified DCs (> 98% CD11c+) generated in the presence of vehicle or SB415286 (Gi: GSK3 inhibitor) were used as control DCs or GiDCs, respectively. SB415286 was used at 10 μm, based on previous studies21,22 and our preliminary dose–response study.
Western blot analysis
BM cells were cultured with or without SB415286 (10 μm) for 1 hr. Reactions were halted by rapidly cooling on ice and washed with ice-cold phosphate-buffered saline (PBS). The whole cell lysates were prepared using cell lysis buffer (Cell Signaling Technology). The cell lysates were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, then blotted onto a nitrocellulose membrane using iBlot™ Dry Blotting System (Invitrogen, Carlsbad, CA). The membrane was probed with primary antibody, and developed with horseradish peroxidase-conjugated anti-rabbit IgG antibody by enhanced chemiluminescence.
Preparation of CD4+ T cells
CD4+ T cells were isolated from spleens or lymph nodes (LN) of BALB/c mice. After lysis of erythrocytes, CD8, CD45R, DX5, CD16, CD32, and MHC class II positive cells in splenocytes were removed using a MACS system (Miltenyi Biotec). Purity of the splenic CD4+ T cells (CD4+ CD3+ cells) was 85–92%.
Lymphocytes were prepared from axial, mesenteric, and inguinal LN. CD4+ T cells were positively selected from the lymphocytes using anti-CD4 (L3T4) MicroBeads and a MACS column (Miltenyi Biotec). In this process, the cells were passed through the MACS column twice to increase the purity. Purity of the LN CD4+ T cells (CD4+ CD3+ cells) was > 98%.
Allogeneic mixed leucocyte reaction (allo-MLR) and cytokine measurement
Allo-MLR was performed using CD4+ T cells from spleens or LN of BALB/c mice as responder cells. Control DCs or GiDCs (1 × 104 to 2 × 104 cells/well) generated from B6 BM cells were cocultured with the CD4+ T cells (2 × 105 cells/well) in 200 μl RPMI-1640 supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 10% FCS and 50 μm 2-ME (96-well plate). To determine cell proliferation activity, after coculture for 68 hr, the cells were pulsed with 0·5 μCi/well [3H]thymidine (Amersham, Tokyo, Japan) for 4 hr and then harvested onto glass fibre. Incorporation of [3H]thymidine was measured with a liquid scintillation counter (MicroBeta Plus; Wallac, Turku, Finland). To evaluate cytokine production, the culture supernatants were collected on day 5 and subjected to enzyme-linked immunosorbent assay (ELISA).
Restimulation of allo-antigen primed T cells
After the allo-MLR for 5 days, the cells were harvested and washed. CD11c+ cells were removed using a MACS system (Miltenyi Biotec). The remaining cell population was > 99% CD4+ T cells (CD4+ CD3+ cells). To stimulate the T cells, wells of 96-well plates were coated with 10 μg/ml anti-CD3 mAb in PBS at 4° overnight and washed with PBS. The allo-antigen-primed CD4+ T cells were cultured on the anti-CD3 mAb-coated well at a density of 1 × 105 cells/200 μl per well in RPMI-1640 supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 10% FCS and 50 μm 2-ME for 24 hr. Cytokine levels of the culture supernatant were measured by ELISA.
Cytokine detection
The protein levels of cytokines in culture supernatants were evaluated by ELISA. The IL-4, IL-6, IL-10, IL-12p40 and IFN-γ levels were determined using OptEIA™ Set for each cytokine (BD Biosciences Pharmingen) according to the manufacturer's instructions.
Flow cytometry
Cells were incubated with 2.4G2 (rat anti-mouse FcII/III receptor, CD32) supernatant to prevent the non-specific binding of antibody to FcRII/III, and then stained using FITC-, PE-, or biotin-conjugated mAb, and streptavidin-PerCP™. Expression of cell surface molecules and the purity of CD4+ T cells were analysed by flow cytometry on EPICS XL (Coulter Co. Miami, FL) as described in previous studies.24,25
Statistical analysis
The Student's t-test was used to analyse data for significant differences. P-values < 0·05 were regarded as significant.
