Changes in nitrogen cycling and retention processes in soils under spruce forests along a nitrogen enrichment gradient in Germany
Abstract
A network of long-term monitoring sites on nitrogen (N) input and output of forests across Germany showed that a number of Germany's forests are subject to or are experiencing N saturation and that spruce (Picea abies) stands have high risk. Our study was aimed at (1) quantifying the changes in gross rates of microbial N cycling and retention processes in forest soils along an N enrichment gradient and (2) relating the changes in soil N dynamics to N losses. We selected spruce sites representing an N enrichment gradient (indicated by leaching : throughfall N ratios) ranging from 0.04–0.13 (low N),≤0.26 (intermediate N enrichment) to≥0.42 (highly N enriched). To our knowledge, our study is the first to report on mechanistic changes in gross rates of soil N cycling and abiotic NO3− retention under ambient N enrichment gradient. Gross N mineralization, NH4+ immobilization, gross nitrification, and NO3− immobilization rates increased up to intermediate N enrichment level and somewhat decreased at highly N-enriched condition. The turnover rates of NH4+ and microbial N pools increased while the turnover rates of the NO3− pool decreased across the N enrichment gradient. Abiotic immobilization of NH4+ did not differ across sites and was lower than that of NO3−. Abiotic NO3− immobilization decreased across the N enrichment gradient. Microbial assimilation and turnover appeared to contribute largely to the retention of NH4+. The increasing NO3− deposition and decreasing turnover rates of the NO3− pool, combined with decreasing abiotic NO3− retention, possibly contributed to increasing NO3− leaching and gaseous emissions across the N enrichment gradient. The empirical relationships of changes in microbial N cycling across the N enrichment gradient may be integrated in models used to predict responses of forest ecosystems (e.g. spruce) to increasing N deposition.
Introduction
The industrialized regions of Europe and North America, which have received chronic and increasing loads of nitrogen (N) input since the Industrial Revolution (Mayewski et al., 1990; Holland et al., 1999), are presently showing elevated rates of N deposition (Galloway et al., 2004; Holland et al., 2005). Since the time that increased N deposition was first reported as a possible threat to forest ecosystems (Nihlgård, 1985), a number of studies have been conducted to investigate the effects of elevated N deposition on forest ecosystems in Europe [e.g. the coordinated studies under the NITREX program (NITRogen saturation EXperiments) published in Forest Ecology and Management 101 (1998) special issue] and in the United States [e.g. individual studies summarized by Aber et al., 1998 and studies in Harvard Forest N saturation experiment published in Forest Ecology and Management 196 (2004) special issue]. The most deleterious effects of chronic high N and acid deposition in many European forests include forest decline (Schulze, 1989), NO3− leaching (Macdonald et al., 2002; Kristensen et al., 2004), and soil acidification (Van Breemen et al., 1982). In Germany, forests that received chronic high N and acid deposition showed a further increase in NO3− leaching and base cation losses to the extent of soil aluminum mobilization (Meesenburg et al., 1995; Meiwes et al., 1998; Xu et al., 1998; Raben et al., 2000), increase in NO and N2O emissions (Brumme & Beese, 1992; Butterbach-Bahl et al., 1997; Brumme et al., 1999; Schulte-Bisping et al., 2003), and impaired vitality of trees (e.g. Lower Saxony Forest Research Station, 2003; Baden-Württemberg Forest Research Station, 2004; Rheinland-Pfalz Research Institute for Forest Ecology and Forestry, 2004; Sachsen State Ministry for Environment and Agriculture, 2004).
Policies to reduce atmospheric pollutants in Germany have decreased sulfur deposition by 50–70%, beginning in the 1990s compared with the 1970s–1980s, but reduction in N deposition was only modest (Matzner & Meiwes, 1994; Meesenburg et al., 1995; Raben et al., 2000). A network of long-term monitoring sites on N input and output of forests across Germany reported an average annual throughfall N deposition from 6.5 to 35.4 kg N ha−1 yr−1 between the period of 1996 and 2001, and 29% of the 57 forest sites included had NO3− leaching losses from 5 to 26.5 kg N ha−1 yr−1 (Borken & Matzner, 2004). The spruce (Picea abies) stands and forest soils with C : N ratios < 25 showed a high risk of NO3− leaching. As a number of Germany's forests are subject to or are experiencing N saturation, there is a need to investigate the changes in microbial N cycling and retention processes in soils with increasing N enrichment.
From our previous studies in Solling, Germany, on N-saturated beech and spruce forest soils, we observed decreased gross N mineralization and increased gross nitrification compared with the low-N forest sites (Corre et al., 2003; Corre & Lamersdorf, 2004). Reduced N mineralization activity under elevated N input could be due to suppression of humus-degrading enzymes and to alteration of chemical bond structures in soil organic matter by long-term high N deposition which, in turn reduce the effectiveness of extracellular catabolic enzymes (Berg & Matzner, 1997; Aber et al., 1998). The N-saturated plots also showed reduced microbial and abiotic N retention and increased NO3− leaching losses. However, the generality of these results to a larger region could not be justified as the investigation was carried out from a site-level manipulation experiment.
To verify our previous findings, we investigated changes in microbial N cycling and retention processes in soils under spruce forests with generally similar stand, climatic, and soil characteristics along an ambient N enrichment gradient, representing a wide range of N leaching to throughfall N deposition ratios. This ratio is the same index of N enrichment used by Emmett et al. (1998) who summarized the results from the NITREX sites, and by Borken & Matzner (2004) who evaluated the data on N input and output from the long-term forest monitoring sites in Germany. Our hypotheses are based on Aber et al.'s model (1998) of a progressive series of responses along N enrichment continuum: N mineralization should show an initial increase followed by a downturn when microbial biomass and activity are reduced under excessive N levels, nitrification should follow that of N mineralization, and NO3− leaching should gradually increase. Our present study uses the approach of space-for-time substitution, and inherent to this approach is the assumption that the sites reflect the different conditions along the N enrichment continuum and that differences in N deposition drive differences in N cycling rates, rather than other site properties. Our objectives were (1) to quantify changes in gross rates of microbial N cycling in forest soils along an N enrichment gradient and (2) to relate the changes in soil N dynamics to N losses via leaching and gaseous emissions. Despite general acceptance that the soil microbial N cycle greatly influences NO3− leaching and gaseous losses (i.e. the conceptual model of Aber et al., 1998) and the fact that a number of German forests are under chronic high N deposition, to our knowledge, our study is the first to investigate changes in soil N-cycling and soil N-retention processes across a regional N gradient in Germany. Results from this regional study, combined with the findings from site-level experiments, should provide a broader mechanistic understanding on the changes of key soil N processes that govern N losses under ambient conditions as opposed to experimental manipulation.
Materials and methods
Site description
We selected spruce forest sites from the European Level II program (Intensive Monitoring of Forest Ecosystems) that have similar previous land use, humus and soil types, climatic conditions, and stand characteristics (Table 1). These site factors were considered analogous in selecting our sites, important to minimizing their confounding effects on responses of forest ecosystems to chronic N deposition (Aber et al., 1998; Lovett & Rueth, 1999; Goodale & Aber, 2001). The major difference among our study sites was the N leaching to throughfall N deposition ratios (Table 1).
