Hereditary haemorrhagic telangiectasia
Abstract
Summary. Hereditary haemorrhagic telangiectasia (also known as Osler-Weber-Rendu syndrome) is a relatively common, under-recognized autosomal-dominant disorder that results from multisystem vascular dysplasia. It is characterized by telangiectases and arteriovenous malformations of skin, mucosa and viscera. This article summarizes the clinical manifestations and the management of this disorder and its management. This review underscores an urgent need to conduct prospective multicentre studies to develop evidence-based management guidelines for this disease.
Introduction
Hereditary haemorrhagic telangiectasia (HHT; also known as Osler-Weber-Rendu syndrome) is a relatively common, under-recognized autosomal-dominant disorder that results from multisystem vascular dysplasia and is characterized by telangiectases and arteriovenous malformations (AVM) of skin, mucosa and viscera [1–3]. HHT affects all ethnic and racial groups and is seen over a wide geographic distribution with an overall frequency of 1 per 5000 to 10 000 persons [4].
Although ancient medical literature from Hippocrates, Galenus and Avicenna record a broad spectrum of diseases and disorders that we recognize today, these writings do not mention a disorder similar to or qualifying for the diagnosis of HHT. In addition, HHT or a description of symptoms, including recurrent epistaxis, is not mentioned in the Bible or in the ancient writings from Egypt, Greece and Rome. The first definitive description of what is now known as HHT was written by Sutton [5] in 1864. He described a case of a man with vascular malformations and recurrent haemorrhage; because this patient was an orphan, no family history was available to determine a familial tendency. Rendu [6] first recognized the complex of hereditary epistaxis and telangiectases in 1896 in a 52-year-old man who had a history of anaemia and recurrent epistaxis since the age of 12 years; this patient’s father also had a history of melena, and both exhibited numerous red spots on the nose, tongue and upper lips owing to dilation of superficial vessels (telangiectases). Rendu clarified this disease entity as distinct from haemophilia. The subsequent decade produced a number of case reports further describing families with hereditary telangiectasia and haemorrhage, including prominent cases by Osler [7] and Weber [8], whose names now appear in the common eponymous labels for this condition. In 1909, Hanes [9] coined the term ‘hereditary hemorrhagic telangiectasia’, in acknowledgement of the theee features, which by then defined the disorder. Subsequently, interest in HHT has increased and the systemic nature of the disorder has been characterized. Table 1 elaborates the historical milestones in defining HHT as a disease entity.
| Year | Milestones in history of HHT |
|---|---|
| 1864 | First description of HHT by Sutton in a man with a vascular malformation and recurrent epistaxis |
| 1896 | Rendu recognized combination of hereditary nature of telangiectasia and epistaxis |
| 1901 | Osler described familial nature and published a syndrome in textbook |
| 1907 | Weber emphasized the association between hereditary telangiectasia and haemorrhage |
| 1909 | Hanes coined the term ‘Hereditary Hemorrhagic Telangiectasia’ |
Materials and methods
This review article on HHT was written using an extensive literature search through PubMed. HHT, Osler-Weber-Rendu syndrome, AVM and vascular malformations were used as search terms. Further references not initially identified in the search but referenced within these articles were also reviewed. Review articles and textbooks were used to discuss the pathology and current understanding of the molecular mechanisms involved in the progression of HHT.
