Dynamics of nitrous oxide in groundwater at the aquatic–terrestrial interface
Abstract
Few data are available to validate the Intergovernmental Panel on Climate Change's (IPCC) emission factors for indirect emissions of nitrous oxide (N2O). In particular the N2O emissions resulting from nitrogen leaching and the associated groundwater and surface drainage (EF5-g) are particularly poorly characterized. In situ push–pull methods have been used to identify the fate of NO3− in the groundwater. In this study, we adapted a previously published in situ denitrification push–pull method to examine the fate of 15N2O introduced into the subsoil–groundwater matrix. Enriched 15N2O was manufactured, added to groundwater via a closed system in the laboratory, and then introduced into the groundwater–subsoil matrix in an upland-marsh transition zone of a salt marsh and a forested alluvial riparian zone. Conservative tracers (SF6 and Br−) and 15N2O were injected into the groundwater and left for 1–4 h after which the groundwater was sampled. Added 15N2O behaved in a conservative manner at one site while the other site showed variability with some injections showing significant consumption (3–8 μg N2O-15N kg−1 soil day−1) of 15N2O. Our results show that the fate and dynamics of N2O in groundwater are complex and variable and that these dynamics should be considered in the development of improved IPCC inventory calculations.
Introduction
The atmospheric concentration of nitrous oxide (N2O) has increased since preindustrial times and continues to do so. The United Nations Framework Convention on Climate Change (UNFCCC) established the Kyoto protocol, which requires participating countries to either reduce or take responsibility for their excess greenhouse gas (GHG) emissions. This necessitates signatories of the Kyoto protocol to develop and publish national inventories of anthropogenic GHG emissions on an annual basis. Guidelines for constructing national inventories have been prescribed by the Intergovernmental Panel on Climate Change (IPCC).
In the IPCC methodology, agricultural sources of N2O are partitioned into three categories: direct emissions from agricultural land, emissions from animal waste systems, and indirect emissions associated with nitrogen (N) that is removed in biomass, volatilized, leached, or exported from agricultural land (Mosier et al., 1998). These categories are thought to contribute an equal 1/3 share of the total estimated agricultural N2O source but with 2/3 of the uncertainty in the total agricultural source due to the wide range of estimates for indirect emissions (Nevison, 2000). Globally, the total agricultural N2O source equals 6.3 Tg N yr−1 (Mosier et al., 1998) with 2.1 Tg N yr−1 coming from indirect emissions. The predominate sources of the indirect emissions, over 75%, are associated with N leaching and runoff (Mosier et al., 1998; Nevison, 2000). N in leachate and runoff enters groundwater, riparian zones, wetlands, rivers, and oceans.
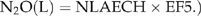
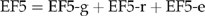
The default value for EF5 is 0.025 kg N2O-N kg−1 NLEACH (Mosier et al., 1998), with component values of 0.015 for EF5-g, 0.0075 for EF5-r and 0.0025 for EF5-e. The value for EF5- is based on observations of supersaturated concentrations of N2O in drainage waters due to leaching of N2O from soil toward groundwaters, or production of N2O in the groundwater via nitrification or denitrification, and the idea that this N2O eventually degasses to the atmosphere. In contrast with direct emissions, there are very few data available to validate the IPCC emission factors for indirect emissions (Groffman et al., 2002). The IPCC model for estimating N2O emissions from groundwater is highly uncertain because the controls on both N2O production and consumption in groundwater are not well understood. Nevison (2000) noted that the default value for EF5-g was based on a literature review of only six studies and concluded that the EF5-g default factor should perhaps be reduced to a value of 0.001. More recent studies have also highlighted uncertainties with the magnitude of the default factors that comprise the EF5. (Reay et al., 2003; Clough et al., 2006). We are not aware of any published studies that have tried to determine the potential for reduction of N2O in groundwater. It is clear that further studies are needed to examine the fate of N2O in groundwater.
In situ push–pull methods have been used to study the fate of nitrate (NO3−) and its potential denitrification rate in the subsoil–groundwater matrix (Istok et al., 1997; Addy et al., 2002), yet there has been no analysis of the fate of intermediate denitrification end-products such as N2O. Addy et al. (2002) used a tracer gas (sulfur hexafluoride, SF6) as a conservative tracer in their push–pull studies, suggesting that gases could be successfully traced with these methods. Few studies have directly measured N2O consumption rates in soils (Kroeze et al., 1989; Hénault et al., 2001; Mei et al., 2004) let alone the vadose zone. The use of 15N labeled NO3− can be used to determine the rate of N2O production. However, the determination of 15N2O consumption rates is hampered by ongoing production of 15N2O from the 15NO3− pool. Thus, only a net measure of N2O is achieved i.e. the difference between production and consumption of N2O. To accurately determine 15N2O consumption rates there is a need to decouple the production of 15N2O from the 15NO3− pool. Clough et al. (2005) recommended the use of 15N2O as a starting substrate in order to better identify the fate of the N2O molecule. The introduction of 15N2O into the subsoil–groundwater matrix, along with a conservative tracer, could potentially provide data on the fate and production of N2O belowground. Thus, the objectives of this study were to adapt the in situ push–pull method of Addy et al. (2002) to allow the introduction of dissolved 15N2O into the subsoil–groundwater matrix and to assess the potential for 15N labeled N2O consumption at two sites with differing denitrification potentials.
