Dose-dependent modulation of CD8 and functional avidity as a result of peptide encounter
Summary
The generation of an optimal CD8+ cytotoxic T lymphocyte (CTL) response is critical for the clearance of many intracellular pathogens. Previous studies suggest that one contributor to an optimal immune response is the presence of CD8+ cells exhibiting high functional avidity. In this regard, CD8 expression has been shown to contribute to peptide sensitivity. Here, we investigated the ability of naive splenocytes to modulate CD8 expression according to the concentration of stimulatory peptide antigen. Our results showed that the level of CD8 expressed was inversely correlated with the amount of peptide used for the primary stimulation, with higher concentrations of antigen resulting in lower expression of both CD8α and CD8β. Importantly the ensuing CD8low and CD8high CTL populations were not the result of the selective outgrowth of naive CD8+ T-cell subpopulations expressing distinct levels of CD8. Subsequent encounter with peptide antigen resulted in continued modulation of both the absolute level and the isoform of CD8 expressed and in the functional avidity of the responding cells. We propose that CD8 cell surface expression is not a static property, but can be modulated to ‘fine tune’ the sensitivity of responding CTL to a defined concentration of antigen.
Abbreviations:
-
- CTL
-
- cytotoxic T lymphocyte
-
- IFN
-
- interferon
-
- LCMV
-
- lymphocytic choriomeningitis virus
-
- LFA-1
-
- lymphocyte function-assisted antigen 1
-
- MFI
-
- mean fluorescence intensity
-
- pMHC
-
- peptide–major histocompatibility complexes
-
- TCR
-
- T-cell receptor.
Introduction
CD8+ cytotoxic T lymphocytes (CTL) are a critical component of the immune system. They exert their function through the lysis of infected cells as well as the secretion of interferon-γ (IFN-γ). Antigen-specific activation of naive or effector CD8+ T cells is the result of T-cell receptor (TCR) binding to peptide–major histocompatibilty complexes (pMHC) on professional antigen-presenting cells or target cells, respectively. Within the polyclonal CTL population elicited to a defined pMHC complex, there exist individual clones with distinct differences in the level of pMHC required for activation.1,2 The amount of pMHC required for activation is termed functional avidity. CTL capable of responding to low levels of pMHC are designated as high avidity, while those that require a high level of pMHC are said to be of low avidity. Importantly, the functional avidity of a cell has been shown to be predictive of their in vivo efficacy, with high-avidity CTL exhibiting an increased capacity for virus and tumour clearance compared to their low-avidity counterpart.2–8 Understanding the control of functional avidity is therefore of significant import.
Although the property of functional avidity has been well documented, the underlying mechanism involved in regulating this property is poorly understood. In addition, there are a number of unresolved questions, for example whether functional avidity is a static or inducible attribute. If functional avidity is a static property, then naive high and low avidity precursor subsets should exist within the naive immune system that are selectively activated following encounter with their required level of pMHC. However, if functional avidity is induced within the responding CTL population, then environmental stimuli, which may include the cytokine environment or the strength or nature of signalling, must drive a responding CTL to differentiate into either a high-avidity or low-avidity CTL.
One molecule that appears to contribute to pMHC sensitivity is the TCR coreceptor CD8, which participates in T-cell activation by facilitating signal transduction following TCR:pMHC engagement.9–17 The CD8 molecule can be expressed on the cell surface as either a CD8αα homodimer or a CD8αβ heterodimer.18 Previous studies have demonstrated that these two isoforms are functionally distinct (reviewed in ref. 19). A study by Renard et al. utilizing transfected T-cell hybridomas that could express only the CD8αα molecule or both CD8αα and CD8αβ molecules showed that the CD8αβ-expressing T-cell hybridomas exhibited a greater sensitivity to peptide compared to the CD8αα-expressing T-cell hybridomas.11 Interestingly, several studies have demonstrated that specific cell lineages can solely express the CD8αα molecule, notably intestinal epithelial lymphocytes, subsets of dendritic cells and natural killer cells.20,21 However, the majority of peripheral CD8+ T lymphocytes express the CD8αβ heterodimer on their cell surface.21
CD8α is associated with the protein kinase Lck, which plays a crucial role in initiating the TCR signalling cascade.22–24 Thus both the αα homodimeric and αβ heterodimeric forms of CD8 have the capacity to promote signal transduction. The basis for the differences in the function of CD8αα homodimers versus αβ heterodimers may lie in their differential localization in the cell membrane. The CD8β cytoplasmic domain contains a palmitoylation sequence that results in trafficking of CD8αβ molecules to lipid raft microdomains in the cell membrane.13,25 Given that TCR signalling components are enriched in these domains following TCR engagement, residence of CD8 associated-Lck at these sites probably facilitates efficient TCR signal transduction.
In our previous studies, we found that both high-avidity and low-avidity CTL can be generated by stimulating cells from P14 TCR transgenic mice with a low versus high concentration of peptide antigen, respectively.14 Differences in avidity were detected regardless of the effector function examined (IFN-γ production or cytolysis) (ref. 14 and data not shown). These established CTL lines exhibited a difference in CD8β cell surface expression. Specifically, the high-avidity CTL expressed more CD8β compared to their low-avidity counterpart, while expressing almost equivalent levels of CD8α, suggesting an increased expression of CD8αβ versus CD8αα molecules in the high avidity cells.14 This led us to hypothesize that the differential expression of CD8 impacted the functional avidity of the cell. Previous studies have supported a role for differential CD8 expression as a mechanism for controlling T-cell responsiveness. For example, male HY TCR transgenic mice have peripheral T cells that bear the self-reactive HY-specific TCR and exhibit low expression of CD8.26–29 While not autoaggressive in vivo, these CD8low cells could produce IFN-γin vitro following stimulation with anti-TCR antibody.27 This finding suggested that the low expression of CD8 resulted in a higher threshold for activation which limited their activation in vivo.