Results
Inhibition of GSK3 activity by SB415286 in BM cells
GSK3 constitutively phosphorylates glycogen synthase. It was reported that SB415286, a specific inhibitor of GSK3, decreased the spontaneous phosphorylation of glycogen synthase.21 To examine the inhibitory effects of SB415286 on GSK3 activity in murine BM cells, we evaluated the level of phosphorylated glycogen synthase in B6 BM cells treated with or without SB415286 by immune blotting. Spontaneous phosphorylation (Ser641) of glycogen synthase was detected in BM cells in the absence of SB415286 (Medium) (Fig. 1). Treatment of the BM cells with SB415286 significantly decreased the spontaneous phosphorylation of glycogen synthase. Thus, it was demonstrated that SB415286 inhibited GSK3 activity in BM cells.

Inhibition of GSK3 activity in BM cells by SB415286. BM cells were cultured in the absence or presence of SB415286 for 1 hr, and whole cell lysates were prepared. Levels of phospho (Ser641) glycogen synthase (p-glycogen synthase) and β-actin in the cell lysates were determined by immunoblotting. A representative immunoblot from three independent experiments is shown (upper). The relative intensity of the specific band is shown (lower). Each column represents the mean ± SE of three independent experiments (*P < 0·05 versus control).
Influence of GSK3 inhibition during DC development on the differentiated DC phenotype
DCs are generated from murine BM cells by culturing with GM-CSF for 6 days.22 We examined the effect of GSK3 inhibition during the development of DCs. BM cells from B6 mice were cultured with GM-CSF for 6 days in the absence or presence of SB415286 and we referred to these cells as control DCs or GiDCs, respectively.
CD11b, B220 and CD8 are representative markers of DC subsets. High levels of CD14 are expressed on monocytes and macrophages but not DCs.21 We first analysed the expression of these molecules on control DCs and GiDCs. Control DCs were positive for CD11c and CD11b and negative for B220 and CD8, a pattern typical of conventional DCs (Fig. 2a). GiDCs exhibited the same expression pattern of these markers as control DCs. Neither control DCs nor GiDCs expressed CD14. Thus, the GSK3 inhibition during the DC generation from BM cells with GM-CSF exerted no significant influences on the surface phenotype of the differentiated DC subset.
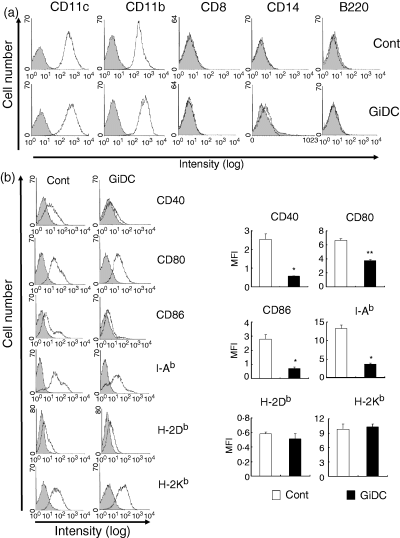
The effects of GSK3 inhibition during DC development on the DC phenotype. BM cells were cultured with GM-CSF for 6 days in the absence or presence of SB415286 and referred as control DCs (Cont) or GiDCs, respectively. Expression of the cell surface molecules was analysed by flow cytometry. Representative FACS profile from three independent experiments (a and b left panel). Mean fluorescence intensity (MFI) subtracted by the level of isotype-matched control antibody is shown in (b, right panel). Each column represents the mean ± SE of three independent experiments (*P < 0·05, **P < 0·01 versus Cont).
