The effects of climate change on species composition, succession and phenology: a case study
Abstract
Climate change and its role in altering biological interactions and the likelihood of invasion by introduced species in marine systems have received increased attention in recent years. It is difficult to forecast how climate change will influence community function or the probability of invasion as it alters multiple marine environmental parameters including rising water temperature, lower salinity and pH. In the present study, we correlate changes in environmental parameters to shifts in species composition in a subtidal community in Newcastle, NH through comparison of two, 3-year periods separated by 23 years (1979–1981 and 2003–2005). We observed concurrent shifts in climate related factors and in groups of organisms that dominate the marine community when comparing 1979–1981 to 2003–2005. The 1979–1981 community was dominated by perennial species (mussels and barnacles). In contrast, the 2003–2005 community was dominated by annual native and invasive tunicates (sea-squirts). We also observed a shift in the environmental factors that characterized both communities. Dissolved inorganic nitrogen and phosphate characterized the 1979–1981 community while sea surface temperature, pH, and chlorophyll a characterized the 2003–2005 community. Elongated warmer water temperatures, through the fall and early winter months of the 2000s, extended the growing season of native organisms and facilitated local dominance of invasive species. Additionally, beta-diversity was greater between 2003–2005 than 1979–1981 and driven by larger numbers of annual species whose life-history characteristics (e.g., timing and magnitude of recruitment, growth and mortality) are driven by environmental parameters, particularly temperature.
Introduction
Ecosystems are under sustained multiple stressors that include climate change, invasive species, and overfishing (Wilcove et al., 1998; Gerten & Adrian, 2000; Harris & Tyrrell, 2001; Jackson et al., 2001; Stachowicz et al., 2002a; Barnes et al., 2006; Crain et al., 2008; Helmuth et al., 2010; Willis et al., 2010). Global Climate change is progressing at a faster rate than previously recorded, particularly in temperate regions (IPCC, 2007). While climate change research has begun to document the effects of these two individual stressors on species and ecosystems (e.g., Gilman et al., 2006; Mieszkowska et al., 2006; Kirby et al., 2007; Reid et al., 2007; Beaugrand et al., 2010; Sorte et al., 2010a), few studies have evaluated the relationship between climate change, invasive species and long-term changes in community function. Yet, there is a need to examine the relationship between climate change (e.g., multiple abiotic factors), shifts in species composition, and native and nonnative phenologies as natural communities are subjected to all of these stressors (Breitburg et al., 1999; Crain et al., 2008). Identifying the potential consequences of climate change as a driver for shifts in species interactions and population growth of introduced species is of one the most pressing goals in ecology (Sala et al., 2000; Zeidberg & Robison, 2007; Gilman et al., 2010; Hillebrand et al., 2010).
While climate change and its role in altering the likelihood of invasion and biological interactions in marine and terrestrial communities have received much attention in recent years (e.g., Stachowicz et al., 2002b; Willis et al., 2010), forecasting the effects of climate change on species interactions and the establishment of invasive species is difficult to predict because climate change refers to changes in many environmental factors. Three environmental factors that may have important roles in marine climate change are rising temperature, dropping pH, and variable salinity levels. Although shifts in these multiple factors are prominent components of climate change (IPCC, 2007), common approaches to predicting the effect of climate change on species interactions have focused on decadal interactions between water temperatures and species composition (Barry et al., 1995; Schiel et al., 2004; Mieszkowska et al., 2006) and the physiological response of species to a single environmental parameter (e.g., Planque & Fredon, 1999; Helmuth et al., 2002; Stachowicz et al., 2002b; Philippart et al., 2003; Gilman et al., 2006; Bibby et al., 2008; Dijkstra et al., 2008).
Studies examining the response of species to changes in single environmental parameters have found that species physiology and reproductive phenology can be affected by changes in temperature, salinity or pH (e.g., growth, reproduction; Vázquez & Young, 2000; Helmuth et al., 2002; Stachowicz et al., 2002b; Fabry et al., 2008; Havenhand et al., 2008; Feder 2010). Rising temperatures have led to earlier starts of marine species [sea urchin, Echinocardium cordatum (Greve et al., 2001; Lindley & Batten, 2002), clam, Macoma balthica (Edwards & Richardson, 2004) polycheate, Nereis virens (Lawrence & Soame, 2004), and limpet, Patella depressa (Moore et al., 2010)]. Further studies examining the relationship between climate warming and species range shifts have focused on decadal changes in species numbers where rising sea surface temperatures led to higher numbers of warmer water species (Sagarin et al., 1999; Mieszkowska et al., 2006; Sorte et al., 2010a). However, the link between warmer waters and changes in species abundance patterns appears to be difficult to predict (Sagarin & Gaines, 2002; Schiel et al., 2004), as the response of species populations to environmental change can differ due to changing interactions between component species (Schiel et al., 2004; Parmesan, 2006; Stachowicz & Byrnes, 2006; Dijkstra & Harris, 2009).