Materials and methods
Study sites and piezometers
Site A was located on the tidally influenced Brushneck Cove of Narragansett Bay, Warwick, RI (41°41′N, 71°24′W) and has been previously described in detail by Addy et al. (2005). In brief, the site was situated in the transition area between a salt marsh and an upland area with an average slope of 10%. There was no organic horizon present and tidal inundation is rare at this site. The soil was classified as a mixed Mesic Typic Psammequents (Soil Survey Staff, 1998) with vegetation dominated by marsh elder (Iva frutescens Pursh var. oraria), sea lavender (Limonium carolinianum), and seaside goldenrod (Solidago sempervirens L.).
A second-order tributary of the Pawcatuck river, ‘Meadow Brook,’ situated in Richmond, RI (41°29′N, 71°41′W) was the location of site B, previously described in detail by Kellogg et al. (2005). Briefly, this site was situated on an alluvial soil classified as a coarse-loamy Mesic Fluvaquentic Endoaquept (Soil Survey Staff, 1998), 7 m from the stream, with vegetation dominated by red maple (Acer rubrum L.), highbush blueberry (Vaccinium corymbosum L.), and summersweet (Clethra alnifolia).
Five years before this study, mini-piezometers [0.8 cm OD; 2 cm screen length (AMS, American Falls, ID, USA)] attached to Teflon tubing (0.7 cm OD) were installed at both field sites. One additional piezometer was installed at site B (replicate 1) immediately before the study, giving a total of three piezometers at each site. Details of the piezometer installation methodology have been reported on previously (Addy et al., 2002). The piezometers at sites A and B were at depths of 125 and 65 cm, respectively, with respective groundwater tables at depths of 90 and 50 cm. Piezometers at site A were 4 m apart while at site B the piezometers were 5 m apart.
Groundwater extraction, 15N2O labeling, and dosing
Groundwater was extracted from the piezometers at sites A and B using a Masterflex L/S portable peristaltic pump (Cole Parmer, Vernon Hills, IL, USA). This water (10 L aliquots) was brought from the field in plastic carboys and stored overnight at 4 °C until it was labeled with SF6. Labeling with SF6 was achieved by purging the water with an SF6 gas mixture (100 μL L−1 SF6 in He; Med-Tech, Medford, MA, USA) via a sparge stone for 15 min. This also served the purpose of lowering dissolved oxygen (DO) concentrations to ambient levels as noted by Addy et al. (2002). Immediately after this, 2 L aliquots of the SF6-dosed water were decanted into 2 L volumetric flasks and sealed with a Suba-seal. A 5 mL volume of potassium bromide (KBr) solution was then injected into the dose water within each 2 L volumetric flask to achieve a Br− tracer concentration of 33 mg L−1.
Water was then labeled with 15N2O that had been previously prepared in the laboratory by adding 15N labeled ammonium nitrate (NH415NO3, min. 98 + atom%15N, Sigma Aldrich, Auckland, New Zealand, Category No. 366536) to unenriched NH4NO3 and distilled water to form a well-mixed uniform salt solution containing NH415NO3 (34 atom%15N excess relative to N2 in air). This solution was then dried at 80 °C to an NH415NO3 salt. The NH415NO3 was then weighed out and placed in glass tubes, sealed and baked at 300 °C according to Friedman & Bigeleisen (1950) generating N2O. The mass of NH415NO3 used was adjusted so that the internal pressure inside the glass tubes did not exceed 2 atm. One glass tube was prepared for each dosing event per peizometer. The resulting N2O used in dosing the groundwater was enriched in 15N, 34.0 atom%15N excess relative to N2 in air [standard error of the mean (SEM) equaled 0.3, n= 9].