| Site characteristics | Idar-Oberstein(Rheinland-Pfalz) | Klingenthal(Sachsen) | Adenau(Rheinland-Pfalz) | Olbernhau(Sachsen) | Heidelberg(Baden-Württemberg) |
|---|---|---|---|---|---|
| Location | 49° 42′N, 7° 18′E | 50° 22′N, 12° 28′E | 50° 24′N, 6° 54′E | 50° 40′N, 13° 20′E | 49° 25′N, 8° 42′E |
| Throughfall (kg N ha−1 yr−1) | 25.99 ± 1.01 c | 27.86 ± 1.42 bc | 30.50 ± 1.03 ab | 31.75 ± 1.57 a | 28.90 ± 0.52 ab |
| % NH4+-N | 39 ± 2 ab | 44 ± 3 a | 44 ± 2 a | 44 ± 2 a | 38 ± 2 b |
| % NO3−-N | 43 ± 1 b | 42 ± 3 b | 43 ± 2 b | 48 ± 3 ab | 50 ± 1 a |
| % dissolved organic N (DON) | 18 ± 2 a | 14 ± 2 ab | 13 ± 2 ab | 8 ± 1 b | 12 ± 1 b |
| Leaching (kg N ha−1 yr−1) | 1.54 ± 0.16 b | 3.51 ± 0.46 ab | 4.86 ± 0.46 ab | 7.29 ± 4.23 a | 7.58 ± 1.33 a |
| % NH4+-N | 14 ± 2 ab | 20 ± 4 a | 9 ± 2 bc | 7 ± 4 bc | 1 ± 0 c |
| % NO3−-N | 44 ± 12 | 64 ± 14 | 67 ± 13 | 76 ± 32 | 86 ± 16 |
| % DON | 42 ± 4 a | 16 ± 7 b | 24 ± 4 b | 17 ± 10 b | 13 ± 3 b |
| Leaching : throughfall ratio | 0.06 ± 0.01 b | 0.13 ± 0.01 ab | 0.16 ± 0.01 ab | 0.23 ± 0.11 a | 0.26 ± 0.04 a |
| NO flux (μg N m2− h−1) | −0.08 ± 0.43 c | −0.44 ± 0.64 c | 0.40 ± 0.93 c | 8.01 ± 2.52 b | 81.02 ± 30.46 a |
| N2O flux (μg N m2− h−1) | 7.72 ± 0.55 b | 5.35 ± 3.90 b | 4.49 ± 1.30 b | 13.84 ± 3.73 a | 12.07 ± 2.60 a |
| Mean annual temperature (° C) | 7 | 5 | 7 | 6 | 7 |
| Mean annual precipitation (mm) | 1095 | 1050 | 874 | 980 | 1093 |
| Elevation (m above sea level) | 660 | 840 | 600 | 710 | 510 |
| Tree specie | Picea abies | P. abies | P. abies | P. abies | P. abies |
| Stand age (year) in 2006 | 132 | 86 | 99 | 114 | 95 |
| Previous land use | Beech forest (Fagus sylvatica) | Forest | Beech forest (F. sylvatica) | Forest | Beech forest (F. sylvatica) |
| Ground vegetation (growing patchily) | Avenella flexuosa | Calamagrostis villosa | A. flexuosa | Deschampsia flexuosa, Calamagrostis villosa | Dryopteris carthusiana, Deschampsia flexuosa |
| Soil classification | Dystic Cambisol | Dystic Cambisol | Dystic Cambisol | Dystic Cambisol | Dystic Cambisol |
| Soil texture | Silt loam | Sandy loam | Sandy loam | Silt loam | Loamy sand |
| Type of organic horizon | Moder | Moder | Moder | Moder | Moder |
- Means (± 1 SE) within each row followed by the same letter indicated no significant difference among sites (one-way anova, Least Significant Difference test at P≤0.05). Throughfall N deposition and N leaching are averaged annual means with n (as number of years) from 4 to 8, depending on the availability of on-site measured data between 1996 and 2003. Leaching losses were measured at 0.8, 1.0, 0.6, 1.0 and 1.0 m depth in Idar-Oberstein, Klingenthal, Adenau, Olbernhau and Heidelberg, respectively.
- NO fluxes (n= 4) were measured in the field at the start of the study in June 2003. N2O fluxes (n= 5) were measured from the soil cores taken from the organic horizon in June 2004.
- The previous land use of the Klingenthal and Olbernhau sites was forest based from the map of the area in 1818, but the forest type was not known.
For the analyses of changes in gross rates of microbial N cycling in the organic horizon, we included three of the NITREX sites (Klosterhede, Gårdsjön, and Solling) and a second Solling site (referred to as the ambient no-roof plot in Corre & Lamersdorf, 2004) that have similar tree species and previous land use as our study sites (Table 2), in order to cover a much wider range of N enrichment gradient. The detailed site characteristics of the Klosterhede- and Gårdsjön-NITREX sites were reported by Gundersen et al. (1998), and the site description of the Solling-NITREX site was taken from Meesenburg et al. (1995) and the Lower Saxony Forest Research Station (2003). The gross rates of soil N cycling for the NITREX sites were measured by Tietema (1998) in 1994 and for the second Solling site by Corre & Lamersdorf (2004) in 2001. There are two main differences in measurement methods employed by Tietema (1998) and by us. First, Tietema (1998) conducted the measurements from disturbed organic horizon samples, while we measured from intact cores of organic horizon. Second, Tietema (1998) calculated the gross transformation rates using a model to fit 15N enrichments of mineral N pools and the net rates measured periodically within 7-day incubation period, while we used the 15N pool dilution technique. The Solling-NITREX site and the second Solling site (Table 2) are located adjacent to each other. The throughfall N deposition in Solling had decreased beginning in the 1990s (Corre et al., 2003; Corre & Lamersdorf, 2004), and the throughfall N deposition rates measured from the Solling-NITREX site and the second Solling site were the annual averages from 1986 to 1994 and from 1995 to 2001, respectively (Table 2). Hence, the soil N cycling rates measured from the Solling-NITREX site in 1994 reflect the influence of the high N deposition period (1986–1994), whereas the N cycling rates reported for the second Solling site in 2001 reflect the declining N deposition phase (1995–2001).
| Site characteristics | NITREX site: Klosterhede, Denmark | NITREX site: Gårdsjön, Sweden | NITREX site: Solling, Germany | Solling, Germany |
|---|---|---|---|---|
| Location | 56° 29′N, 8° 24′E | 58° 04′N, 12° 01′E | 51° 31′N, 9° 34′E | 51° 31′N, 9° 34′E |
| Throughfall (kg N ha−1 yr−1) | 23 | 13 | 42 | 35 |
| Leaching (kg N ha−1 yr−1) | 1 | 1 | 19 | 15 |
| Leaching : throughfall ratio | 0.04 | 0.08 | 0.45 | 0.42 |
| Mean annual temperature (° C) | 9 | 6 | 6 | 6 |
| Mean annual precipitation (mm) | 860 | 1100 | 1090 | 1090 |
| Elevation (m above sea level) | 27 | 113 – 141 | 508 | 508 |
| Tree specie | Picea abies | P. abies | P. abies | P. abies |
| Stand age, year (reference year) | 71 (1994) | 83 (1994) | 110 (1994) | 68 (2001) |
| Previous land use | Forest after heathland | Natural forest | Forest after grass- and heathland | Forest after grass- and heathland |
| Ground vegetation (growing patchily) | Mosses, some Deschampsia flexuosa | Vaccinium sp., and mosses | none | none |
| Soil classification | Haplic Podzol | Ortic Humic Podzol | Dystic Cambisol | Dystic Cambisol |
| Soil texture | Sandy | Sandy loam | Silt loam | Silt loam |
| Type of organic horizon | Mor | Mor | Moder | Moder |
- For the NITREX sites, detailed characteristics of the Klosterhede and Gårdsjön sites were reported by Gundersen et al. (1998) and the Solling site by Meesenburg et al. (1995) and the Lower Saxony Forest Research Station (2003). Throughfall N deposition and leaching losses of the Solling site were the annual means from 1986–1994, and gross rates of soil N cycling were measured by Tietema (1998) in 1994.
- Detailed site characteristics and soil N cycling rates of the second Solling site were reported by Corre & Lamersdorf (2004), of which this site was referred to as the ambient no-roof. Throughfall N deposition and leaching losses were the annual means from 1995 to 2001, and gross rates of N cycling were measured in 2001.
Sampling design
We measured gross rates of microbial N cycling, microbial biomass, and other supporting soil parameters in June 2003 and June 2004. Measurements were carried out separately for the organic horizon (combined Oi, Oe and Oa layers) and 0–5 cm mineral soil in June 2003, while only the organic horizon was sampled in June 2004. In each site, an area of 0.5–1 ha has been delineated for long term, N input and output monitoring. Within this area, we selected five sampling points with a distance from 10 to 50 m from each other. At each sampling point and horizon, four undisturbed core samples were taken using stainless-steel cores of 8 cm diameter and 5 cm length. During transport to the laboratory, the cores were placed in wooden cases with built-in fittings to maintain their integrity. For the organic horizon, all measurements were carried out on intact soil cores. For the mineral soil, however, the samples contained 16–27% stone (site averages), making it difficult to inject 15N solution. The field-moist soil samples were first sieved using a 5 mm sieve before 15N addition. Samples were incubated at 15° C, which is the average summer soil temperature of the sites.