While it is evident that an interaction exists between climate and the appearance and growth of invasive species, relatively little is known of how climate change influences long-term shifts in species composition (see Lindley & Batten, 2002; Lawrence & Soame, 2004; Mieszkowska et al., 2006), and between rate of species turnover, as few studies have concurrent time series of multiple climate related abiotic factors and succession. However, these data are necessary for a mechanistic understanding of the integrated response of species interactions and community function to climate change (Yang & Rudolf, 2010). Therefore, we studied the concurrent effect of climate change and invasive species on long-term changes in species composition and community function by comparing 3-year datasets, separated by 23 years (1979–1981 and 2003–2005) that cover pre- and postinvasion periods allowing for the evaluation of the potential mechanisms underlying changes in species composition. Our pre- and postinvasion datasets are critical to understand potential mechanisms that underlay the relationship between climate change effects on shifts in species composition, life-history characteristics of native and invasive species, and ecosystem function. We predicted that we would find both a shift in species composition and species turnover rate, which would coincide with a shift in abiotic factors, specifically temperature and pH.
Materials and methods
Study site
We replicated an earlier study (Harris and Irons 1982) that documented the development of a marine fouling community on 0.1 m2 Plexiglas® panels from 1979 to 1981 beneath a pier located at the mouth of the Great Bay Estuary in Portsmouth Harbor, Newcastle, New Hampshire (43°4′27″N: 70°45′6″W). Photographs and observations of the 1979–1981 community on the pier indicated the fouling community was dominated by sponges, sea anemones, hydroids, mussels and large barnacles. Soft corals, ascidians (sea-squirts, tunicates), encrusting and erect bryozoans were also found, though not abundant, throughout these assemblages during the course of the study.
Climate-related factors
To understand the impacts of climate change on long-term changes in species composition and species turnover, monthly water samples were collected within 0.5 km of our study site from 1979 to 1981 (Norall et al., 1982). These data included water temperature, salinity, dissolved oxygen, pH, dissolved inorganic nitrogen (DIN), phosphate (PO4), and chlorophyll a (see Norall et al., 1982; Loder et al., 1983 for full description of methodology). Monthly water samples of DIN, PO4, and chlorophyll a were collected from April to December in each of the 3 years (2003, 2004, and 2005) at the Portsmouth Harbor study site. Temperature, salinity, pH, and dissolved oxygen were collected every half hour December 2003 through December 2005 using a YSI environmental data logger. Only data collected between April and December were analyzed as we wanted to compare similar monthly data across all years. All data for individual environmental parameters were collected during each month and averaged. A nonparametric Kruskal–Wallis test was used on log-transformed average monthly data to detect differences between each environmental factor between 1979–1981 and 2003–2005.
To examine which suite of environmental variables characterized the 1979–1981 and 2003–2005 community, we correlated average monthly environmental data collected during the study period to the corresponding Bray–Curtis dissimilarity matrix calculated from average monthly species abundance (e.g., 1979–1981 species abundance was correlated with environmental data collected between 1979 and 1981; Clarke & Gorley, 2006) of biotic data collected between similar months of collected environmental data (e.g., April through December of each year). We used the BIOENV procedure that performs a nonparametric Mantel test and rank correlation coefficients were calculated between the matrix of environmental variables (Euclidean distance) and the matrix of community data (Bray–Curtis dissimilarity, Clarke & Ainsworth, 1993). Randomization tests were subsequently used to test the significance of the correlations. We used P=0.01 as a critical level as autocorrelation in the time series and multiple testing increases the chance of spurious correlations. Analyses were generated using primer 6.0 software.
