Global warming threatens the persistence of Mediterranean brown trout
Abstract
Current climate change exacerbates the environmental restrictions on temperate species inhabiting low latitude edges of their geographical ranges. We examined how temperature variations due to current and future climate change are likely to affect populations’ persistence of stream-dwelling brown trout Salmo trutta at the vulnerable southern periphery of its range. Analysis of 33 years of air temperature data (1975–2007) by time-series models indicated a significant upward trend and a pronounced shift in air temperature around 1986-1987. This warming is associated with an ongoing population decline of brown trout, most likely caused by a loss of suitable thermal habitat in lower latitudes since the 1980s. Population decrease may not be attributed to physical habitat modification or angler pressure, as carrying capacity remained stable and populations were not overexploited. We developed regional temperature models, which predicted that unsuitable thermal habitat for brown trout increased by 93% when comparing climate conditions between 1975–1986 and 1993–2004. Predictions from climate envelope models showed that current climate change may be rendering unsuitable 12% of suitable thermal habitat each decade, resulting in an overall population decrease in the lower reaches of around 6% per year. Furthermore, brown trout catches markedly decreased 20% per year. Projections of thermal habitat loss under the ecologically friendly B2 SRES scenario showed that brown trout may lose half of their current suitable habitat within the study area by 2040 and become almost extinct by 2100. In parallel to the upstream movement of brown trout thermal habitat, warm water species are increasing their relative abundance in salmonid waters. Empirical evidence was provided of how current climate change threatens some of the most healthy native brown trout populations in Southern Europe and how forthcoming climate change is expected to further decrease the conservation status of the species.
Introduction
Global warming is changing the current distribution and abundance of many thermally sensitive species (e.g. Root et al., 2003; Thomas et al., 2004; Lenoir et al., 2008). In particular, one threatened fish family is Salmonidae, which comprises oxygen demanding species that require cold and clean water (Jonsson & Jonsson, 2011). As Elliott & Elliott (2010) emphasize, temperature is often regarded simply as a factor that alters the physiology and behaviour of salmonids, yet it is also a crucial feature of their habitat, being one axis of their multidimensional niche. There are already examples of stock declines due to climate change in many valuable commercial and recreational fisheries from North America (Condron et al., 2005) and Europe (Jonsson & Jonsson, 2004; Hari et al., 2006; Todd et al., 2008). Furthermore, climate change aggravates environmental constraints especially for populations of salmonid species persisting at low-latitude margins. This fact is illustrated by wild brown trout Salmo trutta populations, which are expected to face the greatest risk from climate change at the southern edge of their distribution range (Jonsson & Jonsson, 2009, 2011; Lassalle & Rochard, 2009).
In Southern European rivers, stream-dwelling salmonids are restricted to headwaters, where local environmental conditions mitigate the adverse effects of regional warm climate. Current climate change is projected to worsen freshwater conditions (higher temperatures and longer droughts) in the Mediterranean region, an area already vulnerable to climate variations and decreased water availability (Giorgi & Lionello, 2008). Interannual variations are expected to be more noticeable in summertime and this, together with a gradual warming, would result in more frequent extremely high temperature events. Wild brown trout occurs within a limited range of environmental temperatures, with lower tolerance to high temperatures than other salmonid species (Elliott & Elliott, 2010). In this context, it is a species whose populations, a priori, will experience severe climate change-induced impacts, including shifts in the species distribution range as a consequence of a considerable reduction in their suitable thermal habitat.
Moreover, the Iberian Peninsula shows a strong genetic differentiation among brown trout populations and due to its important role as a glacial refuge is considered a hotspot for the species diversity (Machordom et al., 2000; Suárez et al., 2001; Almodóvar et al., 2006a). These peripheral populations, which naturally occur at low densities, are more prone to local extinctions due to increasingly detrimental human impact and the effects of current climate change (Hampe & Petit, 2005; Nicola et al., 2008, 2009). Furthermore, projected changes in populations will negatively affect sport fishing in a species with a high socioeconomic value.