15 N pool dilution for the measurement of gross rates of N mineralization and nitrification
We followed the 15N pool dilution procedures described by Davidson et al. (1991) and Hart et al. (1994b) for 15N injection into intact cores and subsequent extraction. From the four samples at each sampling point and horizon, two were added with (15NH4)2SO4 solution (for gross N mineralization and NH4+ immobilization) and the other two with K15NO3 solution (for gross nitrification and NO3− immobilization). Each intact core from the organic horizon received five 1 mL injections, containing 30 μg N mL−1 with 98%15N enrichment. The same amount was applied evenly on the sieved, mineral soil samples. This was equivalent to a rate of 2.5 (± 0.1) μg N g−1 for the organic horizon samples and 0.9 (± 0.1) μg N g−1 for the mineral soil samples. One sample of each labeled pair was immediately extracted with 0.5 mol L−1 K2SO4 (approximately a 5 : 1 ratio of solution to dry mass soil). The time elapsed between 15N addition and extraction was 10 min (T0). The T0 samples were used to correct for the reactions that occur immediately after the addition of 15NH4+ and 15NO3−. The other sample of the labeled pair was incubated for 1 day for the 15NH4+-labeled cores and for 2 days for the 15NO3−-labeled cores (T1), and was extracted with K2SO4. Extraction was done by shaking the samples for 1 h and filtering the extracts through K2SO4-rinsed filter papers. Gross N mineralization and nitrification rates were estimated using the modified calculation procedure of Davidson et al. (1991) from the Kirkham & Bartholomew (1954) model.
N concentration and 15N analyses
The NH4+ and NO3− contents of the extracts were analyzed using the same procedures described by Corre & Lamersdorf (2004). Organic N content in the extracts was determined by persulfate digestion (Cabrera & Beare, 1993; Stark & Hart, 1996), which involves oxidation of NH4+ and organic N to NO3− while NO3− remains unchanged. In short, 10 mL of the soil extract was combined with 10 mL of an oxidizing reagent (50 g K2S2O8, 30 g H3BO3, and 100 mL of 3.75 mol L−1 NaOH in a 1 L solution), autoclaved for 1 h at 120° C, and the digest was made to a final volume of 100 mL by adding deionized-distilled water. The N concentration of the persulfate digests was analyzed using continuous flow injection colorimetry (copper–cadmium reduction method; Cenco/Skalar Instruments, Breda, the Netherlands). Extractable organic N is the difference between persulfate-N and NH4++ NO3− concentrations.
To identify the fates of added 15N at T0, part of the T0 extracts was used for serial diffusion of NH4+ and NO3− (Corre & Lamersdorf, 2004) and part was used for persulfate digestion for determination of 15N enrichment in the extractable organic N pool. For the extractable organic N pool, 50 mL of the persulfate digests was added with 2 mL of 10 mol L−1 NaOH (raising the pH to > 13) and left open for 5 days to eliminate any residual NH4+. Another 2 mL of 10 mol L−1 NaOH (maintaining the pH at > 13) and Devarda's alloy were added, converting persulfate-N (in NO3− form) to NH3 which is subsequently trapped in Teflon (polytetrafluoroethylene)-encased acidified disks (Stark & Hart, 1996). The same diffusion procedure and blank correction were followed as described in our previous works (Corre et al., 2003; Corre & Lamersdorf, 2004). We calculated the 15N enrichment of the extractable organic N pool based on isotope mixing equation using the difference in 15N enrichments and N concentrations between the persulfate-N and NH4++ NO3− pools. For the T1 samples, only NH4+ was diffused from the 15NH4+-labeled samples (for gross N mineralization) and only NO3− from the 15NO3−-labeled samples (for gross nitrification). Part of the T1 extracts was reserved for the microbial N immobilization assay. 15N was analyzed using isotope ratio mass spectrometry (Finigan MAT, Bremen, Germany).
Estimation of NH4+ and NO3− immobilization rates and microbial biomass carbon (C) and N by chloroform fumigation
We used the T115NH4+- and 15NO3−-labeled samples to assess NH4+ and NO3− immobilization rates, respectively, as described in our previous works (Corre et al., 2003; Corre & Lamersdorf, 2004). About 25 g of the T115NH4+- and 15NO3−-labeled samples were fumigated with CHCl3 for 5 days and extracted with 0.5 mol L−1 K2SO4 (approximately a 5 : 1 ratio of solution to dry mass soil). From the fumigated T1 extracts and the corresponding unfumigated T1 extracts, extractable organic N and 15N enrichment were determined using persulfate digestion and the diffusion procedures described above. NH4+ and NO3− immobilization rates were calculated using the nonlinear model described by Davidson et al. (1991).
Microbial biomass C and N were determined from undisturbed core samples taken from the same sampling point and layer. We used the fumigation–extraction method (Brookes et al., 1985; Davidson et al., 1989) and following the same procedure described in our previous works (Corre et al., 2003; Corre & Lamersdorf, 2004). Organic C from the extracts was analyzed by ultraviolet (UV)-enhanced persulfate oxidation using a Dohrmann DC-80 Carbon Analyzer with an infrared detector (Rosemount Analytical Division, CA, USA). Organic N was determined using persulfate digestion described earlier. Microbial biomass C and N are calculated as the difference in extractable organic C and persulfate-N between fumigated and unfumigated soils divided by kC= 0.45 (Joergensen 1996) and kN= 0.68 for 5-day fumigated samples (Shen et al., 1984; Brookes et al., 1985).
Calculation of mean residence time (MRT)
The MRT indicates the average length of time an N atom resides in a given pool; a lower MRT indicates a faster pool turnover rate and hence a more dynamic pool. The calculation of MRT (N pool÷flux rate; e.g. microbial NMRT= microbial N pool÷total N immobilization rate) assumed that the NH4+, NO3−, and microbial biomass N pools were at steady state and that the fluxes were equal to gross rates of N mineralization, nitrification and total N (NH4++ NO3−) immobilization, respectively.
Other supporting parameters
NO fluxes (Table 1) were measured in the field in June 2003, at the same time when soil samples were taken for the first measurement of soil N cycling rates. We used a similar dynamic chamber and analytical methods employed by Veldkamp et al. (1998). NO was analyzed with a Scintrex LMA-3 chemiluminescence detector (Scintrex, Ontario, Canada) after conversion to NO2 by a CrO3 catalyst. N2O fluxes (Table 1) and CO2 fluxes were measured from undisturbed soil cores taken from the organic layer in June 2004, at the same time when samples were taken for the second measurement of soil N cycling rates. The soil cores were incubated for 30 min in 1 L glass incubation vessels with a gas sampling port fitting on the lid. Gas samples were analyzed for N2O and CO2 using a gas chromatograph (GC 14A; Shimadzu, Duisburg, Germany) equipped with an electron capture detector (Loftfield et al., 1997). Fluxes were calculated from the increase in N2O and CO2 concentrations during the incubation period minus the background N2O and CO2 concentrations (incubation vessel without a soil core). The CO2 evolution was used for the calculation of total C utilized by microbes ([microbial C : N ratio × total N immobilization rate] + CO2-C evolution rate), which we used as an index of available C similar to that of Schimel (1988) and Hart et al. (1994a). Applying the formula used by the same authors, we estimated microbial growth efficiency as the amount of C assimilated into microbial biomass [microbial C : N ratio × total N immobilization rate] divided by total C utilized.
Soil characteristics (Table 3) were determined at the beginning of the study (June 2003). Total soil organic C and N were measured from air-dried, ground samples using a CNS Elemental Analyzer (Elementar Vario EL, Hanau, Germany). In the organic layer, total Ca, Mg and Al contents were determined from air-dried, ground samples, digested under high pressure in concentrated HNO3, and the digests were analyzed for element contents using an Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP-AES) (Spectro Analytical Instruments, Kleve, Germany). In the mineral soil, exchangeable Ca, Mg and Al contents and cation exchange capacity (from which base saturation was calculated) were determined from air-dried, 2 mm sieved samples, percolated with 1 m NH4Cl, and the percolates were analyzed for element contents using a Flame-Atomic Absorption Spectrometer (Varian, Darmstadt, Germany).
