Plasminogen activator inhibitor type 1 deficiency
Abstract
Summary. Plasminogen activator inhibitor type 1 (PAI-1) is an important component of the coagulation system that down-regulates fibrinolysis in the circulation. Reduced PAI-1 levels may result in increased fibrinolysis and an associated bleeding diathesis. Clear documentation of PAI-1 deficiency as a cause of a bleeding disorder has been rare. PAI-1 was initially identified in the 1980s, and the first reported case of PAI-1 deficiency appeared in 1989. Several reports followed, although only two identified an underlying genetic defect. These reports of PAI-1 deficiency suggest that affected individuals exhibit mild to moderate bleeding symptoms, including epistaxis, menorrhagia, and delayed bleeding after trauma or surgical procedures. Affected individuals rarely exhibit spontaneous bleeding events commonly seen in other procoagulant deficiencies. The majority of bleeding events are controlled with antifibrinolytic agents, such as tranexamic acid and ε-aminocaproic acid. A major issue that contributes to difficulty in establishing an accurate diagnosis of PAI-1 deficiency is that the activity assay is accurate in detection of elevated levels but not at the lowest range. Reported normal ranges begin at zero, thereby making a deficiency state because of a dysproteinaemia difficult to distinguish from that of a normal unaffected individual. Although the antigen assay may be helpful in some circumstances, it assists only with complete quantitative disorders. Because of lack of standardized commercially available PAI-1 activity assay sensitive in the lowest range, the true prevalence of this rare condition has not been established.
Introduction
The plasminogen activation system was a burgeoning field in the early 1980s, with the discovery of the first plasminogen activator inhibitor in 1984 [1,2]. After purification of the inhibitor, the isolation of the c-DNA was completed for what was later termed plasminogen activator inhibitor, type 1 (PAI-1) [3]. Once PAI-1 was isolated, researchers began to study the impact of perturbations of normal levels, either those higher or lower than the established normal range. While numerous investigations have evaluated elevated levels of PAI-1and the documented association of an increased risk of arterial thrombotic events [4], reports of PAI-1 deficiency are limited. The first reported case of low levels of PAI-1 resulting in a lifelong bleeding disorder was published in 1989, when an elderly man was noted to have decreased PAI-1 activity and normal PAI-1 antigen levels, leading to a presumed qualitative defect of the protein [5]. The first person with a quantitative deficiency (undetectable PAI-1 antigen and activity levels) was described two years later [6]. In 1992, the first identified homozygous PAI-1 genetic defect that led to a bleeding disorder was documented [7]. Subsequently, other reports of PAI-1 deficiency leading to a bleeding diathesis have been published, but only one other specific genetic mutation associated with PAI-1 deficiency has been documented [8–17]. PAI-1 deficiency appears to be quite rare, and is associated with a mild to moderate bleeding disorder. Unfortunately, lack of a sensitive PAI-1 activity assay hampers diagnosis of this condition.
Materials and methods
This review was performed by an extensive literature search through PubMed. Further references not initially identified in the search but referenced within these articles were also reviewed. All cases of PAI-1 deficiency reported in the literature were reviewed. Also, prior reviews on the history of the discovery of PAI-1 were evaluated. Finally, documented cases of PAI-1 deficiency within the Indiana Amish population were re-evaluated. Home visits to these patients and their parents were performed within the 2007 calendar year, with an extensive review of personal bleeding histories completed (Rakesh P. Mehta, Amy D. Shapiro, unpublished data).
Incidence
The true incidence of PAI-1 deficiency is unknown in large part because of the difficulty in establishing the diagnosis with present laboratory tests and the rarity of the disorder. We performed a survey within the Federal Network of Hemophilia Centers within the United States to identify documented and/or suspected cases in an effort to determine the prevalence of this disorder. More than 100 surveys were mailed with only six centres responding. Two of the six responders reported PAI-1-deficient patients. These patients were noted to have a history of bleeding with a negative evaluation other than undetectable PAI-1 activity. A recent analysis screened 586 individuals with a bleeding tendency for decreased PAI-1 activity using a modified commercially available assay for the study, and compared them with 200 controls [8]. In the group with a bleeding tendency, 23% had low PAI-1 activity levels compared with 10–13% in the control group; the severity of bleeding was minimal in most of these patients. Another recent report evaluating 66 blood donors revealed 14.6% of the females and 21% of the males to have PAI-1 activity levels <2.0 IU mL−1. Detailed histories of these subjects were not available, but subjects that reported a bleeding history were not included in this analysis [14]. Because the number of case reports of PAI-1 deficiency causing significant bleeding is limited, the true prevalence of this disorder is not established but likely small [5–13,15–17]. Unfortunately, the lack of precision of currently available activity assays within the lowest range of PAI-1 activity continues to hinder accurate diagnosis. Although drawing conclusions from limited populations is often inaccurate, it does not appear that there is an ethnic predilection for PAI-1 deficiency, because cases of PAI-1 deficiency have been reported from North America, Europe and Asia.