Labeling of the water with 15N2O was achieved by scoring the glass tube that contained the 15N2O with a metal file and placing it inside a silicone tube (12.8 mm ID, 2.4 mm wall thickness, Cole Parmer, Vernon Hills, IL, USA: EW-06411-19) that had been stored in a freezer such that one end of the silicone tube was already plugged with a 3 cm length of previously frozen deionized water. The open end of the silicone tube was then capped with a silicone turn-over flange stopper (12.7 mm diameter; Saint-Gobain Verneret, Charny, France: 467013-50). Working rapidly, the Suba-seal was then removed from the 2 L flask, the sealed silicone tube was then bent so that the internal glass tube snapped at the scored mark, releasing 15N2O inside the silicone tube. The silicone tube was then placed into the 2 L flask and the Suba-seal repositioned to seal the flask. Within minutes the ice plug inside the silicone tube melted and released the 15N2O into the dose water. The flask was gently shaken by hand for 2–3 min and then left overnight (12 h) at 4 °C for gases to equilibrate.
The 2 L flasks containing the dose water were transported to the field sites in coolers. At the field sites, the water table depth was recorded prior to pumping 1 L of water from the well. This was discarded before a further 150 mL ambient predose water sample was taken and placed in a nalgene screw-top bottle. These predose samples were transported back to the laboratory in a cooler and stored at 4 °C before chemical analysis. DO in the groundwater was measured on site using a portable DO kit (La Motte, Maryland, BA, USA). The dose water was then readied to be pumped into the peizometers. This was achieved by quickly swapping the Suba-seal on the flask with a rubber stopper fitted with stainless steel (SS) (6 mm OD, 4 mm ID) and copper (3 mm OD, 2 mm ID) tubes. The SS tube was the outlet port for the dose water and was connected to C-Flex tubing (4.8 mm ID; Cole Parmer HV-06424-15) that was then routed through the peristaltic pump to the air tight SS-sampling apparatus (Addy et al., 2002) that was in turn connected to the Teflon tube of the peizometer head (Fig. 1). The copper tubing was attached to a 10 L Tedlar bag filled with He at atmospheric pressure (Fig. 1). Helium entered the flask as the dose water was pumped out, thus preventing a vacuum being created inside the flask, which could lead to rapid degassing of the gases of interest from the dose water and/or the possible implosion of the flask. As the dose water was pumped (8 L h−1) into the well a subsample was taken of the dose water as follows. The first 20 mL of the dose water pumped was discarded to waste with the following 20 mL collected in a gas tight syringe fitted with a two-way stop-cock using the gas tight SS-sampling apparatus. Using this apparatus the pumped water flow could be directed either toward a luer-lok fitting attached to a gas syringe or to the well head. This water sample was then injected into a pre-evacuated (− 0.93 atm.) 160 mL serum bottle sealed with a rubber suba-seal. The serum bottle was inverted so that the water sample covered the septa. Then He gas from a Tedlar bag, was released into the inverted serum bottle via a hypodermic needle until atmospheric pressure was attained, as indicated by the cessation of bubbles coming from the hypodermic needle connected to the He supply. Water samples were then transported back to the laboratory in a cooler and kept at 4 °C until analysis, within 24 h.
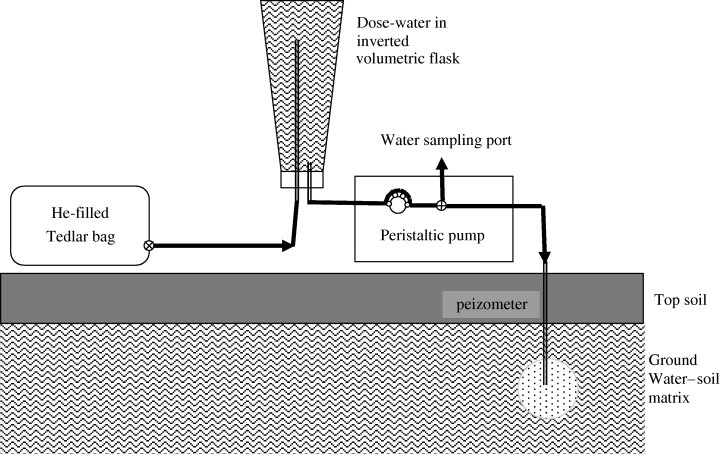
A schematic diagram of the field experimental set up. Showing the dose water flask connected to the He replacement gas and the peristaltic pump in the dose position. During sampling the peristaltic pump was positioned on the other side of the water sampling port.
After the dose water had been pumped into the soil-groundwater matrix it was left to incubate for a total time of 1.5 and 4.3 h at sites A and B, respectively. These incubation times were based on previously measured denitrification rates and tracer recoveries at these sites (Addy et al., 2002; Kellogg et al., 2005). Following the incubation period the dose water was slowly ‘pulled’ out of each peizometer, using the peristaltic pump (6 L h−1). Samples of the extracted groundwater were taken and treated in an identical manner to the water samples previously described. Six samples were taken from each piezometer when the cumulative volume of water extracted reached 0.22, 0.46, 0.70, 0.94, 1.48, and 2.02 L. Site A was dosed and sampled once, on August 19, 2005, while site B was dosed and sampled on two occasions, August 22 and 29, 2005. These sampling dates at site B are subsequently referred to as site B test-1 and site B test-2, respectively.