| N output : inputratio/Sites | pH 1 : 5 H2O | Totalorganic C (g C kg−1) | TotalN (g N kg−1) | TotalC : N ratio | Ca (g kg−1) | Mg (g kg−1) | Altotal (g kg−1) | CEC (μmolc g−1) | Basesaturation(%) |
|---|---|---|---|---|---|---|---|---|---|
| Organic horizon | |||||||||
| 0.06/Idar-Oberstein | 3.6 (0.1) | 484 (3) a | 18.7 (0.2) a | 26 (2) | 1.6 (0.1) | 0.4 (0.0) | 5.6 (0.5) c | ||
| 0.13/Klingenthal | 3.8 (0.0) | 396 (38) b | 18.0 (1.5) ab | 22 (3) | 2.1 (0.5) | 1.4 (0.1) | 22.1 (3.1) a | ||
| 0.16/Adenau | 3.7 (0.0) | 455 (9) a | 17.9 (0.6) ab | 25 (2) | 1.9 (0.0) | 0.6 (0.0) | 8.7 (1.1) c | ||
| 0.23/Olbernhau | 4.0 (0.1) | 408 (10) b | 18.6 (0.4) a | 22 (2) | 4.4 (0.7) | 2.3 (0.3) | 14.2 (0.4) b | ||
| 0.26/Heidelberg | 3.5 (0.0) | 376 (24) b | 15.6 (1.0) b | 24 (1) | 1.2 (0.2) | 0.4 (0.0) | 6.7 (0.1) c | ||
| Mineral soil | |||||||||
| 0.06/Idar-Oberstein | 3.5 (0.1) | 59 (9) a | 2.3 (0.3) a | 26 (2) b | 0.06 (0.01) | 0.01 (0.00) | 0.92 (0.13) a | 155 (11) a | 4 (1) |
| 0.13/Klingenthal | 3.7 (0.0) | 34 (1) b | 1.4 (0.1) b | 24 (1) b | 0.09 (0.02) | 0.04 (0.00) | 0.73 (0.04) ab | 118 (4) ab | 8 (1) |
| 0.16/Adenau | 3.5 (0.1) | 51 (6) ab | 1.6 (0.1) ab | 31 (1) a | 0.05 (0.01) | 0.01 (0.00) | 0.70 (0.08) ab | 125 (9) ab | 4 (1) |
| 0.23/Olbernhau | 3.5 (0.0) | 45 (12) ab | 1.7 (0.4) ab | 26 (2) b | 0.06 (0.01) | 0.02 (0.01) | 0.56 (0.13) bc | 106 (25) c | 7 (1) |
| 0.26/Heidelberg | 3.5 (0.0) | 31 (2) b | 1.2 (0.1) b | 25 (1) b | 0.03 (0.00) | 0.01 (0.00) | 0.34 (0.06) c | 71 (9) c | 5 (1) |
- Ca, Mg and Al were analyzed as total amount in the organic layer and as exchangeable amount in the mineral soil. At each depth, means (± 1 SE; n= 5) within each column followed by the same letter or without letter designation indicated no significant difference among sites (one-way anova, least significant difference test at P≤0.05).
Statistical analyses
We first tested the spatial independence of our sampling points using the rank version of von Neumann's ratio test (Bartels, 1982). This test can be carried out when the sampling points have uniform distance. In the June 2003 sampling, we selected one of our study sites that have sampling points regularly spaced at 10 m and tested their spatial independence using the data on gross N mineralization rates. We found that these sampling points were spatially independent. The sampling points from the rest of our study sites and in the June 2004 sampling were spaced even farther, up to 50 m apart. Based on the results of the spatial independence test, we considered the sampling points at each site as replicates in the succeeding analyses. Tests for normality using Kolmogorov–Smirnov D statistic and for equality of variance using Levene statistic (Sokal & Rohlf, 1981) were first conducted for each parameter. When parameters were heterogeneous, they were log transformed before analysis. Analyses were carried out using one-way analysis of variance (anova) for soil characteristics (Table 3) and microbial N cycling in the 0–5 cm mineral soil and using two-way anova (with sites and sampling periods as factors) for microbial N cycling in the organic horizon. Multiple comparisons of treatment effects were conducted using the least significant difference test at P≤0.05. Correlation analyses were conducted on the mean values of each site (for which N is the number of sites) using Pearson's correlation test.
Results
Space-for-time substitution approach: site characteristics
For our present study sites, throughfall N deposition and N leaching were not correlated with mean annual precipitation and temperature. NH4+ and dissolved organic N (DON) were similar across sites but NO3− increased with increasing leaching losses (Table 1). N leaching was correlated with throughfall N deposition (R= 0.83, P= 0.00, N= 5). The sites have similar soil chemical characteristics: acidic soil pH (≤4) and very low base saturation (≤8%) in which Al dominated over Ca and Mg in the exchange complex (Table 3). With respect to the soil biochemical properties, although the sites differed in total C, total N and total C : N ratios, these were not correlated with the throughfall N deposition and leaching losses (Table 3). Regarding biochemical measures that may reflect the more active microbial processes (e.g. N oxide emissions), NO and N2O fluxes corroborated our index of N enrichment (Table 1) – fluxes increased across the N enrichment gradient. Volumetric moisture contents at the time of measurement did not differ across sites: 36 ± 4% to 40 ± 4% for the organic horizon and 36 ± 3% to 43 ± 6% for the 0–5 cm depth mineral soil.
15 N recovery 10 min (T0) after 15N addition
From the 15NH4+-labeled samples of the organic horizon, 82 ± 2% (averaged across sites and sampling periods, N= 50) was recovered in the NH4+ pool and there was no difference detected among sites and sampling periods (Fig. 1). 15N recoveries in the NO3− pool and extractable organic N pool (on average, 0.7 ± 0.3% and 1.0 ± 0.6%, respectively) were not significantly different from zero (one-sample t-test, P > 0.05). We did not measure total 15N recovery from the whole soil. Assuming that what we did not recover from the extractable N pools remains in the sample as nonextractable (or insoluble) N, this fraction constituted 18% of added 15NH4+. For the mineral soil, 15NH4+ recoveries differed among sites, but its trend did not follow the N enrichment gradient. No 15N above the background level was detected in the NO3− pool and extractable organic N pool (Fig. 1). The fraction that presumably remained in the mineral soil as insoluble N form ranged from 0% (Idar–Oberstien and Heidelberg) to 36% (Adenau) of the added 15NH4+.
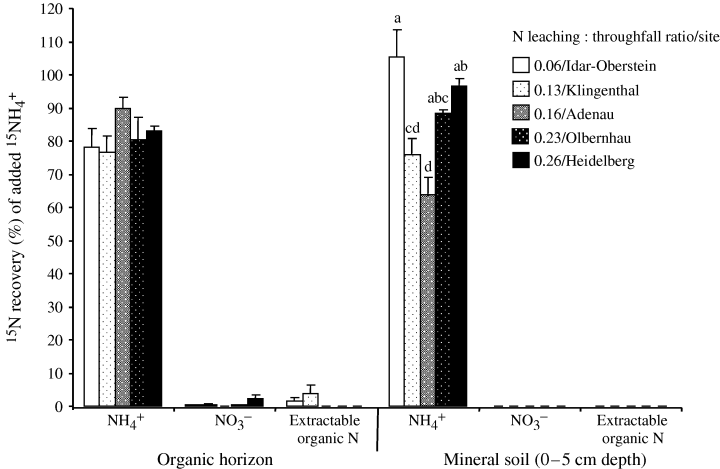
Percent recovery of added 15NH4+ in extractable nitrogen (N) pools after 10 min. 15N (with 98% enrichment) was added at a rate of 2.5 (± 0.1) μg N g−1 for the organic (O) horizon and 0.9 (± 0.1) μg N g−1 for the 0–5 cm mineral soil. For each N pool, means (± 1 SE; n= 10 for O horizon and n= 5 for mineral soil) followed by the same letter or without letter designation indicated no significant difference among sites (two-way anova for O horizon and one-way anova for mineral soil, least significant difference test at P≤0.05).
From the 15NO3−-labeled samples, 15N recoveries in the NH4+ pool at both depths were negligible. 15NO3− recoveries increased with the N enrichment gradient, while 15N recoveries in the extractable organic N pool decreased with the N enrichment gradient (Fig. 2). In all sites, 15N recoveries in the extractable organic N pool did not differ (paired-samples t-test at P > 0.05) between the organic horizon (15 ± 2% of injected 15NO3−) and mineral soil (11 ± 2% of injected 15NO3−). If we assume that what we did not recover in the extractable N pools remained in the samples as insoluble organic N, this contributed on average 35% of the added 15NO3− in the organic layer and 42% in the mineral soil.