Pathophysiology
Plasminogen activator inhibitor, type 1 plays a vital role in regulating fibrinolysis within the circulation and is responsible for the controlled degradation of thrombi [18]. Importantly, fibrinolytic activity is physiologically limited to the immediate vicinity of the thrombus within a blood vessel. Plasmin, the primary protease responsible for fibrinolysis, is formed from the proteolytic cleavage of the zymogen plasminogen. Plasmin formation is catalyzed by the actions of the two major mammalian plasminogen activators: tissue-Plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA). Importantly, the fibrin clot provides a surface to increase the efficiency of plasmin generation through formation of a ternary complex of fibrin, t-PA and plasminogen. Hence, fibrinolysis almost exclusively occurs on the clot surface and not in the circulation [18]. Control of this process is mediated through the plasminogen activator inhibitors, with the primary plasminogen activator inhibitor in plasma being PAI-1 (Fig. 1). This 47 kDa protein is member of a superfamily of serine protease inhibitors, called serpins [19,20]. PAI-1 binds in a stoichiometric manner to plasminogen activators with rapid and irreversible inhibition, leading to the description of PAI-1 as a ‘suicide’ inhibitor.
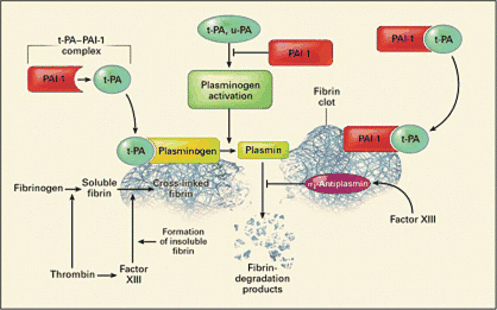
The plasminogen activators circulate in plasma as a complex with PAI-1 as a reversible complex. When the fibrin clot is formed, plasminogen and t-PA (or u-PA) bind to the surface of the clot. These proteins can then interact, and plasmin is formed, which leads to lysis of the cross-linked fibrin into the fibrin-degradation products. PAI-1 also binds to fibrin and, when bound, it can irreversibly bind to inhibitor t-PA(or u-PA) [4].
The source of plasma PAI-1 is unclear. Hepatocytes, endothelial cells, adipocytes, and megakaryocytes all are able to synthesize and secrete PAI-1 into the circulation [1]. The human PAI-1 gene is located on chromosome 7q21.3-22, and its expression may be induced by several factors, including insulin, endotoxin, and transforming growth factor-β. Interestingly, PAI-1 plasma levels exhibit a diurnal variation, with highest levels in the early morning hours and nadir levels in the afternoon [21]. The rate of transcription is assumed to be high, because of the short plasma half-life of approximately 10 min, and a brisk plasma level increase in response to stimuli [20]. A common polymorphism in the promoter region of the PAI-1 gene has been clearly associated with increased PAI-1 levels. A change from five consecutive guanines to four guanines at a position 675 base pairs before the transcription site leads to increased response to various stimuli that increase PAI-1 production [19].
Although plasma PAI-1 exists mostly in an active form, there is also a latent inactive form that results from inherent instability of the molecule [1]. In fact, 80% of the PAI-1 stored in the α-granules of platelets is in the latent form. The latent form can be converted back to the active form with denaturants or negatively charged phospholipids in vitro, but this conversion does not appear to occur within the circulation. Therefore, the physiological significance at this time of the latent form is unclear. Importantly, antigen testing kits may not be sensitive to distinguish between active vs. latent PAI-1.
Like most coagulation protein deficiencies, qualitative or quantitative defects may lead to a deficiency state [5–7,9–13,15,17]. The first case described in 1989 reported normal levels of PAI-1 antigen, with a marked reduction in PAI-1 activity and t-PA:PAI-1 complexes, suggesting that the PAI-1 present lacked activity [5]. Conversely, it is possible that much of the PAI-1 may have been present in the latent form, resulting in a normal antigenic assay but decreased activity assay. Most of the subsequent reports documented true qualitative deficiencies resulting in decreased PAI-1 activity [5–7,9–13,15,17]. Interestingly, two reports detail low plasma levels of PAI-1 activity and antigen, with normal platelet levels [6,10]. Both of these patients had similar lifelong bleeding histories, so that the low plasma levels were believed to be clinically significant and result in the patients’ bleeding symptoms. Neither report explained the discrepancy between the plasma and platelet PAI-1 levels.
