Building our global family – achieving treatment for all
Abstract
Summary. Building our global family by reaching out to women, children and youth and those in sub-Saharan Africa to achieve Treatment for All. The World Federation of Hemophilia (WFH) has committed to recognizing and incorporating the critical and important challenges that are faced by women with bleeding disorders within our global family. The next crucial steps include the development of outreach and registry programmes which can be adapted globally to accelerate the identification of such women, and to educate and guide them to the appropriate clinical care setting. Equally important, awareness must be raised within the broader medical community where women would typically first present with clinical symptoms. Family practitioners, nurse-midwives, obstetricians, gynaecologists and community health clinics will increasingly be strategic and central to WFH outreach efforts, in addition to serving as new care partners essential to the multidisciplinary model of care. Adapting and implementing the WFH development model regionally within Africa is proving to be a successful approach both for the introduction as well as the development of sustainable national care programmes for patients with bleeding disorders. The targeted development of solid national programmes such as in South Africa, Senegal and Kenya has expanded the training capacity of the WFH, as well as providing key regional examples. Local medical professionals are now responsible for providing the training in many regional programmes. Children with bleeding disorders in low-income countries are at great risk of dying young. WFH data demonstrate that among such patients, as the economic capacity of a country decreases so does the ratio of adults to children. The organization of care, training of a multi-disciplinary healthcare team, and education of patients and their families lead to improved mortality independent of economic capacity or increased clotting factor concentrate availability. Additionally, through enhanced youth education, awareness and engagement, we will assure continuity within WFH national member organizations, build greater unity within our global family and capture the innovation and creativity of their ideas to improve Treatment for All.
Introduction
The World Federation of Hemophilia’s (WFH) mission to improve and sustain care goes beyond haemophilia to include advocacy and support for all people with inherited bleeding disorders – regardless of where they might live in the world. The WFH vision of Treatment for All is also for people with von Willebrand’s disease (VWD), rare factor deficiencies and inherited platelet disorders. This means women and men, young and old, and those in developing and developed countries are all important members of our global family. Over recent decades, diagnosis and care have improved dramatically around the world. However, despite the remarkable success achieved to date, much work remains to be carried out. In particular, there are three areas of development that deserve an expanded recognition. These include women with bleeding disorders, patients and their families living in sub-Saharan Africa, and children and youth – the next generation.
Discussion
Women with bleeding disorders
It’s not just about men, women bleed too.
The WFH global family extends beyond haemophilia to also incorporate all inherited bleeding disorders including VWD, rare factor deficiencies and inherited platelet disorders [1].
Although haemophilia disproportionately affects men, other inherited bleeding disorders do not discriminate based on gender. The gender split for other bleeding disorders is relatively equal. Figure 1 shows the proportion of male and female patients for all bleeding disorders [1].
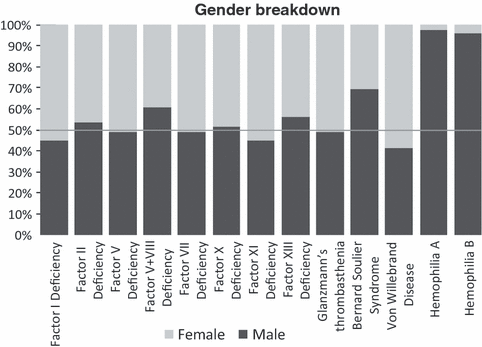
Proportion of male and female patients for all bleeding disorders. The white line indicates 50%. These data are from the 72 countries that provided gender breakdowns to the World Federation of Hemophilia Global Survey 2008.