A limitation to the aforementioned studies examining CD8 expression was that they could not determine whether the detectable differences in CD8 cell surface expression were a consequence of the strength of the TCR signal used to generate the CTL, or alternatively whether the cells expressing disparate levels of CD8β were the consequence of selective outgrowth of distinct precursors from the starting population. Here we directly test the hypothesis that the magnitude of stimulation through the TCR regulates the cell surface expression of CD8 following T-cell encounter with peptide antigen. The results from the current studies indicate that responding CTL can actively modulate their CD8 cell surface expression in a dose-dependent fashion, with increasingly higher concentrations of peptide resulting in decreasing overall levels of CD8 expression as well as altered CD8 isoform expression. Notably, modulation of CD8, especially isoform expression, was associated with changes in functional avidity in these cells, suggesting that CD8 is involved in the control of avidity.
Materials and methods
Mice, cell lines and peptides
C57BL/6 mice were obtained from the Frederick Cancer Research and Development Center (Frederick, MD). Lymphocytic choriomeningitis virus (LCMV) TCR transgenic P14/rag-2 mice were purchased from Taconic (Germantown, NY). All experiments in this study comply with the institutional guidelines approved by the Wake Forest Animal Care and Usage Committee. EL4 cells are a C57BL/6-derived thymoma. The LCMV gp33−41 peptide (KAVYNFATM) was synthesized at the Comprehensive Cancer Center Protein Analysis Core Laboratory at Wake Forest University School of Medicine.
Generation and maintenance of CTL lines
For generation of P14 TCR transgenic CTL lines, 5 × 105 P14 TCR transgenic splenocytes were cocultured with 5 × 106 C57BL/6 splenocytes (2000 rads irradiated) previously pulsed with titrated concentrations of the LCMV gp33−41 peptide, as indicated. Cultures were maintained in 24-well plates containing RPMI-1640 medium supplemented with 2 mm l-glutamine, 0·1 mm sodium pyruvate, non-essential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, 2-mercaptoethanol (0·05 mm), 10% fetal bovine serum, and 10% T-stim (BD Biosciences, San Jose, CA). Responding CTL from primary cultures were maintained by weekly stimulation with splenocytes pulsed with either high (10−5 m) or low (10−9 m) concentrations of peptide antigen as described above.
Flow cytometry
On day 7 post-stimulation, 2 × 105 cells were incubated on ice for 30 min with fluorochrome-labelled antibodies followed by washing. Samples were acquired on a FACSCalibur and analysed using the CellQuest Pro software (BD Biosciences, Mountain View, CA). Anti-CD8α (clone 53.6.7), anti-CD8β (clone H35), anti-TCR-β chain (clone H57), anti-CD44, and anti-Thy-1.2 were all obtained from BD Biosciences (San Diego, CA). Anti-CD2 and anti-lymphocyte function-associated antigen-1 (LFA-1) antibody was purchased from Caltag Laboratories (Burlingame, CA). For determining CD8 expression over several weeks and to standardize the values from week to week, fluorescein isothiocyanate and phycoerythrin directly conjugated beads were utilized and the results were calibrated based on the respective bead values. To determine the CD8β : CD8α ratios the CD8β mean fluorescence intensity (MFI) was divided by the CD8α MFI.
Cell sorting and stimulation
P14 TCR transgenic splenocytes were stained with anti-CD8β and anti-CD44. The CD44low cells were sorted based on their CD8β cell surface expression using the FACSAria (BD Biosciences, Mountain View, CA). Recovered cells were divided into two equal fractions and stimulated with splenocytes pulsed with either high (10−5 m) or low (10−9 m) concentrations of peptide antigen. On day 7 post-stimulation, the cell surface expression of CD8α and CD8β on responding T cells was analysed.
Kinetics of CD8 down-regulation and level of CD8 expressed on CTL in different rounds of proliferation
P14 TCR transgenic splenocytes were stimulated with Thy-1.1+ splenocytes pulsed with either a high (10−5 m) or low (10−9 m) concentration of peptide antigen. On day 1 through day 5 post-stimulation, responding CTL were stained with anti-CD8α, anti-CD8β and anti-Thy-1.2 antibodies. In some cases naive P14 TCR transgenic splenocytes were stained with 5 μm carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Carlsbad, CA) before coculture with peptide-pulsed splenocytes.
RNase protection assay
On day 7 post primary, secondary, or tertiary stimulation, viable P14 TCR transgenic cells were isolated by passing over a Ficoll gradient. RNA was isolated from 2·5 × 106 responding CD8βlow or CD8βhigh cells day 7 post primary, secondary and tertiary stimulation with peptide antigen using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The murine cell surface antigen RNase protection kit (BD Biosciences, San Diego, CA) was used to determine the presence of CD8α and CD8β messenger RNA (mRNA). Briefly, radiolabelled probe was allowed to hybridize overnight at 56°. The following day the samples were treated with RNase and run on a 4·75% acrylamide gel. RNA levels were quantified using a phosphoimager.
Peptide dose–response curve
On day 7 post-stimulation, CTL were plated at 1 × 104/well in a 96-well round-bottom microtitre plate. EL4 cells, previously pulsed with titrated concentrations of peptide and washed three times, were added at 1 × 104/well. The cells were incubated overnight at 37° in a 5% CO2 incubator. Supernatant was harvested and assayed for the presence of IFN-γ. The OptEIA antibody set (BD PharMingen, San Diego, CA) was used according to the manufacturer's directions.
Results
CD8 cell surface expression correlates inversely with the amount of antigen used for stimulation
Previously published data from our laboratory have demonstrated that high-avidity and low-avidity CTL lines can be generated independently of differences in their TCR affinity. Examination of these lines for the cell surface expression of CD8 revealed that high-avidity CTL expressed nearly two-fold more CD8β on their cell surface compared to low-avidity CTL.14 This finding led us to hypothesize that naive CD8+ T cells may possess the potential to alter the expression of the TCR coreceptor CD8 in response to the magnitude of the TCR signal experienced at its initial priming.