Community composition and species turnover
Eight horizontal and eight vertical 0.1 m2 Plexiglas® panels were deployed ∼5 m below mean low water in July 1979 and photographed monthly for ∼2.5 years to document long-term changes in species composition and species turnover. From July 2003 to April 2006, using identical materials, methods and deployment site, a similar study was undertaken to compare changes in community development to the earlier study. Only those data recorded between July 1979 to December 1981 and July 2003 to December 2005 were correlated to environmental parameters because these dates correspond to the period of abiotic data collection. During the 1979–1981 study period, photographs from December 1980 to January 1981, July to August 1981 and November to December 1981 were missing. To examine the development of species composition, slides and photographs of horizontal panels (facing the seafloor only; n=8) and vertical panels (one side only; n=8) taken approximately monthly (∼1200 images) were digitized and downloaded into Adobe Photoshop 7.0©. All individuals on panels were counted and identified to species. Stolonating organisms (e.g., hydroids) were assessed as percent cover by using a point count technique of 100 points overlaying a panel displayed on a computer screen. It was also difficult to differentiate epibionts from their basal individuals. Thus, Molgula spp. /Obelia spp. were recorded as a species complex and as percent cover because Obelia spp. was a common secondary space occupant of the solitary tunicate Molgula spp. Abundances from vertical and horizontal panels were pooled as we wanted to compare total differences in community structure and composition. Kruskal–Wallis on square root transformed average monthly abundance was used to test for temporal changes of epifaunal groups between 1979–1981 and 2003–2005. Statistical analyses were generated using jmp© 8.0 software.
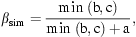
where b and c are the number of species that were found on panels in either one or the other month and a is the number of species present on panels in both months. Beta-diversity (βsim) was calculated between panels between months (e.g., beta-diversity was calculated for panel 1 between January and February, panel 2 between January and February etc.). Calculated values of beta-diversity for individual panels were then averaged and standard error calculated per month. Kruskal–Wallis was used to determine differences in beta-diversity between 1979–1981 and 2003–2005.
Climate change and invasive species
To understand the ecological effects of climate change on the establishment and dominance of an invasive species (Botrylloides violaceus), we calculated relative fitness [growth (blastogenic cycles) and reproductive cycles] as a function of temperature for B. violaceus. Given that blastogenic cycle is dependent on temperature and that duration of brooding period is dependent on both the number and duration of blastogenic cycles (Saito et al., 1981; Grosberg, 1988; Westerman et al., 2009), we used the relationship between temperature and blastogenic cycle to predict the amount of asexual and sexual reproduction that could occur in a given year between 1979–1981 and 2003–2005 at temperatures collected during these time periods.
Using the model developed in Westerman et al. (2009), we estimated asexual growth, using average monthly ambient water temperature, possible in each year between 1979–1981 and 2003–2005. We then calculated the average amount of annual zooid increase at these temperatures assuming an average production of two zooids per zooid per generation. This gave us an estimate annual zooid production via asexual reproduction for B. violaceus at ambient water temperature.
To determine the number of sexual generations possible in temperate waters for this species, we used the estimated duration of brooding period from ambient temperatures above 12 °C (Saito et al., 1981). Brooding period (development time of oocytes within a colony) lasts for the duration of six to seven blastogenic cycles for colonies of B. violaceus that occur in this area (Saito et al., 1981). Thus, brooding period was calculated by multiplying length of blastogenic cycles as a function of temperature by 7 to produce a conservative estimate of annual reproductive cycles. Differences in growth, number of reproductive months and annual reproductive cycles between 1979–1981 and 2003–2005 were tested using Kruskal–Wallis.
Results
Climate-related factors
The environmental time series that correlated most strongly with the 1979–1981 species dissimilarity matrix were DIN and PO4 (Table 1). In contrast, chlorophyll a, temperature and pH were the variables that most strongly correlated with the 2003–2005 community (Table 1). Annual seawater temperature has significantly risen by an annual average of 1.89 °C since 1979 (P<0.001, Fig. 1a). During the 1979–1981 study, monthly average water temperatures ranged from 4.4 °C in April to 15.15 °C in August (Fig. 1a). In contrast, monthly average water temperatures between 2003 and 2005 ranged from 4.22 °C in April to 16.00 °C in August. Between 1979–1981 and 2003–2005, average monthly temperatures between June and November (the period of reproduction and recruitment for most fouling species at the study site) were warmer, with monthly average increases from 0.22 °C to 4.5 °C. Salinity was much lower (P<0.001) with larger fluctuations between 2003 and 2005 than between 1979 and 1981, ranging from a low of 24.0 psu in April to a high of 33.1 psu in September with most salinity values <30 psu during the study period (Fig. 1b). The largest declines in salinity occurred in spring and early summer. Chlorophyll a values were not significantly different (P=0.574) between the study periods. Chlorophyll a values fluctuated both within and across time periods (Fig. 1c).
