Interannual variation and climatic regulation of the CO2 emission from large boreal lakes
Abstract
We studied the interannual variation of surface water partial pressure of CO2 (pCO2) and the CO2 emissions from the 37 large Finnish lakes linking them to the water quality, catchment and climate attributes in 1996–2001. The lake water CO2 was measured three times a year in the study lakes in 1998 and 1999 and for the rest of the years the CO2 was modeled by measured alkalinity. The median annual CO2 emission to the atmosphere ranged between 1.49 and 2.29 mol m−2 a−1. The annual CO2 emission followed closely the annual precipitation pattern with the highest emission during the years when the precipitation was highest (r2=0.81–0.97, P<0.05). There was a strong negative correlation (r2=0.50–0.82, P<0.001) between O2 and CO2 saturation in the lake water during stratification suggesting effective decomposition of organic matter in the lakes. Furthermore, total phosphorus and the proportion of agricultural land in the catchment had significant positive correlations with CO2 saturation.
Introduction
Climate change will affect the northern hemisphere in several ways. Scenarios predict increasing temperature and precipitation for the boreal region (IPCC, 2001). Rising rainfall might enhance the transport of organic matter produced in the terrestrial ecosystem to the freshwaters. The warmer climate may also increase primary production in lakes and on the other hand, accelerate decomposition processes.
Transport of dissolved organic carbon (DOC), from terrestrial to aquatic environments has been suggested to be an important source of carbon in aquatic ecosystems and also a driving force for the CO2 supersaturation of lakes with respect to the atmosphere (Kling et al., 1991; Cole et al., 1994; Hope et al., 1996). Direct evidence of the importance of terrestrial carbon in metabolism of boreal lakes was given by del Giorgio et al. (1999) who showed that in oligotrophic waters the balance of phytoplankton production and community respiration (P/R ratio) is always below unity, suggesting that the systems are heterotrophic and subsidized by allochthonous organic matter. Jonsson et al. (2001) showed that in a boreal humic lake, primary production contributed at most 5% of the total organic carbon (TOC) input and about 20% of the TOC mineralization. The interannual variation in the leaching of TOC from Finnish forest/peatland dominated catchments often exceeds the spatial variation in TOC transport (Kortelainen et al., 1997), which can be reflected in interannual variation of CO2 concentrations in downstream lakes.
In Finland, the small lakes (<100 km2) have been shown to be strongly supersaturated with CO2 relative to the atmosphere (Kortelainen et al., 2000; Striegl et al., 2001). These lakes are typically colored with humic matter and have high TOC concentrations. Huttunen et al. (2002) observed 30 times higher CO2 fluxes from a pond surrounded by peatlands compared with a pond situated in the mineral soil catchment in Finland. In other countries, diel or seasonal variability of CO2 concentrations in surface water has been studied mainly in a few small lakes (e.g. Kratz et al., 1997; Cole & Caraco, 1998; Striegl & Michmerhuizen, 1998; Riera et al., 1999) and the catchment and climate regulation of partial pressure of CO2 (pCO2) in 33 small lakes (Sobek et al., 2003). Interannual variation of pCO2 has been studied by Kelly et al. (2001) concentrating mainly on small lakes. They suggested that weather patterns are important in determining the pCO2 in lakes but found no significant correlation between annual precipitation and pCO2 in their lakes.
This study is based on the 37 large Finnish lakes that cover 43% of the total lake area of Finland and their catchments altogether 137 000 km2, 45% of the total area of Finland. Consequently, their impact on the regional aquatic carbon cycle can be expected to be significant. We linked the surface water CO2 to water quality, catchment and climate attributes. The lakes were investigated over 6 years in order to assess the amount of interannual variation of CO2 in surface waters and further, to find indications of how the changing climate might effect CO2 emissions from large boreal lakes. We show that the annual CO2 emissions from the lakes closely follow the regional long-term precipitation patterns.
Materials and Methods
Study sites and sampling
Altogether 37 of the lakes with lake area greater than 100 km2 were included in this study excluding, however, the artificial reservoirs Lokka and Porttipahta. These lakes are mainly situated in central and eastern Finland (Fig. 1) and their total surface area is 14 128 km2. Water quality of the lakes ranges from oligotrophic to eutrophic and from clear water to humic. Most of the lakes are oligotrophic and the concentrations of organic carbon in the lake water (Table 1) are comparable with smaller lakes (Rantakari et al. 2004). The lakes were sampled three times a year, at the end of the winter stratification (approximately at the end of March), at the end of the summer stratification in August and at fall circulation in October–November in 1996–2001. The profile samples in winter (March) and in summer (August) were taken at the deepest point of the lakes at 5 m depth intervals with the first sample from the depth of 1 m and the last sample 1 m above the sediment. In fall overturn (October–November), the samples were taken from the middle of the water column. In some of the largest lakes there were multiple sampling points (Saimaa 9, Oulujärvi 3, Päijänne 2, Inarijärvi 2, Kallavesi 2 and Viinijärvi 2). All of the sampling points, however, represent the deepest sedimentation areas in the pelagial region. Catchment areas of the lakes were determined on topographic maps and the catchment boundaries digitized using the Arc View georeferencing software. Digitized catchment boundaries were then combined with land use data based on satellite images automatically with ArcInfo software. Proportions of agricultural land, built-up areas (buildings and roads), peatland, forest and water were determined as well as catchment to the lake area-ratio (CA/LA) (Table 2).