The WFH annual global survey reports gender data by disorder and country (see Table 1) [1]. The actual number of known males and females reported by disorder may not equal the total reported because some countries lack gender data on the entire population.
| Disorder | Total | Male | Female |
|---|---|---|---|
| Haemophilia A | 79 820 | 77 859 | 1817 |
| Haemophilia B | 16 976 | 16 318 | 651 |
| Haemophilia type unknown | 662 | 636 | 10 |
| von Willebrand’s disease | 44 731 | 16 235 | 23 207 |
| Factor I deficiency | 806 | 319 | 396 |
| Factor II deficiency | 183 | 61 | 53 |
| Factor V deficiency | 817 | 348 | 363 |
| Factor V + VIII deficiency | 303 | 128 | 82 |
| Factor VII deficiency | 3608 | 1563 | 1630 |
| Factor X deficiency | 891 | 385 | 368 |
| Factor XI deficiency | 3484 | 1382 | 1690 |
| Factor XIII deficiency | 635 | 323 | 254 |
| Bleeding disorder: type unknown | 666 | 180 | 165 |
| Platelet disorders: Glanzmann’s thrombasthenia | 977 | 294 | 310 |
| Platelet disorders: Bernard Soulier syndrome | 206 | 84 | 92 |
| Platelet disorders: other or unknown | 3577 | 1211 | 1944 |
Over the last decades, diagnosis and care for people with haemophilia have evolved considerably [2]. However, for other bleeding disorders the recognition and level of care has not developed similarly. For example, VWD is considered the most common bleeding disorder worldwide. VWD prevalence estimates vary, ranging up to 1.3% of the population [3]. Since the WFH began collecting data on VWD in 1999, only 52 330 individuals worldwide have been reported with VWD. Of those reporting, approximately 41% were men and 59% women. A study from the US Centers for Disease Control and Prevention, published in 2003 [4], reported that the average length of time for diagnosis from onset of symptoms was 16 years. These data show that, too frequently, patients with VWD, in particular women, go undiagnosed, untreated or their care of an underlying bleeding disorder is improperly managed.
However, the number of women reported with bleeding disorders is growing rapidly in developed countries. From 1991 to 2007, the number of female patients treated at US hemophilia treatment centres (HTCs) grew nearly 300%, from 2365 to 9041 (see Fig 2) [5]. Although the 16 years’ lag time to diagnosis is troubling, this rapid increase in diagnosis is encouraging. One of the challenges this now presents is whether the HTCs are equipped to handle this growth in their patient population and how best to replicate this trend globally.
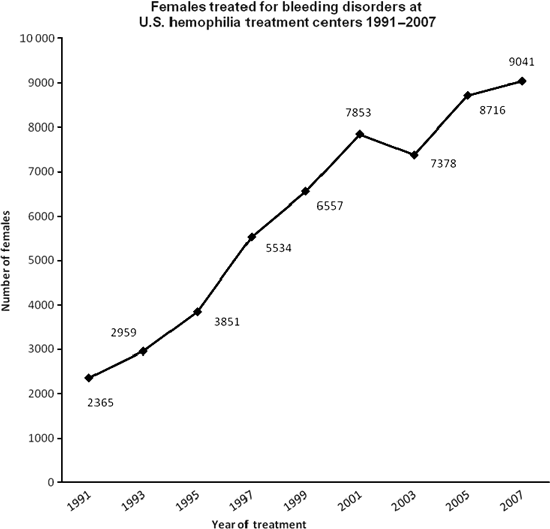
Females treated for bleeding disorders at federally funded treatment centres in the United States, 1991–2007
As with men with haemophilia, bleeding disorders have a significant impact on women’s health and quality of life [6,7]. Women with bleeding disorders suffer reduced quality of life that negatively impacts academic, professional and social experience. Many women are not aware that their symptoms are abnormal and do not seek medical advice. Even when they do seek help, diagnosis of a bleeding disorder is often overlooked and appropriate treatment is not provided because of the lack of awareness among caregivers. Women with bleeding disorders are therefore more likely to have unnecessary surgical intervention, including hysterectomy, at an early age [8].
Another example is that postpartum haemorrhage (PPH) remains the main cause of maternal death and long-term disability for women around the world. PPH occurs in approximately 14 million women worldwide annually. Severe PPH is less common, but is a significant contributor to maternal morbidity and accounts for approximately 150 000 deaths per year [9] with a huge impact on the motherless child. PPH can be prevented or its severity can be moderated by educating women and their providers [8]. While delayed or secondary PPH is rare, occurring after <1% of deliveries [10,11], it has been reported in 20–25% of women with VWD [12,13], 2–11% of haemophilia carriers [14,15] and 24% of women with factor XI deficiency [14].
