The application of genetics to inherited bleeding disorders
Abstract
Summary. Most bleeding disorders encountered in clinical practice will be diagnosed, at least initially, by phenotypic assays. However, since the characterization of the genes that encode coagulation factors in the 1980s, significant progress has been made in translating this knowledge for diagnostic and therapeutic purposes. For the haemophilias, in particular, molecular genetic testing to determine carrier status, prenatal diagnosis and prediction of the likelihood of inhibitor development has now become an established component of comprehensive clinical management. For von Willebrand’s disease (VWD), significant recent advances have allowed for the establishment of genotype–phenotype correlations that have improved our understanding of the disease. The availability of high density single nucleotide polymorphism (SNP) maps will allow investigators to probe the genetic basis of the general symptoms of bleeding and bruising using a comprehensive genome-wide approach. This article will review the state-of-the-art for molecular diagnostics for both haemophilia and VWD and will end with a discussion of plans for an international genome-wide association study (GWAS) designed to improve our understanding of blood coagulation.
The role of genetic testing for von Willebrand’s disease
Von Willebrand’s disease is the most common inherited bleeding disorder known in humans, with prevalence estimates as high as 1% [2,3]. While an objective personal history of excessive mucocutaneous bleeding can usually be obtained from the patient, the documentation of a family history of the disease may not always be possible, and laboratory tests of haemostasis can be variable in their ability to reveal either a quantitative or qualitative defect of von Willebrand factor (VWF), making the diagnosis of VWD challenging in some situations. With these issues as background, this review will consider the role of molecular genetic analysis as a complementary diagnostic modality, particularly where existing clinical and laboratory approaches to diagnosis have failed to provide a definitive answer.
The von Willebrand factor gene
The VWF gene was cloned in 1985 by four groups in the US and Europe [4–7]. The gene is located on the short arm of chromosome 12 at the locus 12p13.3 [4]. It spans approximately 178 kb and contains 52 exons that range in size from 1.3 kb (exon 28) to 40 bp (exon 50) [5]. Analysis of the VWF gene is complicated by at least two other factors in addition to size: (1) there is a partial pseudogene on chromosome 22 with 97% sequence homology to exons 23–34 that necessitates the use of carefully selected gene-specific PCR amplification primers for this region [6] and (2) the VWF locus is highly polymorphic (to date >150 polymorphisms have been reported) (http://www.vwf.group.shef.ac.uk). This makes direct VWF sequencing the methodology of choice for genetic analysis, given that mutation screening approaches such as conformation sensitive gel electrophoresis and denaturing high performance liquid chromatography will be complicated by the frequent sequence variants.
The role of genetic testing for each of the current VWD subtypes (Types 1, 2A, 2B, 2M, 2N and 3 VWD), established by the International Society on Thrombosis and Haemostasis, will be reviewed below [7].
Type 1 VWD
Type 1 VWD, a partial deficiency of qualitatively normal VWF, represents the most common form of the disease and is the most problematic in terms of its diagnosis. The genetic basis of Type 1 VWD has been the focus of much recent investigation and three large multicentre trials have reported consistent results on ∼300 families [11–13]. Mutations (predominantly missense) were identified in ∼65% of index cases and were found more frequently, and with higher penetrance, in cases with lower VWF levels. The most frequently reported genetic variation (10–20% of index cases) identified in all studies was a missense mutation resulting in an amino acid substitution of tyrosine to cysteine at codon 1584 (Y1584C) [14]. Importantly however, some Type 1 VWD patients had no obvious VWF mutation identified and in these (often milder) cases, the genetic determinants are likely to be more complex and could involve other genetic loci. These studies have therefore confirmed prior suspicions that the genetic basis of this condition is highly variable. This genetic complexity precludes the use of molecular genetic testing as a complementary diagnostic aid in the majority of Type 1 VWD cases at the present time.