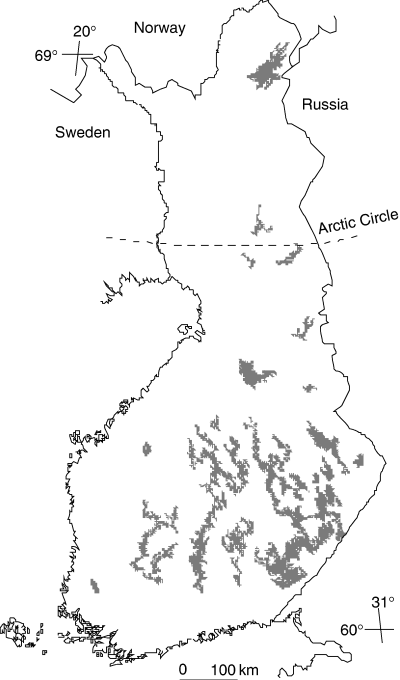
The 37 lakes in Finland having surface area greater than 100 km2.
| Lake area (km2) | Alkalinity (mmol L−1) | pH | Ntot (μg L−1) | Ptot (μg L−1) | TOC (mg L−1) | O2 saturation (%) | |
|---|---|---|---|---|---|---|---|
| Kolimajärvi | 101 | 0.170 (0.160–0.190) | 7.1 (7.0–7.1) | 420 (390–460) | 8.0 (7.0–9.0) | 8.1 (7.3–8.9) | 90 (88–90) |
| Unnukka | 103 | 0.190 (0.180–0.210) | 7.3 (7.2–7.4) | 490 (420–520) | 15 (12–17) | 8.1 (7.2–9.1) | 88 (85–92) |
| Pielavesi | 111 | 0.170 (0.160–0.170) | 7.1 (7.0–7.2) | 400 (390–400) | 7.5 (7.0–8.0) | 6.9 (6.7–7.0) | 87 (86–88) |
| Vesijärvi | 111 | 0.500 (0.490–0.510) | 7.6 (7.5–7.6) | 370 (360–380) | 20 (19–21) | 4.5 (4.5–4.5) | 91 (91–92) |
| Keurusselkä | 118 | 0.110 (0.100–0.120) | 6.7 (6.7–6.8) | 460 (430–500) | 18 (13–24) | 11 (9.9–12) | 88 (85–89) |
| Onkivesi | 120 | 0.200 (0.170–0.220) | 7.1 (6.9–7.3) | 750 (680–810) | 60 (49–76) | 12 (10–13) | 83 (80–87) |
| Pyhäjärvi Pirkkala | 124 | 0.360 (0.350–0.370) | 7.3 (7.3–7.4) | 560 (520–640) | 27 (24–30) | 7.0 (6.5–7.6) | 87 (86–89) |
| Pyhäjärvi Pyhäselkä | 126 | 0.160 (0.150–0.170) | 7.1 (6.8–7.2) | 290 (270–310) | 7.8 (6.0–9.0) | 6.6 (5.9–7.1) | 93 (91–94) |
| Kyyvesi | 133 | 0.160 (0.150–0.170) | 7.0 (6.9–7.1) | 420 (400–450) | 11 (10–13) | 9.5 (8.7–12) | 91 (90–92) |
| Lappajärvi | 142 | 0.190 (0.185–0.200) | 7.1 (6.7–7.5) | 540 (470–750) | 25 (19–36) | 11 (10–12) | 90 (87–93) |
| Viinijärvi 1 | 148 | 0.240 (0.210–0.260) | 6.9 (6.6–7.3) | 280 (240–310) | 8.0 (6.0–10) | 4.5 (3.2–5.7) | 75 (61–89) |
| Viinijärvi 2 | 148 | 0.210 (0.190–0.230) | 7.2 (7.1–7.3) | 330 (310–340) | 13 (11–14) | 6.6 (6.1–7.2) | 92 (90–93) |
| Kivijärvi | 153 | 0.130 (0.120–0.140) | 6.9 (6.8–7.0) | 420 (390–450) | 9.0 (7.0–12) | 8.3 (7.4–8.9) | 89 (87–91) |
| Kiantajärvi | 153 | 0.130 (0.110–0.140) | 6.7 (6.1–7.2) | 300 (250–350) | 11 (8.0–15) | 7.4 (7.0–7.7) | 89 (79–91) |
| Pyhäjärvi Eura | 154 | 0.350 (0.320–0.390) | 7.4 (7.2–7.5) | 430 (330–500) | 21 (16–28) | 5.2 (5.2–5.2) | 92 (88–95) |
| Juurusvesi | 159 | 0.160 (0.150–0.170) | 7.0 (6.7–7.2) | 510 (470–530) | 16 (13–18) | 8.5 (8.0–9.3) | 85 (82–90) |
| Melavesi | 159 | 0.240 (0.200–0.260) | 7.1 (6.4–7.4) | 340 (290–440) | 13 (9.0–16) | 5.5 (5.0–6.7) | 79 (51–93) |
| Koitere | 164 | 0.064 (0.06–0.07) | 6.7 (6.5–6.8) | 280 (270–300) | 8.0 (7.0–9.0) | 7.9 (7.6–8.7) | 91 (88–95) |
| Nilakka | 168 | 0.160 (0.150–0.170) | 7.3 (7.1–7.5) | 340 (330–370) | 12 (10–17) | 7.8 (7.0–8.8) | 90 (88–93) |
| Vanajavesi | 179 | 0.470 (0.440–0.500) | 7.5 (7.4–7.6) | 860 (680–1070) | 34 (31–40) | 7.9 (7.5–8.5) | 89 (88–91) |
| Längelmävesi | 180 | 0.190 (0.180–0.210) | 7.2 (7.1–7.2) | 380 (330–470) | 15 (14–18) | 5.6 (5.4–5.9) | 91 (89–92) |
| Konnevesi | 187 | 0.170 (0.160–0.190) | 6.9 (6.5–7.0) | 350 (340–380) | 5.2 (4.0–6.0) | 5.7 (5.5–5.9) | 82 (62–92) |
| Juojärvi | 228 | 0.100 (0.100–0.110) | 7.1 (7.1–7.1) | 360 (340–370) | 5.5 (5.0–6.0) | 5.2 (5.2–5.2) | 92 (90–94) |
| Yli-Kitka | 240 | 0.260 (0.250–0.260) | 7.3 (7.2–7.2) | 180 (170–190) | 6.0 (4.0–8.0) | 3.6 (3.3–3.8) | 91 (90–92) |
| Pyhäjärvi Kitee | 248 | 0.210 (0.210–0.220) | 7.2 (7.0–7.3) | 220 (200–240) | 6.0 (5.0–8.0) | 3.8 (3.1–4.4) | 93 (91–97) |
| Näsijärvi | 265 | 0.140 (0.130–0.140) | 6.9 (6.9–7.0) | 430 (410–450) | 8.2 (7.0–9.0) | 7.9 (7.3–8.7) | 89 (87–92) |
| Suvasvesi | 276 | 0.180 (0.160–0.190) | 6.7 (6.5–7.2) | 520 (500–550) | 6.2 (6.0–7.0) | 6.2 (5.7–7.1) | 74 (63–85) |
| Höytiäinen | 291 | 0.190 (0.180–0.200) | 6.9 (6.7–7.2) | 380 (350–410) | 5.2 (4.0–6.0) | 5.6 (5.1–6.3) | 92 (91–94) |
| Kemijärvi | 316 | 0.320 (0.240–0.370) | 7.4 (7.2–7.5) | 280 (260–310) | 13 (11–16) | 7.4 (5.5–11) | 91 (88–93) |