We then compared the expression of various activation markers between control DCs and GiDCs (Fig. 2b). The expression of costimulatory molecules (CD40, CD80 and CD86) and MHC class II (I-Ab) on GiDCs was significantly reduced compared to that on control DCs. Especially, CD40 and CD86 were almost negative on GiDCs. In contrast, MHC class I (H-2Kb and H-2Db) levels were comparable between control DCs and GiDCs.
Ability of GiDCs to induce proliferation of allo-CD4+ T cells
We examined the effect of GSK3 inhibition during DC generation on the differentiated DC function to stimulate CD4+ T cells in allo-MLR. We used two types of CD4+ T cells that were isolated from different lymphoid organs, spleens and LN. Purity of CD4+ T cells from spleens or LN was 85–92% and > 98·5%, respectively. These CD4+ T cells from BALB/c spleens or LN were cocultured with control DCs or GiDCs (B6) at various DC : T-cell ratios for 3 days and proliferation activity was evaluated (Fig. 3). No proliferation was observed in the culture of the T cells or each DC type alone (data not shown). Stimulation with each DC type induced significant proliferation of either splenic or LN CD4+ T cells (Fig. 3a,b). The proliferation activity was directly related to the DC : T-cell ratio in both cocultures. Notably, the proliferation activity of splenic or LN CD4+ T cells stimulated with GiDCs was lower than that of cells stimulated with control DCs. Almost the same results were obtained when irradiated DCs were used as stimulator cells in the allo-MLR (data not shown).
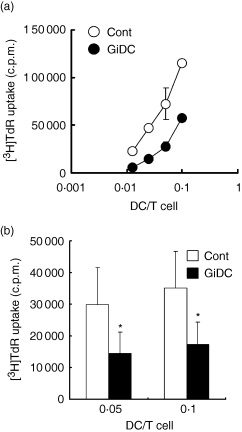
The effect of GSK3 inhibition during DC generation on the DC function to induce proliferation of allo-CD4+ T cells. BMDCs (B6) were generated in the absence or presence of SB415286 and are referred to as control DCs (Cont) or GiDCs, respectively. Abilities of each DC type to activate allo-CD4+ T cells isolated from BALB/c spleens (a) or LN (b) was evaluated. CD4+ T cells (2 × 105) were cocultured with control DCs or GiDCs at various DC : T-cell ratios (spleens: 0·01–0·1; LN: 0·05 and 0·1). After 68 hr of culture, cells were pulsed with [3H]thymidine for 4 hr and harvested on a glass filter. Proliferation activity was determined by measuring [3H]thymidine incorporation. (a) Each symbol represents mean ± SE of triplicate wells. Data were representative of four independent experiments. (b) Each column represents the mean ± SE of four independent experiments (*P < 0·05 versus Cont).
Ability of GiDCs to induce cytokine production by allo-CD4+ T cells
We next evaluated Th1/Th2-cytokine production in the culture of allo-MLR. Control DCs or GiDCs (B6) were cocultured with splenic or LN CD4+ T cells (BALB/c) at a DC : T-cell ratio of 0·05 or 0·1 for 5 days and levels of Th1 (IFN-γ) and Th2 (IL-4 and IL-10) cytokines in each culture were analysed (Fig. 4a,b). Negligible production of these cytokines was shown in the culture of the CD4+ T cells or each DC type alone. Significant production of IFN-γ, IL-4 and IL-10 was observed in the culture of splenic or LN CD4+ T cells stimulated with control DCs at either of the DC : T-cell ratios (0·05 and 0·1). The same level or a significantly increased level of IFN-γ production was observed in the culture of splenic or LN CD4+ T cells with GiDCs, respectively, compared to that with control DCs (Fig. 4a,b). In contrast, IL-4 and IL-10 production in the culture of either splenic or LN CD4+ T cells with GiDCs were markedly reduced compared to those with control DCs. Thus, ability of DCs to induce Th2, but not Th1, cytokine production in CD4+ T cells responding to alloantigens appeared to be impaired by GSK3 inhibition during the development of the DCs.