Although Jonsson & Jonsson (2009) reviewed the eventual impact of climate change on European anadromous populations of brown trout, as far as we are aware studies on stream-dwelling Mediterranean trout populations are still scarce (Lassalle & Rochard, 2009). A thorough study was conducted in a southern Pyrenean basin (River Aragón, Ebro River basin) during a 12-year-period (1993–2004) to assess the conservation status of brown trout. After analysing the influence of a wide range of both environmental and human degradation factors on the deviation of density from carrying capacity (i.e. potential maximum density), we found that the main determinants of the marked decrease of trout abundance and shifts in species distribution were maximum water temperature and stream connectivity (D. Ayllón et al., submitted paper). Furthermore, projections of air temperature and precipitation in this area under A2 and B2 SRES scenarios (Special Report on Emission Scenarios, Nakicenovic et al., 2000) have been made by Brunet et al. (2009). The A2 scenarios focus on a regionally oriented economic development where the global population is expected to increase at a high rate; therefore energy consumption and changes in land use are high and resources become scarce. The B2 scenarios emphasize environmental preservation and social equity, with local solutions to economic, social and environmental sustainability, where the global population is expected to increase continuously, yet more slowly than in A2. Both families of scenarios predict an increase of maximum and minimum air temperatures for 2010–2100, although with no significant changes in precipitation. Therefore, in this study our aim is to examine how current and potential temperature variations due to climate change affect populations’ persistence of brown trout within the warmer boundaries of its distribution. We expected that the increased water temperature would change the distribution and abundance of brown trout due to a reduction of suitable thermal habitat.
Material and methods
Study area
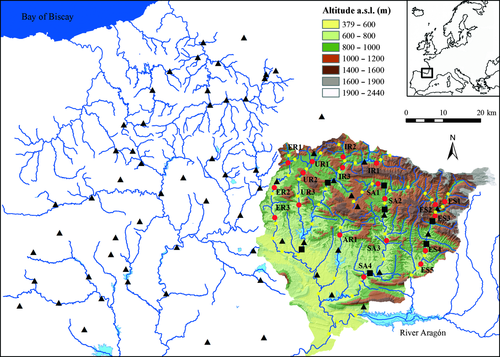
We analysed 19 sites from 12 rivers of the Aragón River basin (Fig. 1), a Mediterranean Pyrenean drainage system. Sampling sites were selected to characterize the full range of environmental and geo-morphological conditions existing within the area. Sampling sites corresponded to second to fourth-order streams and were situated between latitudes 42°30′ and 43°03′N and longitudes 0°43′ and 1°32′W, at an altitude ranging from 540 to 870 m. Brown trout is the prevailing species throughout the study area and its populations consist of exclusively resident individuals. The rivers are not currently stocked and are open to recreational angling except for some reaches, which are preserved sections. The rivers are not impaired by anthropogenic land uses or pollution. The scattered dispersal of power stations and dams on the lower reaches are the main anthropogenic pressures in the study area.
Fish assessment
Brown trout populations were sampled by electrofishing every summer from 1993 to 2004 using a 2200-W generator. Prior to sampling, each site was blocked upstream and downstream with nets. The sampling sites were 71–152 m long, 4–14 m wide and encompassed an area of 356–1909 m2. Individuals were anaesthetised with tricaine methane-sulphonate (MS-222), measured (fork length, to the nearest mm) and weighed (to the nearest g). Scales were taken for age determination. Fish density (individuals ha−1) with variance was estimated separately for each sampling site by applying the maximum likelihood method (Zippin, 1956) and the corresponding solution proposed by Seber (1982) for three removals assuming constant-capture effort. Population estimates were carried out separately for each year class. A qualitative assessment of the abundance of fish species other than brown trout was simultaneously performed at each site. During every brown trout sampling, abundance of nontrout fish species was categorized and ranked as nonpresent (0), rare (1), frequent (2), abundant (3) and very abundant (4). An abundance index was estimated then for every site and year as the average of the individual qualitative abundance index of every species caught in the study area. The total abundance index of nontrout species of a given year was calculated as the average of all sites.
The recreational fishery in the study area is focused on brown trout, which is the only target species. During the fishing seasons (March–August) from 1991 to 2004 creel-surveys were conducted in 52 reaches from 17 streams, where all sampling localities are included (Fig. 1). Monitoring surveys were carefully planned and included any information relevant to the fishery (see full description in Almodóvar et al., 2002). Fishing pressure was estimated by the number of anglers per hectare per day (angler ha−1 day−1). Harvest rate was calculated as the mean number of legal-sized trout kept per hectare per year (individuals ha−1 year−1).
Temporal trends in total brown trout density, abundance of nontrout species, fishing pressure and harvest rate were tested by regressing population parameters against year of observation. Data were log-transformed before the analysis. Significances were obtained by randomization tests and estimates for intercept and slope were obtained by a bootstrapping procedure with 1000 bootstrap samples. Statistical analyses were performed using STATISTICA 6.1 (StatSoft Inc., Tulsa, OK, USA) and SPSS version 18.0 (SPSS, Chicago, IL, USA).