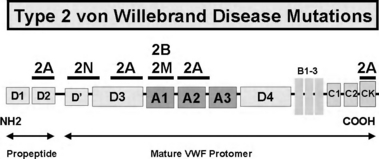
Type 2 VWD
Type 2A VWD accounts for ∼10% of all VWD cases and is characterized by the loss of high and intermediate molecular weight multimers. Type 2A VWD has been associated with more than 50 different missense mutations that result in two types of pathogenetic mechanisms: either aberrant VWF dimer or multimer biosynthesis (group I mutations) or the synthesis of a protein with enhanced susceptibility to A disintegrin-like and metalloprotease with thrombospondin type 1 results (ADAMTS13)-mediated proteolysis (group II mutations) [15,16]. In addition to providing further insights into VWF structure/function, genetic testing for Type 2A VWD can be employed when phenotypic uncertainty exists.
Type 2B VWD is the result of ‘gain-of-function’ mutations within the GpIb binding site on VWF. This leads to an increase in VWF–platelet interactions that result in the selective depletion of high molecular weight (HMW) multimers [8,9] and subsequent thrombocytopenia. The diagnosis of Type 2B VWD is of therapeutic importance given the relative contraindication of desmopressin in managing these patients, and genetic testing can be helpful in this regard, particularly if interpretation of phenotypic assays is difficult.
Type 2M VWD is characterized by decreased VWF–platelet interactions not caused by abnormal multimers. Causative mutations have been localized to the platelet GPIb binding site, in the A1 domain of VWF [19,20], although at distinct locations from Type 2B mutations [10]. Genetic testing can be helpful, although the main therapeutic importance of Type 2M is a poor response to desmopressin, which can usually be identified through a therapeutic trial.
Type 2N VWD was first described as an autosomal form of haemophilia A [11] and is an important differential in the investigation of all individuals (male and female) presenting with a low factor VIII (FVIII) level. The ease of analysis of exons 17–25 of the VWF gene and the relative lack of availability of FVIII binding assays has increased interest in using genetic testing to confirm this diagnosis [12]. With the different pattern of inheritance and different treatments, the distinction between Type 2N VWD and mild haemophilia A is important, and is one that can be definitively resolved with genetic analysis.
Type 3 VWD
In most instances, the severe clinical phenotype, absent plasma VWF and very low FVIII (<0.10 U mL−1) Ievels make the diagnosis of Type 3 VWD straightforward. Despite this, Type 3 VWD individuals may be interested in genetic testing/counselling for future family planning purposes and mutation detection can provide definitive information that can be utilized for prenatal testing. Type 3 VWD has a heterogeneous mutational basis with more than 80 different mutations having been described to date including VWF gene insertions, nonsense and missense mutations as well as partial and total VWF gene deletions [24–26]. In addition to its use in the setting of family counselling, especially for prenatal diagnosis, VWF genotyping may be of value with regard to predicting the likelihood of anti-VWF alloantibody development following exposure to therapeutic concentrates [25,27,28].
New genetic strategies in bleeding disorders (genome-wide association studies)
Over the last 40 years, the remarkable advances in the field of genetics have allowed scientists to identify most of the genes responsible for common and rare Mendelian disorders. The ‘low hanging fruit’ has been picked and we are enjoying the results. Currently, if medically needed, the sequencing of coagulation F8 or F9 genes in the haemophilia patient and the determination of carrier status in the mother is a fairly trivial procedure, which allows for adequate genetic counselling. Interestingly, despite our knowledge of the genes responsible for the most common bleeding disorders, the genetics basis for the variability observed in clinical bleeding is poorly understood. For example, mucocutaneous bleeding disorders without a clear aetiology may represent a complex trait with environmental and genetic influences. The genetic component may be determined by the additive effect of many genes with modest-to-moderate effect for each.
In general, the genetic analysis of these complex traits has proven to be highly challenging. Association and linkage studies have been very successful for single gene conditions but their characterization in complex disorders has had limited success. For reasons of the mix of multiple genetic and environmental contributing factors, large families or populations are needed to identify genes of even modest impact. While linkage studies focus on shared chromosomal segments among affected individuals that are closely related, association studies typically compare the frequency of a specific genetic variant in affected individuals to unaffected controls. This can be performed with known functional variants or with markers that are closely positioned to the causative allele [utilizing a phenomenon known as linkage disequilibrium (LD)] [13].