| Koirus | 316 | 0.200 (0.180–0.210) | 7.3 (7.1–7.4) | 500 (460–540) | 16 (13–18) | 8.1 (7.3–9.0) | 90 (86–93) |
| Puulavesi | 325 | 0.160 (0.150–0.160) | 6.9 (6.6–7.1) | 370 (320–480) | 4.7 (6.6–7.1) | 5.8 (5.5–6.5) | 90 (90–91) |
| Keitele | 502 | 0.160 (0.150–0.170) | 6.7 (6.4–7.0) | 340 (300–390) | 5.6 (12–15) | 6.3 (5.1–7.9) | 80 (64–91) |
| Kallavesi 1 | 517 | 0.180 (0.160–0.190) | 7.2 (6.8–7.4) | 590 (480–660) | 13 (12–15) | 10 (7.5–19) | 87 (84–91) |
| Kallavesi 2 | 517 | 0.210 (0.190–0.220) | 7.2 (7.0–7.3) | 650 (570–760) | 27 (24–33) | 10 (8.9–11) | 84 (80–89) |
| Oulujärvi 1 | 865 | 0.140 (0.130–0.160) | 6.8 (6.4–7.0) | 310 (260–390) | 16 (12–22) | 7.2 (6.5–7.6) | 91 (90–93) |
| Oulujärvi 2 | 865 | 0.140 (0.130–0.140) | 7.1 (6.7–7.5) | 360 (350–360) | 14 (12–16) | 8.4 (8.1–8.7) | 91 (90–92) |
| Oulujärvi 3 | 865 | 0.140 (0.130–0.140) | 6.9 (6.7–7.1) | 350 (320–370) | 17 (16–18) | 7.8 (7.7–7.9) | 92 (92–92) |
| Pielinen | 871 | 0.086 (0.080–0.090) | 6.9 (6.6–7.1) | 370 (340–390) | 6.7 (6.0–8.0) | 6.9 (6.2–7.9) | 92 (90–94) |
| Inarijärvi 1 | 1090 | 0.180 (0.160–0.190) | 7.2 (7.2–7.3) | 170 (160–170) | 5.2 (4.0–7.0) | 3.5 (2.9–4.2) | 96 (92–110) |
| Inarijärvi 2 | 1090 | 0.170 (0.150–0.180) | 7.1 (7.0–7.3) | 160 (140–190) | 4.0 (3.0–5.0) | 3.0 (2.4–3.7) | 93 (90–95) |
| Päijänne 1 | 1110 | 0.200 (0.200–0.210) | 7.1 (7.0–7.1) | 470 (370–510) | 8.3 (7.0–10) | 6.9 (6.2–8.0) | 90 (87–97) |
| Päijänne 2 | 1110 | 0.200 (0.190–0.220) | 7.2 (7.1–7.2) | 470 (450–490) | 6.4 (6.0–7.0) | 6.0 (5.7–6.3) | 91 (90–93) |
| Saimaa 1 | 4400 | 0.310 (0.310–0.310) | 7.3 (7.3–7.4) | 410 (400–410) | 14 (12–15) | 8.2 (8.2–8.2) | 90 (89–91) |
| Saimaa 2 | 4400 | 0.160 (0.150–0.170) | 6.8 (6.6–7.0) | 430 (390–500) | 6.6 (4.0–10) | 5.9 (5.7–6.1) | 87 (80–93) |
| Saimaa 3 | 4400 | 0.160 (0.150–0.170) | 7.0 (6.8–7.1) | 390 (370–430) | 5.5 (5.0–6.0) | 5.9 (5.6–6.0) | 89 (85–92) |
| Saimaa 4 | 4400 | 0.160 (0.150–0.1709 | 7.0 (6.8–7.1) | 390 (330–490) | 6.3 (6.0–8.0) | 5.5 (5.0–5.8) | 90 (88–92) |
| Saimaa 5 | 4400 | 0.160 (0.160–0.160) | 7.1 (7.1–7.2) | 220 (200–260) | 7.0 (6.0–9.0) | 2.5 (2.5–2.5) | 94 (90–92) |
| Saimaa 6 | 4400 | 0.100 (0.090–0.110) | 6.9 (6.7–7.0) | 380 (350–410) | 9.0 (8.0–10) | 7.9 (6.4–9.0) | 91 (90–94) |
| Saimaa 7 | 4400 | 0.120 (0.110–0.130) | 6.9 (6.7–7.2) | 360 (340–370) | 7.5 (7.0–8.0) | 7.0 (6.3–8.0) | 92 (91–94) |
| Saimaa 8 | 4400 | 0.150 (0.140–0.160) | 6.9 (6.7–7.0) | 380 (360–410) | 10 (7.0–22) | 6.3 (5.9–7.3) | 92 (92–92) |
| Saimaa 9 | 4400 | 0.180 (0.170–0.190) | 6.9 (6.6–7.0) | 340 (320–370) | 4.2 (4.0–5.0) | 4.9 (4.5–6.0) | 88 (81–91) |
| Lake area (km2) | CA/LA | Forest (%) | Peatland (%) | Agricultural Land (%) | Water (%) | Built-up (%) | |
|---|---|---|---|---|---|---|---|
| Kolimajärvi | 101 | 15 | 65 | 17 | 4.1 | 14 | 0.21 |
| Unnukka | 103 | 130 | 62 | 16 | 8.4 | 13 | 0.62 |
| Pielavesi | 111 | 10 | 66 | 15 | 3.9 | 15 | 0.27 |
| Vesijärvi | 111 | 4.6 | 59 | 1.9 | 17 | 20 | 3.12 |
| Keurusselkä | 118 | 14 | 75 | 11 | 3.1 | 11 | 0.54 |
| Onkivesi | 120 | 47 | 63 | 19 | 11 | 6.6 | 0.43 |
| Pyhäjärvi Pirkkala | 124 | 140 | 69 | 7.2 | 10 | 13 | 1.16 |
| Pyhäjärvi Pyhäselkä | 126 | 5.4 | 54 | 20 | 4.8 | 20 | 0.52 |
| Kyyvesi | 133 | 11 | 63 | 14 | 6.0 | 16 | 0.33 |
| Lappajärvi | 142 | 11 | 52 | 22 | 15 | 11 | 0.64 |
| Viinijärvi 1 | 148 | 5.3 | 57 | 13 | 11 | 19 | 0.59 |
| Viinijärvi 2 | 148 | 5.3 | 57 | 13 | 11 | 19 | 0.59 |
| Kivijärvi | 153 | 11 | 68 | 18 | 2.3 | 12 | 0.18 |
| Kiantajärvi | 153 | 22 | 61 | 27 | 0.8 | 11 | 0.09 |
| Pyhäjärvi Eura | 154 | 4.0 | 52 | 6.8 | 15 | 25 | 0.94 |
| Juurusvesi | 159 | 34 | 62 | 19 | 6.4 | 12 | 0.44 |