Carriers are another significant and often neglected member of the global bleeding disorder family. Women who have the haemophilia gene are called carriers, and they can pass the gene on to their children. In recent decades, it has been recognized that carriers may also have low levels of factor VIII (FVIII) or factor IX (FIX), meaning by definition that they too have haemophilia. Although most carriers will be asymptomatic in day-to-day life, some experience significant bleeding symptoms, including excessive bleeding during menstruation, childbirth and with surgical intervention.
The reported incidence of carriers per male with haemophilia varies significantly in the literature. The difficulty in accurately defining this figure lies in the fact that most women who are not mothers or daughters of someone with haemophilia do not know whether or not they are or might be carriers. Estimates range from 1.56 [16] to 5 [17] true genetic carriers per male born with haemophilia within a pedigree. Based upon the WFH global estimate of 400 000 people worldwide with haemophilia, this means there are potentially 625 000–2 000 000 carriers worldwide that could require on-going management by an HTC.
It is recognized that the median clotting factor level in carriers is 50%. Thus, half of the carriers are at increased risk of bleeding and, some estimates are higher [18–20]. Twenty percent of true genetic carriers have factor levels under 30% (Carol Kasper MD, Private communication). If one assumes a robust outreach and genetic counselling programme is taking place and utilize the definition that those with a factor level of 30% or less have mild haemophilia, these ratios would suggest that an HTC with 100 patients with haemophilia could have between 30 and 100 carriers* with a low factor level requiring on-going management for possible bleeding problems similar to men with mild haemophilia. Additionally, other carriers with higher factor levels in the range of 40–60% of normal may have an increased bleeding tendency and also require occasional intervention and counselling [19]. To address this need, more refined estimates of the true incidence of carriers are needed.
Additionally, these estimates do not reflect all those in need of carrier testing. For example, in regions of the world where very large families are common, such as Iran, 4000 female relatives for 1500 haemophilia patients have been reported [21]. Because of the unknown carrier status of grandmothers, mothers, aunts, sisters, daughters and nieces of a person with haemophilia must all be considered for screening to establish their carrier status and factor levels. Thus, the number of women who would actually need services of an HTC at least once in their lifetime to assess factor levels or for DNA analysis to determine carrier status is higher than the number of actual carriers requiring on-going support of an HTC. Development of outreach, genetic counselling and registry programmes similar to those offered by the WFH and through our national member organizations (NMOs) for haemophilia is fundamentally important to close this care gap for women with bleeding disorders. In addition, HTCs and NMOs will need to be prepared to support the psychological and social implications associated with carrier testing and diagnosis.
A number of outreach programmes (such as Women Bleed Too in the UK [22], Project Red Flag in the US [23], and the women’s programme of the Canadian Hemophilia Society [24]) have been created to raise awareness about women with bleeding disorders and to improve the quality of care and life for these women. However, to date these programmes have not been tested or optimized for use in developing countries or within diverse cultural communities. Traditional outreach techniques, such as family histories, may not be optimal approaches to identify women with bleeding disorders other than haemophilia. Innovative strategies and tools are needed to reach vulnerable populations. To address this need the WFH is building on a WFH development programme that launched with the Lebanese Haemophilia Association and Ministry of Health in 2006. Following the diagnosis of haemophilia cases in the Bekaa Valley and Akkar regions, a new pilot project to identify VWD patients has been initiated working through the regional community health centres of the Ministry of Health [25]. Other pilot outreach projects are being launched in Egypt and Latin America. A unique pilot outreach effort has been conducted in collaboration with the World Health Organization (WHO) to raise awareness of the risks of PPH and maternal death. In the context of this interregional consultation within the Gulf States on the role of nurses and midwives in ensuring safe clinical transfusion and patient safety, the WFH highlighted the critical role of the clinical management of patients with haemorrhage and bleeding disorders [26]. Given the many settings in which women will present with bleeding complications, multi-faceted outreach and educational approaches are required. The results of these initiatives will be evaluated to inform planning and performance of future WFH global outreach programmes.