In reports of PAI-1 deficiency, a variety of assays have been used, making it difficult to extrapolate a correlation between any specific level and degree of clinical bleeding. Unfortunately, as currently available activity assays include zero within the reported normal range, the sensitivity of these assays at the lowest level is insufficient to provide an adequate diagnostic tool, and makes it difficult to correlate the degree of deficiency with specific bleeding symptoms. A recently published report utilizing a modified PAI-1 activity assay suggests that PAI-1 activity levels <1 U mL−1 in patients with bleeding symptoms was associated with increased plasmin-antiplasmin complexes, thus suggesting that this modified assay and level could be used in the diagnosis of this disorder [22].
Genetic defects
Currently, two documented genetic defects have been reported to be associated with PAI-1 deficiency [7,17,23]. In 1992, a young Amish girl in the United States with a history of bleeding was noted to have absent PAI-1 antigen and activity levels. On genetic analysis, she was found to be homozygous for a dinucleotide insertion within exon 4 of her PAI-1 gene. This insertion led to a frameshift, with subsequent premature stop codon, producing a truncated, non-functional protein [7]. This kindred was further analysed for this mutation, with a total of nine individuals identified to be homozygotes for this mutation, all exhibiting a mild-moderate bleeding disorder (Rakesh P. Mehta, Amy D. Shapiro, unpublished data). More than 100 people within this community have been found to be heterozygotes for this mutation, none of whom have experienced bleeding symptoms.
More recently, a second genetic defect in the PAI-1 gene has been found in a patient from China with a lifelong bleeding disorder [17]. This patient was found to have a G to A substitution at nucleotide position 4497 in exon 2 of the gene, leading to a single amino acid exchange of an Alanine for a Threonine at codon 15 of the signal peptide. Interestingly, this patient was a heterozygote for this mutation, but had an activity level that was 10% of the healthy controls. His father, who had the same mutation had a lower than normal PAI-1 antigen and activity level but much higher than his son’s. In addition, the patient’s mother, who did not have the mutation, had a moderately reduced PAI-1 activity and antigen level. Therefore, the authors concluded that the patient was a compound heterozygote with the documented mutation being partially responsible for the markedly low PAI-1 levels.
Clinical manifestations
The bleeding symptoms associated with PAI-1 deficiency have been fairly consistent and appear compatible with what might be predicted based on the role of this coagulation factor within the haemostatic system. Most reports have documented a mild-to-moderate, delayed bleeding disorder typically associated with injury, trauma or surgery. The initial case of PAI-1 deficiency described a man with a long history of post-traumatic and postsurgical bleeding [5]. Most subsequent reports describe similar symptoms. The Amish population with PAI-1 deficiency has a similar clinical expression [23]. Recent follow-up evaluations reveal a wide variety of bleeding complications, though most were post-traumatic bleeding events, including intracranial haemorrhage and haemarthrosis. Importantly, menorrhagia represented a significant clinical symptom in young women, even resulting in the requirement of packed red blood cell transfusion in one patient because of marked anaemia and iron deficiency from menstrual blood loss (Rakesh P. Mehta, Amy D. Shapiro, unpublished data). Two other reports also document menorrhagia as a significant symptom in PAI-1-deficient females [11,13]. Postsurgical bleeding is a common complication observed in this deficiency state. Of reported events, dental procedures are clearly associated with abnormal bleeding in PAI-1 deficiency [6,11,12,15]. In fact, after having post-traumatic and postsurgical bleeding as a toddler, it was an episode of recurrent oral bleeding in the proband in the Amish population that led to the diagnosis of the bleeding disorder [7]. Easy bruising, epistaxis, and muscle haematomas secondary to injury or trauma have also been reported [6,7,10,17].
The age range of diagnosis is quite wide. The first patient described was 76 years old [5] but a child as young as 4 months has also displayed a bleeding tendency [23]. Thus, there does not appear to be a specific age at which symptoms either typically manifest or a time when they tend to decrease or resolve.
Although most reports of bleeding from PAI-1 deficiency stem from inherited conditions, an acquired deficiency also appears possible [24]. A gentleman with end-stage liver disease presented with spontaneous, deep muscle bleeding. He was found to have detectable PAI-1 antigen but lacked PAI-1 activity. Levels of t-PA were markedly increased; therefore, his bleeding diathesis was believed to have resulted from a profound imbalance of the fibrinolytic system, because of lack of clearance of t-PA by the impaired liver with acquired relative PAI-1 deficiency. Interestingly, elevated levels of urinary t-PA after trans-urethral prostatectomy (TURP) also have been shown to correlate with increased blood loss after these procedures [25]. Potentially, the increased levels of t-PA released lead to a locally acquired deficiency of PAI-1 as well.