Carrying capacity
Carrying capacity was defined as the potential maximum density of fish a river can naturally support during the period of minimum available habitat (Milner et al., 2003). Physical habitat dynamics were modelled using the Physical Habitat Simulation system (PHABSIM; Milhous et al., 1989). PHABSIM simulations determine the potentially available habitat for an aquatic species and its life stages as a function of discharge by coupling a hydraulic simulation model with a model describing the habitat selection patterns of the target species (the habitat suitability criteria, HSC). The standard output of PHABSIM simulations is the curve that relates the Weighted Usable Area (WUA; m2 WUA ha−1, an index of the quality and quantity of available habitat) with stream flow (m3 s−1).
We conducted habitat surveys at every sampling site during the summer of 2004 to collect the topographical, hydraulic and channel structure data necessary to perform PHABSIM simulations, following the procedures described in Ayllón et al. (2010a, 2011). We assessed an average (± SD) length of 99.5 ± 22.9 m and average (± SD) area of 850.9 ± 424.2 m2 per site. To model brown trout habitat selection, reach-type specific HSC for young-of-the-year (YOY, 0+), juvenile (1+) and adult (>1+) life stages were developed (see Ayllón et al., 2010b).
Hydraulic data were calibrated in PHABSIM following procedures set out in Waddle (2001). Habitat competition analyses were performed using the HABEF program within PHABSIM to model habitat partitioning among life stages in the stream areas where physical habitat is suitable for more than one life stage (see Ayllón et al., 2010a and Parra et al., 2011 for further details on competition analyses). Summer habitat time series for the 12-year study period (1993–2004) for each age class were obtained by combining WUA curves as a function of stream flow with historical flow time series provided by the nearest gauging stations.
The area required and so defended (i.e. territory size) by individuals of different life stages provides the link between amount of available habitat and number of individuals of each life stage that can be supported by the habitat associated with a given flow, i.e. carrying capacity. Spatial requirements of individuals were determined by means of an allometric territory size relationship specifically developed for brown trout (Ayllón et al., 2010a). Consequently, to calculate territory size, length at age i was determined for every age class, year, and site. Finally, carrying capacity was estimated for every age class (0+, 1+ and > 1+), year and site through the following ratio, Ki = WUAi/Ti, where Ki is the carrying capacity of age-class i (trout ha−1), WUAi is the mean summer WUA of age-class i (m2 ha−1) and Ti is the area of the territory used by an individual of average body size of age-class i (m2 trout−1).
Temporal trends in carrying capacity and the ratio between density and carrying capacity (D/K ratio) were tested by regressing population parameters against year of observation, following the procedure detailed for fish population assessment.
Air and water temperature modelling
Air temperature data series (1975–2007) were analysed by time-series models to determine their autocorrelation structure. Data came from two meteorological stations close to fish sampling points located at high (Abaurrea, 1050 m) and low (Epároz, 608 m) altitudes, which were representative of upper and lower reaches. Model identification techniques were used to distinguish which time-series model adequately characterized the autocorrelations among the observations. The goal was to identify a model that adequately explained the patterns in the observations and their interdependence. Time-series models were classified as autoregressive, moving average, or autoregressive integrated moving average (ARIMA) (Box & Jenkins, 1976). An interrupted time-series design (i.e., a time-series analysis in which the series is divided, or interrupted, by the intervention into two periods, preintervention and postintervention, which will be compared), based on the models developed by McDowall et al. (1980), was used to examine the temporal trend in air temperature data. The Expert Modeller features in SPSS version 18.0 were used to automatically determine the best-fitting ARIMA model to analyse temperature data.
We developed two different spatial models to predict water temperatures in the study basin during the study period (1993–2004). We developed a model describing maximum water temperature (MAXTw) and a model describing maximum mean water temperature during seven consecutive days (MAX7d-Tw), averaged for the length of the study period. As water temperature data were not available, they were estimated from air temperature data. At a first step, we built a regional model of maximum air temperature (MAXTa) by regressing maximum air temperature to latitude and altitude for 48 stations ranging in altitude from 38 to 1344 m above mean sea level (a.s.l.; Fig. 1). At a second step, we developed a regression model relating average maximum water temperature to average maximum air temperature. To do this, water temperature was recorded daily at seven sites (Fig. 1) by means of data-loggers installed from June of 2004 to November of 2005. A linear and the nonlinear regression model described by Mohseni et al. (1998) were fitted to data and compared by means of the Akaike's Information Criterion adjusted for small samples (AICc; Burnham & Anderson, 2002). The model with the lowest AICc was considered the best fit. The same procedure was used to develop a regional model for maximum mean water temperature during seven consecutive days (MAX7d-Tw) as a function of maximum mean air temperature during seven consecutive days (MAX7d-Ta). We employed MAX7d-Ta as the independent variable as previous studies have shown that weekly and monthly averages of stream temperature and air temperatures are better correlated with each other than are daily values (e.g., Stefan & Preud'homme, 1993; Morrill et al., 2005).
