Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides
Abstract
Ocean acidification (OA) refers to the increase in acidity (decrease in pH) of the ocean's surface waters resulting from oceanic uptake of atmospheric carbon dioxide (CO2). Mounting experimental evidence suggests that OA threatens numerous marine organisms, including reef-building corals. Coral recruitment is critical to the persistence and resilience of coral reefs and is regulated by several early life processes, including: larval availability (gamete production, fertilization, etc.), larval settlement, postsettlement growth, and survival. Environmental factors that disrupt these early life processes can result in compromised or failed recruitment and profoundly affect future population dynamics. To evaluate the effects of OA on the sexual recruitment of corals, we tested larval metabolism, larval settlement, and postsettlement growth of the common Caribbean coral Porites astreoides at three pCO2 levels: ambient seawater (380 μatm) and two pCO2 scenarios that are projected to occur by the middle (560 μatm) and end (800 μatm) of the century. Our results show that larval metabolism is depressed by 27% and 63% at 560 and 800 μatm, respectively, compared with controls. Settlement was reduced by 42–45% at 560 μatm and 55–60% at 800 μatm, relative to controls. Results indicate that OA primarily affects settlement via indirect pathways, whereby acidified seawater alters the substrate community composition, limiting the availability of settlement cues. Postsettlement growth decreased by 16% and 35% at 560 and 800 μatm, respectively, relative to controls. This study demonstrates that OA has the potential to negatively impact multiple early life history processes of P. astreoides and may contribute to substantial declines in sexual recruitment that are felt at the community and/or ecosystem scale.
Introduction
The susceptibility of reef-building corals to increasing carbon dioxide (CO2) levels [ocean acidification (OA)] has been of recent concern with respect to global climate change. Atmospheric CO2 (pCO2) levels are presently estimated to be 387 ppm, 30% higher than the natural range over the last 650 000 years (Siegenthaler et al., 2005). The present day rate of atmospheric CO2 increase is estimated to be 200 times faster than any changes that occurred during the last eight glacial cycles (Siegenthaler et al., 2005) and eight to 15 times faster than any changes in the past 60 Myr, including the Paleo-Eocene Thermal Maximum (PETM) (Zeebe et al., 2009). Approximately, one-third of all CO2 emissions from the past 200 years have been absorbed by the oceans (Sabine et al., 2004). On dissolution in seawater, CO2 reacts with H2O, triggering a series of chemical reactions that alter the seawater carbonate chemistry: [CO2]aq and [HCO3] increase, and [CO32], pH, and the carbonate saturation state (Ω) decrease, causing surface waters to become more acidic (Sabine et al., 2004). Increasing atmospheric CO2 concentrations have already depleted seawater carbonate concentrations by <30 μmol kg−1, simultaneously reducing the pH of the ocean's surface waters by 0.1 U relative to the preindustrial value of 8.18 (a 30% increase in [H+]) (IPCC, 2007). Further reductions of 0.3–0.5 pH units are projected by the end of this century as the oceans continue to absorb anthropogenic CO2 (Sabine et al., 2004; IPCC, 2007).
OA is expected to have negative effects on a variety of marine organisms (Royal Society, 2005), and early life history stages of these organisms may be more sensitive than adults, as has been demonstrated in oysters and echinoderms (reviewed by Kurihara, 2008). The number of studies devoted to the potential impacts on early life history stages of marine invertebrates has risen over the past several years. Mounting experimental evidence now suggests that numerous biological and physiological processes will be negatively impacted as the oceans continue to acidify: sperm motility in urchins (Havenhand et al., 2008), corals and sea cucumbers (Morita et al., 2009); fertilization success in urchins (Kurihara & Shirayama, 2004; Havenhand et al., 2008; Reuter et al., 2010; but see Byrne et al., 2010), mollusks (Parker et al., 2009; but see Havenhand & Schlegel, 2009) and corals (Albright et al., 2010); larval development and/or growth in crustaceans (Arnold et al., 2009; Findlay et al., 2009, 2010; McDonald et al., 2009), mollusks (Kurihara et al., 2007; Ellis et al., 2009; Parker et al., 2009), corals (Albright et al., 2008; Cohen et al., 2009; Albright et al., 2010; Suwa et al., 2010) and echinoderms (Kurihara & Shirayama, 2004; Dupont et al., 2008; Clark et al., 2009; Brennand et al., 2010; O'Donnell et al., 2010); physiology and behavior of mollusks (Ellis et al., 2009); survival of echinoderms (Dupont et al., 2008; Clark et al., 2009) and crustaceans (Findlay et al., 2009); stress response in sea urchins (O'Donnell et al., 2009; Todgham & Hofmann, 2009); and gene expression in sea urchins (Todgham & Hofmann, 2009; O'Donnell et al., 2010). Despite this recent surge, the majority of these studies have been conducted on echinoderms, mollusks, and crustaceans, and comparatively few studies focus on the potential response(s) of early life history stages of corals. As coral larvae do not calcify in the plankton, those studies which have focused on corals (Albright et al., 2008; Cohen et al., 2009; Morita et al., 2009; Albright et al., 2010; Suwa et al., 2010) have primarily investigated the effects of elevated CO2 on postsettlement calcification and growth; only two studies to date have found evidence of impacts before the onset of calcification (Morita et al., 2009; Albright et al., 2010).