To test this hypothesis, naive P14 TCR transgenic splenocytes were stimulated with titrated concentrations of peptide, ranging from 10−5 m to 10−9 m, and on day 7 post-stimulation the expression of CD8α and CD8β was assessed on the responding CTL populations. Day 7 was chosen for analysis because any transient changes in cell surface expression of relevant molecules that occurred as a result of early antigen contact should have returned to baseline. At this time-point, a dose-dependent difference in the cell surface expression of both CD8α and CD8β was detected. Cells stimulated with 10−5 m antigen expressed the lowest level of CD8α and CD8β molecules, whereas CTL stimulated with 10−9 m expressed the highest levels (Fig. 1). This was not a non-specific consequence of the addition of peptide at high levels because an irrelevant peptide had no effect on CD8 expression (data not shown). Interestingly, in both cases the responding CTL showed an overall increased expression of CD8α and CD8β compared with naive cells, suggesting that increased expression of CD8 is a general result of activation. These data show an inverse correlation between the priming concentration of peptide antigen and CD8 expression on the responding CTL.
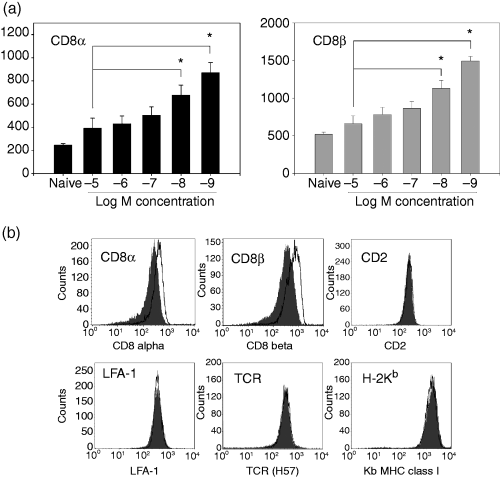
CD8 cell surface expression is modulated in a dose-dependent manner as a result of antigen encounter. Naive P14 TCR transgenic splenocytes were stimulated with C57BL/6 splenocytes pulsed with the indicated peptide concentrations. On day 7 post-stimulation the responding CTL were analysed for the cell surface expression of CD8α (black bars) and CD8β (grey bars) (a). The data shown are the average of at least three independently generated CTL lines. Representative histograms for the cell surface expression of CD8α, CD8β, CD2, LFA-1, H-2Kb, and TCR on P14 TCR transgenic splenocytes on day 7 post-stimulation with the high (10−5 m) (dark grey shaded plot) or low (10−9 m) (bold black line) concentration of antigen (b). *P < 0·05 on paired Student's t-test.
One possibility that could explain the above results was that T cells stimulated with the highest antigen concentration exhibited a generalized down-regulation in the expression of cell surface molecules compared to their counterparts stimulated with the low antigen concentration. To preclude this possibility, we assessed the expression of additional cell surface proteins (TCR, CD2, LFA-1, H-2Kb) on responding T cells at day 7 post primary stimulation with 10−5 m or 10−9 m antigen. In contrast to the differences observed in the CD8α and CD8β profiles, high and low antigen-responding CTL express very similar levels of the other cell surface proteins examined (Fig. 1b). Among the multiple lines that were generated, we occasionally recorded increased cell surface expression of TCR on the CTL generated following stimulation with 10−9 m versus 10−5 m peptide; however, this was not a consistent finding and the fold change was always less than that observed for CD8 expression. Thus the dose-dependent regulation of expression observed following stimulation with peptide antigen occurred selectively for the CD8 molecule.
Responding CTL with decreased expression of CD8 are not the result of selective outgrowth of CD8lowcells present within the naive population
Although the above finding demonstrated that in responding CTL populations, the expression of CD8 correlated inversely with the amount of peptide antigen used for stimulation, they do not discriminate between the selective outgrowth of a CD8low or CD8high population versus modulation of CD8 on individual cells. Our previous analyses had shown that within the naive P14 TCR transgenic T-cell population, the expression of CD8 varied by approximately two-fold (our unpublished data). This left open the possibility that the differences we observed above could be the result of selective activation and/or outgrowth of naive cells with higher or lower expression of CD8. To address this possibility, we sorted naive P14 TCR transgenic splenocytes based on their CD8β expression profile; the sort strategy and the CD8 profile of the sorted cells are shown in Fig. 2(a). Cells were costained with CD44 to ensure that only naive cells (CD44low) were included in the analysis. CD44low naive P14 TCR transgenic splenocytes that expressed either low levels of CD8β (10–15% of the total CD8+ population with the lowest MFI) or high levels of CD8β (10–15% of the total CD8+ population with the highest MFI) were sorted. The recovered CD8βlow and CD8βhigh populations exhibited at least a two-fold difference in their CD8β expression (Fig. 2a). The sorted cells, as well as unsorted cells, were divided and stimulated with 10−5 m or 10−9 m antigen. On day 7 post-stimulation, CD8 cell surface expression was examined. We found that regardless of the initial CD8β expression level, CD8α and CD8β expression levels were higher following stimulation with the low antigen concentration compared to their counterparts stimulated with the high antigen concentration (Fig. 2b). In addition, the CD8 expression patterns on the sorted cells and the unsorted P14 TCR transgenic splenocytes were similar following stimulation with the respective antigen concentration. These data strongly suggested that the CD8 differences observed following stimulation with high or low antigen concentration were not a result of selective outgrowth a CD8βlow or CD8βhigh subset, but rather that the level of CD8 expression present in resting cells could be tuned as a result of the initial encounter with antigen.