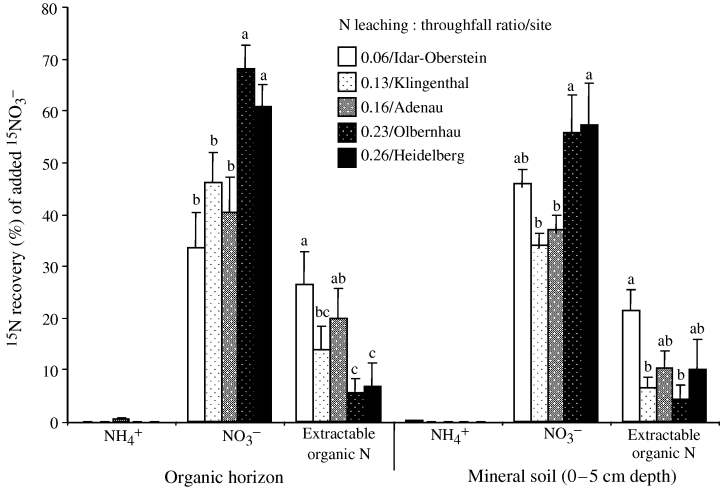
Percent recovery of added 15NO3− in extractable nitrogen (N) pools after 10 min. 15N (with 98% enrichment) was added at a rate of 2.5 (± 0.1) μg N g−1 for the organic (O) horizon and 0.9 (± 0.1) μg N g−1 for the 0–5 cm mineral soil. For each N pool, means (± 1 SE; n= 10 for O horizon and n= 5 for mineral soil) followed by the same letter or without letter designation indicated no significant difference among sites (two-way anova for O horizon and one-way anova for mineral soil, least significant difference test at P≤0.05).
Gross rates of NH4+ transformation and MRT of NH4+ and microbial N pools
For all the processes measured, there was no difference detected between sampling periods (June 2003 and June 2004) for the organic horizon. Gross N mineralization rates in the organic horizon (Fig. 3) increased up to the intermediate N enrichment level (i.e. an N leaching :throughfall ratio of 0.26/Heidelberg), followed by a downturn in the highly N-enriched sites (i.e. N leaching : throughfall ratio of≥0.42/Solling). Microbial NH4+ immobilization rates showed a similar pattern, although we did not detect significant differences among sites (Fig. 3). For mineral soil, gross rates of N mineralization and NH4+ immobilization were much lower and no difference was detected among sites (Table 4).
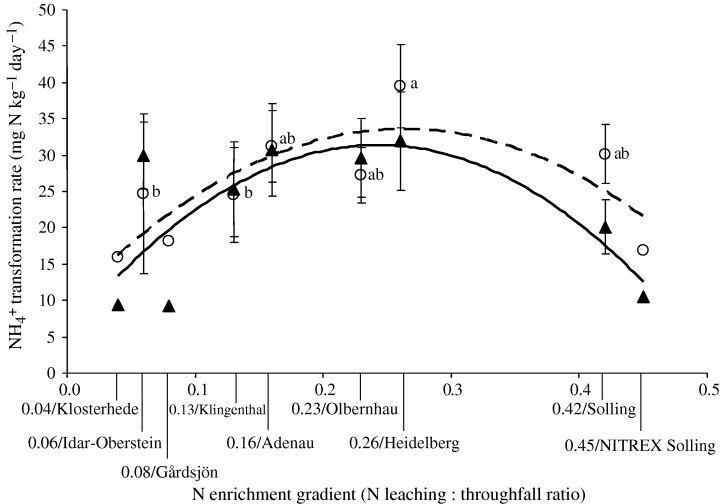
Gross nitrogen (N) mineralization rates (○) and microbial NH4+ immobilization rates (▴) in the organic horizon across the N enrichment gradient. There were no SE values provided for the published data of the NITREX sites (Klosterhede, Gårdsjön Solling; Tietema, 1998), and these were excluded in the test for differences among sites. There was no difference detected among sites for NH4+ immobilization. For gross N mineralization, means (± 1 SE; n= 10 and 6 for 0.42/Solling site) followed by the same letter indicated no significant difference among sites (two-way anova, least significant difference test at P≤0.05). Dashed curve for gross N mineralization, y=−349x2+ 184x+ 9, R2= 0.64, P= 0.05, n= 9; solid curve for NH4+ immobilization, y=−439x2+ 213x+ 6, R2= 0.58, P= 0.07, n= 9).
| N output : inputratio/sites | Gross Nmineralization(mg N kg−1 day−1) | NH4+immobilization(mg N kg−1 day−1) | Grossnitrification(mg N kg−1 day−1) | NO3−immobilization(mg N kg−1 day−1) | Microbial C(mg C kg−1) | Microbial N(mg N kg−1) | MicrobialC : N ratio | Microbial N MRT(day) |
|---|---|---|---|---|---|---|---|---|
| 0.06/Idar-Oberstein | 1.9 (0.5) | 1.2 (0.2) | 0.10 (0.03) | 0.07 (0.04) | 302 (28) a | 30 (3) a | 10 (1) ab | 32.3 (6.7) a |
| 0.13/Klingenthal | 3.2 (3.0) | 0.8 (0.3) | 0.22 (0.08) | 0.25 (0.10) | 224 (22) ab | 21 (3) ab | 11 (1) a | 18.4 (4.7) b |
| 0.16/Adenau | 1.2 (0.3) | 1.7 (0.3) | 0.09 (0.03) | 0.15 (0.08) | 229 (14) ab | 23 (1) ab | 10 (1) ab | 14.9 (2.4) b |
| 0.23/Olbernhau | 2.1 (1.1) | 0.6 (0.2) | 0.17 (0.10) | 0.13 (0.05) | 209 (60) ab | 17 (5) b | 12 (1) a | 15.9 (3.5) b |
| 0.26/Heidelberg | 1.2 (0.1) | 1.0 (0.3) | 0.26 (0.14) | 0.16 (0.03) | 163 (15) b | 20 (2) b | 8 (1) b | 18.0 (3.4) b |
- Means (± 1 SE; n= 5) within each column followed by the same letter or without letter designation indicated no significant difference among sites (one-way anova, least significant difference test at P≤0.05).
For our present study sites (low to intermediate N enrichment), gross N mineralization and NH4+ immobilization were neither related to total C, N and C : N ratio nor to microbial biomass (Table 5). However, the increasing gross N mineralization rates in the organic layer across low to intermediate N enrichment were paralleled with increasing available C (Table 5). The increase in available C in the organic layer also corresponded to a decrease in MRT (fast turnover) of the NH4+ pool (Table 5 and Fig. 4) and the microbial N pool (Fig. 5). In mineral soil, the MRT of NH4+ pools (Fig. 4) and microbial N (Table 4) also declined slightly across our present study sites gradient.
| NH4+immobilization | NH4+ MRT | Grossnitrification | NO3−immobilization | NO3− MRT | Microbialbiomass N | Microbialbiomass C | MicrobialC : N ratio | Available C | |
|---|---|---|---|---|---|---|---|---|---|
| Gross N mineralization | 0.83** | −0.80 | 0.65† | 0.88** | 0.25 | −0.31 | 0.26 | 0.52 | 0.94* |
| NH4+ immobilization | −0.41 | 0.40 | 0.74* | −0.19 | −0.47 | 0.38 | 0.77 | 0.55 | |
| NH4+ mean residence time (MRT) | −0.88* | −0.91* | −0.75 | 0.19 | 0.20 | 0.07 | −0.87† | ||
| Gross nitrification | 0.65† | 0.62 | −0.61 | −0.30 | 0.19 | 0.95* | |||
| NO3− immobilization | 0.74 | −0.55 | −0.44 | −0.02 | 0.84† | ||||
| NO3− MRT | −0.15 | −0.74 | −0.66 | 0.50 | |||||
| Microbial biomass N | 0.45 | −0.36 | −0.48 | ||||||
| Microbial biomass C | 0.67 | −0.08 | |||||||
| Micobial C : N ratio | 0.31 |
- N (sites) = 9 across the entire N enrichment gradient and 5 across our present study sites.
- †,*,** Significant at P= 0.06, P≤0.05 and P≤0.01, respectively.
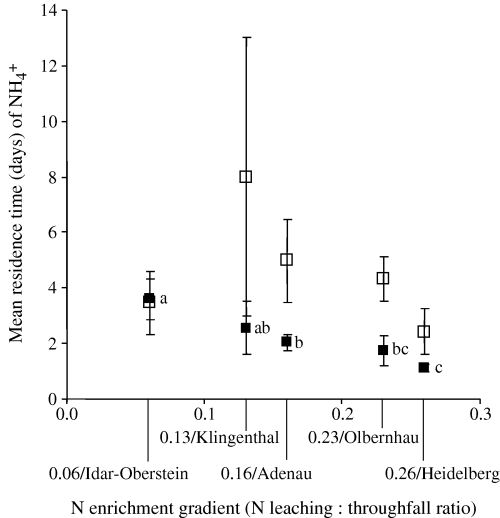
Mean residence time of NH4+ pool in the organic horizon (▪) and in the 0–5 cm mineral soil (□) across our present study sites. There was no difference detected among sites for the mineral soil. For organic horizon, means (± 1 SE; n= 10) followed by the same letter indicated no significant difference among sites (two-way anova, least significant difference test at P≤0.05).