Sexual reproduction in reef-building corals depends on fertilization and the development, survival, and settlement of planula larvae. Coral larvae spend hours to days developing in the water column before they are competent to settle on the reef. Larval settlement involves the recognition of water-soluble and substrate-bound chemical cues, physical attachment to the substrate, and subsequent metamorphosis. Recruitment (identification and inclusion in a population) necessitates survival and growth of the newly settled individual (Harrison & Wallace, 1990). Coral recruitment plays a primary role in: maintaining genetic diversity; populating denuded areas; determining the community structure of coral reefs; and replenishing reefs post disturbances. Environmental factors that disrupt early life history processes can result in compromised recruitment or recruitment failure and profoundly affect marine population dynamics (Gaines & Roughgarden, 1985; Harrison & Wallace, 1990; Doherty & Fowler, 1994; Riegl et al., 2009).
The present study aims to evaluate the effects of OA on sequential early life history processes that are critical to the successful sexual recruitment of corals. The experimental coral chosen for this study was the common Caribbean coral Porites astreoides. P. astreoides is a brooding coral that spawns predictably near the new moon from April through June (McGuire, 1998), rendering larvae easy to collect for use in laboratory experiments. To estimate the potential impact of OA on the sexual recruitment of this species, larval metabolism, settlement, and postsettlement growth were tested at pCO2 levels that represent ambient seawater (380 μatm) and two pCO2 increases that are expected to occur by the middle (560 μatm) and end (800 μatm) of this century (IPCC, 2007).
Materials and methods
Collection of larvae
In 2008 and 2009, 12 adult colonies of P. astreoides were collected from Little Grecian, an offshore bank-barrier reef near Key Largo, FL (USA), several days before the new moon in May and June (2008) and April and May (2009). Colonies were maintained in a flow-through seawater system at the University of Miami's Rosenstiel School of Marine and Atmospheric Science (RSMAS) for approximately 1 week during the predicted period of larval release. Larvae were collected according to the methods outlined by Kuffner et al. (2006). On the mornings following release, larvae from each parent colony were pooled and transferred to sterile containers with filtered seawater for use in experiments. In May, 2010, inclement weather prevented us from directly collecting coral colonies and larvae; we, therefore, obtained ∼800 larvae from eight P. astreoides colonies (∼100 larvae from each of eight colonies), collected by a team of researchers (Smithsonian Marine Station, Fort Pierce) from two shallow (15–20′) patch reefs near Summerland Key, FL. Colonies were maintained at the Mote Marine Laboratory in Summerland Key during the period of larval release, and larvae were pooled upon release for use in experiments.
Seawater chemistry
Seawater chemistry was manipulated via direct bubbling with CO2-enriched air to create three target conditions: 380 μatm (control), 560 μatm (mid CO2), and 800 μatm (high CO2). The control was bubbled with outside air. To verify distinct treatments, water samples were taken and analyzed at the start of respiration experiments and the start and end of settlement experiments; samples were taken weekly during tile conditioning and growth experiments. Water samples were analyzed for total alkalinity (TA) and pH. TA was determined in duplicate (30–40 mL analyses) using an automated, open-cell Gran titration (Dickson et al., 2007, SOP3b), and accuracy was checked against certified seawater reference material (A. Dickson, Scripps Institute of Oceanography). pH was determined on the total scale using an Orion Ross combination pH electrode calibrated at 25 °C against a seawater TRIS buffer (Dickson et al., 2007, SOP6). Concentrations of CO32−, Ca2+, and Ωarag were computed from TA, pH, temperature, and salinity using the program co2sys (E. Lewis, Brookhaven National Laboratory), with dissociation constants for carbonate determined by Mehrbach et al. (1973), as refit by Dickson & Millero (1987) and dissociation constant for boric acid determined by Dickson (1990). pH is reported on the total scale, the scale on which K1 and K2 were determined. Chemical and physical conditions that persisted during each experiment are outlined in Tables S1–S3 of the Supporting Information.