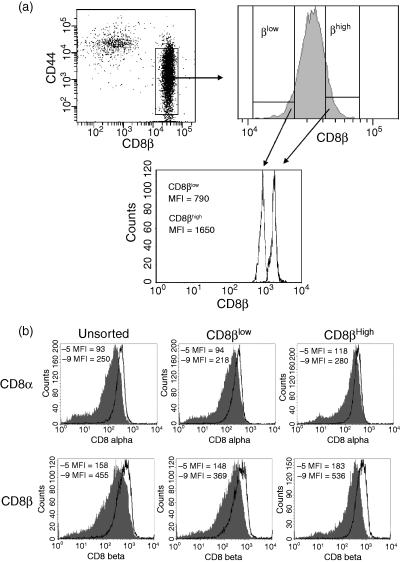
CD8 modulation on sorted CD8βlow or CD8βhigh naive P14 splenocytes following stimulation with a high versus low concentration of antigen. Naive P14 TCR transgenic splenocytes were costained with antibodies to CD44 and CD8β, and CD44low CD8βlow or CD44low CD8βhigh sorted cells. The gating strategy and recovered populations are shown in (a). The sorted cells were then split into two groups and stimulated with either the high (10−5 m) (shaded histogram) or low (10−9 m) (open histogram) concentration of antigen. An unsorted naive P14 TCR transgenic splenocyte sample was included as a control. Day 7 post-stimulation the cell surface expression of CD8α (upper panel) and CD8β (lower panel) was analysed (b). The geometric MFI for CD8 expression is indicated. The sort and post-sort analyses were performed using different instruments (FacsAria versus FacsCalibur). This accounts for the differences in the absolute MFI intensities in A compared with B. The data shown are representative of three experiments.
Kinetics of CD8 expression modulation following peptide encounter
We next determined whether the modulation of CD8 expression occurred immediately following TCR engagement or whether, as has been noted with other molecules, e.g. CD62 ligand,30 altered expression occurred as a function of division. To address this question, CFSE-labelled naive P14 TCR transgenic splenocytes (Thy-1.2+) were cocultured with Thy-1.1+ C57BL/6 splenocytes pulsed with either 10−5 m or 10−9 m peptide. At the indicated day post-stimulation, the responding CTL were stained with antibodies to Thy-1.2, CD8α and CD8β, and the CD8 profile of the CTL in different rounds of proliferation was examined. No proliferation was detected day 1 post-stimulation at any peptide antigen concentration. However, by day 2 post-stimulation, the cells cultured in the presence of antigen-presenting cells pulsed with the high or low concentration of antigen had undergone several rounds of division (Fig. 3a). Interestingly at day 2 post-stimulation, independent of the concentration of peptide antigen and round of proliferation, the responding CTL expressed similar levels of both the CD8α (Fig. 3b) and CD8β (Fig. 3c) proteins. Since we could not detect a difference in CD8 expression as a result of gradual outgrowth of a CD8low/high population during the early proliferative response, we examined the cell surface expression of CD8 at later days post-stimulation to determine when the CD8low and CD8high populations emerged. By day 3 post-stimulation the majority of the 10−5 m and 10−9 m CTL were CFSE-negative (data not shown), thus CD8 regulation as a function of division number could not be determined at this time.
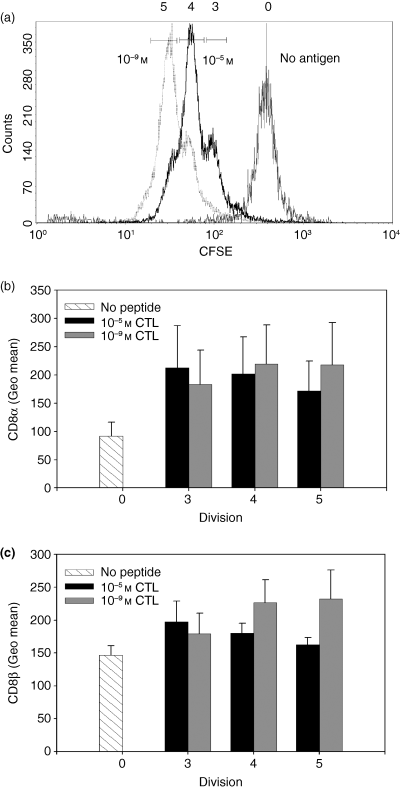
Dose-dependent modulation of CD8 cell surface expression was not evident during the initial rounds of proliferation. (a) Naive P14 TCR transgenic splenocytes labelled with CFSE were stimulated with Thy-1.1+ splenocytes with either unpulsed (thin black line) or pulsed with a high (10−5 m) (bold black line) or low (10−9 m) (grey dotted line) concentration of antigen. On day 2 post-stimulation, the cells were stained with αThy-1.2, αCD8α, and αCD8β antibodies and the cell surface expression of CD8α (b) and CD8β (c) was analysed on responding Thy-1.2+ CTL. The CD8α and CD8β expression on no antigen (stripped bars), 10−5 m (black bars) or 10−9 m (grey bars) stimulated cells in different rounds of proliferation is shown. The data shown are the average of three independent CTL lines.
A subsequent kinetic analysis was performed to address CD8 expression at later time post-stimulation. The data in Fig. 4(a) show that similar to what was observed in the previous analysis, no difference in the expression of CD8α (Fig. 4a) or CD8β (Fig. 4b) could be detected on day 1 or day 2 post-stimulation. However, between days 3 and 4 post-stimulation a difference in CD8α and CD8β cell surface expression became evident. The 10−9 m CTL began to increase cell surface expression of both CD8α and CD8β at day 3 post-stimulation and expressed maximal levels by day 4. However, the 10−5 m CTL only modestly increased CD8α and CD8β expression during this timeframe. These data show that the dose-dependent difference in CD8α and CD8β expression is not present immediately following stimulation, but rather occurs after the initial proliferative burst.