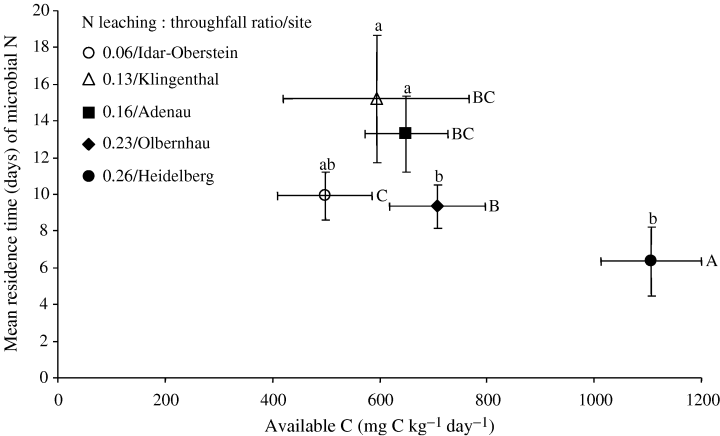
Mean residence time (MRT) of microbial nitrogen (N) vs. available carbon (C) in organic horizon across our present study sites. Means (± 1 SE; n= 5 for June 2004 sampling) followed by the same letter (lower case for MRT and upper case for C) indicated no significant difference among sites (one-way anova, least significant difference test at P≤0.05).
NH4+ immobilization was highly correlated with gross N mineralization across the entire N enrichment gradient (Table 5). NH4+ immobilization rates increased proportionately with increasing gross N mineralization rates (error bars overlap; Fig. 3), but when gross N mineralization rates declined in the highly N-enriched site NH4+ immobilization rates tended to be lower than gross N mineralization rates (error bars do not overlap; Fig. 3). Data from the previous NITREX study (Tietema, 1998) did not contain an error term; therefore, interpreting NH4+ immobilization rates across this N gradient was not determinable with a significant degree of surety.
For our present study sites, microbial C and N in the organic horizon slightly increased toward the site with a leaching : throughfall ratio of 0.16/Adenau (Table 6) followed by a decrease. In mineral soil, these markedly decreased as the sites become more N enriched (Table 4). Microbial C and N were not related to total C and N in both depths. The pattern for microbial C : N ratio in the organic horizon did not follow the N enrichment gradient (Table 6) but was related to pH (R=−0.88, P= 0.05, N= 5). For the organic horizon, sites with pH 3.8–4.0 (Table 3) showed lower microbial C : N ratios (Table 6) than sites with pH≤3.7. The microbial C : N ratios in the mineral soil, with uniformly low pH (pH≤3.7), clearly decreased across the N enrichment gradient (Table 4).
| N output : input ratio/sites | Microbial C (mg C kg−1) | Microbial N (mg N kg−1) | Microbial C : N ratio |
|---|---|---|---|
| 0.06/Idar-Oberstein | 3924 (191) ab | 332 (19) | 11.9 (0.2) a |
| 0.13/Klingenthal | 3638 (280) ab | 364 (27) | 10.0 (0.3) b |
| 0.16/Adenau | 4182 (179) a | 373 (20) | 11.3 (0.2) a |
| 0.23/Olbernhau | 3355 (275) b | 329 (28) | 10.2 (0.2) b |
| 0.26/Heidelberg | 3815 (313) ab | 322 (26) | 11.9 (0.4) a |
- Means (± 1 SE; n= 10) within each column followed by the same letter or without letter designation indicated no significant difference among sites (two-way anova, least significant difference test at P≤0.05.
Gross rates of NO3− transformation and MRT of the NO3− pool
Gross rates of NO3− transformation were three times to an order of magnitude lower than the NH4+ transformation rates (Table 4 and 3, 6). In the organic horizon, there were no differences detected in the gross rates of NO3− transformation between sampling periods (June 2003 and June 2004). For the mineral soil, the rates were much lower; however, no difference was detected among sites (Table 4). Gross nitrification and NO3− immobilization rates in the organic horizon (Fig. 6) increased up to the intermediate N enrichment level (i.e. an N leaching : throughfall ratio of 0.26/Heidelberg) and somewhat declined in the highly N-enriched sites (i.e. N leaching : throughfall ratio of ≥0.42/Solling). The negligible NO3− immobilization reported for the Solling-NITREX site caused a sharp decline in the curve fitting for NO3− immobilization (Fig. 6). Nitrate immobilization rates were higher than gross nitrification rates (paired-samples t-test at P < 0.05; Fig. 6) in the sites with N leaching : throughfall ratios of 0.06–0.16 (where nitrification was low) and 0.42 (where nitrification showed a decline).
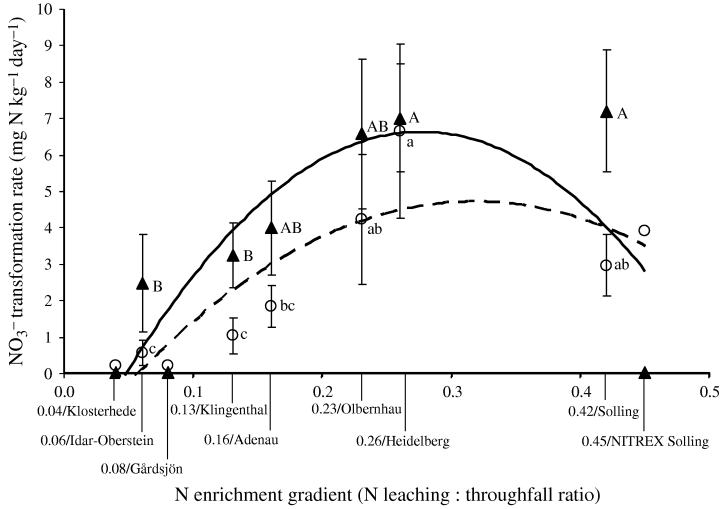
Gross nitrification rates (○) and microbial NO3− immobilization rates (▴) in the organic horizon across the nitrogen (N) enrichment gradient. There were no SE values provided for the published data of the NITREX sites (Klosterhede, Gårdsjön, Solling; Tietema, 1998), and these were excluded in the test for differences among sites. Means (± 1 SE; n= 10 and 6 for 0.42/Solling site) followed by the same letter (lower case for gross nitrification and upper case for NO3− immobilization) indicated no significant difference among sites (two-way anova, least significant difference test at P≤0.05). Dashed curve for gross nitrification, y=−70x2+ 44x−2, R2= 0.74, P= 0.02, n= 9; solid curve for NO3− immobilization, y=−127x2+ 70x−3, R2= 0.65, P= 0.04, n= 9).
Gross nitrification was positively correlated to gross N mineralization across the entire N enrichment gradient (Table 5). The increase in gross nitrification covaried with a decrease in NH4+ pool MRT and an increase in available C across our present study sites (Table 5). NO3− immobilization was correlated with gross nitrification across the entire N enrichment gradient (Table 5). Because NO3− immobilization and gross nitrification covaried, they showed similar correlations with gross N mineralization and MRT of the NH4+ pool. The correlation between NO3− immobilization and NH4+ immobilization (Table 5) reflected their similar pattern across the N enrichment gradient (3, 6). NO3− immobilization and available C share a common parameter in their calculation and their correlation may be a methodological artifact.
The MRT of the NO3− pool increased across our present study sites (Fig. 7). Unlike that of NH4+ pool MRT, we observed no correlation between NO3− pool MRT and available C (Table 5). Instead, we observed an inverse relationship in the organic horizon between NO3− pool MRT and the T015N recovery in extractable organic N from 15NO3−-injected cores across our present study sites (R=−0.96, P= 0.01, N= 5).