| Temperature | pH | Chlorophyll a | PO4 | DIN | Salinity | Phaeophyton | DO | |
|---|---|---|---|---|---|---|---|---|
| 1979–1981 | 0.07 | −0.01 | 0.69 | 0.40 | 0.41 | −0.02 | 0.02 | 0.14 |
| 2003–2005 | 0.34 | 0.28 | 0.19 | 0.12 | −0.01 | 0.18 | −0.07 | 0.07 |
- Numbers in bold were found to significantly correlate with biota.
- PO4, phosphate; DIN, dissolved inorganic nitrogen; DO, dissolved oxygen.
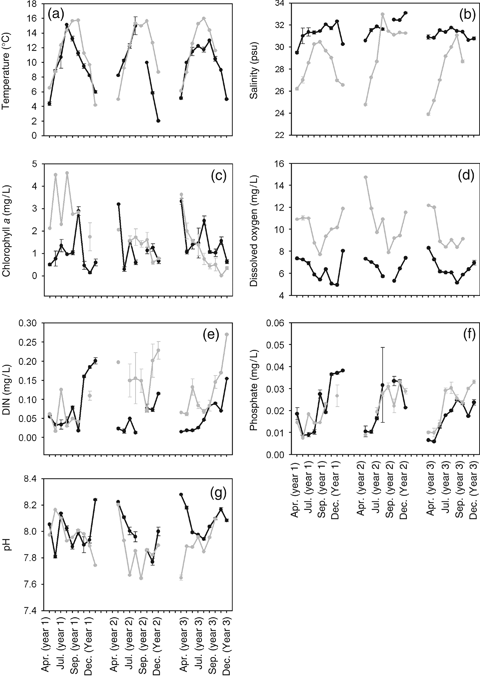
Abiotic factors between April 1979 through December 1981 and April 2003 through December 2005. (a) Temperature, (b) salinity, (c) chlorophyll a, (d) dissolved oxygen, (e) dissolved inorganic nitrogen, (f) phosphate, (g) pH. Black line represents 1979–1981 data and gray line represents 2003–2005 data.
Levels of dissolved oxygen (P<0.001), and DIN have risen (P<0.001) since 1979. Overall, the seasonal pattern of dissolved oxygen has not changed since the earlier study (high levels occurring in spring and winter and low levels occurring in summer), though annual levels have significantly risen by 3.74 mg L−1 since 1979 (Fig. 1d, P<0.01). During the 1979–1981 study, dissolved oxygen levels ranged from 4.96 to 8.32 mg L−1 while they ranged from 7.72 to 14.74 mg L−1 between 2003 and 2005 (Fig. 1d). DIN increased by 0.049 mg L−1 since 1979, rising from an average of 0.067–0.116 mg L−1 (Fig. 1e). PO4 levels declined between the two studies, though not significantly (P<0.0835, Fig. 1f). Annual average pH significantly (P<0.041, Fig. 1g), declined since 1979 from an overall average of 8.02 (1979–1981) to an overall average pH of 7.91 (2003–2005).
Community composition and species turnover
Species composition between 1979 and 2005 was different with apparent shifts in dominance of specific groups (Fig. 2). Overall, bivalves, sponges and crustacean significantly decreased (P=0.002, P=0.001, P=0.037), ascidians increased (P=0.001), hydroids and bryozoans showed no significant differences (P=0.311, P=0.234). Between 1979 and 1981, the groups Crustacea (Balanus spp.) and Mollusca (Mytilus edulis, Anomia simplex and Hiatella arctica) were the dominant groups followed by Ascidiacea (Fig. 2a). During the 1979–1981 period, Botryllus schlosseri was the principal colonial tunicate on the panels; neither the invasive colonial ascidians B. violaceus nor Didemnum vexillum were observed during that period. In contrast, between 2003 and 2005, Ascidiacea were the dominant group followed by Crustacea and Mollusca (Fig. 2b). The large rise in abundance of ascidians stems from the introduction of seasonally abundant invasive colonial ascidians (B. violaceus and D. vexillum, Dijkstra & Harris, 2009) and a rise in the seasonally resident species complex, Molgula spp. (solitary ascidian) and Obelia sp. (hydroid; Fig. 3a).