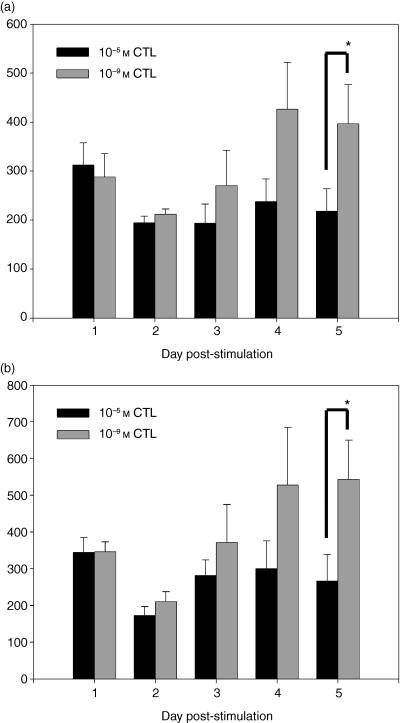
Dose-dependent modulation of CD8 occurred following the initial proliferative burst. Naive P14 TCR transgenic splenocytes were stimulated with Thy-1.1+ splenocytes pulsed with a high (10−5 m) (black bars) or low (10−9 m) (grey bars) concentration of antigen. The expression of CD8α (a) and CD8β (b) was determined on Thy-1.2+ cells on the indicated days post-stimulation. *P < 0·05 as determined by paired Student's t-test. The data shown are the average of the data obtained from three independent CTL lines.
Continued modulation of CD8 level and isoform expression during subsequent antigen encounter
The previous studies demonstrated that naive T cells were capable of modulating CD8 cell surface expression following primary encounter with antigen; however, whether T cells could continue to modulate their CD8 expression following additional peptide encounters was unknown. To address this question, we examined the CD8α and CD8β(Fig. 5a,b) cell surface expression patterns on the 10−5 m and 10−9 m CTL post primary, secondary, and tertiary stimulation. In agreement with the data shown in Fig. 1, T cells initially stimulated with a low concentration of peptide exhibited significantly higher levels of CD8α and CD8β compared to cells stimulated with a high concentration of peptide. Following secondary encounter with antigen, both CTL populations exhibited a decrease in CD8α and CD8β cell surface expression compared to the levels present following primary stimulation; however, the decrease was greater on cells stimulated with a high dose of antigen. This resulted in a widening of the difference in the CD8α and CD8β expression between the CTL populations. Following primary stimulation, the fold difference in the expression of CD8α on 10−9 m CTL versus 10−5 m CTL was 1·8 ± 0·1, whereas following secondary stimulation it had increased to 3·7 ± 0·5. Similarly for CD8β expression, the fold difference changed from 1·7 ± 0·2 following primary stimulation to 3·0 ± 0·4 following secondary stimulation. Subsequent stimulation resulted in a continued decrease in the absolute level of CD8, most dramatically for CD8α expression on the 10−9 m CTL (Fig. 5a,b). The greater decrease in CD8α compared to CD8β on the 10−9 m CTL resulted in significant changes in the ratio of CD8β : CD8α between the 10−5 m and 10−9 m CTL (Fig. 5c). Although the 10−5 m and 10−9 m CTL expressed significantly different levels of CD8α and CD8β post primary stimulation, the CD8β : CD8α ratios were similar. The ratio of the expression of these two molecules is important because it provides insights into the isoform of CD8 expressed by these cells. CD8 can be expressed as either an αα homodimer or an αβ heterodimer.18 Thus as the β : α ratio decreases, CD8β becomes limiting and a greater proportion of CD8 must be in the αα homodimeric form.
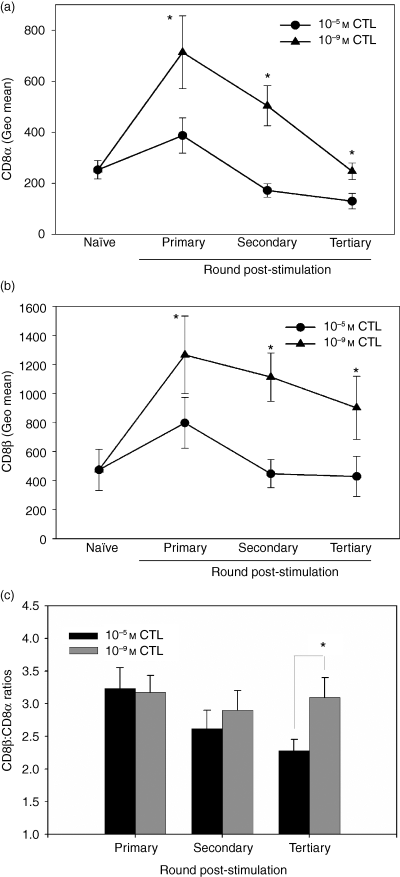
Differential modulation of CD8α and CD8β following multiple stimulations. The CD8α (a) and CD8β (b) expression level was examined on naive P14 splenocytes before their encounter with antigen and following primary, secondary and tertiary stimulation with a high or low concentration of antigen. The CD8β : CD8α ratio was calculated following each round of stimulation; it was determined by dividing the CD8β MFI by the CD8α MFI (c). The data in (a) to (c) are the average of at least five independently generated CTL lines. *P < 0·05 as determined by paired Student's t-test.
In contrast to CD8 expression following primary stimulation, repeated peptide stimulations resulted in an increasingly reduced CD8β : CD8α ratio in the 10−5 m CTL (Fig. 5c), suggesting an increase in the percentage of CD8 molecules of the αα isoform. However the CD8β : CD8α ratio on the 10−9 m CTL remained relatively constant (Fig. 5c). These data show that the responding CTL continue to modulate both their absolute level of CD8 and the relative expression of CD8α versus CD8β molecules with successive peptide encounters.