Larval metabolism
In May 2010, larval metabolic rates were measured twice at each of the three CO2 levels. The first experiment was conducted ∼24 h after spawning (AS), and the second incubation was conducted ∼48 h AS. Each respiration experiment involved four chambers (run simultaneously): three contained filtered treatment water (0.2 μm; ambient, mid CO2, or high CO2) and 20 larvae; the fourth chamber contained filtered ambient seawater and no larvae and was used to correct for background respiration rates. A preliminary experiment was conducted using different numbers of larvae in each chamber to determine the optimal number for the subsequent experiments. Before the experiments, the four chambers were run with filtered seawater alone to ensure that they were reading uniformly; chambers were calibrated in air-bubbled filtered seawater at the measurement temperature (26 °C), and a saturated oxygen value was obtained by computation of the saturation concentration (Benson & Krause, 1984).
Chambers and larvae were dark-acclimated for 2 h before the start of each experiment, and experiments were conducted in a darkened, constant temperature water bath maintained at 26 °C. Respiration was measured over a 2-h interval as oxygen flux using YSI 5750 oxygen electrodes, connected to an ENDECO 1125 four-channel Pulsed DO Sensor (Marion, MA, USA). A PC computer was used to log the temperature and oxygen data output every 10 min from each of the four oxygen electrodes. The oxygen consumption rate was determined by regressing oxygen concentration against time. The oxygen consumption rate determined in each chamber was corrected for the background consumption rate in the control chamber multiplied by the volume of water in the chamber (∼20 mL) and divided by the number of larvae (20) to obtain the respiration rate in nanomoles of oxygen larva−1 h−1. A total of six independent estimates of larval respiration rate were obtained.
Twenty-four hours AS, the experiment commenced at 14:00 hours, and the CO2 levels were close to the target levels of 380, 560, and 800 μatm. Forty-eight hours AS, the experiment commenced ∼2 h earlier in the day, at 12:00 hours. Owing to natural diurnal variation in the seawater system (resulting from photosynthetic uptake of CO2 throughout the day), the ambient and mid-CO2 levels were slightly higher at 48 h AS than 24 h AS. The same should have been true for the high CO2 treatment, but a blocked airstone resulted in a lower than target CO2 level. Owing to both the natural diurnal variability and the airstone blockage, the CO2 levels varied between the two experiments and averaging values from the two experiments for analysis of variance was deemed inappropriate. Rather, CO2 was treated as a continuous variable and data from both experiments was analyzed by linear regression analysis using least squared residuals.
Settlement
In 2008, two settlement experiments were conducted simultaneously. In the first experiment, limestone settlement tiles were preconditioned in ambient seawater (380 μatm), and larvae were settled onto the tiles in treatment seawater (380, 560, or 800 μatm). In the second experiment, settlement tiles were preconditioned in treatment seawater, and larvae were settled in treatment seawater (corresponding to the treatment in which the tiles were conditioned). Details of the tile conditioning and settlement assays are provided below.
Tile conditioning. Before settlement assays, commercially sourced limestone tiles were preconditioned for 40 days in flow-through aquaria with either ambient seawater (380 μatm) or treatment seawater (560 or 800 μatm). Mean tile dimensions were 20.6±0.1 mm × 12.0±0.1 mm × 3.23±0.06 mm (mean±1 SEM), and average tile mass was 1.89±0.04 g. Aquaria turned over approximately once per day. A single source of live rock was divided equally amongst the aquaria to provide a consistent source of crustose coralline algae (CCA) and microfauna.
Settlement assays. Settlement assays were conducted in prerinsed six-well nontreated polystyrene tissue culture plates (BD Biosciences, Woburn, MA, USA). One settlement tile, 10 mL of treatment water and 10 larvae (2 days old) were randomly added to each well. Plates were securely covered and submerged in treatment tanks to ensure temperature control (28 °C) and prevent gas exchange. Sixteen wells were used per treatment. Tiles were examined after 24 h. The number of settled larvae on the top, bottom and sides of each tile was counted using a dissecting microscope. Larvae were scored as ‘settled’ when they had fully metamorphosed (flat/disc-shaped appearance rather than pear-shaped), with little or no possibility of active detachment and further migration (Harrison & Wallace, 1990). Wells in which all 10 larvae could not be accounted for at the end of the experiment were eliminated from the statistical analysis, resulting in the following sample sizes: Ambient Tile Experiment: N=15 (380 μatm); N=16 (560 μatm); N=14 (800 μatm) and Treatment Tile Experiment: N=15 (380 μatm); N=13 (560 μatm); N=13 (800 μatm). Percentage data were arcsine transformed and analyzed using one-way anovas. D'Agostino and Pearson omnibus test and Levene's test were used to verify the underlying assumptions of normality and homoscedacity, respectively. Where significant differences were detected, post hoc Tukey's HSD analyses were used to determine which treatments differed from each other.