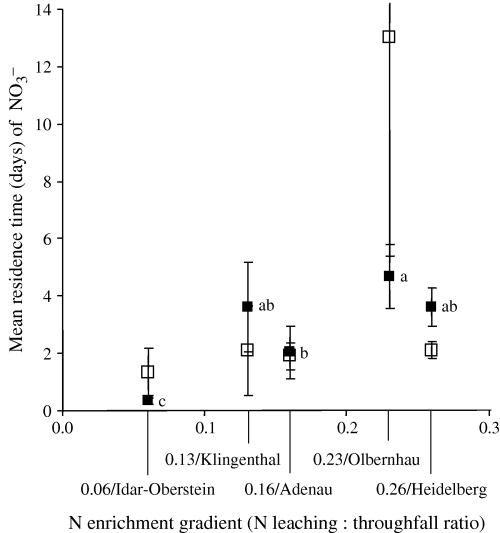
Mean residence time of NO3− pool in the organic horizon (▪) and in the 0–5 cm mineral soil (□) across our present study sites. There was no difference detected among sites for the mineral soil. For organic horizon, means (± 1 SE; n= 10) followed by the same letter indicated no significant difference among sites (two-way anova, least significant difference test at P≤0.05).
Discussion
Space-for-time substitution to represent N enrichment gradient
The extensive review of Aber et al. (1998) showed that the rates at which forest ecosystems progress along the N enrichment continuum are regulated by two main factors: inherent N status of the system (e.g. N supply and demand) and rate of N deposition or N addition. The inherent N status is in turn influenced by forest type (e.g. broadleaf vs. coniferous forests; Kristensen et al., 2004; Magill et al., 2004), land use history and management (Goodale & Aber, 2001; Compton & Boone, 2002), and soil and organic horizon characteristics (e.g. C : N ratio; Dise et al., 1998; Emmett et al., 1998; Macdonald et al., 2002; Borken & Matzner, 2004). We did not observe any correlation between leaching losses and C : N ratios of the organic horizon because our sites encompassed only a narrow range of organic horizon C : N ratios (between 22 and 26; Table 3). Our present sites have generally similar characteristics (stand, climatic, and soil chemical properties), and they mainly differed in throughfall N deposition and losses. These qualities support the implicit assumption of the space-for-time substitution approach that the sites should have similar characteristics such that changes in soil N processes are a consequence of their exposure to chronic and different rates of N deposition. Our index of N enrichment (N leaching : throughfall N ratio) indicates the degree to which sites are able to retain their respective N deposition, and sites should then reflect changes in soil N retention processes over the N enrichment gradient.
Implications of 15N recovery at T0 on abiotic N immobilization
From the 15NH4+-labeled samples, 15NH4+ recoveries from both depths were comparable with our previous findings from similar forest soils in Solling, Germany (Corre et al., 2003; Corre & Lamersdorf, 2004). In the organic layer, a small fraction of injected 15NH4+ was presumably retained as an insoluble N form. Such fast incorporation (within 10 min) of added 15NH4+ into an insoluble N pool has been previously equated to abiotic N immobilization (Fitzhugh et al., 2003) and has been attributed to physical condensation with phenolic compounds in humus (Nömmik, 1970) and clay fixation (Davidson et al., 1991). All sites showed a similar reaction to the added 15NH4+, which suggests that abiotic NH4+ retention may not be affected by N status. A similar result was reported by Johnson et al. (2000) from a variety of forest types with differing N deposition rates (from 0.3 to 27 kg N ha−1 yr−1), whereby abiotic NH4+ immobilization was unrelated to soil N status. In mineral soil, abiotic immobilization of added 15NH4+ can be due to both fixation on clay minerals and reaction with organic compounds. The 15NH4+ recoveries were generally comparable between the organic and mineral soil in each site (paired-samples t-test, P > 0.05), except in the Adenau site (Fig. 1). The low 15NH4+ recovery in the mineral soil of the Adenau site may indicate higher NH4+ fixation by the clay minerals. The differences in 15NH4+ recoveries in mineral soil possibly reflect differences in clay mineralogy, and hence the pattern was not related to the N enrichment gradient.
On the other hand, rapid (within 10–15 min) disappearance of added 15NO3− from the NO3− pool has been equated and further verified to be due to abiotic NO3− immobilization (Berntson & Aber, 2000; Dail et al., 2001). We observed a decrease in the abiotic NO3− immobilization (rapid conversion of added 15NO3− to extractable organic N) with the N enrichment gradient. Berntson & Aber (2000) first reported that abiotic NO3− immobilization from a coniferous forest soil decreased after 11 years of high N addition. This raised an important issue of whether such a change in abiotic NO3− immobilization resulting from a high N fertilization rate is similar to the impact caused by relatively low, but chronic atmospheric N deposition to ecosystems. To our knowledge, our study is the first to affirm that such a decrease in abiotic NO3− immobilization can also occur under an ambient N enrichment gradient. Davidson et al. (2003) put forward a hypothesis explaining the abiotic immobilization of NO3− to organic N. They hypothesized that DOC drives this reaction: DOC reduces Fe(III), producing reactive Fe(II) species that reduce NO3− to NO2− in anaerobic microsites, which in turn reacts readily and abiotically with soil organic matter (Smith & Chalk, 1980; Azhar et al., 1986; Thorn & Mikita, 2000). The NO2− reactions with C-containing compounds may depend on the forms of reactive C, which may affect whether the 15N is recovered in soluble or insoluble organic N. For example, the abiotic NO3− reaction to soluble and insoluble organic N pools differed among temperate forests with differing N status: only 1% of added 15NO3− (from intact soil cores at 10 min) was detected in extractable organic N from an N-saturated spruce forest (Corre & Lamersdorf, 2004), 30% in extractable organic N and 5% (Dail et al., 2001) to 7–10% (Fitzhugh et al., 2003) in insoluble N (from disturbed samples at 15 min and 1-day incubation) from low N deposition (9–11 kg N ha−1 yr−1) deciduous forests, and 11% in extractable organic N and 37% in the insoluble N (at 0.1 day of 15NO3− addition in the field) from an unpolluted/N-limited evergreen mixed-angiosperm forest (Perakis & Hedin, 2001). There is a well-developed literature on the effects of increasing N inputs to changes in structure and decomposition in soil organic matter (Berg & Matzner, 1997; Aber et al., 1998; Neff et al., 2002), which may result to differing reactive C forms in forests with differing degrees of N enrichment. These studies and our present study foster an increasing awareness of the significance of abiotic NO3− immobilization in forest soils. The change in abiotic NO3− immobilization as forests progress along the N enrichment continuum may influence the NO3− retention capacity of forest soils.
Changes in and controls of NH4+ transformation rates
For the organic horizon (Fig. 3), the low-N sites (i.e. an N leaching : throughfall ratio from 0.04 to 0.13) showed comparably low gross N mineralization and NH4+ immobilization rates as has been observed for an N-limited coniferous forest in California (Davidson et al., 1992) and a pine forest at the Harvard Forest site (Venterea et al., 2004). At the intermediate N enrichment level (i.e. an N leaching : throughfall ratio of 0.26) with increased gross NH4+ transformation rates, these rates were higher than those reported for California coniferous and Harvard pine forests. While it has been shown by long-term, high N fertilization that gross N mineralization tended to decrease (Corre et al., 2003; Venterea et al., 2004), our data were the first, to our knowledge, to show that a decrease in gross N mineralization rates can occur under ambient, highly N-enriched conditions (i.e. an N leaching : throughfall ratio of≥0.42). Our study provides compelling evidence of mechanistic changes in soil N cycling under an ambient N enrichment gradient.
For our present study sites (low to intermediate N enrichment), gross rates of NH4+ transformation were not related to total C, N, C : N ratio and microbial biomass. Bulk soil C and N levels, which are a cumulative effect of the historic and recent N deposition, may not reflect the microbially labile C and N fractions. Also, the pattern of microbial biomass across sites (Tables 4 and 6) was not in step with the pattern of NH4+ transformation rates (Fig. 3). The correlation between gross N mineralization rates and available C across low to intermediate N enrichment (Table 5) attested for improved substrate quality in response to increasing N enrichment. The increase in available C, in turn, drove faster turnover rates of the NH4+ pool (Table 5 and Fig. 4) and microbial N pool (Fig. 5), as was demonstrated by Hart et al. (1994a). Our results demonstrated that across low to intermediate N enrichment the parallel increase in gross N mineralization and available C drove a corresponding increase in microbial immobilization of NH4+; this combined with increased turnover rates of the microbial N pool would contribute to efficient retention of NH4+. Furthermore, while abiotic retention of NH4+ is of only minor importance (as shown above), microbial assimilation and turnover appeared to contribute largely to its retention. Such retention of N via microbial assimilation can be supported by the available C; our estimate of microbial growth efficiency in the organic horizon was on average 0.65 ± 0.18 (N= 25 for the June 2004 sampling of our present study sites), which was in the range of those reported for coniferous forests (Hart et al., 1994a; Stark & Hart, 1997).