Pathogenesis
The recognized manifestations of HHT result from abnormalities of vascular structure. The individual lesion in HHT is known as telangiectasis (pl., telangiectases), while the process of formation of telangiectasia or telangiectasis is referred as ‘telangiectasia’ (OMIM#187300). The telangiectasis, small arteriovenous shunt involving both dilated arterioles and venules, appears as 1–2 mm red spot in the skin and disappears upon a slight pressure. The morphogenesis of telangiectasis is not fully understood. Based on histopathological findings of skin telangiectases, Braveman et al. hypothesized that the earliest clinically detectable lesion of HHT is a focal dilatation of postcapillary venules, which continue to enlarge and eventually connect with dilated arterioles through capillaries (Fig. 1) [10]. As the vascular lesion increases in size, the capillary segments disappear and a direct arterio-venous communication is formed. This entire sequence of morphological events is associated with a perivascular mononuclear cell infiltrate in which the majority of cells are lymphocytes and minority are monocytes/macrophages by ultrastructure. In fully developed telangiectases, the venules are markedly dilated and convoluted, extend through the entire dermis, have excessive layers of smooth muscle without elastic fibres and often connect directly to dilated arterioles. Although Braverman’s hypothesis is generally accepted, the real evolution of skin telangiectases over time is unknown at the moment owing to a lack of new studies concerning this issue and also owing to the difficulty to perform ultrastructural studies on skin bioptic samples (which only rarely are symptomatic and they never require surgical interventions). It is important to underscore that the ultrastructural studies have so far been performed in skin samples only, whereas no histological data are available regarding telangiectases of mucosae (oral and nasal), despite their high clinical significance. AVM, the second most prominent lesion of HHT, lack capillaries and consist of direct connections between arteries and veins and are much larger than telangiectases. It is these vascular abnormalities that lead to the common presenting symptoms seen in patients affected with this disorder.
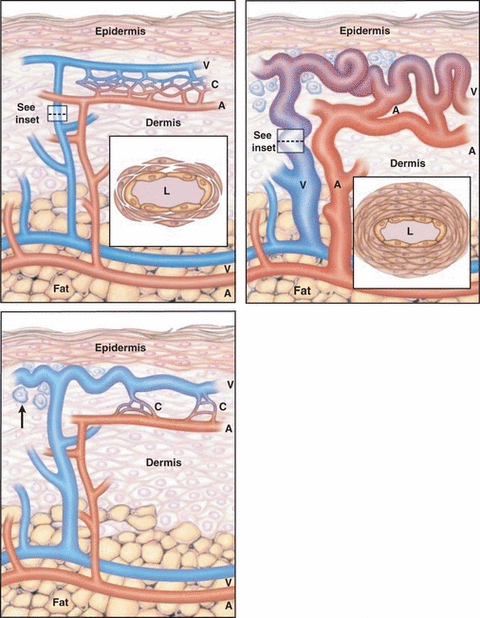
Evolution of a cutaneous telangiectasis in hereditary haemorrhagic telangiectasia. In normal skin (top panel), arterioles (A) in the papillary dermis are connected to venules (V) through multiple capillaries (C). These vessels arise from larger arterioles and venules at the junction of the dermis and fat. The ultrastructure of a normal postcapillary venule (shown in cross section in the inset) includes the lumen (L), endothelial cells and two to three layers of surrounding pericytes. In the earliest stage of cutaneous telangiectasia (middle panel), a single venule becomes dilated, but it is still connected to an arteriole through one or more capillaries. A perivascular lymphocytic infiltrate is apparent (arrow). In a fully developed cutaneous telangiectasis (bottom panel), the venule and its branches have become markedly dilated, elongated and convoluted throughout the dermis. The connecting arterioles have also become dilated and communicate directly with the venules without intervening capillaries. The perivascular infiltrate is still present. The thickened wall of the dilated descending limb (shown in cross section in the inset) contains as many as 11 layers of smooth-muscle cells. (Adapted from Braverman et al. [10]. Reprinted with permission from Guttmacher et al. [2]).