Based on the calculated predictive water temperature regional models, we constructed maps of MAXTw and MAX7d-Tw for the average climate conditions during the study period using ArcGis 9.2 software (ESRI Inc., Redlands, CA, USA). Based on MAX7d-Tw, we represented the spatial distribution of the thermal limit beyond which water temperature becomes stressful for brown trout populations. According to Elliott et al. (1995) and Elliott & Elliott (2010), the upper temperature limits for feeding and growth are above 19.4 °C in brown trout. Therefore, the 19.4 °C isotherm was used to split study sites up into upper and lower localities. Afterwards, we estimated the amount of suitable habitat with MAX7d-Tw at or below the 19.4 °C thermal limit for present air temperatures and for warming scenarios of 0.1–3.5 °C higher than present to quantify projected loss of thermal habitat.
Finally, we calculated the projected loss of salmonid waters on the study area under warming scenarios of 0.1–5 °C air temperature increases. European Water Framework Directive (Council Directive 2006/44/CE) set a maximum water temperature of 21.5 °C as the thermal limit to separate salmonid from warm water fish courses. We followed the same methodology described before, setting MAXTw equalled to 21.5 °C as the thermal limit. The warming scenarios used to calculate the loss of thermal habitat and salmonid waters were selected to include the whole range of air temperature regional projections under the A2 and B2 SRES scenarios presented by Brunet et al. (2009).
Results
Air temperature time series
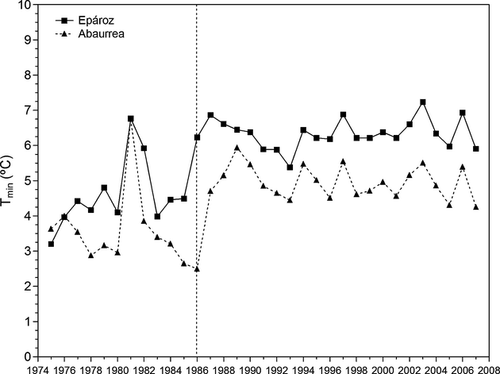
The analysis of 33 years of air temperature data (1975–2007) showed a gradual increase during the study period, which was more apparent in minimum temperatures. The data series fit significant first order vector autoregressive ARIMA models (1,0,0) in both stations (Table 1), thus indicating a significant increasing trend in temperature. The residuals of ARIMA models were not autocorrelated and were normally distributed.
| SE | t-ratio | P | |||
|---|---|---|---|---|---|
| Epároz | AR1 | 0.9583 | 0.0761 | 12.6001 | < 0.0001 |
| Constant | 3.4386 | 1.0631 | 3.2346 | 0.0029 | |
| Φ | 1.6522 | 0.3323 | 4.9725 | < 0.0001 | |
| Abaurrea | AR1 | 0.4154 | 0.1670 | 2.4872 | 0.0185 |
| Constant | 4.4154 | 0.2785 | 15.8350 | < 0.0001 | |
| Φ | 1.1780 | 0.3621 | 3.2537 | 0.0028 |
The yearly plot for the study region suggested a pronounced shift in air temperature in 1986–1987 followed by a levelling off until 2007 (Fig. 2). Thus, the interrupted time-series analysis was done between 1975–1986 and 1987–2007 periods. The analyses showed that the intervention parameter (Φ) was statistically significant in both models (Table 1). We obtained abrupt-permanent functions where the intervention parameter indicated the rate of change in air temperature. That is, air temperature significantly increased by about 1.2 °C in Abaurrea and 1.7 °C in Epároz from 1986–1987. Mean minimum annual temperature in the preintervention period (1975–1986) was 3.5 ± 1.1 °C in Abaurrea and 4.6 ± 0.9 °C in Epároz, whereas in the postintervention period (1986–2007) annual temperature averaged 4.9 ± 0.5 and 6.3 ± 0.4 °C in Abaurrea and Epároz, respectively.