| Melavesi | 159 | nd | nd | nd | nd | nd | nd |
| Koitere | 164 | 10 | 53 | 28 | 0.63 | 14 | 0.04 |
| Nilakka | 168 | 13 | 64 | 15 | 3.8 | 17 | 0.26 |
| Vanajavesi | 179 | 15 | 63 | 7.5 | 19 | 8.5 | 1.56 |
| Längelmävesi | 180 | 12 | 70 | 4.5 | 9.6 | 15 | 0.71 |
| Konnevesi | 187 | 31 | 65 | 11 | 4.4 | 20 | 0.43 |
| Juojärvi | 228 | 9.1 | 63 | 12 | 3.8 | 22 | 0.31 |
| Yli-Kitka | 240 | 5.9 | 53 | 24 | 1.2 | 22 | 0.25 |
| Pyhäjärvi Kitee | 248 | 3.2 | 51 | 11 | 8.8 | 29 | 0.30 |
| Näsijärvi | 265 | 29 | 72 | 9.1 | 5.4 | 13 | 0.64 |
| Suvasvesi | 276 | 51 | 62 | 16 | 8.3 | 13 | 0.57 |
| Höytiäinen | 291 | 5.0 | 51 | 21 | 7.5 | 21 | 0.28 |
| Kemijärvi | 316 | 82 | 67 | 28 | 0.52 | 4.7 | 0.06 |
| Koirus | 316 | nd | nd | nd | nd | nd | nd |
| Puulavesi | 325 | 10 | 64 | 10 | 5.2 | 20 | 0.37 |
| Keitele | 502 | 12 | 68 | 12 | 3.2 | 17 | 0.31 |
| Kallavesi 1 | 517 | 25 | 62 | 17 | 8.5 | 12 | 0.60 |
| Kallavesi 2 | 517 | 25 | 62 | 17 | 8.5 | 12 | 0.60 |
| Oulujärvi 1 | 865 | 23 | 58 | 27 | 2.0 | 13 | 0.21 |
| Oulujärvi 2 | 865 | 23 | 58 | 27 | 2.0 | 13 | 0.21 |
| Oulujärvi 3 | 865 | 23 | 58 | 27 | 2.0 | 13 | 0.21 |
| Pielinen | 871 | 9.0 | 61 | 17 | 4.1 | 17 | 0.26 |
| Inarijärvi 1 | 1090 | 13 | 69 | 17 | 0.10 | 13 | 0.04 |
| Inarijärvi 2 | 1090 | 13 | 69 | 17 | 0.10 | 13 | 0.04 |
| Päijänne 1 | 1110 | 24 | 67 | 9.2 | 5.3 | 18 | 0.61 |
| Päijänne 2 | 1110 | 24 | 67 | 9.2 | 5.3 | 18 | 0.61 |
| Saimaa 1 | 4400 | 34 | 59 | 13 | 6.2 | 20 | 0.53 |
| Saimaa 2 | 4400 | 34 | 59 | 13 | 6.2 | 20 | 0.53 |
| Saimaa 3 | 4400 | 34 | 59 | 13 | 6.2 | 20 | 0.53 |
| Saimaa 4 | 4400 | 34 | 59 | 13 | 6.2 | 20 | 0.53 |
| Saimaa 5 | 4400 | 3.1 | 43 | 7.4 | 5.4 | 43 | 0.51 |
| Saimaa 6 | 4400 | 43 | 57 | 19 | 4.6 | 17 | 0.38 |
| Saimaa 7 | 4400 | 35 | 56 | 17 | 5.5 | 19 | 0.39 |
| Saimaa 8 | 4400 | 67 | 59 | 15 | 6.4 | 18 | 0.48 |
| Saimaa 9 | 4400 | 34 | 59 | 13 | 6.2 | 20 | 0.53 |
- nd, not determined, satellite data from the area not available.
All of the lakes over 100 km2 in Finland belong to a water quality monitoring program carried out since 1965 by Regional Environment Centers of Finland. This monitoring program includes also 46 lakes under 100 km2. These 46 lakes have been used in this study as validation data for the regression equations, because the sampling has been carried out in these lakes in similar frequency using the same methods as in the large lakes (>100 km2). The size range of small lakes is 5.9–96 km2. TOC concentrations in both size groups are close to each other, for example in autumn 1998 the average TOC was 7.7 mg L−1 in small lakes (<100 km2) and 7.3 mg L−1 in large (>100 km2) lakes.
Gas concentrations in the water column
Total inorganic carbon (TIC) was measured in 1998 and 1999. Samples were taken into air tight glass bottles approximately between 10:00 and 14:00 hours and samples were placed in coolers while in transit to the laboratories. TIC was measured in the laboratory using infrared spectroscopy. CO2 concentrations were calculated from measurements of TIC and pH with correction for actual water temperature (Stumm & Morgan, 1970; Butler, 1982; Kling et al., 1992). pCO2 was calculated by use of appropriate Henry's law constant, corrected for temperature and atmospheric pressure (Plummer & Busenberg, 1982). The equilibrium pCO2 values in the lake water were calculated with Henry's law, assuming the atmospheric mixing ratio of 361 ppmv for the year 1996 and taking into account the annual increase of 1.5 ppmv a−1 (IPCC, 1996) and the elevation of the lake. Supersaturation rate (ssCO2) was calculated by dividing the lake pCO2 by the atmospheric pCO2.