Having recognized the critical importance and challenges of fully incorporating women with bleeding disorders within the WFH global family, the next crucial steps include the development of outreach and registry programmes which can be adapted globally to accelerate the identification of women to educate and guide them to the appropriate clinical care setting. In parallel, it is important to develop the education and training capacity within WFH NMOs as well as clinical expertise within the HTC network. Equally important, awareness must be raised within the broader medical community where women would typically first present with clinical symptoms. Family practitioners, nurse-midwives, obstetricians, gynaecologists and community health clinics will increasingly be strategic and central to WFH outreach efforts, in addition to serving as new care partners important to the multidisciplinary model of care.
Sub-Saharan Africa
Africa is the second most populous continent. With 53 countries and nearly one billion people the challenges are indeed great. Africa is the most underrepresented geographical area within the WFH. Within Africa, at present, only 15 nations (less than one-third of those in Africa) have NMOs within the WFH national membership. In North Africa, Algeria, Egypt, Morocco and Tunisia are members. Within the three regions of sub-Saharan Africa, WFH has 11 NMOs: West Africa – Cameroon, Ivory Coast, Nigeria and Senegal; East Africa – Eritrea, Kenya and Sudan; Southern Africa – Botswana, Lesotho, South Africa and Zimbabwe (see Fig. 3). During the 2010 WFH Congress, three additional African countries are expected to be accredited as members of the WFH – Ethiopia, Ghana and Tanzania.
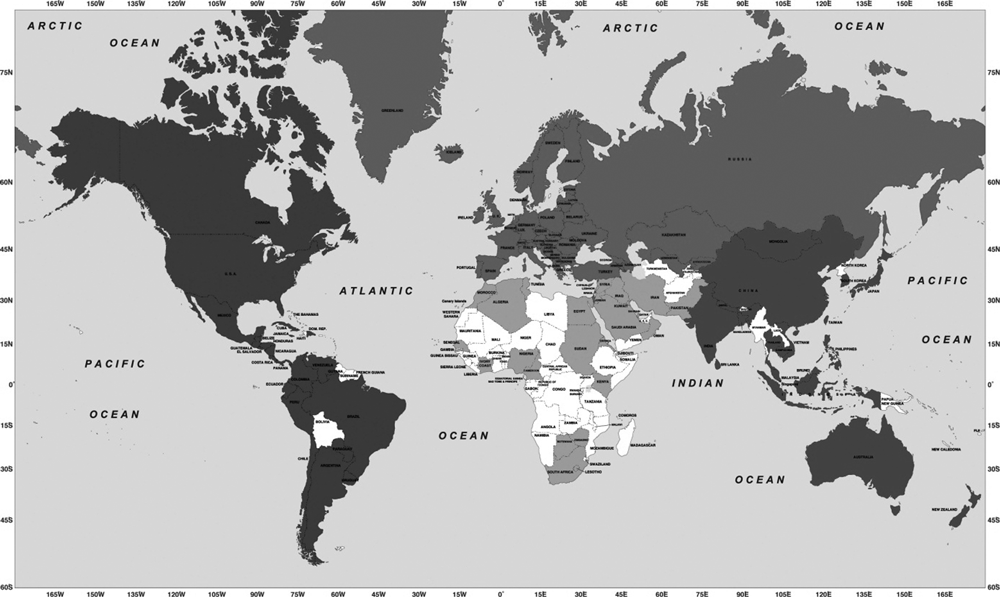
National member organizations of the World Federation of Hemophilia 2010.
There are wide-ranging and disparate needs across the sub-Saharan region. The goal of developing care for people with bleeding disorders must be considered in context with other disease burdens in Africa, such as HIV/AIDS, malaria, tuberculosis, and malnutition.
To maximize the results of WFH work in Africa, WFH is encouraging countries to network with each other and organize programmes on a regional level. Integral to the approach to achieving Treatment for All is building a centre of core expertise within each African region. This then serves as a hub for further regional development activities, as well as a model for what can be achieved. WFH experience has shown that culturally appropriate training that occurs in a setting resembling the level of care within a country maximizes the opportunity for practical learning and achieving sustainable care. Equally important, it is extremely beneficial to have regional role models when communicating with Ministries of Health and cultivating healthcare professionals. To date, WFH regional programming has been primarily based in Senegal (West African region), Kenya (East African region) and South Africa (Southern African region).