For the highly N-enriched Solling sites, reduced gross N mineralization rates and much more reduced NH4+ immobilization rates were attributed to a decrease in available C, microbial biomass (Corre et al., 2003), and DOC (Corre & Lamersdorf, 2004), which point to reduced microbial decomposition and altered soil organic matter structure (Berg & Matzner, 1997; Aber et al., 1998). In particular, the Solling-NITREX site (an N leaching : throughfall ratio of 0.45) showed build-up of organic matter stock from 49 ± 4 Mg ha−1 (C/N = 25; Matzner, 1989) in 1968 (with throughfall N deposition of 43 kg N ha−1 yr−1 between 1969 and 1994) to 121 ± 8 Mg ha−1 (C/N = 26) in 2001 (with throughfall N deposition of 35 kg N ha−1 yr−1 between 1995 and 2001; Meesenburg et al., 1999; Meiwes et al., 2002). This increase of organic matter stock was attributed to the retardation effect of excessive N levels on lignin degradation and on decomposition of recalcitrant organic matter (Matzner, 1989; Berg & Matzner, 1997; Berg & Meentemeyer, 2002). Other studies on chronic, high N fertilization consistently showed reduced microbial biomass (Compton et al., 2004; DeForest et al., 2004), decreased fungal : bacterial biomass and activity ratios (Frey et al., 2004; Wallenstein et al., 2006), and reduced β-glucosidase (a cellulose-degrading enzyme) and phenol oxidase activities (a lignin-degrading enzyme produced by white-rot fungi) (Carreiro et al., 2000; Saiya-Cork et al., 2002; Sinsabaugh et al., 2002; DeForest et al., 2004; Frey et al., 2004). We observed an apparent decrease in microbial C : N ratio in the mineral soil across our present study sites, which possibly indicated decreased fungal : bacterial biomass ratio. Our results and these aforementioned studies indicated that highly N-enriched conditions depress microbial decomposition, and ultimately reduce N mineralization, as a result of changes in microbial biomass, microbial community structure, and enzyme activity.
Changes in and controls of NO3− transformation rates
For the organic horizon (Fig. 6), the N-limited NITREX sites had similarly low NO3− transformation rates as the N-limited Harvard pine forest (Venterea et al., 2004), and the sites with N leaching : throughfall ratios from 0.06 to 0.16 and 0.42 showed comparable intermediate rates with the 13-year N fertilized Harvard pine forest (Venterea et al., 2004) and the coniferous forests in New Mexico (Stark & Hart, 1997). In these sites NO3− immobilization rates were higher than the gross nitrification rates, which was the same case for these cited studies. Our data indicated that in sites with extremely low to intermediate nitrification rates (which also had low to intermediate N mineralization rates), NO3− (and NH4+) produced in the soil may be insufficient to meet microbial demand for N that microbial assimilation was stimulated by the injected 15NO3−. The mechanisms resulting in substantial NO3− assimilation by microbes have been related to the spatial compartmentalization of NH4+, NO3− and C availability. NH4+ depletion may occur at microsites that have high C availability, and the greater diffusion rate of NO3− relative to NH4+ may then lead to significant NO3− assimilation (Davidson et al., 1992; Stark & Hart, 1997). This is supported by our observations where stimulated NO3− immobilization occurred only from the undisturbed cores of the organic horizon, presumably having high microsite heterogeneity, and not from the mixed samples of mineral soil (Table 4).
On the other hand, in sites with the highest gross nitrification rates (i.e. N leaching : throughfall ratios of 0.23 and 0.26, also with the highest N mineralization rates), NO3− immobilization rates did not exceed gross nitrification rates (Fig. 6). Our results further implied that while acid spruce soils are poised to immobilize NO3−, chronic high NO3− deposition on already highly nitrifying sites may possibly result in NO3− excess relative to microbial demand for N. This was depicted by an increasing MRT of the NO3− pool (or NO3− accumulation) across the N enrichment gradient (Fig. 7). The increasing NO3− deposition and MRT of the NO3− pool, which signal high potential for N losses in times of high soil water content, possibly contributed to increasing NO3− leaching and N trace gases emissions across the N enrichment gradient (Table 1). This was the case for the Solling site with an N leaching : throughfall ratio of 0.42; gross nitrification rates (and gross N mineralization rates) had somewhat declined in this site, while microbial NO3− immobilization rates remained high (Fig. 6). However, the extremely high N deposition, combined with low abiotic NO3− retention, in this site resulted in high NO3− pool MRT and high NO3− leaching (Corre & Lamersdorf, 2004).
The correlations of gross nitrification with gross N mineralization and NH4+ pool MRT might indicate that nitrification in these sites depended on availability of NH4+ as substrate (autotrophic nitrification). The correlation between gross nitrification and available C also implied a possible conversion of organic substrate to NO3− with simultaneous release of available C (heterotrophic nitrification). De Boer & Kowalchuk (2001) have discussed in detail that acid-tolerant autotrophic nitrifiers and heterotrophic fungal nitrifiers can operate concurrently in acid forest soils. On the other hand, the similar pattern of NO3− and NH4+ immobilization across the N enrichment gradient precluded the assumption of microbial preference for NH4+, but showed that NH4+ and NO3− are immobilized simultaneously to meet microbial demand for N. Furthermore, the empirical relationship between NO3− pool MRT and abiotic conversion of NO3− to extractable organic N may indicate ecological importance, that the decrease in abiotic NO3− immobilization across the N enrichment gradient (Fig. 2) has in part contributed to the increase in NO3− pool MRT (Fig. 7) and NO3− losses (Table 1).
Conclusions
The key microbial processes that governed the fate of N occurred at much higher rates in the organic horizon than in the mineral soil, indicating the significance of the organic layer in these acidic spruce soils. Gross N mineralization and NH4+ immobilization rates increased from low to intermediate N enrichment, followed by a downturn in highly N-enriched sites. The increasing gross N mineralization rate up to the intermediate N enrichment level was paralleled with increasing available C, attesting to improved substrate quality in response to increasing N enrichment. Across low to intermediate N enrichment, the increase in available C drove faster turnover rates of the NH4+ pool and microbial N pool. Microbial assimilation and turnover rather than abiotic immobilization appeared to contribute largely to the retention of NH4+. The decrease in NH4+ transformation rates in highly N-enriched sites was attributed to retardation effect of excessive N levels on microbial decomposition, as a result of reduced microbial biomass and enzyme activity and changes in microbial community structure.
The patterns of gross nitrification and NO3− immobilization across the N enrichment gradient were similar to those of NH4+ transformation processes, but NO3− transformation rates were lower. In the low-N sites (with low to intermediate nitrification rates), NO3− immobilization was stimulated by the injected 15NO3−, indicating that NO3− (and NH4+) produced in the soil was insufficient to meet microbial demand for N. In sites with intermediate N enrichment (with the highest gross nitrification rates), NO3− immobilization rates did not exceed gross nitrification rates, indicating that chronic high NO3− deposition on already highly nitrifying sites may possibly result in NO3− excess relative to microbial demand for N. This was depicted by decreasing turnover rates of the NO3− pool across low to intermediate N enrichment. On the other hand, our study also showed that abiotic NO3− immobilization was an important process both in the organic horizon and in mineral soil, and that it decreased across the N enrichment gradient. The increasing NO3− deposition and decreasing turnover rates of the NO3− pool, combined with decreasing abiotic NO3− retention, possibly contributed to increasing NO3− leaching and gaseous emissions across the N enrichment gradient.
To our knowledge, our study is the first to report on mechanistic changes in the gross rates of soil N cycling and abiotic NO3− retention under ambient N enrichment gradient. The empirical relationships of changes in microbial N cycling across the N enrichment gradient might be integrated in models used to predict the responses of forest ecosystems (e.g. spruce) to increasing N deposition. Furthermore, other studies and our present study foster an increasing awareness of the significance of abiotic NO3− immobilization in influencing NO3− retention in forest soils. Whether the abiotically immobilized N will ultimately become available or will become part of the recalcitrant soil N sink deserves more attention.
Acknowledgements
This work was funded by the German Research Foundation (BR 1524/4-1). We acknowledge the cooperation of the Forest Research Stations of the states of Rheinland-Pfalz (Dr Joachim Block), Sachsen (Dr Henning Andreae), and Baden-Württemberg (Dr Klaus von Wilpert) for giving us access to the study sites and for willingly sharing the long-term N input–output data and other site characteristics. We also thank the two anonymous reviewers and Dr M. Francesca Cotrufo for the very positive reviews.