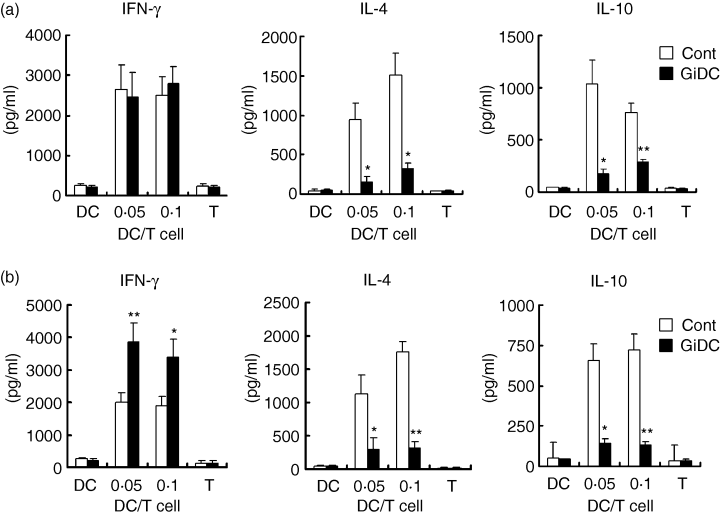
The effect of GSK3 inhibition during DC generation on the DC function to induce cytokine production by allo-CD4+ T cells. BMDCs (B6) were generated in the absence or presence of SB415286 and are referred to as control DCs (Cont) or GiDCs, respectively. The ability of each DC group to activate and induce cytokine production by allo-CD4+ T cells isolated from BALB/c spleens (a) or LN (b) was evaluated. (a,b) CD4+ T cells were cocultured with control DCs or GiDCs at DC : T-cell ratios of 0·05 and 0·1. Each DC type (2 × 104) or CD4+ T cells (2 × 105) alone were cultured as the negative controls (DC or T). Levels of IFN-γ, IL-4 and IL-10 in the culture supernatant on day 5 were determined by ELISA. Each column represents the mean ± SE of five independent experiments (*P < 0·05, **P < 0·01 versus Cont).
Cytokine production by GiDC-primed CD4+ T cells upon stimulation with anti-CD3 mAb
We then analysed cytokine production by GiDC-primed CD4+ T cells upon restimulation through T-cell receptor (TCR). CD4+ T cells purified from BALB/c LN were stimulated with control DCs or GiDCs (B6) for 5 days. After the primary stimulation, CD11c+ cells were removed and the remaining CD4+ T cells (> 99% of CD4+ CD3+ cells) were restimulated with anti-CD3 mAb for 24 hr. In the absence of CD3 stimulation, control DC-primed or GiDC-primed T cells showed negligible production of Th1 (IFN-γ) and Th2 (IL-4 and IL-10) cytokines (Fig. 5). Following the CD3 stimulation, the control DC-primed T cells produced considerable levels of these cytokines. IFN-γ production by the GiDC-primed T cells was slightly but significantly higher than that by the control DC-primed T cells (Fig. 5 left panel). In contrast, these GiDC-primed T cells produced markedly low levels of IL-4 and IL-10 compared to the T cells primed with control DCs (Fig. 5 centre and right panels). Unprimed CD4+ T cells never produced any cytokines upon CD3 stimulation for 24 hr (data not shown). These findings indicated that the GSK inhibition during DC development resulted in severely impaired ability of the DCs to induce Th2 development of responding T cells in the allo-MLR system.