In both the East and West African regions, the WFH started by improving diagnosis through regional laboratory training workshops (e.g. West Africa in 2008 and East Africa in 2009) and by encouraging the development of national patient registries. Five of the 11 sub-Saharan African countries now have patient registries [1]. Diagnosis of Haemophilia and Other Bleeding Disorders, the WFH Laboratory Manual [27], soon to be published in a new and expanded edition, and Guide to Developing a National Patient Registry [28] serve as the primary training tools.
The next development step is to improve knowledge in the multidisciplinary approach particularly the musculoskeletal areas (physical therapy, rehabilitation and orthopaedics) through targeted training programmes (e.g. West Africa in 2010 and East Africa in 2011).
Frequently, WFH work within a country begins with the identification of a core group of medical professionals interested in the provision of care to patients with bleeding disorders. The WFH-funded and facilitated HTC twinning programme pairs emerging HTCs with established ones to help increase the levels of diagnosis and medical attention for people with haemophilia [29]. Encouraging the establishment of medical twinning partnerships allows countries to advance on an individual level as well. At present, WFH has established or plans to establish medical twinning partnerships in all the WFH member countries within the region. To date 11 twinnings have been established†. Twinning has proven to be a highly successful way to introduce care and build the core of medical expertise within a country. Using an example from another region, twinning programmes within China led to the development of a national treatment centre network and have served as the catalyst for the further development of care nationally [30].
The WFH has established that one international unit (IU) of FVIII CFC per capita should be the target minimum for countries wishing to achieve optimal survival for the haemophilia population. Overall, among the WFH NMOs reporting usage of FVIII within Africa, the IU per capita ranges from 0.00036 in Nigeria to 0.715 in South Africa with an overall African average of 0.14. For comparison, globally the FVIII IU per capita for countries with a gross domestic product (GDP) < $US 2000 is 0.024. For countries with a GDP $US 2000 to 10 000 the FVIII IU per capita is 1.03 [1,32,33].
Southern African region
Health authorities in these countries typically provide clotting factor concentrates (CFCs) to people with haemophilia, although at a low level, usually less than one IU of FVIII CFC per capita and utilize appropriate laboratory diagnosis [1]. The WFH has established that one IU of FVIII per capita should be the target minimum for countries wishing to achieve optimal survival for the haemophilia population [31].
As most patients within the Southern African region have some access to CFCs, the morbidity and mortality manifest differently than in other regions. WFH advocacy work within this region has focused on increasing the quantity of CFCs provided and expanding care to include other bleeding disorders as well as targeted support for the introduction of treatment for patients with inhibitors.
From a clinical perspective, WFH work in this region focuses on the development and adoption of treatment guidelines to harmonize management for patients with bleeding disorders across each country. The WFH Guidelines for the Management of Hemophilia, soon to be published in an expanded second edition, serve here as elsewhere as the primary reference point for the development of national/regional treatment guidelines [18].
West African region
Although changing rapidly, typically, diagnosis is quite difficult, governments do not routinely provide treatment products and health professionals have limited knowledge in treating bleeding disorders. Senegal is the most advanced in these respects and therefore provides a solid platform for WFH regional training programmes. WFH development within this region primarily focuses on basic elements in haemophilia care and the introduction of the comprehensive care model [34,35].
Given the economic capacity of many countries within the region, CFCs are for the most part unaffordable in quantities necessary to dramatically improve clinical outcomes. All countries within the region report a heavy reliance on fresh and prepared blood components [whole blood, fresh frozen plasma (FFP) and cryoprecipitate] to treat bleeding disorders. Recent advances in solvent-detergent viral inactivation adapted to the treatment of single plasma donations and cryoprecipitate minipools could present a promising advance in safety for otherwise vulnerable patient populations [36]. The WFH works in parallel with capacity-building programmes for NMOs and medical training programmes to improve transfusion services and educate on best practices to prepare the safest cryoprecipitate possible.