Current understanding of molecular mechanisms involved in HHT
To understand the molecular mechanisms involved in the development of HHT, it is crucial to understand the salient features of transforming growth factor-ß/bone morphogenesis protein (TGF-β/BMP) signalling pathway as the proteins encoded by different mutated loci (ALK1, ENG, BMPRII and MADH 4) in HHT patients share functional roles in TGF-β/BMP pathway (Fig. 2) [11–13]. This pathway regulates a number of biological processes, such as cell cycle control, embryogenesis, growth, development and differentiation of cell types including angiogenesis. The details of this pathway are elaborated in Fig. 1. The TGF-β superfamily consists of TGF-β, BMP, activins and related proteins. The TGF-β/BMP family of ligands mediates its effects by binding specific transmembrane type I and type II Ser/Thr kinase receptors. The type I receptors act downstream of type II receptors and determine the signalling specificity within the receptor complex. Although the core of the TGF-ß receptor complex is formed by the association of RI and RII, it may also contain accessory receptors, such as endoglin and betaglycan [11]. The accessory receptors betaglycan and endoglin can modulate signalling via the type II and type I receptors. Upon ligand-induced heteromeric complex formation, the constitutively activated type II receptor transphosphorylates and activates the type I receptor, which subsequently propagates the signal by phosphorylating specific receptor-regulated (R-) mothers against decapentaplegic (SMAD) transcription factors at the two C-terminal Ser residues [12,14]. On activation, R-SMAD form heteromeric complexes with a related partner molecule, the Co-SMAD [SMAD4 (Drosophila, homologue of, 4) in mammals], and accumulate in the nucleus where they participate in the transcriptional control of target genes. Specificity for response is at least in part determined by the type I receptors that are activated in the cascade. For example, interaction of the type I receptor ALK1 leads to phosphorylation of receptor SMAD 1, 5 and 8 (Fig. 2).
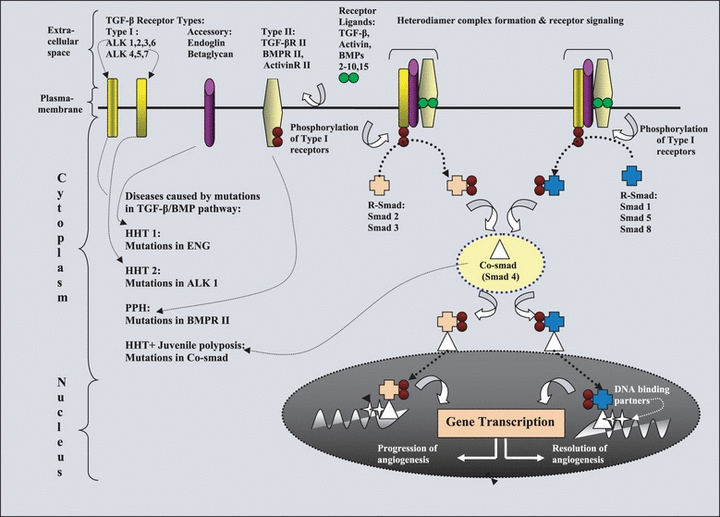
Signalling pathway of transforming growth factor β (TGF-β) superfamily exploring relationship between hereditary haemorrhagic telangiectasia (HHT), primary pulmonary hypertension (PPHN) and juvenile polyposis coli. In the extracellular space, ligands to the TGF-β/bone morphogenetic protein (BMP) superfamily of receptors bind either to an accessory protein, which presents the ligand to the type II receptor, or directly to the type II receptor on the cell membrane. The accessory receptors betaglycan and endoglin can modulate signalling via the type II and type I receptors. The binding of the ligands to the type II receptor then leads to binding of the type I receptor to form a heteromeric receptor complex at the cell surface. This results in phosphorylation and activation of the kinase domain of the type I receptor, which initiates phosphorylation of cytoplasmic signalling proteins termed receptor SMAD (R-SMAD). The pathway only splits into two distinct branches downstream of type I receptors: ALK4, ALK5 and ALK7 specifically phosphorylate SMAD2 and SMAD3, whereas ALK1, ALK2, ALK3 and ALK6 specifically phosphorylate SMAD1, SMAD5 and SMAD8. Phosphorylated R-SMAD binds to a collaborating SMAD (Co-SMAD/ SMAD4), and the resulting complex moves from the cytoplasm into the nucleus. The SMAD complex associates with a DNA-binding partner in the cell nucleus and interacts with various other transcription factors in a cell-specific manner to regulate gene transcription and to mediate the effects of signalling by the TGF-β/BMP superfamily of receptors at the cellular level. This pathway controls the balance between progression and resolution of angiogenesis. Defect in this pathway leads to development HHT, PPH and juvenile polyposis coli.