Gas fluxes
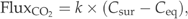

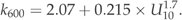

The annual emission of CO2 was calculated using the winter 1 m value for a 2-week period assuming that the excessive CO2 accumulated under ice would be released to the atmosphere during the spring circulation. The summer value was used for a 4.5 months period representing the summer stratification between May and September. The sample taken during the autumn circulation was used for the period of 2 months representing the time after break up of the summer stratification and before ice cover. All of the lakes were not sampled every year. When comparing the annual emission of CO2 between years, the comparison was only made between lakes that were sampled every study year.
Water chemistry
The lakes were sampled in each sampling occasion for oxygen (O2), alkalinity, conductivity, pH, water color, total nitrogen (Ntot), nitrate nitrogen (NO3-N), ammonium nitrogen (NH4-N), total phosphorus (Ptot), phosphate phosphorus (PO4-P), TOC and total iron (Fe). The water chemistry was analyzed from unfiltered samples in the laboratories of the Regional Environment Centers. O2 was determined by taking samples into airtight bottles and adding H3PO4 to the samples in the field and titrating the acidified samples in the laboratory with a Winkler method. Alkalinity was measured by titration with interpoint at pH 4.5 and endpoint at pH 4.2. Conductivity was measured by conductometric determination with temperature compensating cell. Samples for the pH measurements were collected into airtight bottles and transferred to the laboratories in coolers. The pH measurements were carried out electrometrically at 25°C with a pH meter. Water color (milligrams platinum per liter) was determined by comparison with standard platinum cobalt chloride disks. Ntot was determined by oxidation with K2S2O8, reduction of NO3-N to NO2-N in Hg–Cd (Cu–Cd) column and colorimetric determination of azo-color. The sum of NO3-N and NO2-N was measured from the samples by reduction of NO3-N to NO2-N in Hg–Cd (Cu–Cd) column and colorimetric determination of azo-color. NH4 was measured by spectrophotometric determination with hypochlorite and phenol. Ptot and PO4-P were measured with either an autoanalyzer or spectrophotometer. TOC was determined by oxidizing the sample by combustion and measuring total inorganic carbon by IR spectrophotometry. Fe was measured by spectrophotometric determination with TPTZ reagent. The water analysis methods are described in National Board of Waters (1981).
Statistical analysis
In the correlation analysis (Pearson's correlation coefficient r), CO2 concentrations and CO2 supersaturation were connected to water and catchment characteristics. The P-values of the correlations were Bonferroni corrected. For correlation and regression analyses the variables were logarithm or square root transformed if it was necessary to improve the normality of the distribution. The TIC concentrations for the years 1996–1997 and 2000–2001 with missing TIC measurements were modeled by stepwise multiple regression models using alkalinity as an independent variable. The regression models were then applied to the same 37 lakes (altogether 51 sampling points). All statistical analyses were done by SAS 8.2 for Windows software.
The climate in Finland
The mean annual temperature ranges from +5°C in southern Finland to −2°C in northernmost Finland. The long-term average (1961–1990) annual precipitation for the whole country is 660 mm (Table 3), being greater in southern Finland and diminishing towards the north. The permanent snow cover period starts, on average, at the end of October in northern Finland and lasts for 7 months. In southernmost Finland, the snow cover period is considerably shorter starting at the end of December and lasting for 3 months. Large lakes typically freeze in southern and central Finland at the beginning of December, and the ice cover period lasts until the beginning of May. The mean monthly and annual precipitation and temperature for the study years were calculated as an average of the measurements in the 44 weather stations spread evenly throughout the country (Finnish Meteorological Institute 1996–2001).
| 1961–1990 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | |
|---|---|---|---|---|---|---|---|
| Mean temperature (°C) | 2.6 | 1.8 | 1.6 | 2.6 | 2.3 | 3.6 | 3.2 |
| Annual precipitation (mm) | 660 | 566 | 525 | 686 | 574 | 637 | 602 |
| Open water season precipitation (mm) | nd | 430 | 331 | 482 | 346 | 424 | 421 |
| Mean length of ice cover (d) | 165 | 186 | 144 | 165 | 165 | 144 | 130 |
- nd, Data not available.
Results
Concentration of CO2 in the water column and the CO2 emission to the atmosphere
To compare the CO2 concentrations and potential emissions between different years, regression models were developed based on TIC measurements from the years 1998 and 1999 using alkalinity as dependent variable. The surface water alkalinity and TIC were the same in summer and autumn samples (median TIC 185 μmol L−1 and median alkalinity 184 μmol L−1 in summer, and 182 and 185 μmol L−1 in autumn, respectively). Separate equations were developed for different seasons and water depths.
 (1)
(1)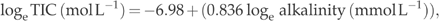 (2)
(2) (3)
(3)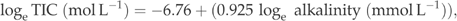 (4)
(4)In the combined data of the years 1996–2001, the highest CO2 concentrations were detected in winter near sediment. There was a strong vertical stratification in many lakes with respect to CO2 during winter and summer, the stratification was lost during circulation. The estimated median CO2 flux at spring thaw (14 days) in different years ranged between 0.218 and 0.266 mol m−2 and the summer flux (135 days) between 0.945 and 1.620 mol m−2. Also the water quality parameters of these lakes varied between the years (Table 4). The annual median flux of CO2 from all 37 lakes larger than 100 km2 in the years 1996–2001 ranged from 1.49 mol m−2 a−1 in 1997 to 2.29 mol m−2 a−1 in 1998 (Table 5). Lake Kemijärvi, situated downstream of large hydroelectric reservoirs Lokka and Porttipahta, has been excluded from the flux estimates because of disturbance of the reservoirs.