In 2009, the second experiment (Treatment Tiles) was repeated according to the previously outlined methodology with the following modifications: 30 wells were used per treatment (with similar omissions when all larvae were not accounted for), resulting in the following sample sizes: N=30 (380 μatm); N=30 (560 μatm); N=29 (800 μatm). Experiments were conducted at 26 °C. Data from the Treatment Tile Experiments (2008 and 2009) were pooled and analyzed by linear regression analysis using least squared residuals.
Spectrofluorometry
To determine whether conditioning settlement substrates at the different pCO2 levels altered the epilithic algal communities (and the availability of potential settlement cues), preconditioned tiles that were not used in settlement assays in 2009 were placed in 15 mL tubes and immediately frozen for use in spectrofluorometry analyses. Epilithic algal communities were extracted from tiles, and concentrations of chlorophylls a, b, c, phycoerythrin (PE) and phycocyanin (PC) were determined by measuring the fluorescent emission of the pigments extracted from the settlement tiles using a SPEX® Fluorolog-3 spectrofluorometer (Horiba Scientific, Edison, NJ, USA). Pigments were extracted using a solution of 10 mL dimethyl sulfoxide (DMSO) and 15 mL 90% acetone for chlorophyll (Chl) analyses; DMSO was added 30 min before the addition of acetone. Ten milliliters of phosphate buffer (0.05 m H2KPO4, 0.05 m HK2PO4, 0.01% mercaptoethanol, pH 6.5) was used for the extraction of PE and PC. Pigment extractions took place overnight. Ten tiles were sampled per treatment (380, 560, 800 μatm) per analysis (PE/PC or Chl). Pigment concentrations were normalized to the weight of the tile and are reported as μg pigment g−1 tile. Emission peaks (wavelengths) for each pigment are as follows: Chl a– 670 nm; Chl c– 635 nm; Chl b– 650 nm; PE – 570 nm; PC – 640 nm.
PE/PC data were square root transformed to meet assumptions of homoscedacity, and all data were analyzed using one-way anovas. Where significant differences were detected, post hoc Tukey's HSD analyses were used to determine which treatments differed from each other.
Juvenile growth
In 2008, once settlement was assessed, juveniles on Ambient Tiles were introduced to treatment aquaria containing water corresponding to the treatment in which they were settled. Individuals were mapped to allow for their identification over time, and growth (increase in cross-sectional area, defined as the outermost extent of visible skeleton) of each individual was quantified according to the methods outlined in Albright et al. (2008). Growth rates (mm2 month−1) were calculated as the rate of change in cross-sectional area over time (49 days in May–June). Data were analyzed using a one-way anova. D'Agostino and Pearson omnibus test and Levene's test were used to verify the underlying assumptions of normality and homoscedacity, respectively. Where significant differences were detected, post hoc Tukey's HSD analyses were used to determine which treatments differed from each other.
Results
Larval metabolism
Larval metabolic rates decreased significantly with increasing pCO2 (F1, 4=32.74, P<0.005) (Fig. 1). Model parameters obtained from regression analysis indicate a 27% and 63% reduction in metabolic rates at pCO2 levels that are projected to occur by the middle (560 μatm) and end (800 μatm) of this century. Initial O2 concentrations in each experiment and treatment were close to 200 μmol kg−1 (the expected saturation concentration at 26 °C and 35 ppt), and absolute O2 concentrations never fell below 160 μmol kg−1, 80% of saturation (2 mg L−1 or ∼60 μmol kg−1 is typically identified as physiologically stressful by the EPA and NOAA). Absolute respiration rates (nmol O2 larva−1 h−1) were as follows (mean±1 SEM), where error represents the analytical precision of the respiration rate (i.e. the standard error of the slope of the regression line): 24 h AS: 2.06±0.09 (368 μatm); 1.7±0.1 (491 μatm); 0.87±0.07 (779 μatm); 48 h AS: 2.0±0.1 (443 μatm); 1.3±0.1 (533 μatm); 1.4±0.1 (633 μatm).
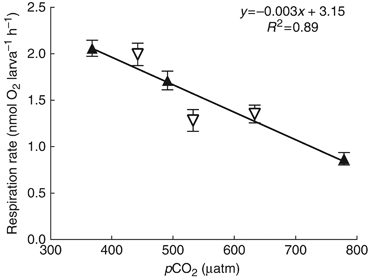
Larval metabolism as a function of pCO2. Data are pooled from two subsequent experiments. Closed triangles represent data collected 24 h after spawning (AS); open triangles represent data collected 48 h AS. Error bars represent the analytical precision of the respiration rates.