Genetic basis of HHT
With the discovery of various genes associated with HHT, it is being realized that HHT is a genetically heterogeneous disorder (Table 2) [15]. Early linkage studies of extended HHT kindreds provided compelling evidence for at least two distinct locations for HHT genes within the human genome: endoglin (ENG) for HHT1 (OMIM#187300) and Activin receptor-like kinase 1 (ALK1) for HHT2 (OMIM#600376). Mutations in the ENG gene (OMIM#131193) localized on the long arm of chromosome 9 (9q33-q34.1) are responsible for HHT1 [16,17], whereas HHT2 results from mutations of the ALK1 gene (OMIM# 601284) localized on the long arm of chromosome 12 (12q11-q14) [18]. Subsequent studies in patients with primary pulmonary hypertension (PPH) and the clinical characteristics of HHT led to the discovery that mutations in bone morphogenetic protein receptor II (BMPRII) contribute for the development of HHT/PPH phenotype [19,20]. Moreover, Gallione et al. [21] reported that mutations in MADH4 or SMAD4 in the 18q21.2 region can cause a syndrome consisting of both juvenile polyposis and HHT phenotypes. In addition, the existence of HHT families without mutations mapping in either ENG or ALK1 genes led to the identification of the third and fourth locus of HHT through linkage analysis. Cole et al. hypothesized a possible third locus at 5q31.3-q32 (OMIM% 601101) in a four-generation HHT pedigree, who presented with uncommon extensive pulmonary involvement [22]. Similarly, Bayrak-Toydemir et al. reported a possible forth locus of HHT at 7p14 (OMIM% 610655) in a family of 12 members, in which eight members were definitely affected with HHT and four were considered ‘suspicious’ for HHT [23]. To date, these third and fourth loci have not been definitely identified. These findings imply that mutations beyond TGF-β/BMP pathway may be contributing for either the development of HHT or modification of HHT phenotype.
| HHT types | Gene | Chromosomal locus |
|---|---|---|
| HHT 1 | Endoglin or ENG | 9q34.1 |
| HHT 2 | Activin Receptor Like Kinase 1 (ACVRL1/ALK 1), | 12q11-q14 |
| HHT 3 | – | 5q31.3-q32 |
| HHT 4 | – | 7p14 |
| HHT + juvenile polyposis coli | MADH4 or SMAD4 | 18q21.1 |
| HHT 2 + primary pulmonary hypertension (PPH) | BMPRII | 2q33 |
It has been observed that levels of vascular endothelial growth factor (VEGF), an important signalling protein involved in both vasculogenesis and angiogenesis, are also increased in HHT patients [24]. Increased VEGF expression was recently demonstrated to cause abnormal microvessels in ENG+/- mice [25]. Hence, conditions that stimulate VEGF expression might affect the HHT phenotype. VEGF expression is increased not only in the plasma, but also in the nasal mucosa of HHT patients irrespective of their clinical presentation with epistaxis [26].
The percentage of ENG and ALK1 mutations thus far reported as causative of HHT is similar (53% and 47%) [27]. There do not appear to be striking mutation hot spots in either gene, and more than 90% of the mutations identified are previously unreported. Mutations of all types, including deletions, insertions, missense mutations and splice site changes, are distributed throughout both genes. Recently, HHT database is established to record all sequence variants identified within these two genes (http://hhtmutation.org/). Information gained from this database suggests that the severity of disease does not correlate with any specific mutations. It is now generally accepted that all endoglin mutations are represented by null alleles and therefore haploinsufficiency is the mechanism underlying HHT1 [28,29]. With regard to HHT2, most mutations seem to give rise to haploinsufficiency, except for very few cases, for which a dominant negative model has been suggested, but not definitely proved [30–33].
Diagnosis and management of HHT
Diagnosis The initial diagnosis of HHT in a family relies on clinical examination, medical history and a vigilant family history (Table 3) [3]. De novo mutations are rare, and penetrance approaches 100% by the age of 40 years; hence, family history is critical. Because of the pattern of inheritance and age of disease penetrance, clinical diagnostic criteria have limitations. Although HHT is an autosomal dominant disorder, it exhibits the property of ‘incomplete penetrance’, an inability to express the full spectrum of disease phenotype despite carrying a defective gene. Furthermore, epistaxis, the most common symptom of HHT, is prevalent in the general population not affected with HHT and is a manifestation of a large number of clinical entities. These factors potentially limit the diagnosis of HHT.