CD8α and CD8β mRNA levels
Having determined that the cell surface expression of CD8 can be modulated following stimulation with different concentrations of antigen, we sought to gain insights into the mechanistic basis for these expression differences. We tested the hypothesis that alterations in CD8 mRNA levels may contribute to CD8 cell surface levels. The mRNA was isolated from responding CTL on day 7 following primary, secondary, and tertiary stimulation and CD8 message levels were determined using an RNase protection assay. Surprisingly, although we observed differences in CD8α and CD8β cell surface expression on responding CTL following their primary stimulation with the high versus low concentrations of antigen, there was no difference in the CD8α or CD8β mRNA levels in the cells stimulated with the two concentrations of peptide antigen (Fig. 6a). However, following secondary stimulation, a significant difference between the T cells in both CD8α and CD8β mRNA levels was detected. CTL stimulated with the low antigen concentration expressed nearly two-fold more CD8α and CD8β mRNA compared to CTL stimulated with the high antigen concentration (Fig. 6b). Similar differences were also apparent in tertiary stimulated cells (Fig. 6c). A similar correlation between the steady-state level of mRNA and cell surface expression of CD8α and CD8β was also observed in established CTL lines (data not shown). As with the analysis of cell surface molecules (TCR, CD2, LFA-1 and H-2Kb), the difference in mRNA levels was specific to CD8, as CD45 and CD3 mRNA levels were in general comparable in the two populations (Fig. 6a–c). Thus following two or more rounds of stimulation, mRNA levels were correlated with the cell surface expression of the CD8α and CD8β proteins. This is consistent with the hypothesis that differences in the steady-state mRNA levels contribute to differences in cell surface expression at these times.
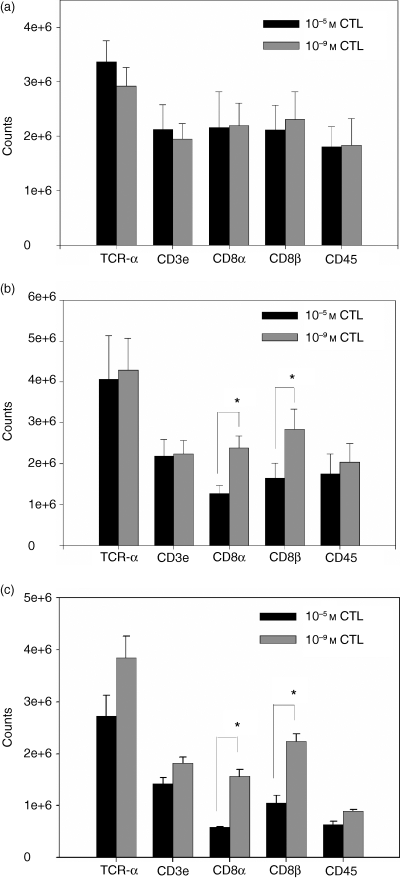
The steady-state mRNA levels of CD8α and CD8β following secondary and tertiary stimulation correlate with CD8 cell surface expression. RNA was isolated from 10−5 m (black bars) and 10−9 m (grey bars) CTL populations following primary (a), secondary (b), or tertiary (c) stimulation and a multiprobe RNase protection assay performed. The amount of RNA loaded between samples was determined by normalization to the RPA standard L32. At least three individual CD8low (10−5 m) and CD8high (10−9 m) CTL lines were analysed at each time-point. *P < 0·05 as determined by paired Student's t-test.
Changes in CD8 isoform expression correlate with differences in functional avidity
We predicted that cells with differences in CD8 would display correlative differences in functional avidity. To determine if this was the case, we examined the production of IFN-γ by CD8low and CD8high CTL over a range of peptide concentrations following primary, secondary and tertiary stimulations. No difference in functional avidity was detected between the CD8low and CD8high responding CTL post primary stimulation (Fig. 7a). However, post secondary stimulation a difference in functional avidity could be detected between the CD8low and CD8high CTL and this difference increased following tertiary stimulation (Figs 7b,c). It was surprising that differences in functional avidity were not apparent following primary encounter with antigen, given the significant differences in CD8 expression on these CTL populations. It is possible that to exhibit low avidity, cells must lower CD8 beyond a discrete threshold that is not yet reached following primary antigen encounter. Alternatively, over multiple encounters with peptide, the ratio of CD8β : CD8α is also changing (Fig. 5c). Thus the changes in isoform, either alone or in combination with absolute changes in the level of CD8, may be the determinant of avidity. Regardless, these data suggest that responding CTL continue to ‘fine-tune’ their sensitivity to peptide over multiple encounters with antigen and that differences in CTL avidity correlate with changing CD8 levels and CD8β : CD8α ratios.

Differences in functional avidity are detected following repeated peptide stimulation. The functional avidity of responding CTL was examined day 7 post primary (a), secondary (b), or tertiary (c) stimulation with 10−5 m or 10−9 m peptide antigen. EL4 cells previously pulsed with the indicated antigen concentrations were cocultured with the CTL overnight and the quantity of IFN-γ produced was determined by enzyme-linked immunosorbent assay. The data in (a) to (c) are each representative of three independent experiments. The values in the inset box are the amount of IFN-γ (pg/ml) produced at the highest peptide concentration.
Discussion
It is well established that the ability of a CD8+ T-cell to recognize an antigen-presenting cell bearing a defined level of pMHC (i.e. the cell's functional avidity) is a critical parameter in predicting in vivo efficacy.2–8 Cells of higher functional avidity are much more effective at reducing viral burden compared to those with lower avidity. However, precisely how the sensitivity of CD8+ T cells to pMHC is determined is poorly understood. One hypothesis is that functional avidity is an inherent property of a cell. In this scenario, individual clones exist within the naive CD8+ T-cell population that can respond to antigen-presenting cells bearing a distinct concentration of pMHC complexes. The alternative hypothesis is that functional avidity is an induced property and is determined by the signals received during activation. It is important to note that these two mechanisms are not mutually exclusive, e.g. the naive repertoire may contain clones with inherently different sensitivities which can be tuned as a result of antigen encounter. Here we tested the hypothesis that the initial encounter with an antigen-presenting cell bearing a defined concentration of pMHC can direct the sensitivity/avidity of the responding CTL.