Settlement
When settled onto Ambient Tiles, percent settlement declined by 11% at 560 μatm and 28% at 800 μatm, relative to controls. Percent settlement was as follows (mean±1 SEM): 65±8 (380 μatm); 58±6 (560 μatm); and 47±9 (800 μatm). Results of anova indicate that these reductions in settlement are not statistically significant (F2, 42=2.78; P=0.07). When settled onto Treatment Tiles, percent settlement decreased by 45% at 560 μatm and 55% at 800 μatm relative to controls (F2, 39=7.05, P<0.005) with percent settlement as follows: 65±8 (380 μatm); 36±10 (560 μatm); and 29±4 (800 μatm) (Fig. 2a). Results of post hoc Tukey's HSD analyses are presented in Table 1.
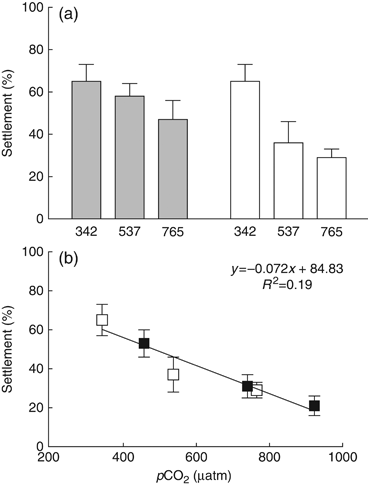
Results of settlement assays (mean±1 SEM). (a) 2008: gray bars represent data from the Ambient Tile Experiment (tiles conditioned in ambient seawater, larvae settled in treatment seawater); white bars represent data from the Treatment Tile Experiment (tiles conditioned in treatment seawater, larvae settled in treatment seawater). (b) Pooled results from both Treatment Tile Experiments (2008 and 2009). Open squares represent data from 2008. Closed squares represent data from 2009.
| anova | df | SS | MS | F-ratio | P |
|---|---|---|---|---|---|
| Settlement (Treatment Tile Experiment, 2008) | |||||
| Treatment | 2 | 1.3988 | 0.699 | 7.0513 | 0.0024 |
| Residual | 39 | 3.8683 | 0.099 | ||
| Total | 41 | 5.2671 | |||
| Tukey's HSD | Meandifference | Q | P<0.05 |
|---|---|---|---|
| 342 vs. 537 μatm | 0.3715 | 4.402 | Y |
| 342 vs. 765 μatm | 0.3890 | 4.701 | Y |
| 537 vs. 765 μatm | 0.0175 | 0.204 | N |
| anova | df | SS | MS | F-ratio | P |
|---|---|---|---|---|---|
| Phycoerythrin | |||||
| Treatment | 2 | 1.079 | 0.5394 | 10.96 | 0.0003 |
| Residual | 27 | 1.329 | 0.0492 | ||
| Total | 29 | 2.408 | |||
| Tukey's HSD | Meandifference | Q | P<0.05 |
|---|---|---|---|
| 357 vs. 555 μatm | 0.4157 | 5.925 | Y |
| 357 vs. 796 μatm | 0.3873 | 5.520 | Y |
| 555 vs. 796 μatm | −0.0284 | 0.405 | N |
| anova | df | SS | MS | F-ratio | P |
|---|---|---|---|---|---|
| Phycocyanin | |||||
| Treatment | 2 | 6.808 | 3.404 | 18.38 | <0.0001 |
| Residual | 27 | 5.000 | 0.1852 | ||
| Total | 29 | 11.81 | |||
| Tukey's HSD | Meandifference | Q | P<0.05 |
|---|---|---|---|
| 357 vs. 555 μatm | 0.9225 | 6.779 | Y |
| 357 vs. 796 μatm | 1.0800 | 7.937 | Y |
| 555 vs. 796 μatm | 0.1576 | 1.158 | N |
| anova | df | SS | MS | F-ratio | P |
|---|---|---|---|---|---|
| Growth | |||||
| Treatment | 2 | 1.0889 | 0.5445 | 12.6033 | <0.0001 |
| Residual | 99 | 4.2768 | 0.0432 | ||
| Total | 101 | 5.3657 | |||
| Tukey's HSD | Meandifference | Q | P<0.05 |
|---|---|---|---|
| 330 vs. 548 μatm | 0.1206 | 3.502 | Y |
| 330 vs. 775 μatm | 0.2571 | 7.094 | Y |
| 548 vs. 775 μatm | 0.1365 | 3.698 | Y |
In 2009, when settled onto Treatment Tiles, percent settlement was reduced by 42% at 560 μatm and 60% at 800 μatm relative to controls (F1, 88=15.87, P<0.0001). Percent settlement was as follows: 53±7 (380 μatm); 31±6 (560 μatm); and 21±5 (800 μatm). Results of settlement experiments with Treatment Tiles from 2008 and 2009 were pooled and analyzed via linear regression using least squares residuals, indicating a significant effect of pCO2 on settlement success (F1, 130=29.58, P<0.0001) (Fig. 2b).