Abundance of epifaunal groups between 1979–1981 and 2003–2005.
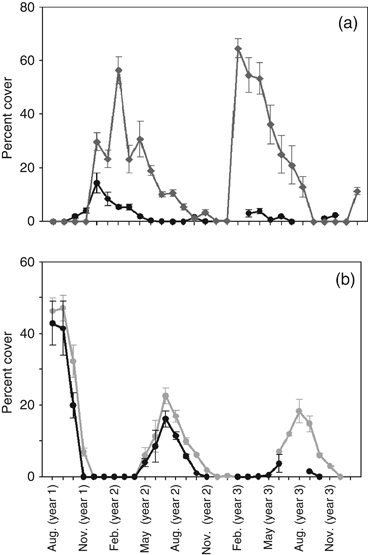
(a) Percent cover of the solitary tunicate and hydroid complex Molgula citrina/Obelia sp. between July 1979–December 1981 (black line) and July 2003–December 2005 (frey line). (b) Percent cover of the hydroid Ectopleura larynx between July 1979–December 1981 (black line) and July 2003–December 2005 (gray line).
We also found shifts in the abundance, appearance and disappearance of dominant members of the community. Two of the resident species, the species complex Molgula spp./Obelia sp. and the hydroid Ectopleura larynx, were seasonally common species in both study periods. The Molgula spp./Obelia sp. complex was more abundant between 2003 and 2005, particularly in January and February, declining in March and April, to be replaced by colonial ascidians in the summer and fall season (Fig. 3a; Dijkstra & Harris, 2009). E. larynx increased in abundance, and remained longer (1 month) in the 2003–2005 community than in the 1979–1981 community (Fig. 3b).
A higher rate of species turnover was observed between 2003 and 2005 than between 1979 and 1981 (P=0.025, Fig. 4). Furthermore, the communities showed contrasting trends in beta-diversity as a function of time (Table 2, Fig. 4). Between 1979 and 1981, annual assemblages of species became increasingly homogeneous during succession as long-lived perennial species such as sponges, mussels and to a lesser extent, anemones, became established (Table 2). The 2003–2005 species assemblages became less homogeneous after their initial development as annual species (e.g., ascidians) were dominant.
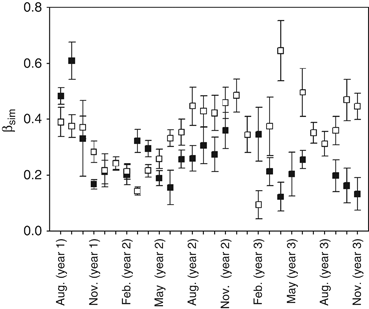
Temporal species turnover (βSim, mean with standard error bars) between 1979–1981 and 2003–2005.
| β sim | Standard error | |
|---|---|---|
| 1979–1981 | ||
| 1 | 0.39 | 0.05 |
| 2 | 0.26 | 0.04 |
| 3 | 0.17 | 0.06 |
| 2003–2005 | ||
| 1 | 0.32 | 0.05 |
| 2 | 0.33 | 0.04 |
| 3 | 0.39 | 0.07 |
- Historical community became more homogeneous with time. In contrast the 2003–2005 community became less homogeneous with time.
Climate change and invasive species
Calculated values of growth and reproduction in the dominant invasive colonial ascidian that was not present in the 1979–1981 study, B. violaceus, revealed rising temperature significantly lengthened the number of annual reproductive cycles (P=0.028) and annual reproductive months of this species (P=0.046; Table 3).
| ZG | # reproductive months/year | # annual reproductive cycles | |
|---|---|---|---|
| 1979 | 22.81 | 2.50 | 0.69 |
| 1980 | 19.59 | 3.00 | 0.62 |
| 1981 | 19.26 | 3.50 | 0.7 |
| 2003 | 26.34 | 4.50 | 1.09 |
| 2004 | 26.73 | 5.00 | 1.21 |
| 2005 | 22.62 | 4.50 | 1.09 |
- Reproductive cycles are based on number of asexual cycles and brooding period.