Analytical procedures
The headspaces of the serum bottles containing the water samples were analyzed for N2O and SF6 concentrations within 12 h. The bottles were brought to room temperature (22 °C) over 1 h, which reduced the ability of the water sample to contain dissolved gases and enhanced the concentration in the headspace. The serum bottles were then shaken vigorously for 1 min to bring water and headspace gases to equilibrium. A 0.1 mL gas sample was then taken and manually injected directly into a Hewlett Packard (Houston, TX, USA) 5890 gas chromatograph (GC) equipped with an electron capture detector (350 °C) fitted with a 5.5 m Poropak Q 50/80 mesh column, N2 carrier gas and a make up gas consisting of Ar (95%) and CH4 (5%). Retention times for N2O and SF6 were 10.5 and 13.7 min, respectively. Reference gas samples were made from gas bottles of known concentration (Med-tech., Meford, MA, USA). A further 15 mL gas sample was also taken from the headspace of the serum bottle, using a 20 mL glass syringe equipped with a three-way tap to prevent the ingress of atmospheric air, and placed in a pre-evacuated 12 mL Exetainer® (Labco Ltd, High Wycombe, UK). This sample was used for determining the 15N enrichment, of the N2O using an automated isotope ratio mass spectrometer (PDZ-Europa Ltd. 20–20, Crewe, UK) as described by Stevens et al. (1993). These gas samples were brought to ambient pressure before analysis by using a double-ended needle and a beaker of water. One needle was consistently placed at a constant depth in the water while the other pierced the Exetainer®. Upon the cessation of gas bubbles entering the water, the sample was considered to be at atmospheric pressure. The N2O reference gas (BOC gases) had an 15N enrichment equal to 0.02122 atom%15N excess relative to ambient N2 in air.
Concentrations of the N2O and SF6 in the water samples were calculated using the headspace gas concentrations, appropriate Bunsen coefficients (Wilhelm et al., 1977; Weiss & Price, 1980) and the equations of Davidson & Firestone (1988). Once headspace gas samples had been taken, the water samples were returned to the 4 °C cooler until the analysis for Br− was performed as described below.
Water samples were analyzed for dissolved organic carbon (DOC), pH, electrical conductivity (EC), Br−, sulfate (SO42−), chloride (Cl−), and NO3− concentrations. Groundwater pH was measured in the field with a hand held meter (Mettler Toledo, Greifensee, Switzerland). The DOC analyses were performed on a Shimadzu organic carbon analyzer fitted with a Shimadzu ASI-5000A autosampler (Shimadzu Scientific Instruments, Columbia, MD, USA). Water sample EC was determined with a conductivity meter at 25 °C (Hanna Instruments, Woonsocket, RI, USA). Ammonium analyses were performed using a flow injection analyzer (Alpkem FS3000 twin channel analyzer, Pulse Instrumentation Ltd, Saskatoon, Canada; application notes P/N A002380 and P/N A002423). Anion analyses were performed using an ion chromatograph (Dionex DX-120 with an AS50 autosampler, Sunnyvale, CA, USA).
Reduction rates of 15N2O, were calculated using the method described by Addy et al. (2002). These calculations utilized data from the water samples pulled at 0.22 and 0.46 L and are based on the difference in N2O concentration and 15N enrichment between the two water samples. Assumptions included a soil bulk density of 1.65 kg m−3 and a porosity of 0.38 m3 pores m−3 soil, as previously reported for this site (Kellogg et al., 2005). Statistical analyses were performed using minitab® statistical software (Minitab, 2000).
Results
Ambient groundwater concentrations of NO3−, NH4+, and Br− were low or undetectable at both sites (Table 1). The groundwater at site B contained more DOC (P < 0.01) and lower DO (P < 0.01) than site A. Dissolved SO42− concentrations were higher at site A (P < 0.01) than site B, while Cl− concentrations did not differ between sites (Table 1). Salinity at site A was four times higher than at site B (Table 1), a reflection of groundwater mixing with sea water that had infiltrated the aquifer in the transition zone, producing groundwater of intermediate salinity (Moore, 1999). Ambient N2O concentrations in the groundwater averaged 4 μg L−1 (Table 1) and were above saturation (0.6 μg L−1), with ambient 15N2O enrichments at site A and B of 0.203 and 0.295 atom%15N excess (relative to ambient N2 in air), respectively. Ambient dissolved CH4 concentrations were significantly higher (P < 0.01) at site B (Table 1).