| 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | |
|---|---|---|---|---|---|---|
| Winter, March | ||||||
| Alkalinity (mmol L−1) | 0.167 | 0.165 | 0.166 | 0.176 | 0.166 | 0.172 |
| pH | 6.8 | 6.8 | 6.8 | 6.8 | 6.8 | 6.7 |
| O2 (%) | 88 | 87 | 92 | 89 | 87 | 89 |
| Ptot (μg L−1) | 11 | 13 | 9.8 | 12 | 12 | 12 |
| PO4-P (μg L−1) | 3.8 | 3.4 | 3.3 | 4.6 | 4.1 | 3.2 |
| Ntot (μg L−1) | 459 | 487 | 445 | 474 | 217 | 482 |
| TOC (mg L−1) | 6.6 | 7.0 | 6.5 | 8.0 | 7.1 | 7.0 |
| Summer, August | ||||||
| Alkalinity (mmol L−1) | 0.166 | 0.162 | 0.190 | 0.170 | 0.176 | 0.185 |
| pH | 7.1 | 7.1 | 7.0 | 7.2 | 7.1 | 7.1 |
| O2 (%) | 97 | 95 | 93 | 92 | 93 | 92 |
| Ptot (μg L−1) | 15 | 12 | 10 | 12 | 12 | 13 |
| PO4–P (μg L−1) | 1.9 | 1.7 | 1.9 | 1.8 | 1.6 | 1.8 |
| Ntot (μg L−1) | 429 | 401 | 351 | 395 | 411 | 450 |
| TOC (mg L−1) | nm | nm | nm | nm | nm | nm |
| Autumn, October | ||||||
| Alkalinity (mmol L−1) | 0.162 | 0.160 | 0.163 | 0.173 | 0.189 | 0.165 |
| pH | 7.1 | 7.1 | 7.1 | 7.0 | 7.0 | 7.0 |
| O2 (%) | 89 | 89 | 89 | 87 | 90 | 87 |
| Ptot (μg L−1) | 15 | 15 | 15 | 12 | 15 | 15 |
| PO4–P (μg L−1) | 3.0 | 3.0 | 3.6 | 3.6 | 2.7 | 3.1 |
| Ntot (μg L−1) | 395 | 435 | 413 | 392 | 430 | 470 |
| TOC (mg L−1) | 6.5 | 6.4 | 7.3 | 7.0 | 7.0 | 7.4 |
- nm, not measured.
| 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | |
|---|---|---|---|---|---|---|
| Winter pCO2 (μatm) | 910 | 880 | 900 | 980 | 880 | 940 |
| Summer pCO2 (μatm) | 630 | 590 | 740 | 630 | 650 | 680 |
| Autumn pCO2 (μatm) | 610 | 540 | 610 | 680 | 760 | 660 |
| Winter flux (mmol m−2 day−1) | 17 | 16 | 16 | 19 | 16 | 17 |
| Summer flux (mmol m−2 day−1) | 8.1 | 7.0 | 12 | 8.1 | 8.6 | 10 |
| Autumn flux (mmol m−2 day−1) | 7.4 | 5.5 | 7.4 | 10 | 12 | 9.0 |
| Annual flux (mol m−2 a−1) | 1.77 | 1.49 | 2.29 | 1.94 | 2.10 | 2.17 |
Median annual CO2 emission followed closely the amount of precipitation. Precipitation measured between January and October in the study year (November and December were excluded, because the last sampling of the year was in October and the precipitation in November and December, being mainly snow in Finland, would not reach the lakes until next year) predicted the annual CO2 emission reasonably well, although the correlation was not significant. However, when the precipitation in the previous autumn (September–December) was added, the compatibility between precipitation and the CO2 emission improved (Fig. 2) except in 1997 with an exceptionally short ice cover period. The precipitation during the open water period (September–October in the previous autumn and June–October in the study year) was capable of explaining the annual CO2 emission from the lakes (r2=0.99, P<0.001) (Fig. 3). Furthermore, mean summer precipitation (June–August) and average surface water pCO2 in August were strongly correlated with each other (Fig. 4). The connection between pCO2 and precipitation was not as pronounced in other seasons. The importance of annual and monthly mean air temperatures on CO2 emission were tested but we did not find significant correlation between the variables.

The combined 14 months precipitation in January–October in the study year and September–December in the previous autumn and the annual emission of CO2 in 1996–2001.

The relationship between combined open water season precipitation in June–October in the study year and September–October in the previous autumn and the annual flux of CO2 in 1996–2001 (y=−0.0000294x2+0.0301x−5.37, r2=0.99, P<0.001).
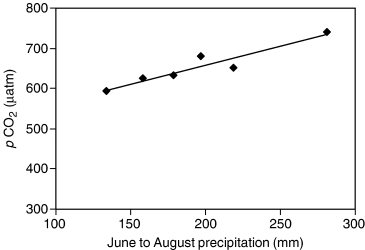
The relationship between average precipitation in June–August and average surface water partial pressure of CO2 (pCO2) in August in 1996–2001 (y=0.940x+470, r2=0.88, P<0.01).
Correlations with water quality
During winter stratification, O2 and Ptot had significant correlations with ssCO2 rates in the data sets for the years 1996–2000. TOC had weak positive correlations with ssCO2 in all studied years, but the correlations were not significant. During summer stratification, O2 was negatively related to ssCO2 in all of the studied years and Ptot had a significant positive relation in 1996 (Table 6). The relationship between CO2 concentration and water quality followed closely the pattern for ssCO2 in all subsets.
| O2% | Ptot | Field % | Built-up % | Latitude | |
|---|---|---|---|---|---|
| Winter 1996 | −0.770** | 0.597** | 0.507* | 0.424 ns | −0.190 ns |
| Winter 1997 | −0.846** | 0.665** | 0.368 ns | 0.237 ns | −0.0139 ns |
| Winter 1998 | −0.905** | 0.779** | 0.429 ns | 0.289 ns | −0.153 ns |
| Winter 1999 | −0.905** | 0.673** | 0.527* | 0.433 ns | −0.228 ns |
| Winter 2000 | −0.830** | 0.757** | 0.609** | 0.456 ns | −0.260 ns |
| Winter 2001 | −0.705** | 0.426 ns | 0.265 ns | 0.185 ns | 0.154 ns |
| Summer 1996 | −0.751** | 0.591** | 0.675** | 0.597** | −0.529* |
| Summer 1997 | −0.825** | 0.186 ns | 0.349 ns | 0.460* | −0.378 ns |
| Summer 1998 | −0.842** | 0.144 ns | 0.355 ns | 0.390 ns | −0.405 ns |
| Summer 1999 | −0.882** | 0.281 ns | 0.396 ns | 0.450 ns | −0.476* |
| Summer 2000 | −0.824** | 0.219 ns | 0.295 ns | 0.375 ns | −0.273 ns |
| Summer 2001 | −0.818** | −0.0769 ns | 0.312 ns | 0.396 ns | −0.485* |
- Bonferroni-corrected significance levels of the correlations:
- ** P<0.001;
- * P<0.05.