Discussion
This study demonstrates that a shift in species composition and community function coincided with shifts in abiotic factors (e.g., temperature) that are closely tied to species life-history characteristics that determine species replacement. Environmental factors that characterized species composition between 1979 and 1981 were DIN and PO4. In contrast, temperature, pH, and chlorophyll a characterized the 2003–2005 community. Concurrently, the 1979–1981 community was dominated by perennial species [mollusks and crustaceans (barnacles)] that persisted over the duration of the study period. The 2003–2005 community was dominated by annual species [(e.g., Molgula spp./Obelia sp. complex, Fig. 2; and invasive colonial ascidians, B. violaceus and D. vexillum, ∼50% cover during periods of dominance (Dijkstra & Harris, 2009)].
One of these environmental factors, sea water temperature, was not only warmer between 2003 and 2005, but was warmer for longer periods of time (warmer through the fall and early winter months), likely extending the phenologies of both native and invasive species. For example, one native species, the native hydroid, E. larynx, which is particularly sensitive to changes in water temperature, experienced a shift towards longer phenology in 2003–2005 than 1979–1981. A decrease of 1–2 °C in autumn months stimulates loss of polyps and tissue regression in E. larynx while a rise in water temperature in the spring stimulates colony development and regeneration (Calder, 1990). This developmental sensitivity to temperature suggests that the observed warmer fall temperatures between 2003 and 2005 (on average 2.5 °C warmer between 2003 and 2005 than between 1979 and 1981) contributed to the observed longer phenology of this species.
This temperature induced increase in E. larynx may have greater community-wide effects as hydroids, particularly E. larynx, accumulate sediment among and beneath the hydraocauli. This accumulated sediment prevents settlement of some species and acts as a refuge against predation for others, allowing local build-up of populations, particularly solitary ascidians (Standing, 1976; Dean, 1981; Schmidt, 1983). Furthermore, studies suggest community succession that begins with hydroids facilitate recruitment of ascidians and leads to a community dominated by solitary ascidians (Dean, 1981). Apparent greater abundance and a shift in the phenology of E. larynx, particularly its continued appearance through October may have facilitated a greater abundance of the solitary ascidian/hydroid complex (Molgula spp./Obelia spp.) observed during the winter months between 2003 and 2005.
Many invasive species appear to benefit from rising temperatures (Stachowicz et al., 2002b; Sorte et al., 2010b; Willis et al., 2010). One marine species commonly used to illustrate this invasive advantage in coastal subtidal communities is B. violaceus, a dominant colonial ascidian in the Gulf of Maine (Dijkstra et al., 2007; Dijkstra & Harris, 2009). Reproduction in B. violaceus is complicated; colonies undergo a number of asexual cycles before sexually reproducing, and reproductive phenology (i.e., asexual and sexual) is closely tied to temperature (e.g., Saito et al., 1981; Stachowicz et al., 2002b; Westerman et al., 2009). When we took this association with temperature into account, we were able to predict an increase in both growth and voltinism (number of annual reproductive cycles) that corresponded to the observed increase in abundance of this species (Table 3). Between 1979 and 1981, water temperatures were warm enough for individual growth, but did not remain above the required temperature that would enable release of competent larvae and facilitate population growth. In contrast, between 2003 and 2005, water temperatures remained above the required temperature for the full development and release of competent larvae. It would appear likely that extended phenology of B. violaceus resulting from prolonged warmer water temperatures enabled a build-up of local populations.
Despite a longer growing season, we observed a higher rate of species turnover and greater heterogeneity in the 2003–2005 community relative to that in the 1979–1981 community (Fig. 4). The increased rate of species turnover and heterogeneity could be the result of an increase in annual species, in species prone to predation [(i.e. Molgula spp./Obelia spp. complex (predated by the wrass Tautologlobus adspersus), Osman et al., 1990)], or in species that create biogenic disturbance (Dijkstra & Harris, 2009). In seasonal environments, where abiotic conditions change predictably, each species thus may have a temporal or seasonal niche. However, the reflection of temporal shifts will likely depend on the identity and longevity of the dominant species (Sutherland, 1978, 1981; Stachowicz et al., 2002a; Stachowicz & Tilman, 2005; Dijkstra & Harris, 2009). Between 1979 and 1981, the blue mussel (M. edulis) was the dominant member of the community and occupied between 40% and 80% of free space for ∼20 months (Dijkstra & Harris, 2009). Our results demonstrate that a community dominated by long-lived species that use limited resources (e.g., free space) may reduce invasion success and therefore dampen temporal shifts in species composition. In contrast, a community dominated by annual species increases the rate of species turnover as the availability of resources is higher (Chesson, 2000; Davis et al., 2000; Stachowicz et al., 2002a; Dijkstra & Harris, 2009). More available resources (e.g., space) enhances invasion (number of species entering a system) success and leads to a shift in species composition. Because the appearance and disappearance of annual species largely depend on abiotic factors (Osman, 1977; Sutherland & Karlson, 1977; Stachowicz & Byrnes, 2006; Dijkstra et al., 2007), an increase in the number of annual species will likely strengthen the correlation between abiotic factors and temporal patterns of species composition.