| Site characteristics | Site A:Warwick | Site B:MeadowBrook |
|---|---|---|
| Depth of mini-peizometers (cm) | 125 | 65 |
| Water-table depth (cm) | 90 | 50 |
| Ground water temperature (°C) | 12 | 13 |
| Dissolved oxygen (mg L−1) | 3.2 | < 1 |
| Dissolved organic carbon (mg L−1) | 2.2 (0.2) | 9.1 (1.4) |
| CH4 (μg L−1) | 5 (1) | 333 (154)–415 (120) |
| N2O (μg L−1) | 4.0 (0.4) | 4.1 (0.2) |
| NO3-N (mg L−1) | 0 | 0 |
| NH4-N (mg L−1) | 0.07 (0.02) | 0.06 (0.03) |
| Br− (mg L−1) | 0.07 (0.03) | 0.27 (0.11) |
| Cl− (mg L−1) | 7.1 (0.5) | 6.3 (0.5) |
| SO42−-S (mg L−1) | 3.8 (0.2) | 0.8 (0.2) |
| PO42−-P (mg L−1) | < 0.1 | < 0.1 |
| Electrical conductivity (mS m−1) | 20.3 | 5.6 |
| Soil texture | Fine sand | Coarse-loamy alluvium |
- Further site details are presented in the text.
When averaged across all nine push–pull tests that were performed the mean (SEM, n= 9) Br− and SF6 concentrations in the initial dose solutions were 33.2 (0.6) mg L−1 and 1.5 (0.1) μg L−1, respectively. Initial concentrations of dissolved N2O in the dose water were well in excess of ambient concentrations and averaged (SEM, n= 3) 150 (44), 65 (12), and 70 (44) μg L−1 for site A, site B test-1, and site B test-2, respectively. The mean 15N enrichment of the N2O gas in the initial dose solutions was as noted above 34.0 atom%15N excess relative to N2 in air.
Concentrations of CH4 in the ambient water at sites A, site B test-1, and site B test-2 were 5 (1), 415 (120), and 333 (154) μg L−1, respectively, (SEM, n= 3). At site B the CH4 concentrations in replicate three were much lower than in replicates one and two, leading to high variability at this site. Immediately before ‘pushing’ the mean (SEM, n= 3) dose water contained CH4 concentrations of 3 (1), 12 (1), and 10 (4) μg L−1 at sites A, site B test-1, and site B test-2, respectively.
After incubation of the pushed dose water in the soil–groundwater matrix the dose-water was withdrawn. There was one particular well where equal volumes of gas were withdrawn along with the water sample during the pull phase of the test. This was at site B, replicate 3, during both tests.
The relationship between C/Co, where C is the pulled groundwater concentration and Co is the original pushed groundwater concentration, and V/Vt, where V is the cumulative volume pulled and Vt is the total volume of water pushed, was plotted for Br− at site A (Fig. 2) and site B (Fig. 3a–c). Recovery of the conservative Br− tracer, averaged (SEM, n= 3) over the three peizometers, was 64 (4)%, 67 (6)%, and 67 (3)% for the push–pull tests at site A, site B test-1 and site B test-2, respectively (SEM in brackets, n= 3). The concentration of Br− in the recovered pull samples had decreased to an average (SEM) 10.7 (0.6) mg L−1 in the final aliquot of the pull phase.
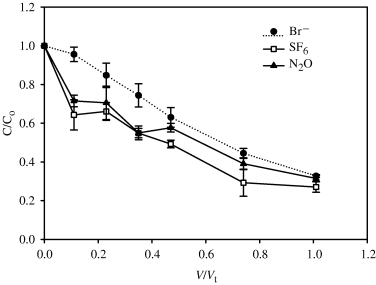
Relationship between C/Co and V/Vt, at site A, for bromide (Br−), nitrous oxide (N2O) and sulfur hexafluoride (SF6). The ratio C/Co is the ratio of the pulled ground water concentration (C) to the original pushed dose water concentration (Co). The ratio V/Vt is the cumulative volume pulled (V) to the total volume of the pushed water (Vt). Error bars are the standard error of the mean (n= 3).