East African region
Like West Africa, these countries are dependent upon whole blood, FFP and cryoprecipitate for treatment. There is limited or no supply of CFCs except for that which is provided as part of a humanitarian donation. Diagnostic capacity varies from one country to another. Although the WFH has facilitated the training of laboratory technicians throughout the region, diagnosis by factor assay is very limited because of the expense and consequent lack of necessary reagents [1]. Similar to West Africa, the WFH focuses on introducing the comprehensive care approach, increasing knowledge in management of haemophilia and improving blood transfusion services. In recent years, Kenya has demonstrated leadership within the region and therefore serves as a focal point for regional training activities in East Africa.
Adapting and implementing the WFH development model [2] regionally within Africa is proving to be a successful approach both for the introduction as well as the development of sustainable national care programmes. Through the targeted development of solid national programmes in South Africa, Senegal and Kenya the WFH training capacity is expanded and provides valuable regional examples. Local medical professionals are now responsible for providing the training in many regional programmes.
The next generation
Child health is one of the United Nation’s (UN) eight core Millennium Development Goals (MDG). According to UN data, in low-income countries, one out of every 10 children dies before the age of five. In wealthier nations, this number is one out of 143. Specifically, by the year 2015 the UN MDG seeks to reduce by two-thirds the under-five mortality rate [37].
Unfortunately, this mortality could be even worse for children born with haemophilia. Children in low-income countries are at great risk of dying young. WFH data suggest that as the economic capacity of a country decreases the ratio of adults to children also decreases (see Fig 4) [1].
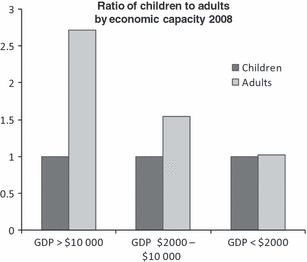
Relationship of economic capacity to number of adults with haemophilia. Comparison of the ratios of the number of adults with haemophilia to the number of children (aged <13 years) with haemophilia in 76 countries grouped according to per capita gross domestic product (GDP) in US dollars. The data were reported by national member organizations in the World Federation of Hemophilia Global Survey, 2008.
The WFH shares the MDG goal to improve child health and has been working in parallel. In 2002, the WFH announced a global development programme, the Global Alliance for Progress (GAP) [38]. One of GAP’s three core objectives is to close the gap between the number of people born with haemophilia and the number who reach adulthood [39].
Since 2002, significant progress has been made among the global haemophilia population. An analysis of data [1,32,33,40–43] collected since the GAP launch demonstrates that WFH programmes have made a dramatic difference in improving care delivery [44] and specifically the mortality of patients with haemophilia. Today, because of WFH interventions more children are surviving into adulthood (see Fig 5). The rise in the number of adults with haemophilia undoubtedly results from the number of children aged 12–18 (those within the 6-year span between 2002 and 2008) that have now reached the age of 19. In addition, a few undiagnosed adults with milder haemophilia who had not been previously known to the health care system in the various countries were identified through the outreach programmes undertaken by the WFH in conjunction with the NMO.
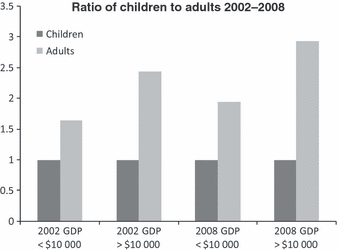
Change over time of relationship of economic capacity to number of adults with haemophilia. Comparison of the ratios of the number of adults with haemophilia to the number of children (aged <13 years) with haemophilia in 39 countries grouped according to per capita gross domestic product (GDP) in US dollars. The data were reported by national member organizations in the World Federation of Hemophilia Global Survey, 2002 and 2008.