Air temperature modelling
Regarding the 1993–2004 time period (48 meteorological stations), MAXTa and MAX7d-Ta were significantly related to altitude and latitude, following the regression models shown in Table 2. We developed regional models of MAXTa and MAX7d-Ta for the 1975–1986 period, when the significant shift in air temperature was detected. These models were developed from the 23 stations where data from that period were available. The obtained models for the 1975–1986 period were also highly significant (Table 2).
| Dependent variable | Independent variables | Coefficient | R2 | F | P |
|---|---|---|---|---|---|
| MAXTa | Altitude | −0.0041 | 0.72 | 37.18 | < 0.0001 |
| 1993–2004 | Latitude | −6.142 | |||
| Intercept | 293.482 | ||||
| MAX7d-Ta | Altitude | −0.0044 | 0.85 | 87.52 | < 0.0001 |
| 1993–2004 | Latitude | −6.914 | |||
| Intercept | 323.253 | ||||
| MAXTa | Altitude | −0.0051 | 0.72 | 23.36 | < 0.0001 |
| 1975–1986 | Latitude | −7.989 | |||
| Intercept | 371.786 | ||||
| MAX7d-Ta | Altitude | −0.0047 | 0.82 | 40.97 | < 0.0001 |
| 1975–1986 | Latitude | −8.645 | |||
| Intercept | 396.336 |
Water temperature modelling
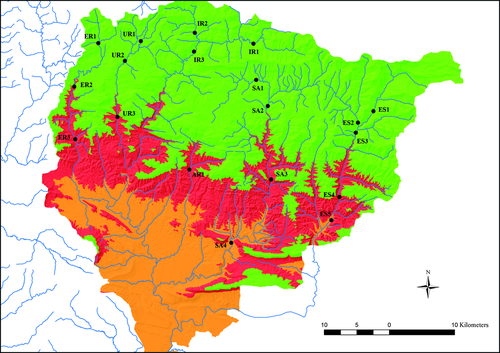
When regressing either MAXTw or MAX7d-Tw, linear regression models were better fitted to data (N = 2682) than nonlinear models. Based on the linear models governing the relationship between air and water temperatures (Table 3), air temperature regional models were converted into regional water temperature models. Study sites were then divided in two groups: the upper localities, presenting MAX7d-Tw lower than 19.4 °C (ES1, ES2, ES3, SA1, SA2, IR1, IR2, IR3, UR1, UR2 and ER1), and the lower localities with MAX7d-Tw above the thermal limit (ES4, ES5, SA3, SA4, AR1, UR3, ER2 and ER3) (Fig. 3). Unsuitable habitat (MAX7d-Tw > 19.4 °C) increased by 92.6% when comparing climate conditions for the 1975–1986 period (49 649 ha of unsuitable habitat, 23.6% of total area) vs. the 1993–2004 period (95 637 ha of unsuitable habitat, 45.4% of total area) (Fig. 3).
| Dependent variable | Independent variables | Coefficient | R2 | F | P |
|---|---|---|---|---|---|
| MAXTw | MAXTa | 0.614 | 0.80 | 10510 | < 0.0001 |
| Intercept | 3.868 | ||||
| MAX7d-Tw | MAX7d-Ta | 0.656 | 0.85 | 14874 | < 0.0001 |
| Intercept | 3.372 |
Fish abundance and carrying capacity
For brown trout, the observed warming is associated with an overall population decrease in the lower reaches. Total mean density gradually declined in the lower localities during the study period at a rate close to 6.0% per year (Log Density = 128.04 (± 42.33) – 0.06 (± 0.02) Year; F1,10 = 8.12, P = 0.0173, R2 = 0.45; Fig. 4a), from a mean 2073.3 trout ha−1 in 1993 to 1339.2 trout ha−1 in 2004. However, in upper reaches populations remained stable, showing no significant population decline (R2 = 0.002, P = 0.8999, mean 5565.1 trout ha−1, range 4171.9–7723.6 trout ha−1).