| Criteria |
| 1 Spontaneous, recurrent epistaxis. Nocturnal nosebleeds heighten concern for HHT. |
| 2 Mucocutaneous telangiectases, especially on lips, tongue, oral cavity, fingers and nose. |
| 3 Internal AVM(s) (pulmonary, cerebral, hepatic, gastrointestinal, spinal). |
| 4 First-degree relative with HHT according to these criteria. |
| HHT diagnosis |
| Definite: 3 or more criteria present |
| Possible: 2 criteria present |
| Unlikely: < 2 criteria present |
- AVM, arteriovenous malformations.
- Source: Derived from [3].
Based on the multisystem nature of this disease, the initial evaluation once the diagnosis of HHT is established should include the following: (i) contrast echocardiography to screen for intrapulmonary shunts and, if identified, computed tomography (CT) of the chest with 3 mm cuts to characterize pulmonary AVM; (ii) magnetic resonance imaging of the brain to screen for cerebral AVM; and (iii) auscultation for a hepatic bruit and medical history for symptomatic liver shunts.
Diagnosis of HHT in children The diagnosis of HHT in children may be particularly difficult, especially if not previously established in the family. Bleeding symptoms consistent with HHT are common clinical problems in children and may be overlooked or ascribed to alternative causes; epistaxis may be associated with a variety of common entities, such as allergic disease, sinusitis and local trauma. Paediatric patients who present with recurrent or persistent clinical bleeding symptoms that are associated with HHT should undergo a detailed physical examination and family history that may uncover a diagnosis, such as HHT, and a thorough evaluation for disorders of haemostasis, such as von Willebrand disease. As the clinical stigmata of HHT may vary widely and in children be absent or sparse, the diagnosis will not be established unless specifically considered.
Role of genetic testing in diagnosis of HHT The fact that mutations that cause HHT have been identified raises the possibility of the use of molecular diagnosis in routine clinical practice. The lack of common alleles or highly recurrent mutations, locus heterogeneity and the presence of mutations in almost all coding exons of the two genes makes the screening for mutations time consuming and costly. However, owing to the availability of advanced molecular techniques like single-strand conformation polymorphisms (SSCP), denaturing high performance liquid chromatography (DHPLC) and subsequent direct sequencing, it is possible to detect the mutations in 70–90% of individuals with HHT [34,35]. Hence, it is likely that in near future genetic testing may become available for this population. In general, an individual who meets the clinical diagnostic criteria for HHT should be tested first in each family to determine whether the family’s HHT mutation can be identified. Genetic testing for HHT in relatives is not helpful unless a definitive mutation is detected in a clearly affected individual. The identification of the genetic defect allows less clinically symptomatic individuals within a family to be identified and receive appropriate preventive care. In addition, once the mutation has been identified in the proband, the choice of prenatal diagnosis may be offered to the family through amniocentesis or chorionic villous sampling.
Imaging studies Advances in imaging studies, including helical CT scanning, magnetic resonance imaging (MRI), magnetic resonance venography (MRV), magnetic resonance arteriography (MRA) and endoscopic imaging, have made it easier to accurately diagnose and treat HHT without performance of invasive procedures, such as surgical exploration or angiograms. A helical CT scan with 3-mm cuts is preferred for evaluation of pulmonary AVM. Table 4 summarizes the clinical approaches and treatment options in the care of persons with HHT.