- ns, nonsignificant correlation.
In autumn, the connection between O2 saturation and CO2 was not as strong as in winter or summer. In 1996–2001, the correlation coefficients between ssCO2 and O2 ranged from r2=0.042 (not significant) in 1996 to r2=0.593 (P<0.001) in 2000. The correlations between ssCO2 and Ptot, TOC and color were not significant.
Correlations with catchment characteristics, maximum depth and latitude
The proportion of agricultural land in the catchment correlated positively with ssCO2 but the correlations were significant only in winters 1996, 1999 and 2000 and in summer 1996 (Table 5). The proportion of built-up area in the catchment had significant positive correlations with ssCO2 in summers 1996 and 1997 (Table 5). In autumn, the correlations were not significant. Proportion of peatland in the catchment had no significant correlations with ssCO2 in any seasons.
Maximum depth correlated with mid-water column ssCO2 in winter (r2=0.217–0.382; P<0.05) but there were no significant correlations between ssCO2 and maximum depth in autumn or in summer. The latitude correlated negatively with ssCO2 in summer. In winter and in autumn, the correlations were not significant.
Discussion
These large, mainly oligotrophic lakes in Finland were persistently supersaturated with CO2 relative to the atmosphere. Surface water pCO2 was at its highest in winter but values were lower than previously reported corresponding pCO2 values for small (0.04–100 km2) Finnish lakes (Striegl et al., 2001). There was a strong vertical stratification with respect to CO2 with the highest concentrations near sediment in winter and summer. In summer, the surface water of the lakes was close to equilibrium, but mainly slightly supersaturated. Like in winter, CO2 concentrations were high in hypolimnion. High concentrations near the sediment indicate that a great part of the decomposition occurs in the sediment. Jonsson et al. (2001) showed that in summer about 40% of the mineralization in a boreal lake occurred in sediment. This might partly explain the higher surface water pCO2 in small lakes than in large lakes. Large lakes in Finland are considerably deeper than small lakes and the distance between the elevated concentrations of CO2 near the sediment and the surface water is large. This is also reflected in winter as a negative correlation between surface water CO2 supersaturation and maximum depth of the lake. Kelly et al. (2001) showed that the ratio of epilimnetic sediment area to epilimnetic volume (Ae/Ve) was smaller in the large lakes, which likely results in lower rates of recycling of fixed carbon to CO2 during summer stratification and thus lower pCO2. As the climate change scenarios predict rising air temperatures in the northern hemisphere (IPCC, 2001), the epilimnetic volume of the large lakes may increase as the thermocline forms deeper than previously. Furthermore, hypolimnion water temperature in deep lakes is probably a rather conservative variable suggesting that the production of CO2 through decomposition in lake deeps will not be enhanced with the rising air temperature.
Surface water pCO2 in August followed closely the average precipitation in June–August (r2=0.88). Large inputs of allochthonous material and high concentrations of TOC in the small Finnish lakes have been reported previously (Kortelainen, 1993). Furthermore, the DOC leaching has been reported to follow the amount of precipitation (Dillon & Molot, 1997; Schindler et al., 1997; Correll et al., 2001). Pace & Cole (2002) suggested that the common temporal dynamics of DOC and color in 20 temperate lakes with different sizes and water quality were the result of climatic conditions that affected both the loading of allochthonous carbon and the losses because of degradation. A strong negative correlation between ssCO2 and O2 saturation in our study lakes indicates efficient decomposition of organic matter in the lakes. The supply of CO2 from groundwater or from surrounding catchments can be assumed to be minor in these large lakes. A linear connection between precipitation in summer months and late summer pCO2 suggests that if a summer precipitation increases with climate change, the lakes will pump more CO2 into the atmosphere than previously, further enhancing the impacts of climate change.
In autumn, the relationship between surface water pCO2 and precipitation was weak, probably because of efficient autumn turnover causing loss of CO2 to the atmosphere. However, the concentrations of CO2 in lake water were highest in winters 1999 and 2001 following the very rainy summer and autumn, suggesting that the high organic matter load to these large lakes during autumn rain is detectable as elevated CO2 concentrations during the next winter. Precipitation was also high in autumn 1996, but CO2 concentrations were rather low in winter 1997, probably because the lakes froze almost a month later than normally in autumn 1996, enabling the effective exchange of CO2 with the atmosphere.
Estimated annual CO2 emission from the lakes to the atmosphere in years 1996–2001 ranged from 1.49 mol m−2 a−1 in 1997 to 2.29 mol m−2 a−1 in 1998. These annual estimates are based only on three measurements per year. Kelly et al. (2001), however, showed that four measurements per year were adequate to describe the CO2 saturation rate of the lakes. The annual CO2 emissions should, anyhow, be comparable with each other because the method used is the same every year. Most of the lakes in our data set are oligotrophic clear water lakes and CO2 emission is of the same magnitude as the results achieved by Cole & Caraco (1998) for a small oligotrophic lake and by Riera et al. (1999) for a clear water lake.
The annual CO2 emission from the lakes followed closely the amount of precipitation. The precipitation measured between January and October predicted the annual CO2 emission reasonably well although the correlation was not significant. However, when the precipitation in the previous autumn was added, the covariability between precipitation and the CO2 emission increased (Fig. 2). The year 1997 made an exception to this pattern, even though precipitation was high in autumn 1996, winter pCO2 was quite low in 1997 due to shorter ice cover period. On the contrary, in 2000 the covariation between precipitation and the CO2 emission was high in spite of a short ice cover period, because the high precipitation periods were concentrated in summer and autumn, not in the previous autumn as in 1997.
Kelly et al. (2001) also found year-to-year variability in pCO2 and linked it to the weather patterns, especially precipitation. Nevertheless, they did not find a significant correlation between average annual rainfall and average open water pCO2. According to our results, the previous autumn precipitation is crucial in determining pCO2 level in the next winter, because of the delay between heavy rainfall and increasing CO2 concentrations. Jonsson & Jansson (1997) observed that in a humic lake in Sweden the gross sedimentation of total particulate matter and organic carbon were highest in the high flow periods. Particulate organic matter supplied by high discharge events rapidly settled out of the water column and formed the major substrate for sediment respiration for the next season. Results from our lakes suggest that organic matter supplied by high discharge in autumn contributes to the CO2 emission during circulation next spring; the highest winter pCO2 values were found after the rainy autumns. There was also a strong negative correlation between O2 saturation and CO2 saturation during winter stratification suggesting efficient decomposition of organic matter in lakes.