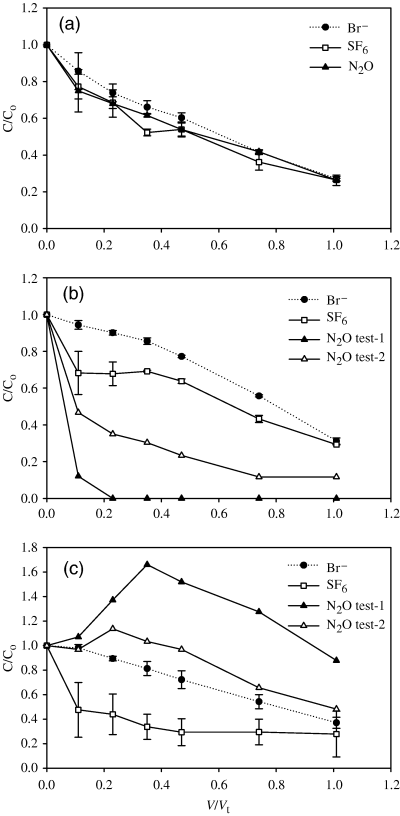
Relationship between C/Co and V/Vt, at site B, for bromide (Br−), nitrous oxide (N2O) and sulfur hexafluoride (SF6). The ratio C/Co is the ratio of the pulled ground water concentration (C) to the original pushed dose water concentration (Co). The ratio V/Vt is the cumulative volume pulled (V) to the total volume of the pushed water (Vt). Graphs (a–c) represent replicates 1–3, respectively. Replicates 1–3 produced varying N2O results but consistent Br− and SF6 results. In graphs (a–c) the data points for the SF6 and Br− are the means of both tests 1 and 2 (n= 2) with error bars the standard error of the mean. Similarly in graph (a) the N2O data points are the mean (n= 2) of both tests 1 and 2 with error bars the standard error of the mean. However, in graphs (b) and (c) N2O data points are from individual tests as noted in the legend.
The conservative tracer SF6 behaved in a similar manner to the Br− anion (2, 3) but with lower average (SEM, n= 3) recoveries, 60 (1)%, 58 (11)%, and 65 (5)% for the push–pull tests at site A, site B test-1, and site B test-2, respectively. Of note however was the divergence between the two conservative tracers at site B, replicates 2 and 3, which was in contrast to site A and replicate 1 at site B. The recovery of N2O, based upon C/Co, was extremely variable and averaged (SEM, n= 3) 55 (3)%, 63 (36)%, and 57 (17)% for the push–pull tests at site A, site B test-1 and site B test-2, respectively. This variability was due to both intersite variability, between sites A and B, and intrasite variability at site B. At site A the mean N2O C/Co values closely tracked those of the mean C/Co values for the conservative tracers (Fig. 2). At site B, replicate 1 behaved in a conservative manner, similar to site A with N2O C/Co values closely tracking the conservative tracers Br− and SF6 (Fig. 3a). However, in replicate 2 at site B the N2O C/Co values decreased rapidly with respect to the conservative tracers during both tests (Fig. 3b) while for replicate 3 the N2O C/Co values increased over and above those of the conservative tracers for both tests. N2O concentrations in the final water sample withdrawn averaged (SEM, n= 3) 46 (13), 25 (15), and 32 (10) μg L−1 for sites A, B test-1 and B test-2, respectively.
There was no significant decrease in the 15N enrichment of the N2O during incubation for all replicates at site A and for replicates 1 and 3 at site B (Fig. 4). However, for replicate 2 at site B the 15N enrichment of the N2O decreased exponentially in both dosings, as V/Vt increased, to a mean of 1.634 atom%15N excess relative to N2 in air (Fig. 4).
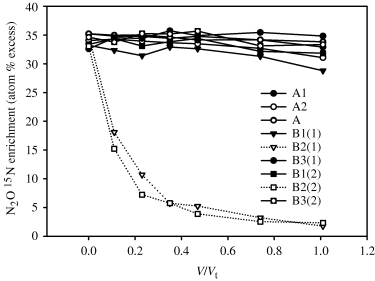
Relationship between the N2O 15N enrichment (atom%15N excess relative to ambient N2 in air) of the sampled waters and V/Vt is shown for each of the nine dosings that were performed, where V is the cumulative volume pulled and Vt is the total volume of the pushed water. Data points are from individual replicates. Legend shows site (A or B) with the following numeral indicating the replicate while the numeral in brackets indicates either test 1 or test 2 at site B.
Denitrification rates, i.e. the N2O reduction rates, were only determined where N2O concentrations decreased significantly, (i.e. for site B, replicate 2, and equated to 8 and 3 μg N2O-15N kg−1 soil day−1 for tests 1 and 2, respectively).
Dissolved CH4 concentrations in the pulled samples varied between sites (Fig. 5) with concentrations in the final aliquots sampled of 3 (1), 256 (37), and 199 (33) μg L−1 for sites A, site B test-1, and site B test-2, respectively. Thus, there was no significant change in the C/Co ratio for dissolved CH4 at Site A. However, at site B the dissolved CH4 concentrations increased significantly as the cumulative volume of water pulled increased (Fig. 5), although these concentrations were still below ambient levels recorded at the start of the experiment.