We hypothesized that one mechanism by which cells could tune their sensitivity was through active modulation of CD8 expression. CD8 was an attractive candidate given its well-established role in facilitating TCR signal transduction.9–17 In our study, naive P14 CD8+ T cells were stimulated with graded concentrations of peptide antigen and CD8 cell surface expression was assessed. These analyses revealed an inverse correlation between CD8 cell surface expression and the concentration of antigen used for stimulation. Naive P14 TCR transgenic CD8+ T cells stimulated with the highest concentration of antigen exhibited the lowest cell surface expression of CD8α and CD8β in comparison to cells stimulated with lesser antigen concentrations. Dose-dependent modulation of CD8 was not restricted to the P14 system, as similar studies in the OT-1 TCR transgenic model yielded comparable results (data not shown). The change in CD8 expression was not a transient effect following antigen encounter, as these analyses were performed on day 7 post-stimulation. In fact dose-dependent differences in cell surface expression were not immediately apparent following antigen encounter, but instead occurred at ≥ day 3 post-stimulation. While it is unknown at this time, this result suggests that a differentiation event occurs following or near the end of the proliferative burst. In contrast to the changes in CD8 expression, examination of the cell surface molecules LFA-1, CD2 and H-2Kb indicated that the responding CTL stimulated with the high or low antigen concentration exhibited similar levels of these molecules.
Our initial studies left open the possibility that the differences in CD8 expression were the result of the selective outgrowth of cells with disparate CD8 expression. However, stimulation of naive cells sorted for high versus low levels of CD8β expression showed that regardless of the initial level of CD8β cell surface expression, stimulation with a high antigen concentration yielded a responding population expressing a low level of CD8, whereas stimulation with a low antigen concentration resulted in a responding population expressing a high level of CD8. Further subsequent stimulation resulted in modulation of CD8β : CD8α ratios, suggesting changes in isoform expression. These results established the ability of naive CD8+ T cells to actively modulate their CD8 cell surface expression as a result of encounters with high versus low levels of peptide antigen. These data significantly expand our understanding of CD8 modulation. While it has been shown previously that repetitive stimulation with high levels of antigen could result in down-modulation of CD8 and functional non-responsiveness,27,28 to our knowledge this is the first report where escalating amounts of presented pMHC have been shown to result in corresponding dose-dependent modulation of both the level and isoform of cell surface CD8 on peripheral resting naive T cells that correlates with differences in the sensitivity of the T cell for recognition of its cognate peptide. Importantly this is not simply modulation to a high or low expression of CD8, but across a range that allows dose-dependent tuning to a discrete level based on the amount of antigen encountered. Thus previous studies where CD8 was down-regulated to the point of non-responsiveness may be the extreme end of the phenomenon observed in our studies. However, it is critical to note that CD8 down-modulation is not a mechanism solely to turn off cells, but appears to be a mechanism to generate highly functional cells that respond optimally to a discrete level of peptide.
A previous report by Konno et al. examined the CD8β expression pattern of peripheral human CD8+ T cells, and detected three different CTL subsets denoted as CD8α+ CD8βhigh, CD8α+ CD8βlow and CD8α+ CD8β– (CD8αα) in adults. The fraction of CD8α+ CD8βlow and CD8αα increased with age whereas the CD8α+ CD8βhigh fraction decreased. Performing TCRVβ CDR3 spectratyping, they detected clones in each CD8β fraction with identical sequences, suggesting that an individual T-cell clone gave rise to the CD8α+ CD8βhigh, CD8α+ CD8βlow and CD8αα cells. Additionally they determined that the CD8α+ CD8βlow and CD8αα cells had undergone more rounds of proliferation than the CD8α+ CD8βhigh subset.31 Our data are consistent with the notion that CD8 expression levels are induced as a result of antigenic stimulation; however, we do not observe the programmed differentiation of CD8α+ CD8βhigh→CD8α+ CD8βlow→CD8αα cells following peptide encounter. In our studies, CD8 can remain high if peptide presentation levels are minimal. The reasons for these differences are unknown but based on our results, we would speculate that they might reflect the antigen exposure history of the cells studied.
CD8 down-regulation has also been observed following stimulation of naive T cells in the presence of IL-4 and neutralizing IFN-γ antibody.32,33 The latter suggested that one possibility to explain low CD8 expression in our system was that high levels of peptide were inducing the production of IL-4. However, this was not the case because addition of neutralizing IL-4 antibody did not affect CD8 modulation (data not shown). Furthermore, a study by Chidgey et al. demonstrated that the percentage of thymocytes that expressed high levels of CD8β was decreased following stimulation with a high level of antigen, suggesting that in the presence of a strong agonist these cells appeared to be shifting the isoform of CD8 expressed from CD8αβ to CD8αα.34 This study agrees with the data presented here that T cells modulate their CD8 expression following encounter with different antigen concentrations. While we did not observe down-regulation of only the CD8β molecule, we did find that CD8β expression levels decreased to a greater extent than CD8α in cells stimulated with high concentrations of antigen, presumably resulting in a shift in isoform expression such that more CD8αα molecules are expressed. Thus there may be similarities in CD8 regulation in CD8+ thymocytes undergoing developmental maturation and mature T cells in the periphery.