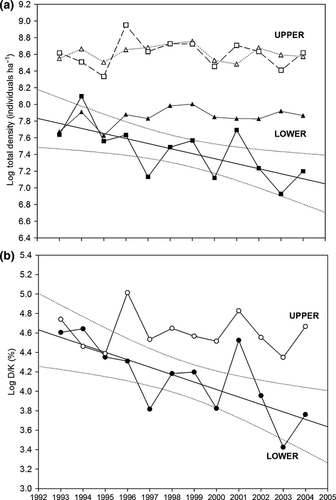
In many cases, climate change would exacerbate declining suitable physical habitat; however, total carrying capacity did not decrease during the monitoring period in either upper or lower reaches (upper, R2 = 0.004, P = 0.8446, mean 5546.7 trout ha−1, range 4835.2–6353.1 trout ha−1; lower, R2 = 0.16, P = 0.2036, mean 2579.0 trout ha−1, range 2051.6–2992.0 trout ha−1). Conversely, the density/carrying capacity (D/K) ratio significantly decreased in populations at lower altitudes at a rate about 8% per year (Log D/K = 157.38 (± 45.69) – 0.08 (± 0.02) Year; F1,10 = 11.25, P = 0.0073, R2 = 0.53; Fig. 4b), from a mean 95.5% in 1993 to 41% in 2004. The D/K ratio averaged 98.5% in the upper reaches and was nearly constant during the study period (R2 = 0.02, P = 0.6860).
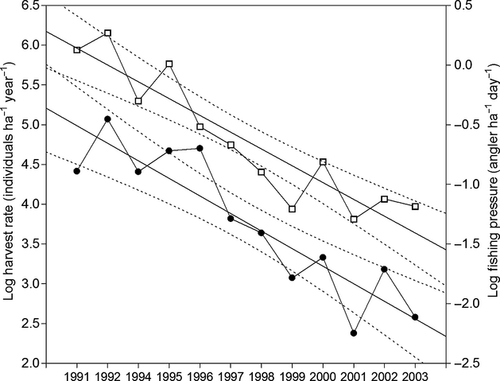
Fishery data showed that anglers are homogeneously disseminated throughout the study area, so that the fishing effort is uniformly distributed both geographically and with respect to site altitude (Fig. 1). During the study period average fishing pressure (angler ha−1 day−1) was significantly higher (anova, F1,174 = 47.52, P < 0.0001) in upper localities (mean 0.81 angler ha−1 day−1, range 0.03–3.82) than in lower ones (mean 0.21 angler ha−1 day−1, range 0.01–1.26). Moreover, a significant decrease of fishing pressure was observed in the whole area during the study period, both in lower (Log Fishing pressure = 318.26 (± 64.99) – 0.16 (± 0.03) Year; F1,9 = 24.22, P = 0.0008, R2= 0.73) and upper localities (Log Fishing pressure = 214.59 (± 40.21) – 0.11 (± 0.02) Year; F1,10 = 28.54, P = 0.0003, R2 = 0.74). From 1991 to 2003 a significant decline in the mean annual catch of brown trout in the studied rivers occurred (Log Harvest rate = 410.45 (± 65.43) – 0.20 (± 0.03) Year; F1,10 = 38.63, P < 0.0001, R2 = 0.79; Fig. 5). Harvest rate decreased at a rate of 20% per year, from an average 82.8 trout ha−1 year−1 in 1991 to 13.2 trout ha−1 year−1 in 2003.
The abundance index of nontrout fish species significantly increased during the study period in both lower (Abundance index = −73.87 (± 21.25) + 0.0375 (± 0.011) Year; F1,9 = 12.42, P = 0.0065, R2 = 0.58) and upper reaches (Abundance index = −33.97 (± 11.38) + 0.0172 (± 0.006) Year; F1,9 = 9.13, P = 0.0144, R2 = 0.50), although the rate at which abundance of nontrout species increased with time was significantly higher in the lower reaches.