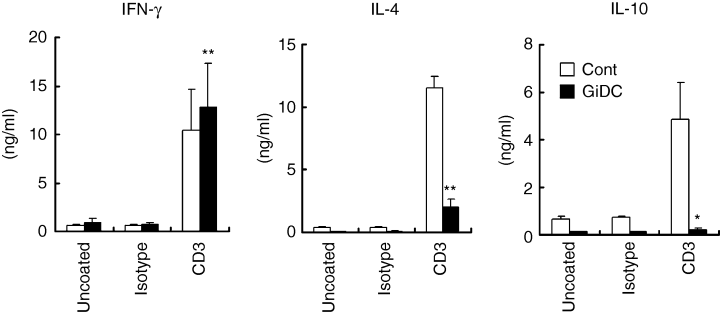
The effect of GSK3 inhibition during DC generation on the DC function to polarize Th1 and Th2 cells in allo-MLR. BMDCs (B6) were generated in the absence or presence of SB415286 and referred as control DCs (Cont) or GiDCs, respectively. Each DC type (2 × 104) was cocultured with CD4+ T cells (2 × 105) from BALB/c LN. After the allo-MLR for 5 days, the cells were harvested and CD11c+ cells were removed. The remaining CD4+ T cells (1 × 105) were cultured on anti-CD3 mAb (CD3) or isotype-matched IgG (Isotype) coated or uncoated wells for 24 hr. Cytokine production in the culture supernatant was determined by ELISA. Each column represents the mean ± SE of four independent experiments (*P < 0·05, **P< 0·01 versus Cont).
Cytokine production by GiDCs upon CD40 ligation
During antigen presentation, DCs are activated via interaction of the CD40 with CD40 ligand (L) expressed on T cells and thereby produce inflammatory cytokines such as IL-6 and IL-12. These cytokines appear to affect the subsequent Th1/Th2 differentiation.9,11 We evaluated the ability of GiDCs to produce IL-6, IL-10 and IL-12 upon CD40 ligation. Control DCs and GiDCs were stimulated with anti-CD40 mAb (IgM) for 24 hr. The CD40 ligation significantly increased IL-6 and IL-12p40 production by control DCs (Fig. 6a). However, the CD40 ligation never induced production of these cytokines by GiDCs beyond the base line level. IL-10 production was unaffected by CD40 ligation in both types of DC, control DCs and GiDCs.
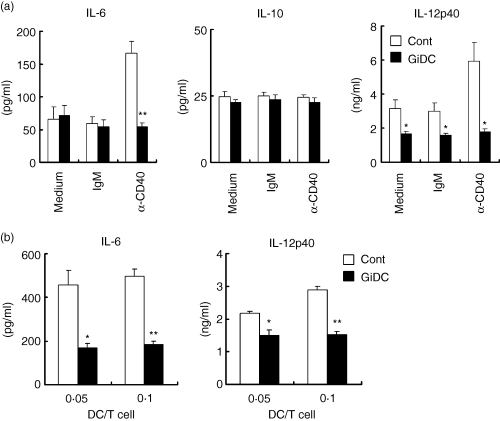
The effect of GSK3 inhibition during DC generation on the DC function to produce IL-6, IL-10 and IL-12p40. BMDCs (B6) were generated in the absence or presence of SB415286 and are referred to as control DCs (Cont) or GiDCs, respectively. (a) Each DC type (4 × 104) was treated with anti-CD40 mAb (α-CD40) or isotype-matched IgM (IgM) or cultured with medium alone (Medium) for 24 hr. (b) Each DC type (2 × 104) was cocultured with CD4+ T cells (2 × 105) from BALB/c LN for 5 days. Cytokine production in the culture supernatant was determined by ELISA. Each column represents the mean ± SE of four independent experiments (*P < 0·05, **P < 0·01 versus Cont).
Then, IL-6 and IL-12p40 productions were evaluated in allo-MLR cultures where LN T cells from BALB/c mice and control B6 DCs or GiDCs were present at DC : T-cell ratios of 0·05 and 0·1. After 5 days, cytokine production was evaluated in the culture supernatants. Considerable production of IL-6 and IL-12p40 was detected in the culture of T cells and control DCs (Fig. 6b). By contrast, levels of IL-12p40 and IL-6 in the culture with GiDCs were significantly lower than those with control DCs. IL-12p70 production was not detected in any of the cultures tested (data not shown).