Spectrofluorometry
Epilithic algal communities of tiles that were preconditioned in ambient seawater (380 μatm) had significantly higher concentrations (μg g−1) of PE (F2, 28=10.96, P<0.0005) and PC (F2, 28=18.38, P<0.0001). PE concentrations were reduced by 78% and 74% at 560 and 800 μatm, respectively, compared with controls, while PC concentrations were reduced by 73% and 83% (Fig. 3a and b). Results of post hoc Tukey's HSD analyses for PE/PC data are presented in Table 1. No significant differences were observed in the concentrations of Chl a and Chl c (Fig. 3c and d). Chl b was not detected.
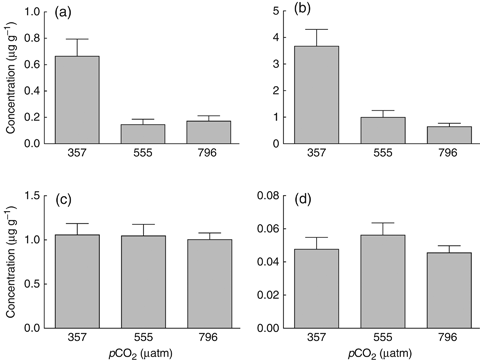
Concentrations (mean±1 SEM) of (a) phycoerythrin (570 nm), (b) phycocyanin (640 nm), (c) chlorophyll a (670 nm) and (d) chlorophyll c (635 nm) in biofilms of settlement tiles preconditioned for 40 days (May 2009) in treatment seawater (380, 560, 800 μatm). Concentrations were determined by measuring the fluorescent emission of the pigments extracted from the settlement tiles using a spectrofluorometer.
Absolute pigment concentrations (μg pigment g−1 tile) by treatment were as follows (mean±1 SEM): PE: 0.7±0.1 (357 μatm); 0.15±0.04 (555 μatm); 0.17±0.04 (796 μatm). PC: 3.7±0.6 (357 μatm); 1.0±0.3 (555 μatm); 0.6±0.1 (796 μatm). Chl a: 1.1±0.1 (357 μatm); 1.0±0.1 (555 μatm); 1.00±0.07 (796 μatm). Chl c: 0.048±0.007 (357 μatm); 0.056±0.007 (555 μatm); 0.046±0.004 (796 μatm).
Growth
Postsettlement growth significantly declined with increasing pCO2 (F2, 99=12.60, P<0.0001). Results of post hoc Tukey's HSD analyses are presented in Table 1. Growth rates decreased by 16% and 35% at 560 and 800 μatm, respectively, compared with controls (Fig. 4). Absolute growth rates (mm2 month−1) by treatment were as follows [mean±1 SEM (N)]: 330 μatm, 0.74±0.04 (38); 548 μatm, 0.62±0.03 (35); 775 μatm 0.48±0.03 (29).
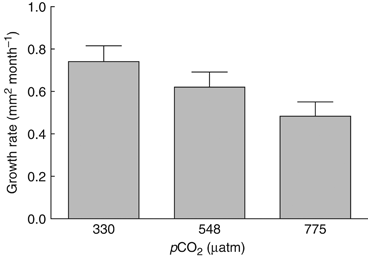
Juvenile growth rate (increase in cross-sectional area) as a function of pCO2 over 49 days in 2008 (mean±1 SEM). Individuals exhibiting partial or full mortality were excluded from the analysis, resulting in fewer individuals in the mid and high pCO2 treatments: N=38 (380 μatm); N=35 (560 μatm); N=29 (800 μatm).
Discussion
This study demonstrates that OA has the capacity to affect coral recruitment by impacting several early life history processes, including: larval metabolism, larval settlement, and postsettlement growth. Results of the respiration experiments demonstrate that near-future OA scenarios significantly depress larval metabolic rates. Metabolic suppression resulting from exposure to acidified conditions has previously been reported to occur in a variety of adult marine invertebrates, including: crabs (Metzger et al., 2007), squid (Rosa & Seibel, 2008), worms (Pörtner et al., 1998), bivalves (adult and juveniles, Michaelidis et al., 2005), pteropods, and amphipods (reviewed in Fabry et al., 2008). Recent work, conducted on sea urchin larvae, demonstrated that culturing larvae in acidified conditions resulted in the downregulation of several genes involved in aerobic metabolism (Todgham & Hofmann, 2009; O'Donnell et al., 2010). Metabolic suppression is considered an adaptive strategy for the survival of short-term hypercapnia and hypoxia (reviewed in Fabry et al., 2008); however, slowed metabolism is generally achieved by halting energy-expensive processes, such as protein synthesis (Hand, 1991; Langenbuch et al., 2006), and therefore, if sustained, will lead to reductions in growth and reproductive potential (Fabry et al., 2008). Thus, metabolic suppression is not considered to be advantageous under chronic elevations of CO2, such as OA (Langenbuch & Pörtner, 2004; Langenbuch et al., 2006).