Diagnosis
The assays to establish the diagnosis of PAI-1 deficiency have been reviewed. Several antigen tests are available, but these are not able to detect qualitative deficiencies [8]. Unfortunately, the currently available PAI-1 activity assays are designed to detect increased activity rather than a deficiency state and subsequently are not adequately sensitive at the lowest levels. Because a PAI-1 activity level of zero is often reported as being within the normal limit of these assays, they cannot reliably discriminate a deficiency state from a non-deficient individual. Interestingly, correlating plasmin-antiplasmin levels with markedly low PAI-1 activity levels (<1 IU mL−1) may help distinguish patients with clinically significant PAI-1 deficiency [22].
Therefore, the diagnosis of this disorder remains challenging. However, it is worthwhile to analyse both antigen and activity levels when evaluating a patient for this deficiency. If PAI-1 activity levels are reported as <1 IU mL−1 and the patient has characteristic delayed post-traumatic or -surgical bleeding, then the diagnosis should be considered and a trial of antifibrinolytic agents entertained. Furthermore, because of the rarity of this condition and the difficulty making an accurate diagnosis, it is important to consider other more common bleeding conditions; disorders such as von Willebrand disease and platelet function defects are far more common and should be firmly ruled out prior to making the diagnosis of PAI-1 deficiency.
Management
Antifibrinolytic agents remain the mainstay of treatment for this disorder with all published reports documenting the benefit of EACA or tranexamic acid for bleeding control or prevention [5–7,12,13,15,23]. These agents function to control inappropriate plasmin generation, and subsequently minimize bleeding once it has occurred or prevent bleeding when instituted prophylactically before interventions or invasive procedures. For example, a young Amish boy developed recurrent bleeding of his subdural haematoma after he discontinued use of the prescribed EACA upon discharge from the hospital. Reinstitution of this agent helped stop the recurrent bleeding and led to a full recovery (Rakesh P. Mehta, Amy D. Shapiro, unpublished data). The efficacy of these agents has also been documented as prophylactic medications in prevention of bleeding in association with surgical procedures [5,23]. In patients with menorrhagia, EACA has been effective in limiting the amount of menstrual blood loss and appears most effective when instituted a few days before the anticipated onset of the menstrual cycle [13,23].
Prognosis
Patients with PAI-1 deficiency may do quite well, as bleeding once recognized can be controlled with currently available agents. In fact, 1 patient was 76 years of age before the diagnosis was established and had lived a relatively long life without prior specific treatment. However, some patients experience life-threatening bleeding events in association with injury or menses. Therefore, early diagnosis and prompt initiation of therapy are required to achieve optimal outcome. The identified PAI-1-deficient Amish kindred is still relatively young with none of the identified homozygous deficient patients currently older than 25 years. Consequently, longevity with complete absence of PAI-1 remains unestablished. Of interest, almost 100 heterozygotes in this Amish population have been identified with no adults identified to be homozygosity-deficient despite approximately 500 members of the kindred tested. (Rakesh P. Mehta, Amy D. Shapiro, unpublished data) It is anticipated that with early identification of PAI-1-deficient patients and appropriate therapy, a normal lifespan may be achieved. However, there are numerous unanswered questions for this population, including fertility, ability to carry a pregnancy to term, and development of atherosclerosis. The link between PAI-1 and cancer prognosis has been firmly established [26]. Increased levels of PAI-1 expression in breast cancer tissue are associated with a worse prognosis [27]. Theoretically, PAI-1-deficient patients may have improved survival with cancer because of decreased PAI-1 levels; in PAI-1 knockout mice, there was significantly less tumour invasion then in the controls [28]. Only long-term follow-up of these patients will provide the information necessary to answer these unresolved questions.
Conclusion
Plasminogen activator inhibitor, type 1 deficiency is a rare bleeding disorder whose mainstay of treatment is antifibrinolytic agents. The accurate diagnosis of this disorder remains a challenge and the development of a readily available standardized sensitive activity assay capable of differentiation between low normal levels and a true deficiency state is needed. Once available, the correlation of specific levels of deficiency with particular clinical symptoms could be established. Because this disorder results from either a qualitative or quantitative defect, an accurate PAI-1 activity assay is critical to establish the diagnosis. Also, with improved assays, the true prevalence of clinically significant PAI-1 deficiency could be determined. Although rare, PAI-1 deficiency should be considered in patients with delayed post-traumatic or postsurgical bleeding after other more common bleeding disorders have been excluded. If PAI-1 deficiency is established, then a trial of antifibrinoltyic agents should be considered. In the future, a database of patients with this disorder should be created to establish the range of clinical symptoms experienced, the genetic defects leading to a deficiency state, and the association of specific levels with clinical events.
Research Interests
Most research regarding PAI-1 currently pertains to elevated levels. The laboratory of Dr Douglas Vaughan and Dr Mohan Sathyamoorthy of the Cardiovascular Medicine Section at Vanderbilt University is very interested in the long-term effects of PAI-1 deficiency on the development of atherosclerosis. Dr Sathyamoorthy can be contacted at [email protected].