Thermal habitat loss projections
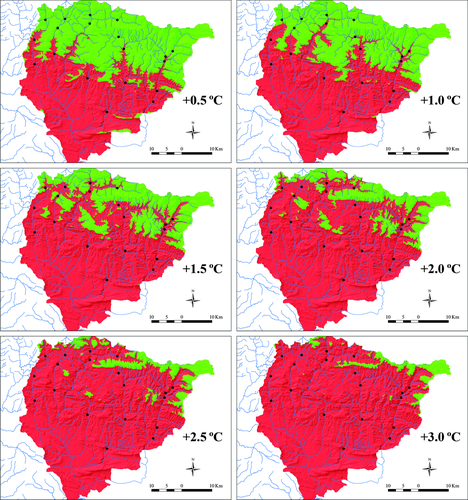
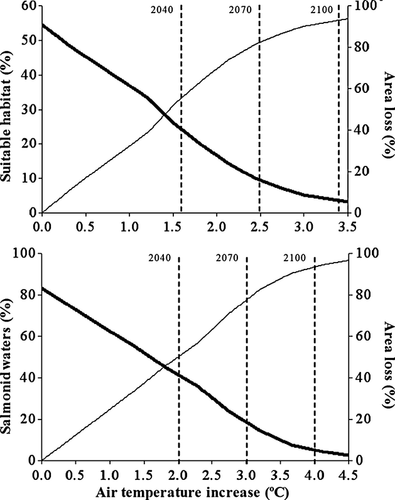
Projected brown trout suitable thermal habitat area for different warming scenarios decreases until being almost inexistent at air temperature increases above 3 °C (Figs 6 and 7). All study sites will be located in areas of unsuitable thermal habitat when air temperature is warming above 1.5 °C. According to regional projections for the SRES scenario B2, this will occur before the year 2040. By 2040, more than half of the present area of suitable thermal habitat will have been lost under the SRES scenario B2. The proportion of suitable thermal habitat will decrease down to 3.5% of total area by the year 2100. Under warming projections in the study area for a higher-emissions-scenario (A2), no suitable thermal habitat will exist by 2085. According to the model's predictions, at maximum air temperature increases higher than 4.5 °C (by year 2085 under the SRES scenario A2), all the salmonid waters within the study area will become warm water fish courses (Figs 6 and 7). By the year 2040 and under the SRES scenario B2, almost half of the present area of the salmonid waters will be more suitable for warm water fish.
Discussion
We observed an ongoing population decline in brown trout at the southern periphery of its distribution range. This decrease may be explained by a loss of thermal habitat induced by the gradual increase in water temperature in lower altitudes dating from the 1980s. Similar to our findings, overall results on salmonids certainly indicate that global warming is especially critical at the southern edge of the species’ ranges, causing a gradual decline in populations primarily due to a generalized loss of suitable thermal habitat (e.g. Keleher & Rahel, 1996; Flebbe et al., 2006; Mather et al., 2008; Winfield et al., 2010; Horreo et al., 2011). In Europe, Buisson et al. (2008), Jonsson & Jonsson (2009) and Lassalle & Rochard (2009) suggested that expected climate change will shift the thermal habitat of Atlantic salmon Salmo salar and brown trout northwards, with decreased production and population extinction at the southern part of their distribution areas. This study has provided empirical evidence for this prediction in the southern limits of brown trout distribution, thus lending support to previous studies on the species.
The abrupt shift in air temperature observed in 1986–87 in the study area followed by a levelling off until the present probably caused a stream warming which may have led to an effective loss in potential thermal habitat for brown trout below an altitude of 600 m. This exceptional warming in the 1980s, which peaked near 1990, was global (Myneni et al., 1997), and its effects were observed both in aquatic (Gerten & Adrian, 2000; Hari et al., 2006) and terrestrial ecosystems (Lenoir et al., 2008). Therefore current climate change may be rendering unsuitable 12% of suitable thermal habitat each decade within the study area, resulting in an overall population decrease in lower altitudes of around 6% per year. This trend may not be attributed to physical habitat modification or angler pressure, as carrying capacity remained stable during the study period and the populations were not overexploited. Average annual angler exploitation was around 20% in both upper and lower reaches and fishing pressure actually decreased significantly downstream.
Brown trout catches from Western European rivers have been declining sharply since the 1990s, presumably linked to an increase in Northern Hemisphere air temperatures (EIFAC, 2002). Our study showed that brown trout catches markedly decreased 20% per year from 1991 to 2003. Furthermore, Clews et al. (2010) observed a 67% reduction of brown trout populations in the Wye River basin in Wales between 1985 and 2004, with climatic factors being the most plausible explanation for the observed trend. Hari et al. (2006) similarly found an upward shift in thermal habitat of stream-dwelling brown trout in Alpine rivers, due to a general warming which probably caused the decline of 66% of brown trout catches in Switzerland between 1978 and 2001.