Depressed metabolic rates in coral larvae may hold implications for larval fitness and motility, thereby limiting dispersal and settlement rates. Recent work demonstrated that oxygen consumption and energy use in Acropora intermedia peaks ∼5 days AS, when larvae begin actively swimming and exploring (Okubo et al., 2008). During the planktonic dispersal phase, larvae actively explore and change their position in the water column to: locate ideal settlement sites (Mundy & Babcock, 1998; Raimondi & Morse, 2000) and possibly influence horizontal transport and dispersal (Szmant & Meadows, 2006). If metabolic suppression during the planktonic stage translates into decreased larval motility, the ability of larvae to regulate their vertical position in the water column may be compromised, thereby impacting dispersal and settlement potential. P. astreoides is a brooding species, and the larvae contain symbiotic algae during the planktonic dispersal stage (as opposed to larvae of spawning species which generally do not contain symbiotic algae until after settlement/metamorphosis). These algae are likely providing the larvae with an additional source of energy in the form of translocated metabolites (Richmond, 1982; Harrison & Wallace, 1990), which may render them less susceptible to stressful environmental conditions. Recent work suggests that the nutritional status of a coral may play a role in its sensitivity to acidified conditions, with decreased sensitivity in individuals with supplemental food and/or nutrients (Cohen & Holcomb, 2009). It is, therefore, possible that larvae of broadcast-spawning species, devoid of symbionts, may be more heavily impacted during the planktonic dispersal phase than larvae of brooding species such as P. astreoides.
The effect of pCO2 on larval settlement may indicate either a direct (physiological disruption of settlement and/or metamorphosis) or indirect (interference with benthic habitat/settlement cues) effect. By conditioning tiles in ambient seawater and settling larvae onto those tiles in treatment seawater, we assessed the potential for acidification to directly impair larval settlement success. Alternatively, by conditioning tiles in treatment seawater and settling larvae in treatment seawater we assessed the potential for OA to indirectly affect larval settlement by altering the substrate community composition and the availability of biological and chemical settlement cues. Results of the Ambient Tile experiment indicate a trend of decreasing settlement with increasing pCO2 (Fig. 2a). However, these results are not statistically significant, and it is not possible to determine whether the reductions in settlement are due to a nonsignificant effect of acidified water on larval physiology or whether the chemistry and microbiology of the settlement tiles were altered by acute pH shifts that occurred as tiles were moved to treatment water for the 24 h settlement experiment. A significant effect of pCO2 on larval settlement was only observed when tiles were conditioned in acidified seawater, with significant reductions in both 2008 and 2009. The results of these experiments indicate that OA has the capacity to impact larval settlement but may primarily do so indirectly, by affecting the chemistry and microbiology of the substrata.
Acidification has been shown to negatively impact larval settlement and/or metamorphosis in other marine invertebrates, including at least three species of marine bivalves (Talmage & Gobler, 2009) and a broadcast spawning coral (Albright et al., 2010). Two prior studies (Albright et al., 2008; Anlauf et al., 2011) have reported no effect of acidified seawater on the ability of coral larvae to successfully settle and metamorphose. However, it is important to note that both of these studies tested only for direct effects of pCO2 on larval settlement and did not address the potential for indirect effects by conditioning substrates in acidified seawater. Kurihara (2008) observed no effect of acidified seawater on the ability of Acropora tenuis larvae to successfully settle; however, materials and methods were not provided for these experiments, and direct comparisons with our results are, therefore, invalid.