When looking at individual countries, the results are equally impressive. It is noteworthy that the reduction in mortality is not exclusively dependent upon an increase in the availability of CFCs. For example, in two of the first GAP countries, Georgia and the Philippines, significant progress in mortality is evident despite differences in improvement in the availability of CFCs over a similar period of time (see Fig 6). A base year of 2003 is used here for comparison as age data were not reported to the WFH for 2002 for Georgia and the Philippines. Equally noteworthy, the per capita economic capacity of the country does not necessarily correlate to the potential for improvement. In Georgia, which has a GDP per capita of US$4500 [45], between 2003 and 2008 the IU per capita of FVIII CFCs increased from 0.017 to 0.424, a 2394% increase. In the Philippines, with a GDP per capita of US$3300 [45], CFC usage only slightly increased from 0.010 to 0.015. Clearly, other factors contribute to the reduced patient mortality. One reasonable conclusion is that the intensive development work of the WFH and NMO within the GAP project over this period of time has contributed to improved and sustainable outcomes. Most notably, the better organization of care, training of a multidisciplinary team of health care professionals, and education and psycho-social support of patients and their families may lead to improved mortality independent of economic capacity or increased CFC availability. Future research correlating severity of haemophilia with the age of patients over time is needed to assess if outcomes differ based on the severity of haemophilia.
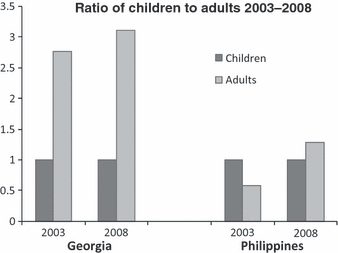
Comparison of the ratios of the number of adults with haemophilia to the number of children (aged <13 years) within Georgia and the Philippines before entry into the World Federation of Hemophilia (WFH) Global Alliance for Progress project and year-end 2008. The data were reported by the national member organizations in the WFH Global Survey, 2003 and 2008.
To sustain and build upon WFH success, we must also cultivate and integrate these youth into the work of the WFH and NMOs. The first 3 years of the WFH strategic plan focused on building our global family to include more fully those with VWD, rare factor deficiencies and inherited platelet disorders. The WFH is now looking to expand our youth programmes to ensure that a future generation is ready to assume the mantle of leadership [46]. Additionally, through enhanced youth education, awareness and engagement, we will assure continuity within WFH NMOs, build greater unity among our global family and perhaps most importantly capture their innovative and creative ideas to help realize our vision of Treatment for All.
World Federation of Hemophilia programmes are evolving to incorporate an integrated approach to youth leadership development with supplementary components such as youth specific publications, youth opportunities in WFH development programmes such as twinning, utilization of social media forums such as Facebook and Twitter [47], expanded youth fellowship programmes and a summer camp programme ‘Journey Around the World’ (JAW) [48]. JAW is an innovative game for children and youth attending summer camps to raise their awareness of the needs of people with haemophilia in developing countries. JAW engages youth in more developed countries to give back to the global community, as well as recognize the importance of continued advocacy to sustain their own care. We are now working across the spectrum to establish a wide range of programmes and opportunities for youth leadership and involvement.
Conclusion
Although this article has selected three specific segments of our global family for visibility and emphasis, it should not be concluded that these are the only ones with challenges or deserving of attention. The particular emphasis placed on women, children and youth, and those in sub-Saharan Africa is due to their critical importance and previously lagging visibility relative to other segments and regions. To fully achieve our vision of Treatment for All, we must learn from our experiences, build upon all of our many successes, embrace the diversity of bleeding disorders and construct a future where all patients regardless of who they are or where they might live realize the hope and promise of Treatment for All.
Footnotes
Acknowledgements
The WFH gratefully acknowledges the support of so many for their commitment and dedication – the national member organizations, WFH volunteers and staff, the healthcare professionals and governments committed to building national care programmes and the WFH’s partners and donors that provide financial support. I would like to thank the following individuals for their input and assistance in developing this paper: Dr Paula Bolton-Maggs, Gordon Clarke, Dr Bruce Evatt, Dr Andra James, Dr Carol Kasper, Shirin Ravanbod, Dr Johnny Mahlangu, and Dr Alison Street.
Disclosures
The author stated that he had no interests which might be perceived as posing a conflict or bias.