| Organ/system | Type of lesion | Sites | Clinical symptoms | Emergencies | Screening tool | Diagnostic modality | Treatment* |
|---|---|---|---|---|---|---|---|
| Nose | Telangiectasia | Nasal mucosa | Epistaxis, iron deficiency anaemia | Massive epistaxis | Medical history | Clinical examination | Humidification, packing, antifibriolytic therapy (EACA and Tranexamic acid), pork salt chop sticks, topical oestrogen/progesterone ointments, local cauterization, Septal dermoplasty, laser, embolization of external carotid artery iron therapy |
| Skin | Telangiectasia | Lips, tongue, palate, face, conjunctivas, trunk, nail beds, finger pad | Cosmetic disfigurement, bleeding (usually minor) | Nil | Medical history and Clinical examination | Clinical examination | Topical agents, laser ablation |
| Lung | AVM | Often multiple; predilection for lower lobes | Asymptomatic, cyanosis, clubbing, migraine, cerebral abscess, embolic stroke, polycythemia, pulmonary hypertension | Massive haemoptysis, hypovolumic shock, haemothorax | Medical history, auscultation of bruit over chest, blood gas measurement, orthodeoxia† pulse oximetry, chest X-ray, linkage to 9q3, | High-resolution helical CT scan, Angiography | Transcatheter or stereotactic embolization of AVM, surgical resection of AVM, ligation of arterial supply of AVF, iron therapyNote: Require infective endocarditis prophylaxis prior to dental and surgical intervention to reduce the risk of brain abscess. |
| Central nervous system | AVM | Brain, spinal cord, meninges | Asymptomatic, headache, subarachnoid haemorrhage | Transient Ischemic Attack (TIA)/ischaemic stroke, haemorrhagic stroke, brain abscess | Medical history, auscultation of bruit over the skull | MRI/MRV/MRA CT scan | Neurovascular surgery, ligation of the feeding artery, stereotactic surgery, transcatheter embolization of the feeding artery, radiosurgery |
| Gastro- intestinal tract (except liver) | AVM, telangiectases, angiodysplasias | Stomach, duodenum, small bowel, colon, liver | Asymptomatic, bleeding, iron deficiency anaemia | haematemesis, melaena, haematochezia, hypovolumic shock, high output failure | Medical history, stool guiac examination for the occult blood | Endoscopy, angiography, CT scan | Blood transfusion, endoscopic application of photocoagulation, ethinyl estradiol/norethindrone, iron therapy for anaemia, |
| Hepatic | AVM | Liver parenchyma | Asymptomatic, portal hypertension, biliary disease | Hepatic encephalopathy | Medical history, auscultation of bruit over the liver | CT scan, MRI/MRV/MRA angiography, | No intervention, liver transplantation only for life-threatening lesions |
- AVM, arteriovenous malformation; CT, computed tomography; EACA, epsilon amino caproic acid; MRI, magnetic resonance imaging; MRV, magnetic resonance venography; MRA, magnetic resonance arteriography.
- *In life-threatening emergencies, ABC should be established first.
- †Greatest deoxygenation occurs in upright position.
- Source: Derived from [2].
Principles of treatment In general, treatment of HHT is aimed at: (i) control of local and systemic symptoms; (ii) surveillance for and of lesions; and (iii) measures to prevent complications associated with AVM.
In general, pulmonary and central nervous system (CNS) AVM require monitoring vigilance. Patients with pulmonary AVM should be given infective endocarditis prophylaxis before dental and surgical procedures. As these lesions grow with time, life-long periodic surveillance is recommended. Currently, no guidelines are available regarding the frequency and choice of imaging modality of surveillance for these lesions. Because of the systemic nature of this disease, a multidisciplinary team approach involving an otolaryngologist, pulmonologist, interventional radiologist, neurologist, neuroradiologist, neurosurgeon, geneticist, cardiologist, gastroenterologist, hepatologist, and haematologist should be considered to provide optimal patient care. Recognizing this need, multidisciplinary specialty clinics for HHT have been established in North America and in other parts of the world. The list of these centres and the various resources for the patients, their families and medical professionals are available at the Website of the HHT Foundation International (http://www.hht.org).
The role of medical therapy in preventing progression of vascular lesions in HHT is not very encouraging. There are anecdotal reports of efficacy of antiangiogenic medications, such as VEGF antibodies (Avastin/Bevacizumab) [36], oestrogens [37], thalidomide [38] and interferon [39]. Clinical studies are underway to clarify the role of these agents in treating HHT (http://www.ClinicalTrials.gov). The subsequent section details the clinical manifestations based on the organ of involvement and its management.