Both positive settlement cues from CCA and settlement interference by turf algae have been previously documented (Morse et al., 1988; Webster et al., 2004; Birrell et al., 2005; Kuffner et al., 2006; Vermeij & Sandin, 2008; Ritson-Williams et al., 2010). Red or blue phycobiliproteins such as PE and PC are major pigment characteristics of red algae (e.g. CCA) and/or cyanobacteria. Chlorophylls a and c are major pigment characteristics of chromophytes, such as Bacillariophyceae (diatoms), Dinophyceae (dinoflagellates), Prymnesiophyceae (coccolithophores), etc. (Rowan, 1989; Jeffrey & Vesk, 1997). Biofilms that developed on tiles conditioned in ambient seawater contained significantly higher concentrations of PE and PC (Fig. 3). Using PE/PC concentrations, we were unable to differentiate between red algae and cyanobacteria; however, visual differences in the tiles (noticeably more CCA present on tiles conditioned at ambient CO2), led us to believe that the differences in PE/PC measured by spectrofluorometry were indicative of CCA abundance as opposed to cyanobacteria. Therefore, the prevalence of PE and PC on control settlement tiles may partially explain the higher settlement rates that were observed. The data suggest that, as CO2 levels increase, changes in the algal community occur as red algae are outcompeted by other algal types, such as diatoms and other chromophytes. These data are in agreement with previously published studies indicating that CCA recruit and calcify more slowly at elevated CO2 (Anthony et al., 2008; Kuffner et al., 2008). These findings indicate that OA has the potential to alter coral recruitment dynamics by shifting epibenthic/epilithic algal community composition away from taxa known to facilitate larval settlement of certain coral species (e.g. CCA) and towards alternate algal species (e.g. consortiums dominated by diatoms and other chromophytes).
The observed reductions in juvenile growth rates are consistent with the hypothesis that calcification and, ultimately, growth decline as pCO2 increases and saturation state decreases (Albright et al., 2008; Jokiel et al., 2008; Marubini et al., 2008; Cohen et al., 2009). Larval and juvenile calcification may be more sensitive to acidification than adults, as has been shown for at least two marine invertebrates (one bivalve, one echinoderm; reviewed by Kurihara, 2008). This may, in part, be due to the presence of amorphous calcium carbonate (ACC) precursors that can occur at the onset of calcification and later stabilize into less soluble forms of CaCO3. ACC is 30 times more soluble than calcite (Brecevic & Nielsen, 1989; Politi et al., 2004), rendering it particularly vulnerable to acidified conditions. Larval spines of urchins form via an ACC precursor that later stabilizes into calcite (Beniash et al., 1997; Politi et al., 2008); similarly, shell formation in mollusk larvae involves an initial, transient ACC phase (Weiss et al., 2002; Marxen et al., 2003), and it has been suggested that the same may be true for corals (Meibom et al., 2004).
Slowed postsettlement growth resulting from exposure to acidified conditions has been documented in a number of scleractinian coral species (Albright et al., 2008; Kurihara, 2008; Cohen et al., 2009; Albright et al., 2010; Suwa et al., 2010) and may translate into increased juvenile mortality, as risk of mortality is inversely proportional to juvenile growth rate and colony size (Hughes & Jackson, 1985; Babcock, 1991; Vermeij & Sandin, 2008). Additionally, for corals and other species that exhibit a direct relationship between colony size, onset of sexual maturity (Szmant, 1986) and fecundity (Babcock, 1991), reduced growth will substantially diminish reproductive potential. Slowed growth will result in longer time spent in juvenile, nonreproductive life stages, which, in combination with adult loss, would shift population structures toward dominance by smaller size classes, ultimately reducing effective population sizes, population fecundity, and the resilience of reef-building corals (Done, 1999).
Coral recruitment and early postsettlement survivorship are critical to the persistence and resilience of coral reefs. Recent research using artificial settlement substrates indicates that recruit survivorship during the first year is extremely low, generally reported to be as low as 0.2–6.0% survivorship, depending on the species and environment (Fairfull & Harriott, 1999; Wilson & Harrison, 2005). Stochastic events or chronic stressors that further reduce survivorship during these critical stages have the potential to significantly alter future population sizes (Gosselin & Qian, 1997; Vermeij & Sandin, 2008). Results of this study demonstrate that OA has the potential to interfere with recruitment by negatively impacting multiple early life history processes, including larval metabolism, settlement and postsettlement growth, with implications for survivorship. The compounding nature of successive impacts may translate into a substantial decline in recruitment that is felt at the community and/or ecosystem scale.
Acknowledgements
We would like to thank B. Mason for field and laboratory assistance and for constructive review of the manuscript, M. Miller for field assistance, L. Brand and C. Alvarez for assistance with spectrofluorometry analyses. We also thank V. Paul, R. Ritson-Williams, and the Smithsonian Lab at Ft. Pierce for the provision of larvae in 2010. This project was funded in part by Mote Marine Laboratory's Protect Our Reefs specialty license plate and by the NOAA-Coral Reef Conservation Program with logistical support from the Florida Keys National Marine Sanctuary (FKNMS). Additional support was provided by National Science Foundation Grant (NSF OCE 0547169) and the Korein Foundation. Collection of coral spawn in 2008 and 2009 was conducted under permits FKNMS-2008-023 and FKNMS-2009-022.