Influence of other environmental factors on community composition
Despite rising temperatures, dissolved oxygen levels have dramatically and unexpectedly risen since 1979. The amount of dissolved oxygen in a body of water is linked to temperature where more oxygen is found in colder waters than warmer waters. In recent years, precipitation and intensity of storms has risen and have led to more freshwater flooding events across the region (Wake et al., 2008), which could lead to increased mixing, particularly in estuaries, and a rise in oxygen concentrations. Additionally, despite warmer sea water temperatures and rising nutrient levels at this site, chlorophyll a levels were not consistently higher in the 2003–2005 study. The introduction of large numbers of suspension feeding species (e.g., B. violaceus, D. vexillum) may mask the effects of increasing nutrients on plankton i.e., algal biomass (Alpine & Cloern, 1992; Dzialowski & Jessie, 2009). Species richness and abundance at this site has increased since 1979 (Dijkstra & Harris, 2009), and higher oxygen concentrations may facilitate further establishment of individuals and species in this area as higher oxygen levels support larger numbers of individuals.
The decline in pH at our study site may have harmful effects on the biology of animals in our system. Biological consequences of even a slight decline in pH (0.1) negatively effects calcification, reproduction and physiological processes in a wide variety of species, particularly those that build calcified shells (see reviews by Harley et al., 2006; Fabry et al., 2008; Widdicombe & Spicer, 2008; Ellis et al., 2009). Additionally, fertilization and survival of calcified organisms can be severely reduced at low pH (Havenhand et al., 2008; Miller et al., 2009). Our results of shifting species composition from organisms with calcareous shells towards organisms that are soft bodied are in agreement with models that project substantial shifts in species composition in rocky intertidal habitats towards soft-bodied organisms (Wootton et al., 2008). Consequently, lower pH observed at our study site may account for fewer barnacles and mussels in the 2003–2005 community, though further examination of the long-term effects of low pH on bivalve populations and on soft-bodied organisms is required (see Hendriks et al., 2010).
Conclusion
Few long-term datasets have multiple abiotic factors and biotic sampling frequent enough to incorporate relative changes in phenologies, individual fitness or changes in species interactions, essential factors that determine the effects of climate change on species turnover and community function (see review Dupont & Thorndyke, 2009; Parker et al., 2009). Our study indicates that key changes in abiotic factors, particularly temperature, have driven long-term changes in species composition through increased phenology of both native and invasive species (facilitating annual reproduction). Greater rate of species turnover observed in this study are likely attributed to changes in dominant species (from perennial species to annual species), whose continued appearance and disappearance is driven by interannual changes in abiotic factors, particularly temperature. Furthermore, our study demonstrates the importance of incorporating life-history characteristics (e.g., temperature-induced reaction norms of growth and reproduction) to evaluate the relationship between climate and the success of invasive species. Before substantive predictive power in climate change models is possible, we need to determine the response of multiple species to multiple climate parameters through collection of data that reflects annual and interannual changes in species interactions.
Acknowledgements
Field assistance was provided by L. Kintzing, N. Rennels, N. Carlson, K. Frick and R. Toppin. We also thank W. Lambert and T. Lee for their comments on previous drafts of the manuscript. Finally, we thank the members of the University of New Hampshire's Coastal Marine Laboratory for the use of their dock during the study. J.A.D. was partially funded by a University of New Hampshire Graduate School Teaching Fellowship, a research grant from the Marine Program, a Dissertation Fellowship as well as a grant provided by the New Hampshire Estuaries Project for J.A.D., E.L.W. and L.G.H.