Open water season precipitation (June–October in the study year and September–October in the previous year) was adequate to explain the variation of the annual CO2 emission (Fig. 3), although half of the annual run-off and TOC leaching from forested catchments has been estimated to occur during spring flooding (Kortelainen et al., 1997). The relationship was best modeled by the second-order curve (Fig. 3), suggesting that with the higher precipitation the dilution effect diminishes the response of CO2 emission. The importance of the open water period precipitation to the CO2 emissions may, however, be specific to this data set because there were no major differences in January–May precipitation between the study years. The differences in precipitation were more pronounced in summer and autumn. Furthermore, high precipitation in winter as snow does not automatically mean high spring runoff. Instead, the intensity of spring floods depends on weather conditions in spring. For example, the length of the snowmelt period and the evaporation rate during this period affect the intensity of spring floods. There also may be variation in the quality of organic matter and its availability to degradation in different seasons. According to our results, open water season precipitation is more important considering the lake water CO2 content than precipitation during snow cover period. Consequently, our results suggest that the climate change alteration of winter precipitation, may have only a small effect on lake CO2. If the climate change simultaneously increases winter temperatures then the snow cover period may be shorter, and winter precipitation may result in increasing CO2 emissions.
The close connection between annual CO2 emission and precipitation implies that the organic carbon produced in the terrestrial ecosystem is transported to the lakes and decomposed. Sobek et al. (2003) found a strong correlation in open water seasons between TOC and pCO2 in small, unproductive Swedish lakes. They suggested that landscape characteristics determine accumulation and subsequent supply of allochthonous organic matter from boreal catchments to lakes. Sinsabaugh & Findlay (2003) suggested that the metabolism of small upstream systems is dominated by allochthonous dissolved organic matter (DOM) that originates from terrestrial systems. With high inputs and short hydraulic residence times, DOM dynamics often exceed microbial community response times and all but the most reactive material passes downstream unaltered. A strong correlation between average precipitation over several months and annual CO2 emission or surface water pCO2 suggests that there is a delay between the TOC supply and elevated CO2 concentrations in the surface water.
We did not find significant correlation between TOC and ssCO2 in winter, probably because of a long stagnant period with a negligible supply of fresh organic matter and exchange of CO2 with the atmosphere. No correlation between TOC and pCO2 was found either in autumn. Unfortunately, we did not have enough summertime TOC measurements to be able to calculate the correlations, but the nonsignificant correlations between ssCO2 and color in summer suggest that the relationship between ssCO2 and TOC would probably have been weak (in other seasons the correlation between color and TOC ranged between r2=0.58 and 0.74). In small lakes, shorter turnover times presumably result in better connection between CO2 and TOC concentrations.
The correlation between annual and monthly mean air temperatures and CO2 emission was not significant. Hypolimnion water temperature in deep lakes is, however, probably a rather conservative variable and not markedly affected by changes in mean annual air temperatures similarly, Sobek et al., (2004) found no correlation between in-situ water temperature and pCO2 in a global-scale database consisting of 4902 lakes. There was a negative correlation between ssCO2 and latitude in summer samples. The growing season is significantly shorter in northern Finland than in southern Finland (the mean effective temperature sum 1300°C at the southern coast vs. 200°C in the northernmost Finland) and thus both primary production and decomposition can be expected to be less efficient in northern Finland in terrestrial and aquatic ecosystems. Therefore, it can be assumed that a raise in annual mean temperature might have an indirect effect on lake ecosystems through changes in their catchments. The Finnish lake surveys carried out in 1987 (Kortelainen, 1993) and 1995 (Rantakari et al., 2004) have shown a decreasing TOC concentrations towards the north. Also, in the present data set, the negative correlation between TOC and latitude could be detected in some seasons and in some years.
The positive correlation between ssCO2 and Ptot and between ssCO2 and agricultural land or habitation percentage in the catchment imply a positive connection between eutrophication and CO2 production. Prairie et al. (2002) found that degree of O2 undersaturation increased with lake trophic status suggesting that net heterotrophy can also be found in eutrophic lakes. Undersaturation of O2 and supersaturation of CO2 suggest that the lakes are net heterotrophic (Prairie et al., 2002). The decomposition of organic matter may furthermore be more efficient in eutrophic lakes because of availability of other nutrients. On the other hand, it has been reported that notable amounts of organic matter and DOC can be transported from agricultural lands (Correll et al., 2001; McTiernan et al., 2001), contributing to CO2 content of downstream lakes. In our data set, the strongest correlation with Ptot was found in winter. Decomposition of organic matter releases nutrients. In winter, immobilization of nutrients is minor because of low primary production resulting in elevated concentrations. With our sampling arrangements, we cannot rule out the possibility that the close connection between summer surface water pCO2 and precipitation partly originates from enhanced primary production. Increasing rainfall also increases the transport of nutrients from terrestrial ecosystems and additionally enhances primary production, thus transporting easily degradable matter to lakes.
Our results from the largest Finnish lakes give an interesting perspective into the possible impacts of climate change on the carbon balance of lakes. Recent climate change scenarios predict that annual precipitation will increase by 8% over 40 years and by 12% over 70 years in Finland (Carter et al., 2002). Increasing precipitation results in increasing runoff and leaching of organic carbon (Dillon & Molot, 1997; Schindler et al., 1997). Organic carbon export simulations based on climate change scenarios and neural networks indicate increasing DOC fluxes in Canadian rivers (Clair et al., 1999) and in Finnish headwater streams (Holmberg, 2003). Lakes have been shown to act as pumps of carbon from the terrestrial ecosystem to the atmosphere (e.g. Cole et al., 1994). Climate change may enhance this phenomenon in the boreal landscape because CO2 emissions from large lakes appear to closely follow the annual precipitation pattern.
Acknowledgements
We thank the personnel of the Regional Environmental Centers of Finland for sampling and analyzing the water chemistry, and Jari Huttunen, Rob Striegl and Jon Cole for their kind help with CO2 flux calculations, and S. Siitonen for babysitting. Financial support was provided by the Academy of Finland.