One possibility to explain the differential cell surface expression was intracellular retention of CD8 in cells stimulated with high antigen concentrations. However, staining for CD8α and CD8β following permeabilization suggested that this was not the case (data not shown). Thus we investigated the possibility that differences in the steady-state level of CD8α and CD8β mRNA contributed to differences in cell surface expression. We found that the steady-state CD8α and CD8β mRNA levels in cells following multiple rounds of stimulation (Fig. 6) and in established cell lines (data not shown) correlated with cell surface expression. These differences in the level of CD8 mRNA could be the result of alterations in the rate of gene transcription or the rate of mRNA turnover. One surprise arising from these studies was that there was no difference in CD8 mRNA levels following primary stimulation with a high compared with a low concentration of antigen, even though there was a significant difference in surface expression. This finding indicated that cell surface expression was controlled by an alternative mechanism following primary activation. It is tempting to speculate that this alternative regulatory mechanism allows cells to retain maximum flexibility for the regulation of CD8 expression. For example, if a cell had initially down-regulated CD8 as a result of high antigen exposure, it may retain the ability to up-regulate CD8 if a cell bearing a lower level of antigen were subsequently encountered. Once regulation at the mRNA level occurs, it may be harder to reverse the down-regulation of CD8, resulting in a loss of plasticity. It is clear that at some point, cells can no longer adapt to stimulation with different levels of pMHC. Established CTL lines of high or low avidity cannot alter their avidity when stimulated with inappropriate levels of peptide.35 High avidity cells undergo tumour necrosis factor-α-mediated death as a result of supraoptimal stimulation, while low avidity cells will die by neglect if stimulated with a suboptimal amount of antigen. When cells reach this fixed state is an area of active investigation.
In addition to changes in the level of CD8α and CD8β at the cell surface, it is possible that other modifications contribute to avidity. Changes in CD8 glycosylation occur as a result of activation and have been shown to affect binding to pMHC.36 It was feasible that differential glycosylation of CD8 was contributing to the avidity of the CTL in our study. However, treatment of CD8high or CD8low cells with neuraminidase resulted in a similar affect on tetramer binding in that the increase in tetramer binding was equivalent in the two populations (data not shown). Therefore, glycosylation differences do not appear to be the mechanism employed by the CTL studied here to modulate peptide sensitivity. Furthermore, in addition to CD8α and CD8β, several other signalling molecules, such as LCK, Zap-70 and LAT, either localize in or are recruited to lipid rafts following TCR engagement. Changes in the expression or localization of these molecules could contribute to the peptide requirement. In this regard analyses of the expression of Lck15 and LAT (our unpublished data) in established lines suggested that levels of these proteins do not differ significantly in the cells stimulated with high versus low peptide concentrations (data not shown).
Previous work from our laboratory showed that while established high and low avidity CTL lines expressed similar levels of CD8α on their cell surface, the high-avidity CTL expressed nearly two-fold more CD8β compared to their low-avidity counterpart.14 These data, together with the observation reported here that expression continues to be modulated over time, suggest that the control of CD8 expression is complex. Specifically the way in which CD8 is modulated appears to change over time, with CD8α and CD8β similarly modulated in the initial stimulation and CD8α more highly modulated with subsequent stimulation. At some point CD8 expression stabilizes in the high-avidity versus low-avidity cells with relatively similar CD8α levels in the face of divergent β levels.
As noted previously, an increased ratio of CD8β : CD8α is consistent with enrichment for CD8αβ heterodimers compared with CD8αα homodimers in cells stimulated with the low antigen concentration compared to cells stimulated with the high antigen concentration. Overall, therefore, as T cells undergo multiple encounters with peptide, the absolute level of CD8 is decreasing and the isoform expression is changing. Together these data suggest that the contribution of CD8 to avidity may be through changes in both the absolute level of CD8 (i.e. cells must decrease expression past a defined threshold to exhibit low avidity) and the ratio of CD8αα versus CD8αβ on the cell (i.e. increased expression of αα homodimers is necessary for low avidity).
The finding that the avidity of the effector cells generated as a result of a single stimulation with the high antigen concentration compared with the low antigen concentrations did not differ in spite of significant differences in CD8 expression was surprising. A number of possibilities could account for this finding. One is that CD8 levels do not solely account for the control of avidity. Given the large body of work showing a role for CD8 in signal transduction and in controlling peptide sensitivity,1,9–17,37,38 it is hard to discount altogether the role of CD8. Instead, in this scenario another molecule, i.e. one involved in signal transduction or membrane targeting of CD8, must also be regulated for the avidity changes to become apparent. For example, differential palmitoylation of CD8, which would alter its localization to lipid rafts,25 could impact the ability of CD8 to function efficiently. Alternatively it is possible that the ratio of CD8β : CD8α is the critical determinant of avidity. This would be in keeping with the finding that differences in avidity increase as differences in the ratio of CD8β : CD8α widen. Discriminating between these possibilities will require further study.
The changes in CD8 expression and peptide sensitivity appear to be the only difference between the cells stimulated with the high versus the low concentrations of antigen. Analysis of molecules associated with effector versus memory cell differentiation, e.g. CD44, CD43, CD62L, CD123 and CCR7, showed similar expression patterns (data not shown). There was no indication that these cells differentiated into TC2 cells39 or CD8low suppressor cells40 because no IL-4 production was detectable (data not shown).
In summary, the work presented here shows that dose-dependent modulation of CD8 cell surface expression can occur following stimulation with peptide antigen. Following the initial encounter of naive cells with antigen, cells up-regulate CD8 expression; the extent to which this occurs is determined by the level of peptide encountered. Modulation of CD8 occurred subsequent to the initial proliferative burst, consistent with a differentiation event, and continued through subsequent encounters with antigen, with increasing antigen concentrations resulting in decreasing CD8 at the cell surface. Changes occurred in both the absolute level and the relative isoform of CD8 expressed. Together, these results support the ability of responding CTL to ‘fine tune’ their sensitivity to different antigen concentrations by active modulation of CD8.
Acknowledgements
We thank Dr Griff Parks, Dr Beth Hiltbold, Dr Jason Grayson and Dr Drew Cawthon for helpful discussions with regard to this manuscript. This work was supported by National Institutes of Health grant AI43591 (to M.A.A.-M). C.J.K. was supported by a National Research Training Award: Grant AI07401.