Cytokine production by GiDCs upon TLR stimulation
LPS is a major cell wall component of Gram-negative bacteria, binds to the TLR4–MD2 complex expressed on DCs and induces vigorous production of inflammatory cytokines.26 We noticed that the expression of TLR4–MD2 on GiDCs was significantly increased compared with that on control DCs (Fig. 7a). Then, to compare the ability for cytokine production in response to LPS between control DCs and GiDCs, these DCs were stimulated with LPS for 24 hr. LPS stimulation induced vigorous production of IL-6, IL-10 and IL-12p40 in both types of DC (Fig. 7b). The LPS-induced productions of IL-6 and IL-10 by GiDCs, however, were more vigorous than those by control DCs. In contrast, LPS-induced IL-12p40 production by GiDCs was significantly reduced compared to that by control DCs. After the LPS stimulation, GiDCs exhibited similar levels of MHC class I and II, CD80 and CD86, but a low level of CD40 compared to those on control DCs (data not shown). Thus, GSK3 activity during DC development appeared to differentially regulate the ability of the DCs to produce cytokines upon stimulation through TLR4.
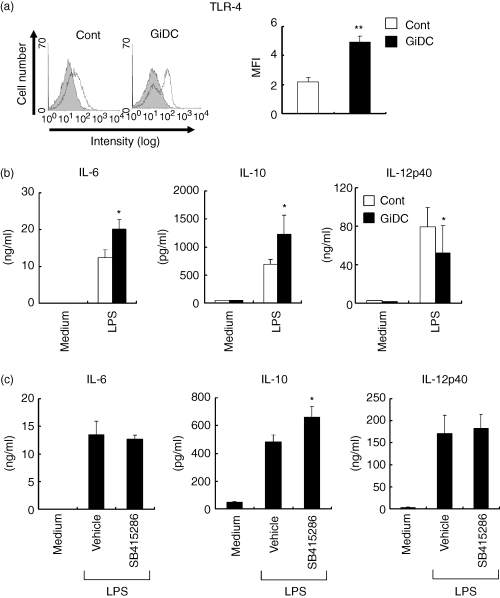
The effect of GSK3 inhibition on cytokine production by DCs. BMDCs (B6) were generated in the absence or presence of SB415286 and are to referred as control DCs (Cont) or GiDCs, respectively (a,b). (a) Surface expression of TLR4-MD2 was analysed by flow cytometry. Data were representative of three independent experiments with similar results (left). Mean MFI subtracted by the level of isotype-matched control antibody was shown (right). Each column represents the mean ± SE of three independent experiments (**P < 0·01 versus Cont). (b) Each DC type (4 × 104) was treated with LPS or cultured with medium alone (Medium) for 24 hr. Cytokine production in the culture supernatant was determined by ELISA. Each column represents the mean ± SE of four independent experiments (*P < 0·05 versus Cont). (c) BMDCs were generated by culturing lineage-marker-negative BM cells with GM-CSF for 6 days. The differentiated BMDCs were pretreated with vehicle or SB415286 for 1 hr and then stimulated with LPS for 24 hr in the presence of medium (Vehicle) or the inhibitor. As the negative control, cells were cultured with medium alone (Medium). Each column represents the mean ± SE of four independent experiments (*P < 0·05 versus Vehicle).
We next examined the role of GSK3 activity during LPS-induced DC activation. DCs (BMDCs cultured for 6 days with GM-CSF) were pretreated with or without SB415286 for 1 hr and then stimulated with LPS for 24 hr in the presence or absence of the inhibitor. SB415286 showed no effects on LPS-induced IL-6 and IL-12p40 production, but substantially enhanced LPS-induced IL-10 production by DCs (Fig. 7c).