Clinical manifestations and prognosis of HHT according to the organ of involvement
Abnormal vessel formation and subsequent bleeding form the basis of most clinical manifestations of HHT. Although the number and location of lesions vary widely, even within the same family, most telangiectases are found in the oral, nasal and gastrointestinal mucosa and the fingertips, whereas AVM occur most commonly in the lungs, liver and CNS. In general, smaller telangiectatic lesions usually present with symptoms of recurrent bleeding, whereas symptoms of the larger, internal AVM do not result from haemorrhage. Complications of AVM most often occur as a result of shunting of blood, thrombosis or embolus [40,41].
Nose
Epistaxis owing to telagiectases in the nasal mucosa is the most common and often the earliest symptom of HHT. As many as 95% of affected individuals eventually experience recurrent epistaxis, with a mean age of approximately 12 years and a mean frequency of 18 episodes per month. Although severe epistaxis may cause chronic anaemia in some patients, others have mild, infrequent nosebleeds that do not require treatment. In general, most patients experience an increase in frequency and severity of epistaxis with advancing age, but some patients report no particular change in their episodes over time, and some even experience improvement [42,43].
Besides implementation of local measures, including application of local pressure and ice, therapeutic options consisting of high-dose antifibrinolytic agents, such as administration of tranexamic acid at 1–2 g three times daily either orally or intravenously at the time of epistaxis [44], or combined oestrogen–progesterone preparations [45], have shown efficacy. If these measures are insufficient and the frequency and duration of episodes impair the patient’s quality of life, a photocoagulation laser or a septal mucosal dermoplasty may be recommended [45,46]. Embolization of the external carotid artery branches has been performed for short-term relief of symptoms, although it is ineffective for long-term management and can lead to complications related to ischemia [47]. Chemical cauterization should always be avoided because it may harm nasal structures. In the presence of anaemia, oral iron supplementation or rarely parenteral iron therapy with or without blood transfusion, depending on the level of anaemia and clinical symptoms, may be required. An old local remedy using ‘salt pork’ may also be used with some success. Salt pork is cut into plugs the size of the nostril, a loop of thick thread is placed through one end and each plug is wrapped in wax paper and frozen until required. Application of this material provides haemostasis through vessel constriction owing to cold and presence of tissue thromboplastin to enhance local coagulation, with the oily texture allowing easy removal without disruption of the clot once formed.
Skin
Multiple telangiectases of the hands, face and oral cavity occur in similar percentages of patients, but the age at onset is generally later than for epistaxis [43,48]. It is common for patients to report first noticing telangiectases in one or more of these locations in the decade between 30 and 40 years of age (Fig. 3). Telangiectases in these locations are not commonly a source of troublesome bleeding. Most patients receive treatment for cosmetic reasons. Painful cutaneous telangiectases can be treated with laser therapy [2].

Typical facial telangiectasia in a 60-year-old woman with hereditary haemorrhagic telangiectasia, who presented with severe iron deficiency anaemia owing to gastrointestinal blood loss related to bleeding from intestinal telangiectasia.
Gastrointestinal tract
The prevalence of intestinal telangiectasia varies from 10% to 33% [49] in patients with HHT. They occur anywhere in the gastrointestinal tract, most commonly in the stomach and upper duodenum. Approximately 25% of individuals older than 60 years present with melena or anaemia. Bleeding tends to be slow but persistent and may increase in severity with age [50]. Endoscopy and, less commonly, angiography can demonstrate the presence of large telangiectases, AVM or angiodysplasia. Use of photocoagulation using bipolar electrocoagulation or laser techniques is useful for control of bleeding in the short term. In clinical trials, medical treatment with oestrogen and progesterone has shown to be beneficial in reducing the incidence of bleeding in these patients [51]. Anecdotal reports exist regarding resolution of gastrointestinal HHT lesions after treatment with interferon-α that was used for chronic hepatitis [52].