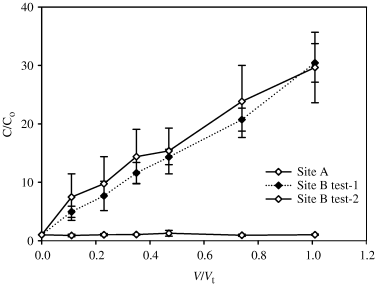
Relationship between C/Co and V/Vt, for dissolved methane (CH4) from site A to site B during tests 1 and 2. C is the pulled ground water CH4 concentration, Co is the original pushed dose water CH4 concentration, V is the cumulative volume pulled and Vt is the total volume of the pushed water. Data points are the average of three replicates with error bars the standard error of the mean.
Discussion
There are several possible fates for N2O injected and incubated in the subsoil–groundwater matrix in terms of both concentration and 15N enrichment (Table 2). These fates range from conservation of the added N2O, [i.e. C/Co behaves in a similar manner as the conservative tracers over time, with constant N2O 15N enrichment (scenario A, Table 2), to a decrease in both the N2O C/Co ratio and its 15N enrichment (scenario E, Table 2)].
| Scenario | Initial 15N enrichment | C/Co for N2O relativeto tracer | Potential reason(s) |
|---|---|---|---|
| A | Maintained | Conservative | No 15N2O reduction occurringNo advective influx of ambient N2O orin situ N2O production |
| B | Maintained | Decreasing over time | 15N2O reduction occurringNo advective influx of ambient N2O orin situ N2O production |
| C | Decreasing with time | Increase over time | Ambient N2O input rate(s) (advective influx or in situ production) > reduction rate of incubated 15N2O |
| D | Decreasing with time | Conservative | Ambient N2O input rate(s) (advective influx or in situ production) equal reduction rate of incubated 15N2O |
| E | Decreasing with time | Decreasing over time | Ambient N2O input rate(s) (advective influx or in situ production) < reduction rate of incubated 15N2O |
- The assumption is made that the associated tracers (SF6 and Br−) shows good recovery in all cases, behaving in a conservative manner.
The data from sites A and site B, replicate 1, show that the N2O concentration behaved in a conservative manner with no significant decrease in the 15N enrichment of the N2O (Fig. 4), (i.e. scenario A in Table 2). We know that the ambient groundwater contained some antecedent N2O but the N2O concentration of this groundwater was insignificant when compared with the added 15N labeled N2O. Therefore, hydrodynamic dispersion or advective groundwater flow that occurred, either during the actual dosing event or during the incubation period, did not significantly affect the N2O 15N enrichment. Had we used a lower concentration of N2O or 15N enrichment in our original dose water then we may have observed a significant decrease in 15N enrichment.
For site B, replicate 2, we observed a different result with both the N2O concentration and 15N enrichment decreasing more rapidly than the conservative tracers. This leads us to consider scenario E in Table 2 where a decrease in 15N enrichment must be due to either an advective influx or in situ production of N2O. While a higher advective flux is possible at this replicate, a comparison of the recovery of the conservative tracers at site B replicates 1 and 2 suggests that groundwater conditions were similar between these two replicates and that in situ denitrification was responsible for both the depletion of the 15N2O enrichment as well as the overall decline in the N2O concentration. Site B, replicate 2, appears to be a relative ‘hot-spot’ for denitrification. We assume N2O production did not occur via nitrification due to the low DO concentrations and the lack of any measured NH4+ substrate. This assumption could easily be verified by using 15NH4+ as a substrate in future studies.
At site, B replicate 3, we observed marked increases in N2O concentration, (i.e. C/Co, increases, but the 15N enrichment of the N2O remained constant). The increase in N2O concentration would suggest that active net production of N2O was occurring, but the constant 15N enrichment is difficult to explain. It is possible that production of low enrichment N2O was balanced by isotopic discrimination in N2O reduction, resulting in no net change in the 15N enrichment of N2O in the incubation. A further compounding factor at this peizometer was the occurrence of significant gas bubbles, during the extraction of the dose water after its incubation. This gas had a negligible N2O content but a considerable CH4 concentration. In theory it is possible that the gas bubble presence caused an error in the calculations of V, but had this been the case the results for the tracer (e.g. Br− would have been erroneous and this was not the case). We do not believe that this particular result draws the method into question since another eight piezometers were successfully sampled providing data that was interpreted in a sensible and logical fashion. However, further field work is required to fully understand the processes at site B, replicate 3.