The general technique mostly used to forecast changes in distributions of salmonids to warming scenarios is the ‘climate envelope model’ (CEM), defined as the ecological conditions a species needs for survival based on its physiological tolerances (Thuiller, 2004; Araújo & New, 2007). As climate change modifies the ‘envelope’, the species distribution shifts accordingly (e.g. Scott & Poynter, 1991; Rahel et al., 1996; Jager et al., 1999; Flebbe et al., 2006; Preston, 2006). Some authors have suggested that there are sources of uncertainty in CEM models which need consideration (Thuiller, 2004; Araújo et al., 2005; Pearson et al., 2006). Firstly, the models rely on the accuracy of climatic models and climate change scenarios, which are subjected to a high level of uncertainty. Secondly, the models do not consider biotic interactions, such as competition, predation or parasitism, all of which may change the patterns of species persistence (Berg et al., 2010). Notwithstanding possible uncertainties, our findings and previous work on salmonids are a useful approach to illustrate the potential impact of climate change on species distribution. Specifically, the CEM method is best suited to predict changes in the distribution of salmonids with limited ranges, dispersal capabilities and narrow physiological tolerances. For example, Kennedy et al. (2009) demonstrated that under a scenario where atmospheric CO2 reaches a concentration of about 710 ppm by the end of the 21st century, regional climate warming may cause a shift in the altitudinal distribution of endangered Gila trout Oncorhynchus gilae, resulting in a 70% loss in suitable summer habitat. Likewise, in the Columbia River basin, Rieman et al. (2007) found that populations of threatened bull trout Salvelinus confluentus may lose 18–92% of thermally suitable habitat over the next 50 years as a result of a projected warming of 1–5 °C. The CEM approach used in the present study showed that if recent trends continue in the future, in the regional and more ecologically friendly B2 SRES scenario brown trout may lose half of their current habitat within the study area by 2040 and become almost extinct in the area by 2100.
The upstream movement of the thermal habitat of brown trout may have caused a significant increase in the relative abundance of nontrout fish species, at a significantly higher rate in the lower reaches of the salmonid waters. Less common species such as Pyrenean gudgeon Gobio lozanoi, Ebro nase Parachondrostoma miegii, Pyrenean minnow Phoxinus bigerri, Pyrenean stone loach Barbatula quignardi, Ebro barbel Barbus graellsii and Iberian red fin barbel Barbus haasi, which were limited in their altitudinal distribution by temperature, are moving upwards. Furthermore, temperature increases in lower habitats may allow alien species, which are broadly established in downstream areas, to invade upstream localities as observed elsewhere (e.g. Rieman et al., 2007; Rahel & Olden, 2008). In a similar way, Matulla et al. (2007) assessed the impact of an IS92a emission scenario on the fish fauna in an Alpine river, concluding that salmonid species (brown trout and grayling Thymallus thymallus) are becoming restricted to increasing higher elevations due to a loss of thermal suitable habitat, while the cyprinid zone is elongated.
Predicted water temperature increases will certainly influence the physiological characteristics of brown trout, such as rates of development and growth, and associated life-history traits. There is no empirical evidence of this species adapting to increasing temperature, even in geothermal rivers (Elliott & Elliott, 2010). Furthermore, laboratory measures of thermal tolerance to high temperatures are usually quantified without other stressors which nearly always occur in the field (e.g. toxins, diseases, lower food availability, higher predation) that could decrease the extreme temperatures fish can tolerate (Hari et al., 2006; Mather et al., 2008). Although salmonids have varied life histories and some plasticity in habitat use that confer resilience to changing environments (Isaak et al., 2010), the capacity for adaptation or migration in response to a changing climate is limited within our study area. Stream-dwelling populations face more severe environmental restrictions compared with their migratory relatives. Our study populations live in landlocked linear aquatic habitats where upward movement is impeded by dams which severely limit their ability to seek refuge upstream from adverse extreme temperatures (Almodóvar & Nicola, 1999). Hence, to improve the resistance and resiliency of brown trout in light of climate change, mitigation and restoration measures should focus on creating or facilitating access to thermal refuges.
To sum up, our findings illustrate how current and future climate change threatens some of the most healthy native brown trout populations in Southern Europe, which have considerable conservation value given their peripheral location at the southern part of the species’ range. Because of their isolation, marginal populations of brown trout contain a large proportion of the species’ genetic, life history and ecological diversity (e.g. Suárez et al., 2001; Nicola & Almodóvar, 2004; Almodóvar et al., 2006b), thus contributing to speciation and evolutionary divergence. Reduction in population size and loss of interpopulation connectivity as a consequence of potential climate change are expected to increase the risk of local extinction, due to increasing vulnerability to demographic and environmental stochastic events. Therefore, the observed and projected reduction in suitable thermal habitat in Mediterranean-type streams may have relevant consequences for the ecological and evolutionary species success.
Acknowledgements
This study was supported and funded by the project ‘Study of brown trout populations of Navarre’ in the framework of the agreement between Complutense University of Madrid and Navarre Government.