Lung
Pulmonary AVM occur more frequently in patients with HHT1 compared with HHT2 – 75% and 44%, respectively [33] . They can occur as discrete lesions vs. diffuse pattern. They are thought to be congenital but may enlarge over time [53]. They are commonly located in the posterior lower lobes. They may be asymptomatic for many years and present insidiously or dramatically with respiratory symptoms, such as exercise intolerance, cyanosis or pulmonary haemorrhage, migraine headaches, polycythemia and clubbing [54]. Approximately 30–40% of individuals with HHT, who have pulmonary AVM, will have a CNS presentation with thrombotic and embolic events, such as stroke, brain abscess, or transient ischemic attacks, owing to right-to-left shunting [53,54] that can occur even in the presence of near normal pulmonary arterial oxygen tension [57]. It is common for several adverse events to occur before a pulmonary AVM is identified as the source of the CNS events [57]. Pregnant women with untreated pulmonary AVM are at high risk of pulmonary haemorrhage [58]. Pulmonary disease indistinguishable from PPH has been reported in multiple patients with HHT with mutations in the ALK1 gene, indicating that close monitoring for the development of PPHN is required [20].
Pulmonary AVM with a feeder vessel of more than 3 mm should be treated using transcatheter embolization to reduce the risk of embolic events [57]. Pulmonary AVM may grow in size over time; hence, smaller lesions that are detected need to be followed [54]. To prevent cerebral abscess, antiobiotic prophylaxis is recommended for dental and surgical procedures in any patient with evidence of an intrapulmonary shunt. Patients with diffuse pulmonary AVM may require lung transplantation if the patient is severely hypoxic. The morbidity and mortality associated with lung transplantation could be the limitation to offer this therapeutic modality [59].
Central nervous system
Central nervous system AVM, including the brain, meninges and spinal cord, are also thought to be congenital. Cerebral AVM occur more frequently in individuals with HHT1 compared with HHT2 – 15–20% vs. 1–2%, respectively [33,40,55,60]. Although the risk of neurological symptoms increases with multiplicity of pulmonary AVM, CNS lesions may present at any age with seizure, headache or intracranial haemorrhage [55,56]. Complications, including stroke, transient ischemic attacks and brain abscess, have been reported owing to paradoxical embolization of thrombus or bacterial emboli that bypass pulmonary capillaries. CNS lesions may present in the neonatal period, infancy or childhood in otherwise asymptomatic children [61]. Spinal AVM are less common, occurring in approximately 1% of individuals with HHT, and may manifest as subarachnoid haemorrhage, progressive myelopathy, radicular pain or sphincter disturbance [62,63]. Techniques currently used to treat CNS AVM include transcatheter embolization, resection and stereotactic radiosurgery [64,65].
Hepatic AVM
Hepatic vascular lesions include a variety of intrahepatic shunts and disseminated intraparenchymal telangiectases [66,67]. The prevalence of hepatic involvement in HHT is unknown, but in one study, hepatic vascular abnormalities were identified by CT in 74% of consecutive HHT patients [68]. In most patients with HHT, liver involvement remains clinically silent, but hepatic vascular lesions (shunts between portal hepatic artery and hepatic vein) can present as high-output heart failure, portal hypertension, biliary disease and portosystemic encephalopathy [68].
Hepatic AVM are not treated in asymptomatic patients because they rarely present with catastrophic emergencies, and unlike pulmonary AVM, treatment with embolization results in high mortality rates owing to liver infarction. At present, liver transplantation is the treatment of choice for patients with otherwise life-threatening symptoms secondary to hepatic shunts [69]. Recently, there is a case report of successful treatment of massive hepatic AVM with VEGF antibody (Bevacizumab; dose: 5 mg kg−1 over a 12-week period), suggesting potential role of targeted therapies in treating HHT [36].
Rare sites of AVM
Arteriovenous malformation have been described only rarely in other locations, including coronary arteries [70,71] and the vessels of the eye [72], spleen [73], urinary tract [73] and vagina [74]. These lesions are treated with embolization, ligation or surgical resection.
Future directions
As HHT is a heterogenous multisystem disorder, it is critical to improve awareness among clinicians so as to consider this entity in the differential diagnosis for patients presenting with symptoms associated with this disorder. Multicentre multidisciplinary collaborative studies are required to develop evidence-based guidelines for the management of visceral HHT. Better understanding of the molecular mechanisms is crucial for the development of targeted therapy or gene therapy to cure this disease.
Disclosures
The authors stated that they had no interests which might be perceived as posing a conflict or bias.