Discussion
DCs induce polarization of naive CD4+ T cells to Th1 or Th2 effector cells.1,2 However, the mechanism underlying the DC regulation of Th1 or Th2 differentiation is not fully understood. In particular, the DC-derived factors responsible for the initiation of Th2 differentiation are poorly characterized compared to those for Th1 differentiation. In the present study, we examined the role of GSK3 activity during the development of DCs from BM cells to functional DCs. We could demonstrate that DCs developed under conditions in which GSK3 activity was significantly inhibited (GiDCs) showed severely impaired ability to induce Th2 polarization in responding CD4+ T cells in an allo-MLR system. On the other hand, the GiDC capability to induce Th1 differentiation was sustained or rather augmented. Thus, GSK3 appeared to play a pivotal role in DC development of the capability to induce Th2 polarization in the allo-MLR system.
Human CD14+ monocytes differentiate into DCs after culturing with GM-CSF plus IL-4. Recently, Rodionova et al.22 showed that GSK3 inhibition by SB415286 during the culture of human CD14+ monocytes in the presence of GM-CSF plus IL-4 resulted in differentiation into macrophage-like cells rather than DCs. CD14 expression is generally down-modulated during the differentiation of DCs from monocytes. However, these macrophage-like cells of Rodionova et al. expressed a high density of CD14 compared to conventional human monocyte-derived DCs, suggesting that GSK3 activity was required for the differentiation of DCs from human monocytes.
In the present study, we examined the effect of GSK3 inhibition on DC generation from lineage-marker-negative BM cells with GM-CSF alone. DCs generated in the presence of SB415286 (GiDCs) exhibited normal expression of a DC marker (CD11c) and had a conventional DC phenotype (CD11b+ B220– CD8–) similar to those on the control DCs developed in the absence of SB415286. Neither control DCs nor GiDCs expressed CD14. In addition, the absolute number of CD11c+ DCs generated from BM cells was unaffected by the GSK3 inhibition (data not shown). Thus, GSK3 inhibition seemed to exert no considerable influence on the development of the DCs and their subset. The difference between the previous study using human monocytes22 and our present results may be attributable to the difference in the origin of the differentiated DCs (human monocytes versus lineage-marker negative murine BM cells) and/or the culture condition (with or without IL-4).
CD28-mediated signals are induced by interaction with the ligands, CD80 and CD86, expressed on DCs during T-cell priming.27 It has been shown that, compared with Th1 differentiation, Th2 polarization strictly depends on CD28 costimulation.6–8 Expression of CD80 and CD86 on GiDCs was significantly lower than on control DCs. In particular, the GiDCs were almost negative for CD86, whereas CD80 expression was substantially sustained on the GiDCs compared to control DCs. It has been reported that CD28 signals via interaction with CD86 preferentially promote Th2 differentiation compared with those via interaction with CD80.28,29 Thus, the marked deficiency of CD86, but not CD80, expression on GiDCs may be associated with the impaired function to induce Th2 polarization. In addition to CD28 costimulation, OX40 and inducible costimulatory molecule (ICOS) signals have been implicated in Th2 promotion.30–32 However, we could not detect significant differences in the expression of the ligands for these molecules, OX40L and ICOSL, between control DCs and GiDCs (data not shown).
DC-derived factors that promote Th2 differentiation are not well characterized. Diehl et al.11,12 have postulated that IL-6 promotes Th2 differentiation by activation of nuclear factor of activated T cells c2 (NFATc2), while inhibiting IFN-γ mediated-signalling and Th1 differentiation. Consistent with this postulate, mouse pulmonary DC that produced abundant IL-6 showed reduced ability to promote Th1 differentiation.13 Furthermore, the addition of IL-6 into mouse allo-MLR resulted in enhanced Th2 differentiation and reduced Th1 response.13 We found that CD40 expression on GiDCs was severely reduced compared with control DCs and stimulation of the GiDCs with anti-CD40 mAb failed to induce IL-6 production. The impaired IL-6 production appeared to be attributable to the deficiency of CD40 expression on GiDCs. Furthermore, the IL-6 level in the culture of allo-MLR with GiDCs was significantly reduced compared with that with control DCs. From these observations, we considered that the impaired ability of GiDCs to promote Th2 differentiation might be attributable to their reduced CD40 expression and the low production of IL-6 during the antigen presentation, in addition to the altered interaction with the CD4+ T cells via CD28. The precise mechanism underlying the impaired function of GiDCs for Th2 polarization remains unclear and should be pursued in further studies.