Association studies are known to provide greater statistical power than linkage studies for complex disorders. However, the traditional case-control approach is limited by the low number of candidate genes available, and also by the lack of replication in subsequent independent studies [14]. The availability of high-density SNPs maps now allows investigators to perform the search of gene variants involved in disease through whole genome association. This particular approach has sparked a large number of GWAS. These kinds of studies are somewhat limited by their substantial cost. However, the fast decrease in cost of SNP genotyping has made them much more attainable in recent years [15–17].
A significant weakness in current genetic investigations of haemostasis and its complications represented by bleeding or thrombosis is their dependence on a candidate gene approach. A comprehensive genome-wide search is the only way to identify those genes that would not be suspected based on our current understanding of haemostasis. This non-biased approach should focus on the identification of common variants contributing to the variability of the bleeding phenotype.
A disease that has been proposed as a model of a complex bleeding disorder is VWD type 1, which is characterized by incomplete penetrance and variable expressivity. The extent of clinical bleeding in patients with VWD type 1 does not always correlate with VWF levels. Patients with mild or moderate deficiencies may show considerable variation in bleeding tendency even within the same family. Conversely, mild bleeding and bruising are common in the general population without an identifiable bleeding disorder and some symptoms may overlap between bleeders and healthy controls [18]. One could test the hypothesis that aetiological studies of variable phenotype of VWD type 1 can be carried out most effectively using a population from a large unrelated cohort with VWD type 1 with a common phenotypic characterization (bleeding). In recent years, the validation of a new bleeding score in the adult and paediatric population that has been increasingly utilized worldwide, has allowed for a more homogeneous characterization of the bleeding phenotype [19–21].
Such a genetic study is being started through a collaborative consortium that includes several investigators from around the world. Taking advantage of a large collection of recruited individuals with VWD type 1 and also with a mucocutaneous bleeding disorder without a clear aetiology, and many extended families with multiple cases, the investigators propose to search for causal genes by carrying out a GWAS. This will be done using a multi-stage study design that utilizes available patient material to maximize statistical power and efficiency while minimizing cost.
An initial genome-wide discovery stage will be carried out in caucasian patients and controls. Two subsequent sequential follow-up stages will test selected candidate association signals, first in a second caucasian case-control cohort and then by family-based association analysis in a large collection of caucasian multiplex families. Finally, an extension stage will test association signals confirmed in the first replication phase in case-control cohorts from several different non-caucasian ethnic groups or other bleeding cohorts. This multi-stage approach has demonstrated to provide enough stringency to ‘pick up’ true signals and eliminate false positives.
Recent genome-wide association studies have identified several gene variants involved in platelet size and function as well as myocardial infarction and thrombosis [22–24]. However, most variants affecting bleeding phenotypes remain undiscovered. Therefore, this study may provide new genetic variants involved in bleeding. It is expected that with the discovery of genetic determinants of bleeding, the care of patients with these types of disorders will improve not only by the ability of practitioners to determine bleeding risk but also by the potential therapeutic alternatives that will rise as a result of these new findings.
Conclusion
Given the recent significant expansion of our knowledge about human genetics, and in particular, of the molecular basis of coagulation factors, we are now in a position to consider the appropriate role for the inclusion of this knowledge into clinical care. Molecular testing for haemostatic disorders requires access to appropriate expertise, which is not typically available in routine clinical haemostasis laboratories. However, the incorporation of tests based on this knowledge can be done quite easily in specialized centres and aid in patient diagnosis and management. In addition, the possibility of investigating coagulation through new strategies, such as genome-wide association testing, opens up exciting possibilities for the acquisition of new knowledge with the ultimate goal of improving the care of patients with inherited bleeding disorders.
Disclosures
The authors stated that they had no interests which might be perceived as posing a conflict or bias.