Our CH4 data suggest that conditions are sufficiently anaerobic at site B to support production of this important GHG. At site A, ambient groundwater concentrations of CH4 were relatively low and there was no change over the course of the incubation. However, at site B, the process of SF6 labeling stripped out the high ambient CH4 concentrations in the dose water so that when the dose water was injected at the start of the incubation there was a large differential between the CH4 concentration in the dose water and the ambient groundwater. The increase in dissolved CH4 concentration in the pulled water samples (Fig. 5) was likely driven by diffusion of CH4 from the surrounding groundwater matrix. The differences in CH4 dynamics between sites A and B are consistent with the differences in ambient DO levels between the sites and support the results showing that some peizometers at site B are located in denitrification hotspots (Yu & Patrick, 2004).
A previous study in the fringe area of site A recorded a denitrification rate of 2 μg NO3−N kg−1 soil day−1 following the addition of NO3− (Addy et al., 2002), while a previous study at site B has measured much higher but more variable rates of NO3− denitrification (61–40 μg NO3−N kg−1 soil day−1 at 65 cm depth, (Kellogg et al., 2005). However, these previous studies did not present information on the relative production of N2O and N2 production during denitrification. While the relative N2O reduction rates in the present study are consistent with these previous studies (site B higher than site A), the rate of N2O reduction that we measured at site B is much lower than the total denitrification rates measured in the previous study. The magnitude of the N2O reduction rates that we measured are also considerably lower than rates measured in surface soils. Hénault et al. (2001) reported a lag phase of 48 h in a gley soil before N2O was reduced, with measured N2O reduction rates from a variety of soils that were > 3360 μg N kg−1 soil day−1 while Blackmer & Bremner (1976) measured N2O consumption rates > 570 μg N kg−1 soil day−1. A possible reason for the low N2O reduction rates that we observed could be the time required for the denitrifying community to generate N2O reductase. Although there were ambient levels of N2O present in our sites, the higher concentrations injected with the dose water may not have been able to be immediately processed by the denitrifier community. Other studies have shown the denitrification enzymes and communities to be highly responsive to factors such as the temperature and water regime and carbon availability (Chèneby et al., 1998; Hénault et al., 2001) and in some instances denitrification may reduce NO3− in preference to N2O. Previous studies have observed significant lag times between addition of NO3− and denitrification activity (Aelion & Shaw, 2000; Addy et al., 2002). A similar lag may also occur for N2O. If so, it may be necessary to expose the peizometers to elevated N2O to condition the microbes to record true potential N2O reduction rates.
The divergence of the conservative tracers at site B, replicates 2 and 3, could possibly have been due to the relative physical states of the tracers and their respective interactions with the soil–groundwater matrix. It is possible that the peizometers at replicates 2 and 3 were in a soil matrix that was less dense or denser than the other peizometers. Thus, the resulting physical turbulence or mixing of the dose water with the groundwater may have resulted in the gas tracer behaving differently to the anion tracer as a result of varying pressure during injection.
Further modifications of this method are possible to facilitate measurement of N2O dynamics at multiple sites. While we used highly enriched 15N2O, the use of N2O that is closer to levels of natural abundance 15N enrichment could be used if there was a sufficient difference between the 15N enrichment of any dissolved ambient N2O and the N2O supplied in the dose water. When ambient N2O concentrations are low, it may even be possible to use commercially manufactured N2O, with a sufficiently different 15N signature from that of the ambient dissolved N2O, so that the dose water could be simultaneously labeled with SF6 and N2O by bubbling a tank-gas mixture of these gases through the dose water for a suitable period. While we have used a 2 L dose water volume there is the potential for a greater volume of N2O labeled dose water to be used (e.g. 10 L as used by Addy et al., 1999). This would allow the integration of N2O dynamics to occur over a greater volume of soil and reduce the potential impact of ambient groundwater diffusing into the dose plume. This could be achieved by replacing the flask holding the dose water with a gas impermeable bag. Then there would also be no requirement for the He gas, as the gas impermeable bag would deflate as the dose water was injected into the groundwater. A further modification could be the inclusion of a pressure gauge to note the pressure of the dose water as it is injected into the groundwater. This could indicate the relative densities of the soil matrix. Modified methods that allow for collection of data at multiple sites could allow for information on N2O dynamics that could be scaled to address questions about the importance of these dynamics to IPCC inventories.
Acknowledgements
Financial assistance for T. J. C. in the form of the ‘America/New Zealand Soil Science Professional Exchange Program Award,’ a ‘Trimble Agricultural Research Fellowship,’ and a grant from the ‘Lincoln University Foundation,’ are gratefully acknowledged and without which this work would not have been possible. T. J. C. would also like to thank staff at both the University of Rhode Island and the Institute of Ecosystem Studies for useful discussions.