In addition to the CD40-mediated cytokine production, we examined the ability of GiDCs for cytokine production in response to TLR stimulation. In striking contrast to the CD40 expression, TLR4 expression was significantly increased on GiDCs compared to control DCs. IL-6 and IL-10 production by GiDCs in response to LPS was higher than that by control DCs. It seems that the increased production of these cytokines by GiDCs is associated with the augmented expression of TLR4. In contrast, the LPS-induced IL-12p40 production by GiDCs was reduced compared to that by control DCs. Machinery such as intracellular signalling for IL-12p40 production in response to the LPS may be impaired in GiDCs. It has been reported that GSK3 negatively or positively regulates CAMP response element binding protein (CREB) or nuclear factor-κB (NF-κB) activity, respectively.21 On the other hand, activation of CREB or NF-κB upon TLR stimulation appears to promote IL-10 or IL-12 production, respectively. Increased and decreased production of IL-10 and IL-12 by GiDCs, compared to control DCs, may be attributable to the altered balance of CREB and NF-kB p65 activity upon TLR4 stimulation. Thus, GSK3 activity during the generation of DCs appears to be involved in the regulation of the DC ability to not only induce Th2 polarization in allo-MLR system, but also cytokine production in response to LPS.
Recently, Martin et al.21 demonstrated that GSK3 plays a pivotal role in regulating the balance of pro-inflammatory and anti-inflammatory cytokine production in human monocytes upon TLR stimulation. Blocking the GSK3 activity during LPS stimulation resulted in enhanced IL-10 and reduced IL-12p40, IL-6 and TNF-α production by monocytes. We also examined the effect of GSK3 inhibition during the stimulation of differentiated DC with LPS on subsequent cytokine production. LPS-mediated IL-10 production was slightly but significantly increased by treatment with SB415286. Unlike human monocytes, however, LPS-mediated IL-6 and IL-12p40 production was unaffected by the treatment with SB415280. In contrast, Rodionova et al.22 showed that SB415286 treatment reduced the secretion of not only pro-inflammatory cytokines (IL-12p40, IL-12p70, IL-6 and TNF-α) but also an anti-inflammatory cytokine (IL-10) by human monocyte-derived DCs upon E. coli stimulation. Thus, the role of GSK3 in cytokine production may be different depending on the type of cells (monocytes versus DCs) and stimuli (LPS versus E. coli).
In the present study, we used BALB/c CD4+ T cells as responder cells and C57BL/6 DCs as stimulator cells. In this culture system, Th2 differentiation is clearly induced as well as Th1 differentiation. We have not examined the role of GSK3 using T cells from strains other than BALB/c mice. Strain difference might influence Th1 versus Th2 differentiation with control DCs or GiDCs and should be pursued in future studies.
We described here a novel role of GSK3 activity during DC generation from mouse BM cells in the development of DC function. The GSK3 activity appeared to be required for the establishment of DC function to induce Th2 differentiation in responding T cells of the allo-MLR system. Inappropriate Th1/Th2 balance is thought to be associated with a variety of immune disorders including allergy and autoimmunity. In addition, Th1/Th2 balance affects infectious and cancer immunity and allograft rejections. Thus, the DC regulation of Th1/Th2 balance is crucial to control these immunological diseases and the immune reactions. Our present findings may lead to the development of clinical applications exploiting the new regulation system of DCs via GSK3 for the treatment of transplantation, cancer, and various immune disorders.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (S), Grant-in Aid for Exploratory Research and a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS), and a Grant-in-Aid for Scientific Research on Priority Areas by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan. This study was also supported by the Akiyama Foundation and the Uehara Memorial Life Science Foundation. We wish to thank Ms Mayumi Kondo for her assistance in the preparation of this manuscript.




